Abstract
Degradable hydrogels to deliver bioactive proteins represent an emerging platform for promoting tissue repair and vascularization in various applications. However, implanting these biomaterials requires invasive surgery, which is associated with complications such as inflammation, scarring, and infection. To address these shortcomings, we applied microfluidics-based polymerization to engineer injectable poly(ethylene glycol) microgels of defined size and crosslinked with a protease degradable peptide to allow for triggered release of proteins. The release rate of proteins covalently tethered within the microgel network was tuned by modifying the ratio of degradable to non-degradable crosslinkers, and the released proteins retained full bioactivity. Microgels injected into the dorsum of mice were maintained in the subcutaneous space and degraded within 2 weeks in response to local proteases. Furthermore, controlled release of VEGF from degradable microgels promoted increased vascularization compared to empty microgels or bolus injection of VEGF. Collectively, this study motivates the use of microgels as a viable method for controlled protein delivery in regenerative medicine applications.
Keywords: VEGF, microfluidics, biomaterials, hydrogels, protein delivery
1. Introduction
Synthetic hydrogel microparticles (microgels) have broad biomedical applications including cell encapsulation and transplantation [1-8], wound healing [9], imaging tools [10], and protein and drug delivery [11-15]. Microgels offer additional advantages to the attributes of bulk hydrogels for cell and protein delivery, including delivery via catheters or injection via small diameter needles, which minimizes complications associated with surgery (e.g. trauma, infection, scarring), and preserves native tissue structure without in situ gelling considerations that often limit biomedical applications of bulk hydrogels. Furthermore, when appropriately sized, microgels conform to the geometry of the application site, which facilitates uniform distribution of biomolecules to target sites. Importantly, microgels with different characteristics (e.g., different proteins, release rates) can be synthesized in separate batches and simple co-delivery of the microgels in the desired ratios will result in a “mosaic” formulation resulting in complex or multi-component materials.
Of various synthesis routes available to generate synthetic microgels, microfluidics-based polymerization is particularly well-suited for preparing microgels containing proteins and cells because of the aqueous, cytocompatible nature and precise control over particle size of this continuous process [7]. Microgels for protein delivery rely on passive diffusion of the protein through a non-degradable microgel network, and therefore the release kinetics are solely dictated by protein size and microgel mesh size [16]. This inability to modulate protein delivery rate severely hinders the application of microgels to regenerative medicine, immunoengineering, and cancer therapy. We present a strategy to engineer synthetic microgels with protease-degradable crosslinks and tunable protein release kinetics. Furthermore, we demonstrate that these protease-degradable microgels promote in vivo vascularization by controlled release of vascular endothelial growth factor (VEGF) and complete degradation of microgels that allows for tissue ingrowth and remodeling.
2. Materials and Methods
2.1 Microfluidic device fabrication
PDMS microfluidic flow focusing devices were fabricated using soft lithography from silicon and SU8 masters. Devices were plasma treated and then bonded directly to glass slides. Microfluidic devices were then heated to 110 °C for 30 minutes to improve PDMS-glass sealing. Prior to use, devices were infused with Aquapel™ for 30 seconds and then purged with nitrogen to render surfaces hydrophobic.
2.2 4-arm poly(ethylene glycol)-maleimide (PEG-4MAL) microgel generation
PEG-4MAL (20 kDa, Laysan Bio) was dissolved in phosphate-buffered saline (PBS) at 5% (w/v) then filtered through a 40 μm cell strainer (Corning). For experiments involving the injection of microgels in vivo, microgels were functionalized with GRGDSPC (RGD, Genescript). PEG-4MAL was reacted with 2.0 mM RGD for 30 minutes at 37 °C to create RGD-functionalized macromer. For all other experiments, RGD was not used in the formation of microgels. Crosslinker solutions (DTT (Sigma) or GCRDVPMSMRGGDRCG (VPM, Genescript) or combinations of both) were prepared at predetermined molar concentrations and then adjusted to a pH of 4.5 to slow down gelation kinetics in order to prevent the device from clogging. PEG-4MAL and crosslinker were then infused into the flow-focusing microfluidic device to form polymer droplets. Droplets were formed within an oil solution consisting of light mineral oil (Sigma) mixed with 2% SPAN80 (Sigma) and then collected into a 15 mL conical tube (Falcon). After formation, microgels were washed in PBS five times by centrifugation to remove mineral oil and surfactant.
2.3 Microgel degradation
Two hundred microgels were loaded into each well of a 96-well plate. Collagenase or PBS was then added to each well and microgels were incubated at 37 °C for 20 hours. After incubation with protease or PBS, images of each well were acquired using a fluorescent microscope and the total number of microgels in the well was quantified.
2.4 Protein release kinetics
Prior to microgel formation, PEG-4MAL was reacted with AlexaFluor488-labeled IgG (rat anti-mouse, Thermo Fisher), AlexaFluor555-labeled IgG (rat anti-mouse, Thermo Fisher), or VEGF165 homodimer (Thermo Fisher) pre-labeled with NHS-Dylight488 (Thermo Fisher). All proteins were reacted with PEG-4MAL at 20 μg/mL for 30 minutes at 37 °C protected from light. After washing, 100 μL of 200 μm diameter microgels were added to transwells with 8 μm pore sizes in a 48 well plate (Corning) then treated with 3.9 or 39 units/mL of type 1 collagenase in 500 μL of PBS (Worthington). The microgels were then maintained in an incubator at 37 °C with gentle shaking. At indicated time points, the supernatant was sampled and analyzed on a plate reader (Biotek). Images of the microgels were acquired on an inverted microscope (Nikon TE 300) with a fluorescent camera (Hamamatsu Orca ER II).
2.5 VEGF bioactivity assay
We have previously shown that PEGylation of VEGF homodimer primarily results in a VEGF molecule conjugated to two PEG-4MAL macromers [17]. To confirm PEGylated VEGF maintained bioactivity, human umbilical vein endothelial cells (HUVEC, Lonza) were grown in endothelial growth media (EGM-2, Lonza) and synchronized in growth factor free basal media (EBM-2, Lonza) with 1% fetal bovine serum overnight followed by addition of VEGF, PEG-4MAL conjugated VEGF, PEG-MAL only, or EGM-2 for 24 h. Cell metabolism was assayed by the CellTiter 96 MTS Aqueous Cell Proliferation Assay (Promega). To confirm VEGF released from microgels maintained bioactivity, microgels containing VEGF were incubated in MMP-2 (50 nM) (R&D Systems) for 30 minutes at 37°C followed by addition of TIMP-1 (50 nM). HUVEC were then exposed to released VEGF (100 ng/mL) or soluble VEGF (100 ng/mL) for 24 h and cell metabolism was assayed.
2.6 Microgel vascularization
To track microgel retention in vivo, RGD was conjugated with Dylight750 for IVIS imaging or Dylight555 for microscopy imaging then tethered to PEG-4MAL macromer. Under protocols approved by Georgia Tech’s Institutional Animal Care and Use Committee, C57BL/6J mice (Jackson Labs) were anesthetized with 2.5% isoflurane during microgel injection and image acquisition. Backs of mice were shaved, dilapidated with Nair™, and sterilized with 70% ethanol. A 1 mL syringe with a 23 gauge 0.5” needle was loaded with 100 μL of microgels. The tip of needle was then inserted into the subcutaneous space of the dorsum and microgels were slowly injected, taking care not to disturb the native tissue structures. A total of 16 mice received two of the following microgel formulation chosen for this study: VPM + VEGF, VPM – VEGF, DTT + VEGF, VPM + sVEGF. Experimental groups were designed such that 4 samples were used for each group. IVIS Spectrum CT (Perkin Elmer) imaging system was used to track microgel position and persistence over time. At 14 day, following injection, functional vasculature was labeled by perfusing anesthetized mice with 1.0 mg/mL Dylight649-labeled tomato lectin (Vector Labs) via tail vein injection. To wash out excess fluorescent lectin, mice were perfused with saline solution. Mice were then euthanized with CO2 and the regions of the skin where microgels were injected were excised. Microgels and vasculature were imaged using a confocal microscope (Nikon Ti-E with Perfect Focus System and C2-Plus Confocal System) and analyzed with ImageJ software.
3. Results
3.1 Generation of microgels using microfluidics
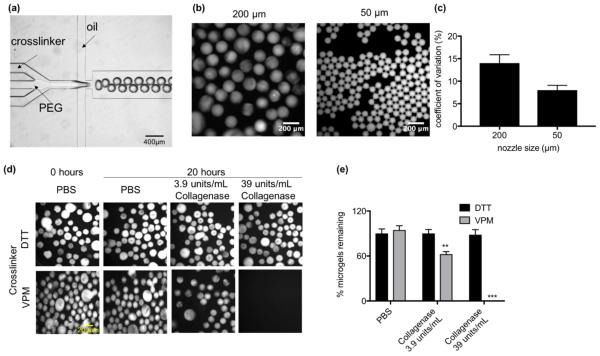
To engineer synthetic microgels, we used a PEG-4MAL macromer, which is crosslinked into a network via a Michael-type addition reaction with thiols. The PEG-4MAL platform outperforms other PEG-based polymers in generating structurally defined hydrogels with stoichiometric incorporation of ligands and improved crosslinking efficiency [17]. In addition, PEG-4MAL exhibits minimal local and systemic inflammation and toxicity and is rapidly excreted in the urine [18], important criteria for in vivo applications. We designed a microfluidic flow focusing device to produce droplets of PEG-4MAL and crosslinker (Fig. 1a). Three independent flow inlets (PEG-4MAL, crosslinker, and mineral oil containing SPAN80 surfactant) were used to produce droplets. After the PEG-4MAL and crosslinker flow streams merge, the solution is focused into an oil-covered droplet where it crosslinks and is then collected at the outlet (Video S1, Supporting Information). Microgels of defined diameters with homogeneous size distribution can be simply produced by changing the inlet flow rates and nozzle size (Fig. 1b,c). To generate protease-degradable microgels, we used the crosslinking peptide GCRDVPMSMRGGDRCG (VPM), which is rapidly cleaved by matrix metalloproteinase (MMP)-1 and MMP-2 proteases [19]. To confirm protease-dependent degradation, we first reacted PEG-4MAL with AlexaFluor488-labeled IgG (AF488-IgG) to form an AF488-IgG-functionalized macromer and then generated 200 μm diameter microgels crosslinked with VPM or dithiothreitol (DTT). Microgels were incubated in type 1 collagenase at 39 units/mL or 3.9 units/mL in buffer solution and imaged on a fluorescence microscope (Fig. 1d). After 20 hours, 100% of the VPM-crosslinked microgels in 39 units/mL collagenase degraded whereas only 25% of VPM-crosslinked microgels in 3.9 units/mL collagenase degraded (Fig. 1e). The DTT-crosslinked microgels did not degrade in the presence of the protease. The degradation of VPM-crosslinked microgels was dependent on the concentration of collagenase used (Fig. S1, Supporting Information). These results show that VPM-crosslinked microgels are degradable by proteases in a concentration-dependent fashion.
Fig. 1. Generation of protease degradable microgels using flow focusing microfluidics.
(a) Image of microfludic flow focusing device with 200 μm nozzle. (b) Image of microgels generated using a 200 μm (left) or 50 μm (right) nozzle. (c) Coefficient of variation of diameter for microgels generated from 200 μm or 50 μm nozzles. (d) Images of microgels crosslinked with DTT or VPM in the presence of collagenase or PBS. (e) Percent of DTT or VPM crosslinked microgels remaining after 20 hour incubation with type 1 collagenase or PBS (n = 3 independent experiments). Significance compared to PBS control was determined using two-way ANOVA with Dunnett’s post-test, **p<0.01, ***p<0.005.
3.2 Tuning microgel release kinetics
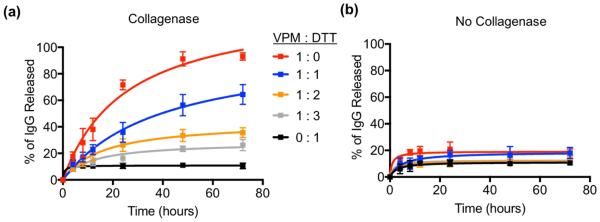
In order to engineer microgels with tunable sensitivity to proteases, and therefore tunable release kinetics, we prepared microgels that were crosslinked with varying VPM to DTT molar ratios. Fluorescent IgG-loaded microgels were incubated in the presence of proteases, the supernatant was sampled at indicated intervals, and fluorescent signal was measured. As expected, protein released most quickly from microgels that were crosslinked exclusively with protease-sensitive VPM crosslinker, while microgels crosslinked with protease-insensitive DTT did not release significant protein after one hour in protease, suggesting that the ~20% protein release observed resulted from passive diffusion of untethered protein (Fig. 2a). By varying the crosslinker ratio of MMP-sensitive VPM to protease-insensitive DTT, the degradation rate of capsules in collagenase can be controlled, and therefore the release rate of encapsulated protein can be controlled. Protein release in all groups had plateaued after 3 days, suggesting that remaining crosslinks were protease-insensitive. Importantly, microgels incubated in the absence of protease did not undergo degradation for any crosslinker formulation tested, as evidenced by minimal protein release over 80 hours (Fig. 2b). These results demonstrate that the degradation and release rate of proteins encapsulated within microgels can be engineered. Furthermore, mixtures of different microgels, in this case protease-degradable and non-degradable, can be prepared in order to co-deliver bioactive molecules with different release rates (Video S2, Fig. S2, Supporting Information).
Fig. 2. Controlled degradation of microgels and release of protein.
Release kinetics of fluorescent IgG from microgels crosslinked with different molar ratios of VPM to DTT. (a) Microgels were treated with type 1 collagenase (3.9 units/mL) or (b) PBS (n = 3 independent experiments).
3.3 In vitro VEGF release kinetics
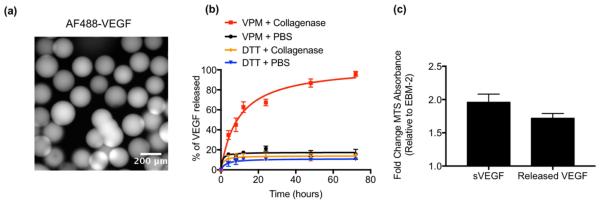
An important application for hydrogels is delivery of VEGF protein to promote vascularization [20]. We generated VEGF-containing microgels that degrade in the presence of protease to explore the application of the microgel delivery platform in regenerative medicine. Consistent with our previous observations [18], covalently tethering of VEGF to PEG-4MAL through its free cysteine does not affect its bioactivity (Fig. S3, Supporting Information). To measure the release kinetics, VEGF was labeled with a fluorescent dye and then reacted with PEG-4MAL. The microfluidic focusing device was then used to generate 200 μm diameter VPM- or DTT-crosslinked microgels (Fig. 3a). VEGF was released for VPM-crosslinked microgels incubated in protease, whereas minimal VEGF release occurred over 3 days from DTT-crosslinked microgels incubated in protease as well as from VPM-crosslinked microgels incubated in saline (Fig. 3b). In a separate set of experiments, unlabeled VEGF was released from VPM crosslinked microgels by incubating microgels in MMP-2. Upon complete microgel degradation, VEGF was incubated in TIMP-1 in order to inhibit MMP-2 activity. VEGF released from the microgels exhibited similar bioactivity levels compared to soluble VEGF (Fig 3c). These results confirm protease-dependent release of bioactive VEGF from VPM-crosslinked microgels, consistent with observations seen in IgG-loaded microgels. Notably, the release kinetics were similar to that observed for AF488-IgG-containing microgels, indicating release kinetics independent of protein size.
Fig. 3. Release kinetics and bioactivity of VEGF.
Prior to formation into a gel, PEG-4MAL was functionalized with fluorescently labeled VEGF (10 μg/mL). (a) Representative image of fluorescent VEGF bound within the microgels. (b) Percent of VEGF released over time in the presence of type 1 collagenase (3.9 units/mL) or PBS (n = 4 independent experiments). (c) Endothelial cell metabolic assay for soluble VEGF (100 ng/mL) or VEGF released from protease-degradable microgels treated with MMP-2 (50 nM) and TIMP-1 (50nM) (n = 3 independent experiments). A total dose of 15 ng was used for both soluble and released VEGF conditions.
3.4 Microgel injection and vascularization in vivo
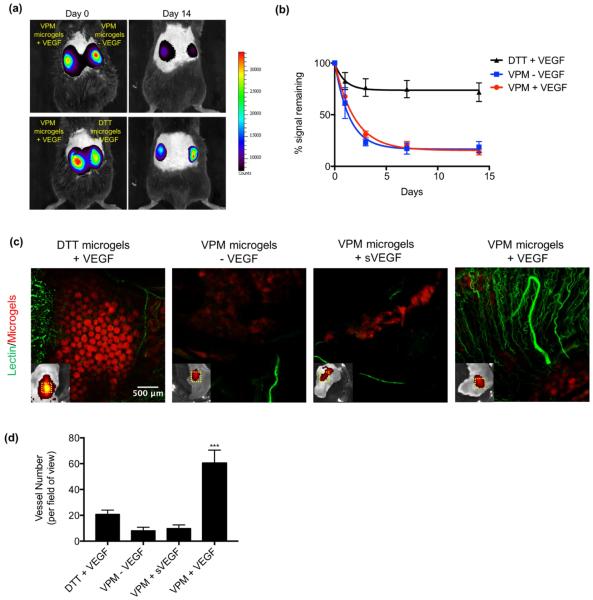
To examine the ability of VEGF-releasing microgels to promote vascularization in vivo, we injected different microgel formulations in the subcutaneous space in the dorsum of mice and measured retention of VEGF and functional vascular ingrowth at the site of injection (Fig. 4). Microgel suspensions were simply injected using a standard tuberculin syringe with no incision required, and none of the time constraints due to crosslinking kinetics that accompany injectable bulk gels. The simplicity of microgel injection lends itself to clinical applications where more complex schemes could produce heterogeneous material properties (due to inadequate mixing) or more trauma at the implant site (due to incision), especially in non-expert hands. In order to track microgels and promote tissue repair at the injection site, microgels were covalently functionalized with 2.0 mM of the fluorescently labeled adhesive peptide GRGDSPC (‘RGD’) by co-incubation of labeled RGD and PEG-4MAL before microgel generation. Using an intravital imaging system (IVIS), we monitored the position and fluorescence intensity of injected microgels over 14 days in vivo (Fig. 4a). The fluorescent signal decreased exponentially over time for the VPM-crosslinked microgels (half-life = 1.3 days). In contrast, the signal from DTT-crosslinked gels decreased initially but stabilized to ~80% of the original signal after day 1 and remained relatively unchanged thereafter. (Fig. 4b). This result indicates that VPM-crosslinked microgels degrade in a well-defined pattern in vivo, while DTT crosslinked microgels do not degrade significantly over 14 days. At day 14, the circulatory system of the mice was perfused with fluorescently labeled lectin to stain functional vasculature. Confocal images of explanted skin regions where microgels were injected show significant increases in the number of blood vessels for VEGF-encapsulated VPM-crosslinked microgels compared to empty (no VEGF) VPM-crosslinked microgels and to DTT-crosslinked gels loaded with VEGF (Fig. 4c,d). Importantly, a bolus of soluble VEGF (sVEGF) co-delivered with empty VPM-crosslinked microgels resulted in minimal vascularization, indicating that controlled release of VEGF at the treatment site is required to achieve robust vascularization. Degradation of VPM-crosslinked microgels promoted host tissue ingrowth, while DTT-crosslinked microgels were still present at the injection site and prevented tissue ingrowth and remodeling (Fig. 4c). These results demonstrate that controlled VEGF delivery from protease-degradable microgels promotes robust vascularization and tissue remodeling in vivo.
Fig. 4. Protease degradable VEGF microgels promote vascularization in vivo.
Microgels functionalized with a fluorescent RGD molecule were injected into the subcutaneous space on the back of C57BL/6 mice. (a) Images of microgel fluorescence at day 0 and day 14. (b) Percent of signal remaining over time compared to day 0. Data was fit with a simply decay model. (c) Representative fluorescent images of skin explants perfused with lectin to label vasculature. Fluorescent images of microgels in skin explants (bottom left). Dotted white line indicates the area represented in the large image. (d) Number of lectin labeled vessels per field of view (n = 4 mice per group). Significance was determined using one-way ANOVA with Tukey post-test, ***p<0.005.
4. Discussion
Implantation of devices via surgical incisions is often associated with complications such as inflammation, infection, trauma, and scarring. Injectable biomaterials mitigate these complications and are thus an attractive means for cell and drug delivery applications. Here, we present an injectable microgel platform to deliver VEGF and promote vascularization. Mice that received degradable microgels releasing VEGF exhibited increased vessel formation compared to mice that received empty microgels or a bolus injection of VEGF. We attribute this increase in vascularization to 1) sustained release of VEGF from the microgels, and 2) host cell binding to RGD within microgels that provide a scaffold for tissue ingrowth and vascularization. Previous reports have shown that sustained release of VEGF combined with a cell-adhesive biomaterial scaffold are critical driving factors for improved vascularization [21-23]. However, the need for invasive surgery to implant bulk, pre-formed devices limits the translation of these tools to the clinic. In situ gelation offers a solution for noninvasive delivery of biomaterials, though the choice of polymer and crosslinker is often limited by physiological conditions that affect gelling such as temperature, pH, and the presence of ions [24]. Our results support a microgel-based drug delivery system as an effective method for delivering VEGF and potentially other bioactive molecules.
In a previous report, we utilized a microfluidic device to generate non-degradable PEG-4MAL microgels crosslinked in a DTT/oil emulsion [6]. This microfluidic device, however, could not produce microgels crosslinked with peptides due to the limited solubility of these peptides in oil. We therefore designed a unique microfluidic device to generate protease-degradable microgels by reacting PEG-4MAL with VPM. This new microfluidic device brings together streams of PEG-4MAL macromer and VPM and the mixture is pinched off into a droplet by an oil stream. The new device incorporates a serpentine channel which is necessary for allowing sufficient crosslinking of the microgels before collection into a conical tube. We demonstrate precise control over microgel size using the PDMS based microfluidic flow focusing device. For subcutaneous injections, relatively large diameter particles (~200 μm) and needles did not significantly damage native structures. For more delicate procedures which would require a smaller diameter needle, smaller diameter microgels (<50 μm) would be preferable.
We demonstrate protease-dependent degradation of these microgels to release the incorporated IgG or VEGF. We also show control over the in vitro degradation rate of the microgels via tuning the ratio of protease-degradable (VPM) to non-degradable (DTT) crosslinker. In addition to the VPM peptide used in this study, other protease-cleavable peptides with faster or slower degradation rates could be employed as gel crosslinkers in order to more precisely control the degradation and protein release kinetics [19]. In this way, the release kinetics of proteins from the microgels are dependent on local cellular demand rather than release initiated by hydrolysis or other chemical stimuli. Microgels labeled with a near infrared dye were used to track microgel degradation in vivo. VPM-crosslinked microgels exhibited an exponential decay in fluorescence signal to low levels, which is attributed to microgel degradation. DTT-crosslinked microgels exhibited a small decrease in signal initially that stabilized and remained relatively constant for the duration of the observation period. Although we cannot rule out in vivo degradation of DTT-crosslinked microgels by collagenases or other mechanisms, the signal loss for these microgels could be related to release of unincorporated dye or oxidation of the fluorochrome.
A key advantage of the 4-arm PEG-maleimide material is the ability to easily conjugate molecules presenting free thiols for efficient tethering of biomolecules within the microgel. We show that ~80% of fluorescent IgG or VEGF was maintained within the microgels in the absence of collagenase, with the remaining 20% of untethered proteins released within 4 hours. Importantly, the bioactivity of the VEGF tethered within the microgels remained similar to non-PEGylated VEGF. In vivo experiments also suggest VEGF remains bioactive over the course of the study. We posit that cell-controlled degradation of the microgels and sustained release of VEGF improves vascularization compared to materials that do not allow cellular based remodeling. Non-degradable microgels containing VEGF exhibited vascularization around the perimeter of the injection site, however, vessels were unable to penetrate within areas occupied by the microgels. Furthermore, without the sustained release of VEGF, vessels did not form even in the presence of a degradable microgel scaffold.
5. Conclusion
Synthetic microgels offer significant advantages as protein delivery vehicles, including minimally invasive, injectable delivery, control of protein release rates, control of particle size, and the ability to deliver multiple proteins with independently controllable release rates simultaneously. We demonstrate that protease-degradable microgels promote in vivo vascularization by controlled release of the vasculogenic protein VEGF and complete degradation of microgels that allows for tissue ingrowth and remodeling.
Supplementary Material
Acknowledgements
This work was funded by the U.S. National Institutes of Health (R21 EB020107) and the Juvenile Diabetes Research Foundation (2-SRA-2014-287-Q-R). C.G.G. was funded by the IOF-Marie Curie Post-Doctoral Fellowship (331655). M.S.S. is funded by ERC (306990).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Allazetta S, Hausherr TC, Lutolf MP. Microfluidic Synthesis of Cell-Type-Specific Artificial Extracellular Matrix Hydrogels. Biomacromolecules. 2013;14:1122–1131. doi: 10.1021/bm4000162. doi:10.1021/bm4000162. [DOI] [PubMed] [Google Scholar]
- [2].Siltanen C, Yaghoobi M, Haque A, You J, Lowen J, Soleimani M, et al. Microfluidic fabrication of bioactive microgels for rapid formation and enhanced differentiation of stem cell spheroids. Acta Biomaterialia. 2016;34:125–132. doi: 10.1016/j.actbio.2016.01.012. doi:10.1016/j.actbio.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chan HF, Zhang Y, Leong KW. Efficient One-Step Production of Microencapsulated Hepatocyte Spheroids with Enhanced Functions. Small. 2016;12:2720–2730. doi: 10.1002/smll.201502932. doi:10.1002/smll.201502932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cha C, Oh J, Kim K, Qiu Y, Joh M, Shin SR, et al. Microfluidics-Assisted Fabrication of Gelatin-Silica Core–Shell Microgels for Injectable Tissue Constructs. Biomacromolecules. 2014;15:283–290. doi: 10.1021/bm401533y. doi:10.1021/bm401533y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rossow T, Heyman JA, Ehrlicher AJ, Langhoff A, Weitz DA, Haag R, et al. Controlled Synthesis of Cell-Laden Microgels by Radical-Free Gelation in Droplet Microfluidics. J. Am. Chem. Soc. 2012;134:4983–4989. doi: 10.1021/ja300460p. doi:10.1021/ja300460p. [DOI] [PubMed] [Google Scholar]
- [6].Headen DM, Aubry G, Lu H, Garcia AJ. Microfluidic-Based Generation of Size-Controlled, Biofunctionalized Synthetic Polymer Microgels for Cell Encapsulation. Adv. Mater. 2014;26:3003–3008. doi: 10.1002/adma.201304880. doi:10.1002/adma.201304880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu AL, Garcia AJ. Methods for Generating Hydrogel Particles for Protein Delivery. Ann Biomed Eng. 2016;44:1946–1958. doi: 10.1007/s10439-016-1637-z. doi:10.1007/s10439-016-1637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vegas AJ, Veiseh O, rtler MGU, Millman JR, Pagliuca FW, Bader AR, et al. Long-term glycemic control using polymer-encapsulated human stem cell–derived beta cells in immune-competent mice. Nature Medicine. 2016:1–10. doi: 10.1038/nm.4030. doi:10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, Segura T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat Mater. 2015;14:737–744. doi: 10.1038/nmat4294. doi:10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang QM, Wang W, Su Y-Q, Hensen EJM, Serpe MJ. Biological Imaging and Sensing with Multiresponsive Microgels. Chem. Mater. 2016;28:259–265. doi:10.1021/acs.chemmater.5b04028. [Google Scholar]
- [11].Zhang X, Lü S, Gao C, Chen C, Zhang X, Liu M. Highly stable and degradable multifunctional microgel for self-regulated insulin delivery under physiological conditions. Nanoscale. 2013;5:6498–9. doi: 10.1039/c3nr00835e. doi:10.1039/c3nr00835e. [DOI] [PubMed] [Google Scholar]
- [12].Lai W-F, Susha AS, Rogach AL. Multicompartment Microgel Beads for Co-Delivery of Multiple Drugs at Individual Release Rates. ACS Appl. Mater. Interfaces. 2016;8:871–880. doi: 10.1021/acsami.5b10274. doi:10.1021/acsami.5b10274. [DOI] [PubMed] [Google Scholar]
- [13].Stukel J, Thompson S, Simon L, Willits R. Polyethlyene glycol microgels to deliver bioactive nerve growth factor. J. Biomed. Mater. Res. 2014;103:604–613. doi: 10.1002/jbm.a.35209. doi:10.1002/jbm.a.35209. [DOI] [PubMed] [Google Scholar]
- [14].Tibbitt MW, Han BW, Kloxin AM, Anseth KS. Student award for outstanding research winner in the Ph.D. category for the 9th World Biomaterials Congress, Chengdu, China, June 1-5, 2012 Synthesis and application of photodegradable microspheres for spatiotemporal control of protein delivery. J. Biomed. Mater. Res. 2012;100A:1647–1654. doi: 10.1002/jbm.a.34107. doi:10.1002/jbm.a.34107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sivakumaran D, Maitland D, Hoare T. Injectable microgel-hydrogel composites for prolonged small-molecule drug delivery. Biomacromolecules. 2011;12:4112–4120. doi: 10.1021/bm201170h. doi:10.1021/bm201170h. [DOI] [PubMed] [Google Scholar]
- [16].Wang K, Lin S, Nune KC, Misra RDK. Chitosan-gelatin-based microgel for sustained drug delivery. Journal of Biomaterials Science, Polymer Edition. 2016;27:441–453. doi: 10.1080/09205063.2016.1143673. doi:10.1080/09205063.2016.1143673. [DOI] [PubMed] [Google Scholar]
- [17].Phelps EA, Templeman KL, Thulé PM, Garcia AJ. Engineered VEGF-releasing PEG-MAL hydrogel for pancreatic islet vascularization. Drug Deliv Transl Res. 2015;5:125–136. doi: 10.1007/s13346-013-0142-2. doi:10.1007/s13346-013-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Phelps EA, Headen DM, Taylor WR, Thulé PM, Garcia AJ. Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials. 2013;34:4602–4611. doi: 10.1016/j.biomaterials.2013.03.012. doi:10.1016/j.biomaterials.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. doi:10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- [20].Briquez PS, Clegg LE, Martino MM, Mac Gabhann F, Hubbell JA. Design principles for therapeutic angiogenic materials. Nature Reviews Materials. 2016;1:15006. doi:10.1038/natrevmats.2015.6. [Google Scholar]
- [21].Phelps EA, Landázuri N, Thulé PM, Taylor WR, Garcia AJ. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci U S A. 2010;107:3323–3328. doi: 10.1073/pnas.0905447107. doi:10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Seliktar D, Zisch AH, Lutolf MP, Wrana JL, Hubbell JA. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J. Biomed. Mater. Res. 2004;68:704–716. doi: 10.1002/jbm.a.20091. doi:10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- [23].Zhu S, Segura T. Cell-Demanded VEGF Release via Nanocapsules Elicits Different Receptor Activation Dynamics and Enhanced Angiogenesis. Ann Biomed Eng. 2016;44:1983–1992. doi: 10.1007/s10439-016-1581-y. doi:10.1007/s10439-016-1581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymers with Aligned Carbon Nanotubes: Active Composite Materials. 2008;49:1993–2007. doi:10.1016/j.polymer.2008.01.027. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.