Abstract
The interactions between corneal nerve, epithelium, and stroma are essential for maintaining a healthy cornea. Thus, corneal tissue models that more fully mimic the anatomy, mechanical properties and cellular components of corneal tissue would provide useful systems to study cellular interactions, corneal diseases and provide options for improved drug screening. Here a corneal tissue model was constructed to include the stroma, epithelium, and innervation. Thin silk protein film stacks served as the scaffolding to support the corneal epithelial and stromal layers, while a surrounding silk porous sponge supported neuronal growth. The neurons innervated the stromal and epithelial layers and improved function and viability of the tissues. An air-liquid interface environment of the corneal tissue was also mimicked in vitro, resulting in a positive impact on epithelial maturity. The inclusion of three cell types in co-culture at an air-liquid interface provides an important advance for the field of in vitro corneal tissue engineering, to permit improvements in the study of innervation and corneal tissue development, corneal disease, and tissue responses to environmental factors.
Keywords: silk, cornea, innervation, tissue model, stroma
II. Introduction
The cornea is the outermost layer of the human eye and is an important part of the ocular light path. The cornea has three distinct layers: the epithelium, stroma, and endothelium. The external epithelial layer has 3–5 layers of epithelial cells and protects the inner structures [1, 2]. The middle stromal layers are composed of aligned corneal stromal cells guided by parallel collagen lamellae [2]. The innermost layer of the cornea is the endothelial layer. Current tissue models fail to incorporate each of these distinct anatomical layers and are not able to realize disease conditions of corneal tissue. Corneal opacity is one of the principal causes of bilateral blindness, affecting 7 million people around the world [3]. Among them, 2.85 million people have diminished or an absence of sensation due to corneal nerve dysfunction or degeneration [4]. Every year 46,000 patients in the USA receive corneal transplantation surgery.
The cornea is the most densely innervated surface in the human body [5]. Neuronal innervation is closely related to the health or disease state of the corneal epithelium and stroma. Innervation is distributed throughout the epithelium and stroma but is absent in the endothelial layer [6]. Stromal nerve trunks with a density of 33–71/mm2 [7] arise from the limbal plexus and enter the peripheral corneal stroma radially. In the stroma, nerves are organized parallel to the collagen lamellae and branch into smaller fascicles as they proceed toward the superficial stroma [8]. The nerve fibers then penetrate the epithelial layer with a density of approximately 600 terminals/mm2 [6, 8]. The nerves interact physically and chemically with corneal tissue, providing sensing and releasing trophic factors including neurotransmitters and neuropeptides to maintain homeostasis [6]. During corneal development, nerve growth is modulated by many growth factors, including brain derived nerve growth factor (BDNF), nerve growth factor (NGF), glial cell derived neurotrophic factor (GDNF), and neurotrophic (NT-3) [9–12]. Among these growth factors, NGF is critical for corneal nerve survival, axonal branching, elongation, sprouting and regeneration [10]. The lack of these trophic factors can lead to neurotrophic keratopathy, a disorder that make cornea more susceptible to injury [13].
Despite the importance of corneal innervation, the interactions between nerve and corneal tissue in healthy and diseased cornea are not fully understood [5]. This is partially due to the limitations with rabbit, mice, pig and human in vivo models, including the complexity of the in vivo environments, differences between human and animal corneal tissues, and the challenges with studying human embryo cornea. These drawbacks underscore the need for new research tools.
In vitro corneal tissue models have unique advantages for studying cellular interactions including the ability to simplify the complex in vivo environment, utilize human cells, be cost effective when compared with animal and human studies, and be designed for high throughput analysis. An in vitro tissue model of human corneal innervation can also support studies of corneal nerve functions. Current corneal tissue in vitro models mainly focus on corneal epithelial and stromal cells and use collagen as substrates [14, 15]. Among the few co-culture studies that used corneal cells and neurons [15, 16], layers of collagen hydrogel were used to resemble the lamellar structure of cornea but failed to recapitulate the alignment of the stromal cells and the multi-layer features of the epithelial cells. Further, the native density of nerve endings and branches has also not been achieved through in vitro cultures. Collagen as the substrate also poses significant limitations due to low mechanical stiffness, leading to mismatched mechanical properties and contraction in long-term culture [17, 18].
Silk is a biodegradable protein material with highly tunable mechanical properties that can be cast into optically clear films [18, 19]. Silk films, physically crosslinked through water vapor annealing, provided elastic moduli of 67.7 kPa [20] that matched the stiffness of the cornea, which is 40–60 kPa [21, 22]. Silk films with surface patterns and functionalized with RGD supported the alignment, growth, and matrix synthesis by human corneal stromal cells [23]. These films also did not contract and slowly degraded in vitro, providing support for sustained in vitro tissue models. The silk can also be formed into sponges which can support neuron growth and the formation of neuronal connections [24–26]. In our previous study [16], 2D human corneal stromal stem cells (hCSSCs) and dorsal root ganglion neurons (DRG) in a co-culture system was generated and we observed that axon length increased when the DRGs were co-cultured with the hCSSCs.
The goal of the present study was to generate a 3D silk protein based co-culture system including the corneal stromal layer, epithelial layer, and DRG neurons, to further understand the interactions between corneal innervation and corneal tissues. The scaffold design was to closely mimic corneal anatomy, with silk film stacks for corneal epithelial and stromal cell growth surrounded by a silk sponge seeded with the DRG simulating the limbus tissue. The guidance for neuronal extensions was generated by the addition of NGF in the epithelial layer scaffold. An air-liquid interface was also designed for a bioreactor support system to house the corneal tissues and to better mimic the native corneal environment. This new corneal tissue construct supported dense innervation in the epithelial and stromal regions as well as sustained cultivation in vitro, critical outcomes for the system utility toward the further study of corneal development, function and dysfunction.
III. Materials and methods
3.1 Preparation of silk solution
Silk solution was prepared from cocoons of Bombyx mori silkworm based on the procedure developed in our previous studies [20, 27]. Silk cocoons were purchased from Tajima Shoji Co. (Yokohama, Japan) and boiled for 30 min in 0.02 M Na2CO3 solution (Sigma-Aldrich. St Louis, MO). The boiled silk was rinsed with deionized water 6 times and dried overnight. The extracted silk was then dissolved in a 9.3 M LiBr solution and dialyzed against distilled water for 2 days to obtain a silk aqueous solution (5–7% w/v).
3.2 Preparation of growth factor stamped flat silk films
Flat, optically clear, porous silk films were prepared by casting 120 µL of 1% w/v silk solution with 0.05% w/v of polyethylene oxide (PEO, MW = 900,000, Sigma–Aldrich) on a 12 mm diameter glass coverslip (Electron Microscopy Science, Hatfield, PA) [28]. The films were then dried overnight. High and low concentration NGF inks were used for stamping the silk films. The inks were composed of 50 µl (4 mg/mL) acetic acid-type I collagen solution (rat-tail tendon, BD, Franklin Lake, NJ) containing 100 ng/ml keratinocyte growth factor (KGF) (Sigma), 100 ng/ml hepatic growth factor (HGF) (Sigma), 200 ng /ml epithelium growth factor (EGF) (Thermo Fisher, Waltham MA), and either a high concentration of NGF (400 ng/ml) or a low concentration of NGF (200 ng/ml) (R&D Systems, Minneapolis, MN). Multi-circular, radial and uniform stamp patterns were employed (Supplementary Figure 1). The multi-circular stamps were formed by dipping a 12 mm outside diameter and a 6 mm inside diameter donut shape polydimethylsiloxane (PDMS) (Fisher Scientific Co. Fair Lawn, NJ) stamp in the low NGF ink and pressing onto the dried silk film. The center was stamped with a 6 mm PDMS cylinder carrying the high concentration NGF ink. The radial pattern was stamped with the high NGF ink with its shape indicated in Supplementary Figure 1. The whole surface of the uniformly stamped silk film was covered with high NGF ink. The silk films were annealed in water filled desiccators at −25 mmHg for 2.5 h for physical cross-linking. Before use, the silk films were exposed to UV light for 30 min on each side and the soaked in DI water for 48 h to extract any residual PEO to form the pores.
3.3 Preparation of patterned silk films
Patterned silk films were also prepared based on the procedures developed in our previous studies [29, 30]. Briefly, 1% w/v silk solution with 0.05% w/v of PEO (MW = 900,000; Sigma–Aldrich) was cast onto patterned PDMS molds with 600 lines/mm grating. The PDMS molds were prepared using our previously reported methods [30]. The silk films were dried at room temperature overnight and water annealed with the same methods used with the stamped silk films. The patterned silk films were then peeled off from the molds using established methods [30]. The silk films were immersed in DI water for 2 days to extract the PEO to generate the pores (0.5–5µm) [28], and then immersed in 70% ethanol for 8 min and washed with PBS (5×) before cell seeding.
3.4 Preparation of silk sponges
Salt leached silk scaffolds with 500–600 µm pores were prepared using our previously reported procedure [31]. The scaffolds were mounted in a custom designed well fabricated to be 1 mm depth depressed into a Delrin sheet (McMaster-Carr, Robbinsville, NJ). The scaffold was sliced into 1 mm thick layers using microtome blade and cut into donut shapes (15 mm outer diameter, 12 mm inner diameter) with a biopsy punch (McMaster-Carr, Robbinsville, NJ). The silk sponge donuts were sterilized by autoclave before cell seeding.
3.5 RGD and PDL surface modification
Arginine-Glycine-Aspartic acid-Serine (RGD) peptide (Bachem, Torrance, CA) functionalized patterned silk films were prepared using methods from our previous work [29]. Stamped flat silk films and salt leached silk scaffolds were soaked in 1 ml of 10µg/ml poly-D-lysine (PDL) solution overnight at 4°C.
3.6 Preparation of collagen hydrogels
Collagen gels were prepared by adding 100 µl of 10× DMEM (Sigma) to 900 µl (4 mg/mL) acetic acid-type I collagen solution (rat-tail tendon, Corning, Corning NY), followed by neutralization with 20 µl 1M NaOH (Sigma).
3.7 Human corneal stromal stem cell (hCSSCs) culture
Human corneal stromal stem cells (HCSSCs) were isolated from collagenase digests of limbal stromal tissue dissected from de-identified human corneas, obtained from the Center for Organ Recovery and Education, (CORE; Pittsburgh, PA), as described previously [32]. Ethical aspects of the research protocols were approved by the Committee for Oversight of Research Involving the Dead (CORID) Protocol #161. HCSSCs were passaged 4 times before seeding. Cells were detached with 0.25% trypsin (GIBCO) solution and seeded on the surface of the sterilized patterned porous silk films at a concentration of 15,000 cells/cm2. Cell seeding was accomplished by adding the cell suspension dropwise on top of the films. The films were incubated for 30 min to allow time for cell attachment. Seeded silk films were cultured in proliferation medium containing DMEM/MCDB-201 in the ratio of 3 to 2(v/v) with 2% fetal bovine serum, 10 ng/mL platelet-derived growth factor, 1 mg/mL lipid-rich bovine serum albumin (Albumax, Life Technologies, Grand Island, NY), 10 ng/mL epidermal growth factor, 5 mg/mL transferrin, 5 ng/mL selenous acid, 0.1 mM ascorbic acid-2-phosphate, 10−8 M dexamethasone, 100 IU/mL penicillin, 100 µg/mL streptomycin, 50 µg/mL gentamicin, and 100 ng/mL cholera toxin until confluent (2 days). After cells reached confluency, hCSSCs were differentiated on the silk films into keratocytes with differentiation medium composed of advanced DMEM (Life Technologies), containing 1.0 mM L-ascorbic acid-2-phosphate (Sigma), 50 µg/mL gentamicin (Life Technologies), 2 mM L-alanyl-L-glutamine (Life Technologies), 100 µg/mL penicillin,100 µg/mL streptomycin (Mediatech, Manassas, VA), 0.1 ng/mL transforming growth factor-beta3 (TGFβ-3, Sigma), and 10 ng/mL basic fibroblast growth factor (FGF-2, Sigma)[33].
3.8 Human corneal epithelial cell (hCECs) culture
Primary hCECs (C0185C, Thermo Fisher) were passaged 5 times before seeding. Cells were detached with 0.25% trypsin (GIBCO) and seeded on top of sterilized stamped silk films at a density of 150,000 cells/cm2. The films were then incubated for 4 hours to allow time for cell attachment and then cultured in keratinocyte SFM medium (Thermo Fisher) for 2 days to reach confluency.
3.9 Chicken dorsal root ganglion (DRG) cell culture
DRG explants were dissected from day 8 chicken embryos following protocols developed in our prior study [34]. The explants were then carefully placed on the surface of the salt leached silk scaffolds with forceps and incubated for 2 h to allow time for cell attachment. The scaffolds were then flipped over and cultured in DMEM containing 20% FBS and 50 ng/ml NGF.
3.10 Co-culture of hCSSCs hCECs and DRG neurons
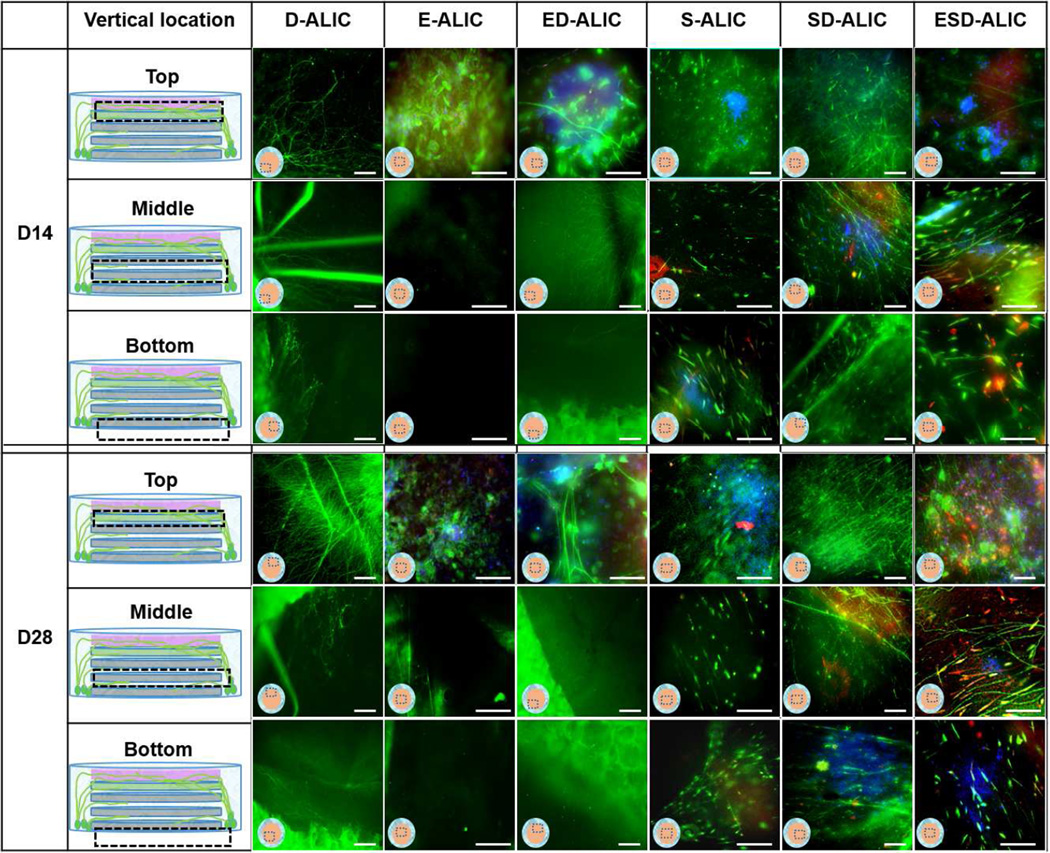
The scaffolds for co-culture were designed to mimic corneal anatomy (Figure 1). For convenience, E, S and D will be used to represent hCECs, hCSSCs and DRG neurons, respectively. To prepare the co-culture scaffolds, 3 layers of patterned silk films seeded with S were stacked with their patterns in a crisscross pattern. Then, the silk film stacks were cut to 12 mm diameter with a biopsy punch (McMaster-Carr) and transferred to the center of the silk sponge donuts. The flat silk films seeded with E were then transferred with forceps and gently placed on top of the film stacks. To achieve integrity of the scaffold, 500 µl type I rat tail collagen was casted on top and absorbed into the scaffold. In order to guide axons toward the top of the scaffolds, 50 µl of collagen hydrogel containing 400 ng/ml of NGF was cast on top of the film stack. The scaffolds were then incubated at 37°C for 30 min to complete the crosslinking. After this, the whole scaffold was immersed in hCSSCs differentiation medium and cultivated for 2 days. A customized designed waffle-shaped PDMS floating shelf (5 mm thick, 5 cm diameter, with 16 × 1 mm2 holes) was prepared by casting PDMS on top of Delrin ® molds (McMaster-Carr). This PDMS shelf allowed the top of the scaffold to remain at the air-liquid interface (ALIC) while the bottom was immersed in hCSSCs differentiation medium. The cultivation in liquid (LC) and air-liquid interface (ALIC) lasted 28 days. The co-cultures of E and D (ED-LC and ED-ALIC), S and D (SD-LC and SD-ALIC), and single cultures (E-LC, E-ALIC, S-LC, S-ALIC, D-LC, D-ALIC), were also processed as comparisons for the three cell types in tri-cultures (ESD-LC, ESD-ALIC). The scaffolds for two types of cell co-cultures and single cultures were prepared with the same methods as the ESD tri-cultures but only contained the respective cellular components.
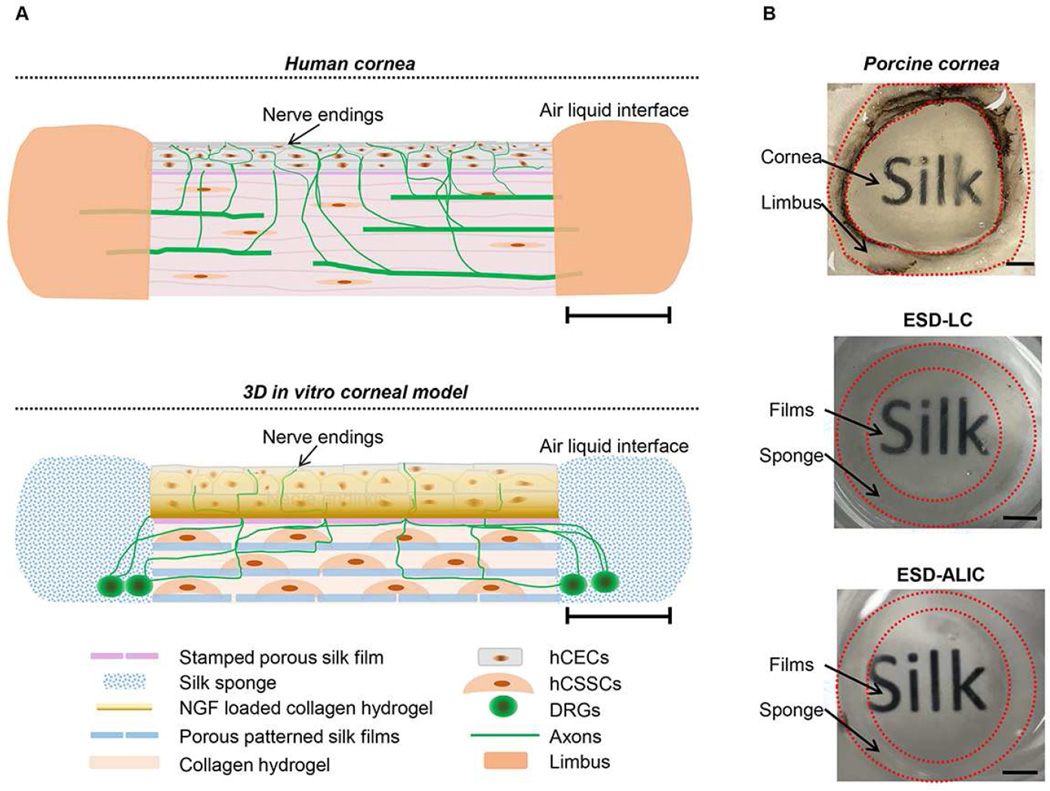
Figure 1.
A) Side view schematics of human cornea and in vitro 3D corneal tissue model. Scale bars=3mm. B) Pictures of porcine cornea, day 28 co-culture of hCECs, hCSSCs and DRG explants in liquid (ESD-LC) and day 28 co-culture at air-liquid interface (ESD-ALIC) soaked in glycerol. Scale bars=3mm.
3.11 Immunohistochemistry
The single cultured and co-cultured samples were fixed at days 14 and 28. Samples were fixed in 4% paraformaldehyde in PBS (Affymetrix, Cleveland, OH) for 45 min and then treated with 5% BSA for 30 min. Cellular morphology was revealed with anti β tubulin III staining. Keratocan was stained to reveal hCSSCs ECM secretion while involucrin was stained to reflect the maturity of hCECs. The dilution of antibodies is indicated in Supplementary Table 1. The samples were treated with primary antibodies for 12 h at 4°C and then washed with PBS 3 times, 15 min each. The samples were stained with secondary antibodies for 8 h at 4°C and washed with PBS 3 times, 15 min each. DAPI was diluted 1:1000 in 5% BSA solution at the same time as the primary antibodies. Images were taken on a BZX-700 microscope (Keyence Corporation, Itasca, IL) at 10× and 4×. Maximum intensity projection images were generated using Advanced 3D Analysis software (Keyence Corporation, Itasca, IL). In order to compare transparency, the fixed samples for ESD-LC, ESD-ALIC and the porcine cornea were immersed in glycerol for 1h and placed over a paper with the word “SILK” printed. Photos were taken to indicate transparency.
3.12 Quantitative Reverse transcript PCR (qPCR)
Gene expression levels for keratocan (KERA), lumican (LUM), smooth muscle actin (ACTA2) aldehyde dehydrogenase 3A1 (ALDH3A1), involucrin (IVL), connexin 37 (GJA4), and cytokeratin 3 (KRT3) were quantified by RT-PCR (qPCR) [23, 35, 36]. In brief, total RNA was extracted using Trizol with single step acid-phenol guanidinium method [37], adsorbed onto a silica-gel membrane using the Qiagen RNeasy Kit protocol (Qiagen, Valencia, CA), eluted, and quantified [38]. The RNA extracted from the scaffolds was reverse transcribed to cDNA in a 20 µl reaction using high-capacity cDNA reverse transcription kit (Thermo Fisher). Quantitative RT-PCR of cDNA (~30 ng/µl) was performed using assays containing fluorescent hybridization probes (Taq Man: Thermo Fisher). Reactions were incubated at 95°C for 10 min and amplification was carried out on samples with 2 min incubation at 50°C, followed by 50 cycles of 15 seconds at 95°C and 1 min at 60°C. The reaction for RT-PCR was processed in a 15 µl solution containing 1× Universal PCR Master Mix (Thermo Fisher) with 6 µl cDNA samples. RNA expression at day 28 was compared to day0 samples using 18s as a reference gene.
3.13 Calcium imaging
Calcium imaging was conducted on day 28 ESD-ALIC scaffolds to observe neuronal function. For the assay, 50µg Fluo-4 (Invitrogen) was reconstituted with 50µl pluronic F-127 (Invitrogen) and then diluted in DMEM at a 1:1000 dilution. The scaffolds were incubated in 1 ml of the DMEM-Fluo-4 solution for 1h at 37°C, and then washed with PBS solution to remove excess dye. The stimulation solution consisted of 250µM menthol in DMEM and was added dropwise onto the scaffolds while images were taken every 60ms with a BZX-700 microscope (Keyence Corporation, Itasca, IL) at 10× magnification.
3.14 Neuronal extension measurement
Positive staining of β tubulin III was determined at 4× magnification. The images were collected from n=3 samples from 3 independent experiments. All the stitched images were then converted into 8- bit tiff files using Image J (NIH). The neuron J routine [39] was then applied to measure axon length. The density of axons on 4× images was counted using Image J cell counter.
3.15 Statistical analysis
Data analysis was performed using Student’s T test. The significance level was set at p<0.05. All experiments were run in at least triplicates for two independent experiments.
IV. Results
4.1 Guidance of neuronal innervation
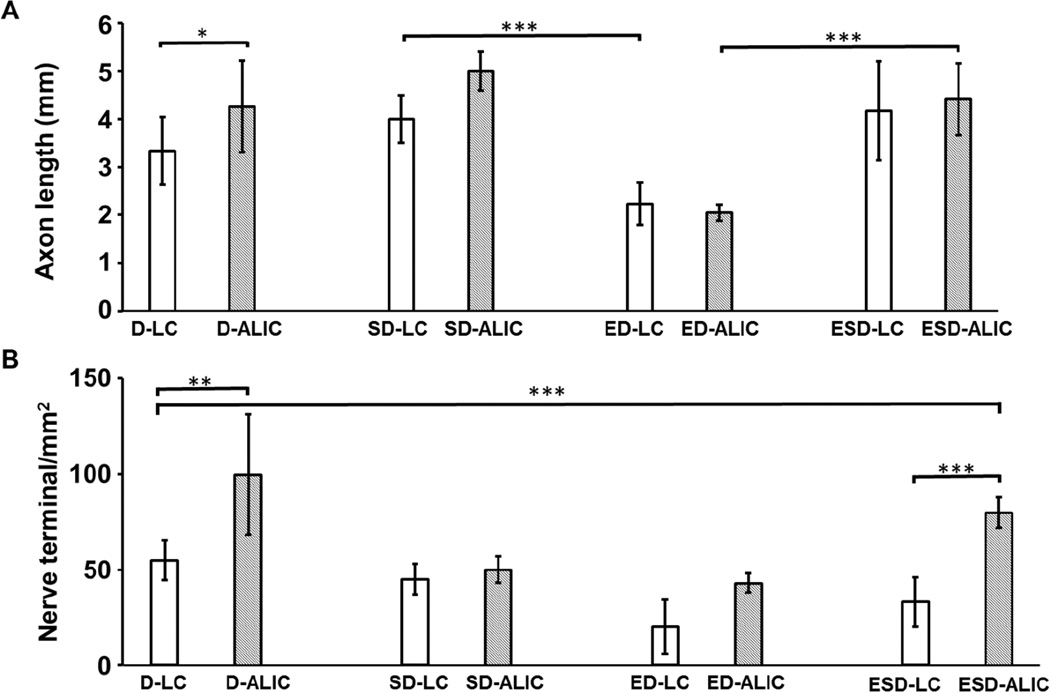
After 14 days of cultivation, the uniformly stamped silk films provided higher axon density (112 ± 34 termini/mm2) than multi-circular (26 ± 5 termini/mm2) and radial stamped films (33 ± 8 termini/mm2) (Supplementary Figure 1). Thus, this strategy was selected to guide neuronal innervation towards the center of the scaffolds in the remaining studies. After 28 days of cultivation the axons were mostly located on the top surface of the scaffolds (Figure 2 A, B) indicating successful guidance of innervation. This guidance was also studied at the ALIC (Figure 2 C, D) where the top surface of scaffolds had twice the density of axons than in the LC. The length and density of axons reached an average of 3 ± 0.7 mm and 55 ±10.61 termini/mm2 in the LC versus 4 ± 0.5 mm and 99 ± 13.5 termini/mm2 in the ALIC. Thus, the combination of stamped silk films and NGF loaded collagen supported the effective guidance of neuronal innervation towards the top center of the scaffolds.
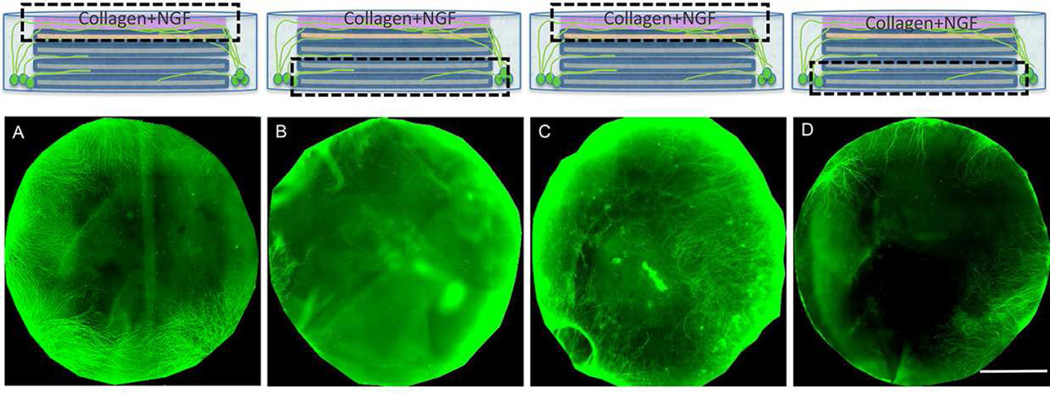
Figure 2.
Immunocytochemistry staining of β III tubulin (green) showed axons being guided towards the top center of the scaffolds in the LCs and ALICs. A) The top surface of day 28 D-LC sample. B) The bottom surface of day 28 D-LC sample. C) The top surface of day 28 D-ALIC sample. D) The bottom surface of day 28 D-ALIC sample. Scale bars =6mm.
4.2 Co-culture and single cultures in the liquid phase
The scaffolds for tri-culture in liquid remained intact and transparent through 28 days (Figure 1B). When hCECs were innervated in the ESD-LC and ED-LC groups, the morphology of the hCECs adopted a healthy polygonal epithelial cell morphology whereas in the E-LC group the cells were elongated (Figure 3). HCECs aggregation was observed in the liquid phase single cultures and co-cultures (Figure 3). The hCSSCs retained alignment at both cultivation time points (Figure 3). Innervation was developed in all the co-culture groups, with the SD-LC group resulting in axons that were ~2 times longer than in the ED-LC group (Figure 5 A). The innervation was located on the top surface of scaffolds and between each layer of silk films (Figure 3, Supplementary Figure 4 B). There was no significant difference in length and density of axons between the SD-LC and ESD-LC (Figure 5).
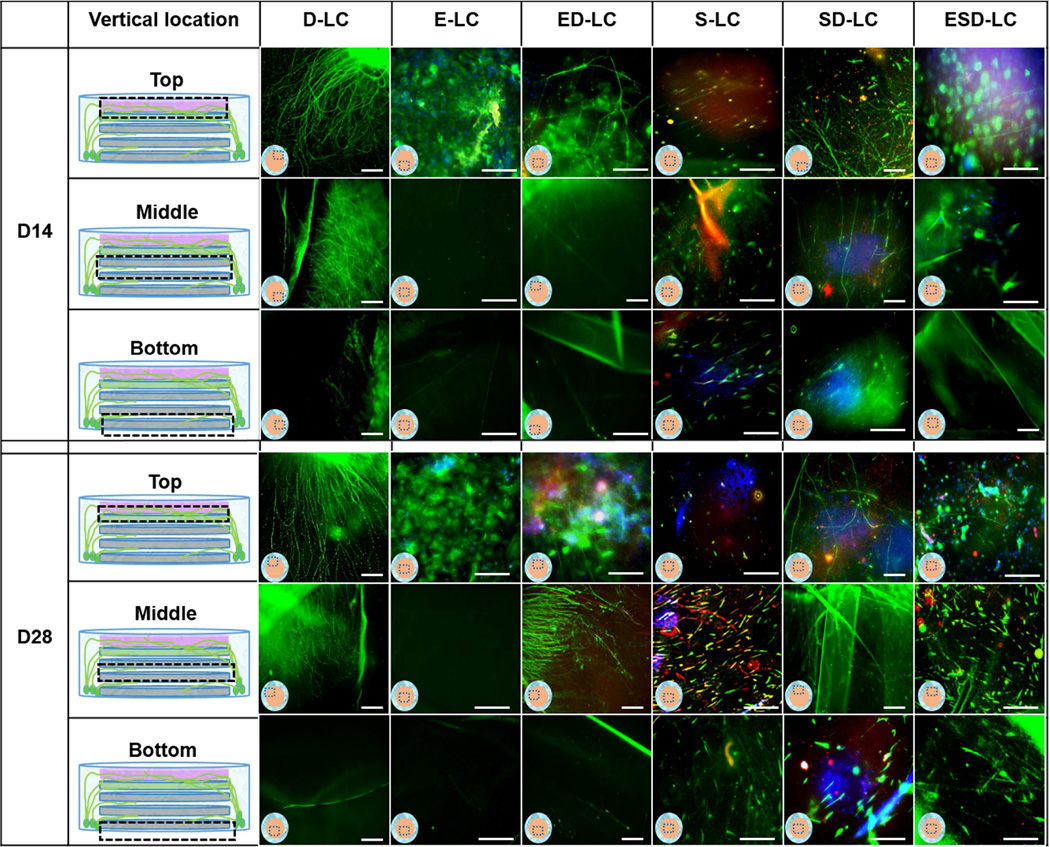
Figure 3.
Immunocytochemistry staining of DRGs, hCECs, and hCSSCs cultured alone (D-LC, E-LC, S-LC); hCECs, DRG co-culture (ED-LC); hCSSCs, DRG co-culture (SD-LC); hCECs, hCSSCs, DRG tri-culture (ESD-LC) in liquid phase. β III tubulin was stained green in all the samples. Involucrin was stained red in E-LC, ED-LC groups and the top layer of ESD-LC sample. Keratocan was stained red in S-LC, SD-LC groups, middle and bottom layers of ESD-LC sample. The dashed boxes indicate the location of images. Scale bars =100µm
Figure 5.
Quantification of density and length of axons in day28 DRG single cultures in liquid phase (D-LC) and at air-liquid interface (D-ALI); DRG and hCSSCs co-culture in liquid phase(SD-LC) and at air-liquid interface (SD-ALIC); DRG co-culture with hCECs in liquid phase (ED-LC) and at air-liquid interfaces (ED-ALIC); DRG neuron, hCSSCs and hCECs tri-culture in liquid phase (ESD-LC) and at air-liquid interfaces (ESD-ALIC). The air-liquid interface culture supported significantly longer axons compared to the liquid cultures. D-ALIC and ESD-ALIC groups provided the densest axons among the groups reaching an average of ~100 termini/cm2. Data was collected from n>3 from three independent experiments. Standard deviation of each group is indicated as error bars. ***P<0.0001; **P<0.00; *P<0.01.
4.3 Co-culture and single cultures at the air-liquid interface
The integrity of the scaffolds and the transparency of the stacked films was maintained through 28 days of cultivation (Figure 1B). Immunostaining showed that the hCECs formed polygonal epithelial cell morphology (Figure 4) and developed into multicellular layers in the ESD-ALIC group (Supplementary Figure 2). The presence of involucrin in the day 28 ESD-ALIC group was highest among the different conditions in ALIC and higher than the day 28 ESD-LC samples (Figure 3, 4). The alignment of hCSSCs remained through the cultures in the S-ALIC, SD-ALIC and ESD-ALIC systems (Figure 4). The secretion of keratocan was also observed in the hCSSCs single cultures and co-cultures. The DRGs cultured alone at the ALIC had an axon density that was approximately two-fold higher than in the LC (Figures 5). Innervation was observed at the top surface and between the silk film layers in the scaffolds (Figure 4 SD-ALIC, ED-ALIC, ESD-ALIC, Supplementary Figure 4 A). The dense innervation appeared in the ESD-ALIC group (80 terminal/mm2), which was 3 and 2 times higher than the ESD-LC and D-LC groups respectively (Figure 5 B). Calcium imaging (Supplementary Figure 3) showed more firing when menthol was added into the medium, supporting neuron function in the tissue constructs.
Figure 4.
Immunohistochemistry staining of DRGs, hCECs, and hCSSCs cultured alone (D-ALIC, E-ALIC, S-ALIC); hCECs, DRG co-culture (ED-ALIC); hCSSCs, DRG co-culture (SD-ALIC); hCECs, hCSSCs, DRG tri-culture (ESD-ALIC) at air-liquid interface. β III tubulin was stained green in all the samples. Involucrin was stained red in E-ALIC, ED-ALIC groups and the top layer of ESD-ALIC sample. Keratocan was stained red in S-ALIC, SD-ALIC groups, middle and bottom layers of ESD-ALIC sample. The dashed boxes indicate the location of images. Scale bars =100µm
4.4 Q-PCR analysis
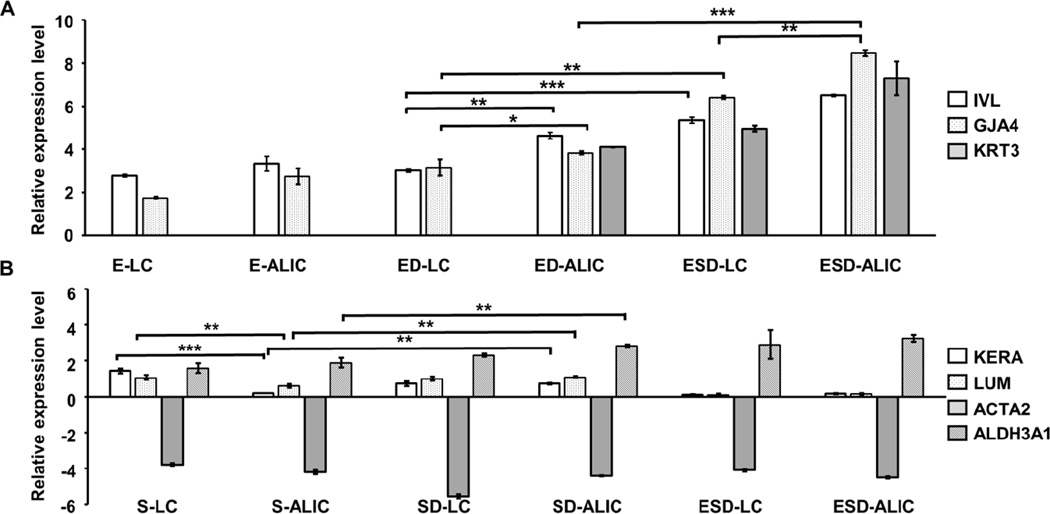
In order to investigate the impact of innervation on the hCECs, expression of mRNA for involucrin (IVL), connexin 37 (GJA4), and cytokeratin-3 (KRT3) was quantified by qPCR. IVL is a marker for maturity whereas GJA4 documents accumulation of cell-cell junctions in the epithelial layer. IVL and GJA4 were expressed in all groups, whereas KRT3 was only expressed in ED-ALIC, ESD-LC and ESD-ALIC samples (Figure 6 A). ED-ALIC had significantly higher expression of IVL and GJA4 compared to ED-LC. Also, the ESD-LC and ESD-ALIC groups had significantly higher IVL and GJA4 expression compared to ED-LC and ED-ALIC groups. The expression of mRNA for keratocan (KERA), lumican (LUM), aldehyde dehydrogenase (ALDH3A1) and smooth muscle actin (ACTA2) was quantified to analyze the functional state of the hCSSCs (Figure 6 B). KERA and LUM are corneal stroma ECM proteins that were previously shown to be expressed by keratocytes differentiated from hCSSCs [36]. ALDH3A1 is highly expressed in both epithelium and stromal cells of differentiated cornea and important for cornel transparency[40]. Smooth muscle actin (ACTA2) is a marker of myofibroblasts, cells involved in secretion of fibrotic non-transparent corneal tissue [41]. The expression of ACTA2 was down regulated in all the culture groups (Figure 6 B). The S-LC group had the highest KERA and LUM expression compared to all the other groups. When hCSSCs were innervated (SD-ALIC), the expression of KERA and LUM were not different from the SD-LC and were significantly higher than the S-ALIC. The expression of ALDH3A1 was significantly greater in groups with innervation compared to non-innervated samples
Figure 6.
Gene expression of hCSSCs (A) and hCECs (B). The expression levels of mRNA for involucrin (IVL), connexin 37 (GJA4) and cytokeratin 3 (KRT3) were quantified through Q-PCR and normalized with day 0 samples of hCECs. The expression levels of mRNAs for keratocan (KERA), lumican (LUM), aldehyde dehydrogenase 3A1 (ALDH3A1) and smooth muscle actin (ACTA2) were quantified through Q-PCR and normalized with day 0 samples of hCSSCs. The expression level of IVL and GJA4 were significantly higher in ED and ESD co-cultures compared to hCECs single cultures. hCSSCs expressed the most keratocan when cultured in the liquid phase. SD-ALIC groups had significantly higher keratocan expression compared to the S-ALIC groups. The data were collected from n>3 from three independent experiments. Standard error of each group is indicated as error bars. ***P<0.0001; **P<0.001; *P<0.01.
V. Discussion
Compared to collagen-based corneal tissue models with innervation [15, 42], the silk protein provided tunable materials to match the mechanical properties of human cornea [17, 26, 43]. Films and sponges prepared from silk supported aligned hCSSCs growth and improved neuronal extensions [23, 24]. In collagen-based tissue models, NGF was loaded into hydrogels to create a concentration gradient to guide neuronal growth [12, 32]. However, the density and length of innervation were not quantified, and collagen undergoes consistent contraction over time that impacts cell functions. In our present study of corneal tissue models, NGF loaded collagen gels were combined with NGF stamped silk films to guide the axons towards the top center of the scaffolds, while concurrently avoiding the problem of material contraction. Interactions between cells sourced from chickens in contact with human cells has been previously reported [44]. Dorsal root ganglion neurons were used to mimic corneal innervation, due to its roles as a critical component as sensory neurons [45]. The average terminal density and axon length reached 100 termini/mm2 and 4 mm in the ALICs. The guided, dense, and long axons establish an essential foundation to for innervated corneal tissue models. Further, these systems remained functional for at least one month in culture, supporting sustained cultivation to allow both acute and chronic studies with these new corneal tissues.
Previously, an air-liquid interface was achieved by culturing tissue constructs in trans-wells [14, 46, 47]. HCSSC survival in trans-wells was not robust in some of our preliminary experiments. Thus, PDMS shelves were designed to maintain a fluid environment for the stroma while the epithelium was positioned at the ALIC. As a result, the hCSSCs survived well in these systems. After the scaffold design for neuronal innervation guidance and air-liquid cultivation was completed, the hCSSCs and hCECs were included in the cultures. In our previous studies, we developed patterned corneal stoma construct using RGD functionalized silk film stacks [26], which supported the secretion of aligned ECM from hCSSCs. In a more recent study, 2D co-culture systems with silk films and silk collagen hydrogels improved DRG axonal development when co-cultured with hCSSCs. In order to include the epithelium, an important barrier layer for the cornea [3], HGF, KGF and EGF were stamped on the top silk film layer and hCECs survived through 28 days of cultivation in the LC and ALIC. Multilayer growth of hCECs was achieved in the ALIC systems, reflecting the importance of the air-liquid environment to generate suitable outcomes for these cornea tissues.
The expression of IVL, GJA4 and the number of epithelial cellular layers in the innervated and air-liquid interface cultured samples were significantly higher than in the non-innervated samples cultivated in liquid phase, suggesting innervation and the air liquid interface contributes towards achieving cell and tissue maturity of the corneal epithelium.
During the sustained cultivation in LC, the survival of hCSSCs appeared to decrease when hCECs were included in the system. This outcome was likely due to the hCECs remaining proliferative throughout the cultivation which created competition for nutrients. This issue did not appear in the ALICs, which again supported the key role of this environment maintaining a healthy epithelium and stroma. However, when hCSSCs were cultured alone, the LC provided better KERA expression compared to the ALIC, indicating the liquid environment enhanced the secretion of ECM in the stroma.
The highly expressed ALDH3A1 and KERA in the innervated stroma showed the essential role of innervation on corneal stromal transparency and function [48]. In humans, it was observed that the impairment of corneal innervation can cause corneal ulcers (neurotrophic keratitis) [49]. Patients with neurotrophic keratitis present decreased corneal sensitivity with alterations in corneal epithelium, nerve, keratocyte, and endothelium. Our findings corroborate that corneal sensory nerves play a critical role in maintaining the vitality, metabolism, and replenishment of corneal cells [50]. While preliminary in outcome in the present study, the data suggest that this new corneal 3D tissue model has potential to help to explore and address these types of corneal diseases.
The interaction between corneal cells and innervation was also observed through the morphology. The axons developed from the bottom of silk sponge and grew towards the top of the scaffold in single culture and co-culture groups. In the tri-cultures, the axons branched at the edge of scaffold and sprouted thin and long axons that grew in between stromal layers and on the epithelial layer. Epithelial innervation developed through cultivation formed close connections with hCECs in the ALIC. In stromal layer, the axons were guided by the pattern on the silk film and grew parallel with hCSSCs. Through the longer and denser axons in the ESD-ALIC compared to the ED-ALIC, a synergistic effect by the hCSSCs was revealed. This finding is in agreement with our previous study which illustrated the importance of collagen type I and BDNF secreted by hCSSCs in improving neuronal extensions [16]. Interestingly, we observed longer axons in all the ALIC groups. A similar effect was shown in other DRG neuron single cultures and co-cultures with skin tissue at the air-liquid interface [51, 52]. The results indicate the importance of the ALIC in supporting long and dense neuronal innervation at in vitro environment.
Cultivation time for current cornea tissues models was limited to 1–2 weeks [15, 42]. Cornea development takes 2 months in the human embryo [6]. Interactions between innervation and corneal tissue in longer-term cultivation are critical in this study, the robust silk protein based scaffold retained integrity and transparency through 28 days of cultivation. These results support the utility of these scaffold designs and bioreactor support for sustainable cultivation of 3D cornea tissues for a range of studies in the future.
VI. Conclusions
Innervated silk-based corneal tissue models were developed which supported long and dense neuronal innervation with multi-layer hCECs for the epithelium and aligned hCSSCs for the stromal layers. The impact of innervation on the corneal stroma and epithelium in sustained culture was demonstrated. With these advances in relevance, innervation, and time in culture, these corneal tissue models can be considered as supplements to animal models in areas such as drug development, disease intervention and in general corneal physiology research.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health [Grant number: R01 EY02085]. J Funderburgh was supported by R01 EY016415. The authors thank Erica P Kimmerling, James D White, and Nicole Raia for the insightful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.McMenamin PG, Steeleand C, McGhee CN. Excimer lasers in Ophthalmology. London: Martin Dunitz; 1997. Cornea: anatomy, physiology and healing; pp. 41–63. [Google Scholar]
- 2.DelMonte DW, Kim T. Anatomy and physiology of the cornea. Journal of Cataract & Refractive Surgery. 2011;37(3):588–598. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Whitcher JP, Srinivasanand M, Upadhyay MP. Corneal blindness: a global perspective. Bulletin of the World Health Organization. 2001;79(3):214–221. [PMC free article] [PubMed] [Google Scholar]
- 4.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. British Journal of Ophthalmology. 2011 doi: 10.1136/bjophthalmol-2011-300539. p. bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen BS, Bakirand M, Jain S. Corneal nerves in health and disease. Survey of ophthalmology. 2014;59(3):263–285. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marfurt CF, Cox J, Deekand S, Dvorscak L. Anatomy of the human corneal innervation. Experimental eye research. 2010;90(4):478–492. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Al-Aqaba MA, Fares U, Suleman H, Loweand J, Dua HS. Architecture and distribution of human corneal nerves. British Journal of Ophthalmology. 2010;94(6):784–789. doi: 10.1136/bjo.2009.173799. [DOI] [PubMed] [Google Scholar]
- 8.He J, Bazanand NG, Bazan HE. Mapping the entire human corneal nerve architecture. Experimental eye research. 2010;91(4):513–523. doi: 10.1016/j.exer.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müller LJ, Marfurt CF, Kruseand F, Tervo TM. Corneal nerves: structure, contents and function. Experimental eye research. 2003;76(5):521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 10.Madduri S, Papaloïzosand M, Gander B. Synergistic effect of GDNF and NGF on axonal branching and elongation in vitro. Neuroscience research. 2009;65(1):88–97. doi: 10.1016/j.neures.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 11.You L, Ebnerand S, Kruse FE. Glial Cell–Derived Neurotrophic Factor (GDNF)–Induced Migration and Signal Transduction in Corneal Epithelial Cells. Investigative ophthalmology & visual science. 2001;42(11):2496–2504. [PubMed] [Google Scholar]
- 12.You L, Kruseand FE, Volcker H. Neurotrophic factors in the human cornea. Investigative Ophthalmology and Visual Science. 2000;41(3):692–702. [PubMed] [Google Scholar]
- 13.Okada Y, Reinach PS, Kitano A, Shirai K, Kaoand WW-Y, Saika S. Neurotrophic keratopathy; its pathophysiology and treatment. Histology and histopathology. 2010;25(4):771. doi: 10.14670/HH-25.771. [DOI] [PubMed] [Google Scholar]
- 14.Reichl S, Bednarzand J, Müller-Goymann C. Human corneal equivalent as cell culture model for in vitro drug permeation studies. British journal of ophthalmology. 2004;88(4):560–565. doi: 10.1136/bjo.2003.028225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suuronen EJ, Nakamura M, Watsky MA, Stys PK, Müller LJ, Munger R, Shinozakiand N, Griffith M. Innervated human corneal equivalents as in vitro models for nerve-target cell interactions. The FASEB journal. 2004;18(1):170–172. doi: 10.1096/fj.03-0043fje. [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Ghezzi CE, Whiteand JD, Kaplan DL. Coculture of dorsal root ganglion neurons and differentiated human corneal stromal stem cells on silk - based scaffolds. Journal of Biomedical Materials Research Part A. 2015;103(10):3339–3348. doi: 10.1002/jbm.a.35465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roeder BA, Kokini K, Sturgis JE, Robinsonand JP, Voytik-Harbin SL. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. Journal of biomechanical engineering. 2002;124(2):214–222. doi: 10.1115/1.1449904. [DOI] [PubMed] [Google Scholar]
- 18.Elsheikh A, Wang D, Brown M, Rama P, Campanelliand M, Pye D. Assessment of corneal biomechanical properties and their variation with age. Current eye research. 2007;32(1):11–19. doi: 10.1080/02713680601077145. [DOI] [PubMed] [Google Scholar]
- 19.Jin HJ, Park J, Karageorgiou V, Kim UJ, Valluzzi R, Cebeand P, Kaplan DL. Water - Stable Silk Films with Reduced β - Sheet Content. Advanced Functional Materials. 2005;15(8):1241–1247. [Google Scholar]
- 20.Hu X, Shmelev K, Sun L, Gil E-S, Park S-H, Cebeand P, Kaplan DL. Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules. 2011;12(5):1686–1696. doi: 10.1021/bm200062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng Y, Yang J, Huang K, Leeand Z, Lee X. A comparison of biomechanical properties between human and porcine cornea. Journal of biomechanics. 2001;34(4):533–537. doi: 10.1016/s0021-9290(00)00219-0. [DOI] [PubMed] [Google Scholar]
- 22.Last JA, Liliensiek SJ, Nealeyand PF, Murphy CJ. Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. Journal of structural biology. 2009;167(1):19–24. doi: 10.1016/j.jsb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Rnjak-Kovacina J, Du Y, Funderburgh ML, Kaplanand DL, Funderburgh JL. Corneal stromal bioequivalents secreted on patterned silk substrates. Biomaterials. 2014;35(12):3744–3755. doi: 10.1016/j.biomaterials.2013.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang-Schomer MD, White JD, Tien LW, Schmitt LI, Valentin TM, Graziano DJ, Hopkins AM, Omenetto FG, Haydonand PG, Kaplan DL. Bioengineered functional brain-like cortical tissue. Proceedings of the National Academy of Sciences. 2014;111(38):13811–13816. doi: 10.1073/pnas.1324214111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coulombre AJ, Coulombre JL. The role of intraocular pressure in the development of the chick eye: IV. Corneal curvature. AMA archives of ophthalmology. 1958;59(4):502–506. doi: 10.1001/archopht.1958.00940050058005. [DOI] [PubMed] [Google Scholar]
- 26.Orssengo GJ, Pye DC. Determination of the true intraocular pressure and modulus of elasticity of the human cornea in vivo. Bulletin of mathematical biology. 1999;61(3):551–572. doi: 10.1006/bulm.1999.0102. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Kluge JA, Leiskand GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29(8):1054–1064. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence BD, Marchant JK, Pindrus MA, Omenettoand FG, Kaplan DL. Silk film biomaterials for cornea tissue engineering. Biomaterials. 2009;30(7):1299–1308. doi: 10.1016/j.biomaterials.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gil ES, Mandal BB, Park S-H, Marchant JK, Omenettoand FG, Kaplan DL. Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering. Biomaterials. 2010;31(34):8953–8963. doi: 10.1016/j.biomaterials.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gil ES, Park SH, Marchant J, Omenettoand F, Kaplan DL. Response of human corneal fibroblasts on silk film surface patterns. Macromolecular bioscience. 2010;10(6):664–673. doi: 10.1002/mabi.200900452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao D, Dong S, Lu Q, Hu X, Kaplan DL, Zhangand B, Zhu H. Salt-leached silk scaffolds with tunable mechanical properties. Biomacromolecules. 2012;13(11):3723–3729. doi: 10.1021/bm301197h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu S, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du Y, Lathrop KL, Syed-Picard FN, Adamsand SM, Birk DE. Human limbal biopsy–derived stromal stem cells prevent corneal scarring. Science translational medicine. 2014;6(266):266ra172–266ra172. doi: 10.1126/scitranslmed.3009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du Y, Funderburgh ML, Mann MM, SundarRajand N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23(9):1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leclere PG, Norman E, Groutsi F, Coffin R, Mayer U, Pizzeyand J, Tonge D. Impaired axonal regeneration by isolectin B4-binding dorsal root ganglion neurons in vitro. The Journal of neuroscience. 2007;27(5):1190–1199. doi: 10.1523/JNEUROSCI.5089-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Du Y, Mann MM, Funderburghand JL, Wagner WR. Corneal stromal stem cells versus corneal fibroblasts in generating structurally appropriate corneal stromal tissue. Experimental eye research. 2014;120:71–81. doi: 10.1016/j.exer.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J, Du Y, Mann MM, Yang E, Funderburghand JL, Wagner WR. Bioengineering organized, multilamellar human corneal stromal tissue by growth factor supplementation on highly aligned synthetic substrates. Tissue Engineering Part A. 2013;19(17–18):2063–2075. doi: 10.1089/ten.tea.2012.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical biochemistry. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Altman GH, Karageorgiou V, Horan R, Collette A, Volloch V, Colabroand T, Kaplan DL. Human bone marrow stromal cell and ligament fibroblast responses on RGD - modified silk fibers. Journal of Biomedical Materials Research Part A. 2003;67(2):559–570. doi: 10.1002/jbm.a.10120. [DOI] [PubMed] [Google Scholar]
- 39.Meijering E, Jacob M, Sarria JC, Steiner P, Hirlingand H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry Part A. 2004;58(2):167–176. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- 40.Jester JV. Corneal crystallins and the development of cellular transparency. Seminars in cell & developmental biology. 2008;19(2):82–93. doi: 10.1016/j.semcdb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du Y, Roh DS, Funderburgh ML, Mann MM, Marra KG, Rubin JP, Liand X, Funderburgh JL. Adipose-derived stem cells differentiate to keratocytes in vitro. Molecular Vision. 2010;16:2680–2689. [PMC free article] [PubMed] [Google Scholar]
- 42.Suuronen EJ, McLaughlin CR, Stys PK, Nakamura M, Mungerand R, Griffith M. Functional innervation in tissue engineered models for in vitro study and testing purposes. Toxicological Sciences. 2004;82(2):525–533. doi: 10.1093/toxsci/kfh270. [DOI] [PubMed] [Google Scholar]
- 43.Rockwood DN, Preda RC, Yücel T, Wang X, Lovettand ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nature protocols. 2011;6(10):1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salehi H, Karbalaie K, Razavi S, Tanhaee S, Nematollahi M, Sagha M, Nasr-Esfahaniand M-H, Baharvand H. Neuronal induction and regional identity by co-culture of adherent human embryonic stem cells with chicken notochords and somites. International Journal of Developmental Biology. 2011;55(3):321–326. doi: 10.1387/ijdb.103185hs. [DOI] [PubMed] [Google Scholar]
- 45.Chen C-C, England S, Akopianand AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proceedings of the National Academy of Sciences. 1998;95(17):10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang J-E, Basuand SK, Lee VH. Air-interface condition promotes the formation of tight corneal epithelial cell layers for drug transport studies. Pharmaceutical research. 2000;17(6):670–676. doi: 10.1023/a:1007569929765. [DOI] [PubMed] [Google Scholar]
- 47.Tegtmeyer S, Papantoniouand I, Müller-Goymann CC. Reconstruction of an in vitro cornea and its use for drug permeation studies from different formulations containing pilocarpine hydrochloride. European journal of pharmaceutics and biopharmaceutics. 2001;51(2):119–125. doi: 10.1016/s0939-6411(01)00123-0. [DOI] [PubMed] [Google Scholar]
- 48.Lambiase A, Sacchetti M, Mastropasquaand A, Bonini S. Corneal changes in neurosurgically induced neurotrophic keratitis. JAMA ophthalmology. 2013;131(12):1547–1553. doi: 10.1001/jamaophthalmol.2013.5064. [DOI] [PubMed] [Google Scholar]
- 49.Semeraro F, Forbice E, Romano V, Angi M, Romano M, Filippelli M, Di Iorioand R, Costagliola C. Neurotrophic keratitis. Ophthalmologica. 2014;231(4):191–197. doi: 10.1159/000354380. [DOI] [PubMed] [Google Scholar]
- 50.Blanco-Mezquita T, Martinez-Garcia C, Proença R, Zieske JD, Bonini S, Lambiaseand A, Merayo-Lloves J. Nerve Growth Factor Promotes Corneal Epithelial Migration by Enhancing Expression of Matrix Metalloprotease-9NGF Promotes Epithelial Migration. Investigative ophthalmology & visual science. 2013;54(6):3880–3890. doi: 10.1167/iovs.12-10816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gingras M, Bergeron J, Déry J, Durhamand HD, Berthod F. In vitro development of a tissue-engineered model of peripheral nerve regeneration to study neurite growth. The FASEB journal. 2003;17(14):2124–2126. doi: 10.1096/fj.02-1180fje. [DOI] [PubMed] [Google Scholar]
- 52.Bryce G, Ribchester R. Culture of isolated embryonic chick dorsal root ganglia at an air-liquid interface: a simple method for studying the mechanism and control of neurite outgrowth. Journal of neuroscience methods. 1993;48(1):89–97. doi: 10.1016/s0165-0270(05)80010-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.