Abstract
NF-κB plays an essential role in the initiation and progression of pancreatic cancer and specifically mediates the induction of epithelial-mesenchymal transition and invasiveness. In this study we demonstrate the importance of activated NF-κB signaling in EMT induction, lymphovascular metastasis, and neural invasion. Modulation of NF-κB activity was accomplished through the specific NF-κB inhibitor (BAY 11-7085), triptolide, and Minnelide treatment, as well as overexpression of IKBα repressor and IKK activator plasmids. In the classical lymphovascular metastatic cascade, inhibition of NF-κB decreased the expression of several EMT transcription factors (SNAI1, SNAI2, ZEB1) and mesenchymal markers (VIM and CDH2) and decreased in vitro invasion, which was rescued by IKK activation. This was further demonstrated in vivo via BAY 11-7085 treatment in a orthotopic model of pancreatic cancer. In vivo NF-κB inhibition decreased tumor volume; decreased tumor EMT gene expression, while restoring cell-cell junctions; and decreased overall metastasis. Furthermore, we demonstrate the importance of active NF-κB signaling in neural invasion. Triptolide treatment inhibits NGF mediated and neural-tumor co-culture in vitro invasion and dorsal root ganglia (DRG) neural outgrowth through a disruption in tumor-neural cross talk. In vivo, Minnelide treatment decreased neurotrophin expression, nerve density, and sciatic nerve invasion. Taken together, this study demonstrates the importance of NF-κB signaling in the progression of pancreatic cancer through the modulation of EMT induction, lymphovascular invasion, and neural invasion.
Introduction
Pancreatic cancer is now the 3rd leading cause of cancer related death in the United States and is projected to be the 2nd by 2030 1. The overall 5 year survival rate of this disease is a dismal 7%, making it one of the very few cancers which have this in single digits. 2 The primary reason for the poor prognosis is mainly due to the late diagnosis, limited therapeutic options, early metastatic spread, and the aggressive nature of pancreatic cancer.
Tumor cell dissemination early in development has been demonstrated in pancreatic cancer is considered to be one of the causes of recurrence even after surgical resection 3. The process of lymphovascular metastasis is a multistep cascade, in which tumor cells invade the basement membrane, intravasate into the vascular or lymphatic circulation, extravasate out of the circulation at a distant site, and develop into a metastatic lesion. The initiation of this process is orchestrated by the induction of the epithelial-mesenchymal transition (EMT), stimulating cells to lose cell-cell adhesions and thus, allowing tumor dissemination throughout the body.
In addition to the lymphovascular metastasis, the pancreatic tumor is also known to undergo neural invasion, in which the tumor uses the neural network for the spread of disease 4. Dissemination of tumor cells into the nerves, rather than the vascular or lymphatic circulation, has been shown in up to 100% of pancreatic cancer patients 5, 6. Invasion into the nerves, or perineural invasion (PNI), is characterized as the invasion of tumor cells “in, around, or through” the nerves 4. Neural invasion in pancreatic cancer has been contributed to the severe abdominal pain experienced by patients and tumor recurrence following curative surgical resection 4, 7.
Contributing to the cross-talk between nerve and tumor, is the upregulation of neurotrophin and neurotrophin receptors in pancreatic cancer. Overexpression of nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), and glial derived neurotrophic factor (GDNF) provide additional growth signals for nerves. Increased density of nerves and thickness of nerve bundles has been observed histologically in pancreatic ductal adenocarcinoma 7, 8. In addition to the growth of nerves within the pancreatic tumor, these signals activate proliferative signaling pathways in the tumor and have been shown to increase invasiveness in pancreatic cancer cells 9-12.
Induction of EMT and increased cellular invasiveness has been attributed to the induction of NF-κB in many different cancer types, including pancreatic cancer 13, 14. Up to 70% of PDAC tumors show a constitutive activation of NF-κB and this has shown to be required for tumor development 15, 16. Inhibition of this pathway may prove clinically effective in decreasing metastatic spread. Minnelide™, a pro-drug derived from triptolide, has been shown in several cancers to downregulate NF-κB activity 17-19.
In this study, we show the role of NF-κB in invasion and metastasis and that its inhibition using Minnelide treatment decreases EMT, invasion, and metastasis. We also examine the effect of Minnelide on neural invasion and show a disruption of neural-tumor cross talk via decreased expression of neurotrophin and neurotrophin receptors and neural invasion both in vitro as well as in vivo.
Materials and Methods
Cell line culture and maintenance
MIA PaCa-2 and AsPC-1 were obtained from ATCC. S2-VP10 was derived from the metastatic cell line, SUIT-2 (a gift from Professor Masato Yamamoto, University of Minnesota). KPC cell line was derived from LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre transgenic mouse tumor as described by Banerjee et al, 2014 20. Briefly, tumors were digested by collagenase B and dispase II and plated in medium (supplemented with growth factors and 2% serum) for 2-3 weeks to remove fibroblasts. Established primary cell line was further grown in DMEM containing 10% serum. Human pancreatic ductal epithelial cells were a gift from Professor Anil Rustgi and cultured as described in Reichert et al, 2013 21.
MIA PaCa-2 and KPC cell line were grown in DMEM-high glucose, supplemented with 10% fetal bovine serum; S2-VP10 cultured in RPMI with 10% FBS; and AsPC-1 was cultured in RPMI with 20% RPMI. All media was supplemented with 1× penicillin and streptomycin.
Boyden chamber invasion assay
Invasion inserts (Corning BioCoat) were hydrate in serum-free medium; the bottom of the chamber contained the attractant (10% FBS, recombinant NGF, or neural/cancer cells plated on the bottom of the well); 25,000 cells were plated on the top of the insert. At 24 hours, non-invaded cells were scrubbed from the top chamber via cotton swab, invaded cells were fixed in methanol, and stained by crystal violet. Invaded cells were counted by microscopy.
Migration assay
Assay was conducted by Electric Cell-substrate Impedance Sensing (ECIS) (Applied Biophysics) and performed as described in Banerjee et al, 2014 20.
Immuohistochemistry
Resected tissues were fixed in 10% formalin followed by 80% ethanol, paraffin embedded, sectioned, and deparaffinized in xylenes and hydrated through graded ethanol solutions. Antigen retrieval was performed in Reveal Decloaker (Biocare Medical) and background staining was minimized by blocking in Sniper Universal Blocking Sera (Biocare Medical). Slides were stained using anti-vimentin, anti-ncadherin, and anti-MMP9 antibodies (Cell Signaling) and incubated overnight. Alexefluor fluorescent antibody conjugates were used following primary antibody staining. Slides were counterstained by DAPI Prolong gold mounting medium (Life Technologies) and visualized by Leica fluorescent microscope. Negative controls were incubated with mouse IgG1 isotype control and did not demonstrate and specific staining.
NF-κB assay
Activity of NF-κB was determined by p50 binding ELISA (Thermo Scientific) performed according to manufacturer's protocol of whole cell lystates. Values were normalized to microgram protein, as measured by protein estimation (Thermo Scientific). Activation of NF-κB was stimulated by 20 ng/mL TNFα (Sigma).
NF-κB modulation plasmids
Modulation of NF-κB signaling utilized transient plasmid overexpression by attractene transfection. Plasmids: IKK (IKK-2 S177E, S191E) and IKBα (pBabe-Puro-IKBalpha-mut(superrepressor)) plasmids (Addgene).
Animals models
All experiments were performed according to the University of Minnesota Animal Care and Use Committee guidelines.
KPC murine model
LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre transgenic mice were treated with 0.42 mg/kg body weight/day Minnelide starting at 4-6 weeks until moribund. Tumor tissues were utilized for primary tumor cell line establishment, immunohistochemistry for nerve staining (PGP9.5), and gene expression levels via quantitative PCR.
PDX subcutaneous model
Deidentified clinical pancreatic adenocarcinoma tumor samples (PDX1 and PDX2) were obtained from the University of Minnesota Department of Surgery. Samples were implanted subcutaneously into the flank of NOD/SCID mice. When tumors measured ∼700-800 mm3, they were removed; cut into approximately 1-2 mm pieces; and implanted subcutaneously into the right flank of age matched NOD/SCID mice. Propagated tumors were used for experiments. PDX1 and PDX2 are two patient derived xenografts. When tumors measured 750 mm3, 0.42 mg/kg Minnelide treatment was started. Animals were sacrificed at 10 days and tumor was harvested.
Orthotopic animal model
100,000 MIA PaCa-2 cells suspended in 1:1 PBS/Matrigel were injected into the tail of the pancreas of athymic nude female mice via Hamilton syringe. At 2 weeks, 7.5 mg/kg BAY 11-7085 was administered IP every other day for 3 weeks. Control animals received DMSO.
Sciatic nerve invasion assay
50,000 MIA PaCa-2 cells were injected into the right sciatic nerve using a dissecting microscope and 33 gauge needle in 3 μL. Minnelide treatment started at day 7 after cell injection. Mice were observed for 6 weeks for gross behavior, limb function, and sciatic nerve function, as determined by paw spread distance.
DRG outgrowth assay
Dorsal root ganglia were isolated from the lower lumbar section of an adult mouse and placed in a droplet of Matrigel (Corning) in a 24 well plate. DRG/Matrigel were maintained in DMEM/F12 medium supplemented with 10% FBS. Conditioned medium was removed from KPC cells cultured for 48 hours and diluted 1:1 in with fresh medium. DRGs were imaged via microscope daily.
Statistical methods
Values are expressed as mean ± standard error of mean. In vitro experiments were all independently performed at least three times. Statistical significance was calculated by Student's test or ANOVA analysis and significance (*) is defined as p <0.05.
Results
NF-κB is activated in pancreatic cancer and imparts invasiveness
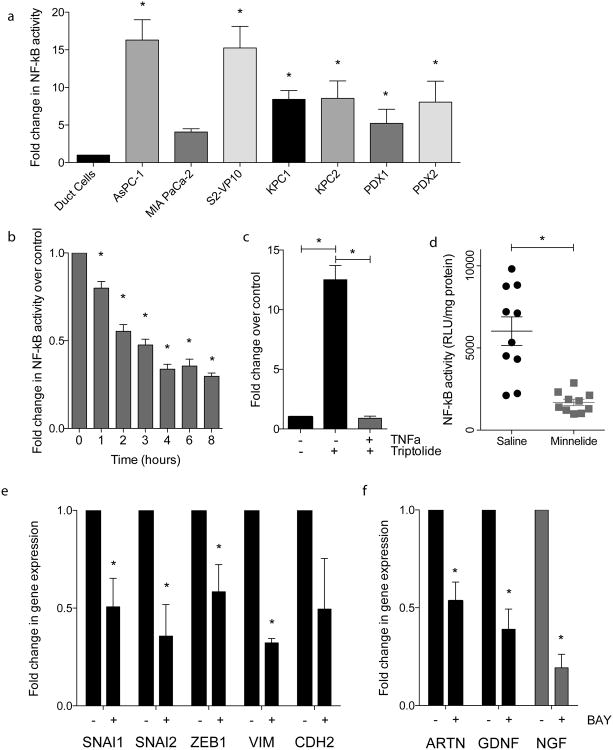
NF-κB pathway is a major pro-proliferative pathway in a number of cancers including pancreatic cancer 16. Our results show that the NF-κB pathway is constitutively activated in pancreatic cancer compared to the normal ductal cells. Amplification of NF-κB activity was exhibited in several established pancreatic cancer cell lines: AsPC-1 (16.31 fold ± 2.70); MIA PaCa-2 (4.08 fold ± 0.43); and S2-VP10 (15.26 fold ± 2.87) and tumors KPC1 (8.43 fold ± 1.16); KPC2 (8.56 fold ± 2.32); PDX1 (5.22 fold ± 1.89); and PDX2 (8.06 fold ± 2.78) (Figure 1a). Along with regulating proliferation of tumor cells, NF-κB pathway also plays a significant role in regulating the EMT as well as invasion in pancreatic cancer 22-24.
Figure 1.
NF-κB is activated in pancreatic cancer and imparts invasiveness: (a) increased p50 binding activity in several cell lines and tumors, as compared to cell lines; (b) triptolide treatment inhibition of NF-κB activity in a time dependent manner; (c) triptolide inhibits TNFα induced NF-κB activity; d) decreased NF-κB activity in vivo in MIA PaCa-2 tumors treated with Minnelide; (e) BAY 11-7085 treatment decreased (e) EMT gene expression and (F) neurotrophin gene expression in S2-VP10 cell line. Each bar is representative of three or more independent experiments; error bars are represented in SEM; and the asterisk (*) indicates a p value < 0.05.
In addition, our results also show that treatment with Minnelide results in significant downregulation of NF-κB activity both in vitro (Figures 1b and 1c) as well as in vivo (Figure 1d). Downregulation of NF-κB also results in the downregulation of key EMT players (Figure 1e) as well as key genes involved in tumor-neural cross talk (Figure 1f).
NF-κB inhibition also resulted in decreased EMT gene expression (Figure 1e). Treatment by BAY 11-7085 for inhibition of NF-κB signaling decreased several EMT genes: SNAI1 (0.507 fold ± 0.146), SNAI2 (0.357 fold ± 0.161), ZEB1 (0.584 fold ± 0.139), VIM (0.322 fold ± 0.022), and CDH2 (0.495 fold ± 0.259). BAY 11-7085 treatment also decreased neurotrophin gene expression: ARTN (0.534 fold ± 0.097), GDNF (0.390 fold ± 0.103), and NGF (0.139 fold ± 0.069) as compared to untreated samples.
NF-κB activity is required for pancreatic cancer invasiveness
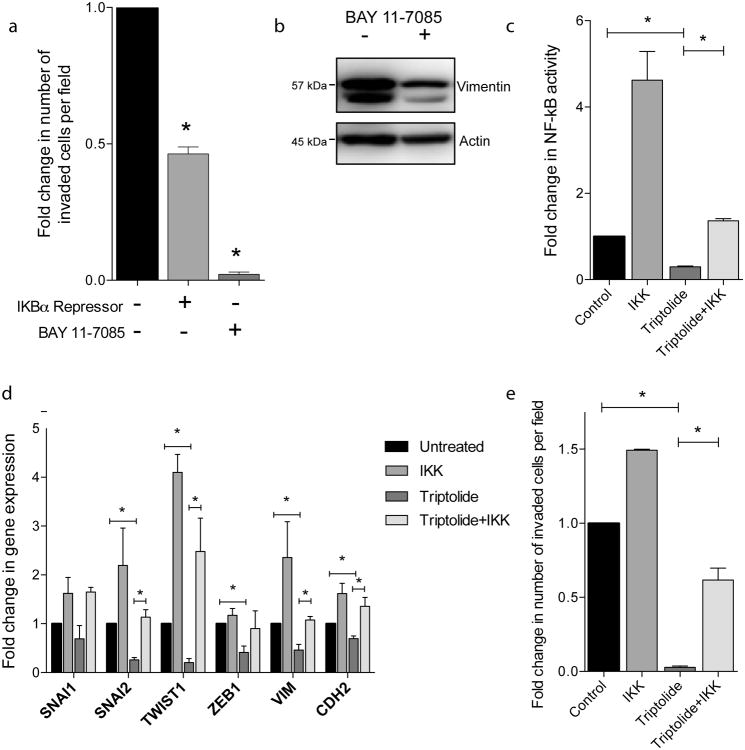
Next we wanted to determine if inhibition of NF-κB signaling does indeed decrease cellular invasiveness in pancreatic cancer. Through inhibition of NF-κB activity by IKBα repressor plasmid and BAY 11-7085 pharmacological inhibition we saw a decrease in Boyden chamber invasion as compared to untreated control (Figure 2a). IKBα repressor plasmid expression decreased invasion to 0.46 (± 0.025) of control and BAY 11-7085 treatment decreased invasion to 0.02 (± 0.008) of untreated control. Pharmacological inhibition via BAY 11-7085 also decreased EMT marker, vimentin, at the protein level (Figure 2b).
Figure 2.
NF-κB activity is required for invasion: (a) Inhibition of NF-κB through IKBα repressor plasmid expression or BAY 11-7085 treatment decreased cellular invasiveness via Boyden chamber invasion assay; (b) BAY 11-7085 treatment decreased vimentin protein expression; expression of IKK plasmid rescues triptolide inhibition of (c) NF-κB activity; (d) EMT gene expression; (e) Boyden chamber invasion. Each bar is representative of three or more independent experiments; error bars are represented in SEM; and the asterisk (*) indicates a p value < 0.05.
Next we wanted to confirm that NF-κB was responsible for the effect of triptolide on EMT and invasion. Triptolide treatment decreased NF-κB activity to 0.66 fold (± 0.089) of untreated MIA PaCa-2 control. Cells expressing the IKK enhancer plasmid treated with triptolide more than restored the NF-κB activity that triptolide diminished (4.104 fold ± 0.701) (Figure 2c).
To determine if NF-κB signaling is mediating the downregulation of EMT gene expression from triptolide treatment, we expressed the IKK enhancer plasmid to rescue the effects of triptolide treatment. Triptolide treatment decreased SNAI1 (0.693 fold ± 0.270), SNAI2 (0.259 fold ± 0.048), TWIST1 (0.205 fold ± 0.080), ZEB1 (0.407 fold ± 0.187), VIM (0.589 fold ± 0.160), and CDH2 (0.695 fold ± 0.055) gene expression as compared to untreated control. With the expression of the IKK enhancer in addition to triptolide treatment, expression of these genes is rescued to approximately untreated levels or higher: SNAI1 (1.650 fold ± 0.095), SNAI2 (1.135 fold ± 0.154), TWIST1 (4.099 fold ± 0.367), ZEB1 (0.900 fold ± 0.364), VIM (3.554 fold ± 0.607), and CDH2 (1.357 fold ± 0.181), as compared to untreated control (Figure 2d).
We then wanted to determine if this rescue was functionally significant in the context of invasion. Control, triptolide treated, and IKK enhancer expressing triptolide treated cells were examined for invasive potential. Triptolide decreased Boyden chamber invasion (0.0282 fold ± 0.009) and cells expressing the IKK enhancer plasmid treated with triptolide rescues this effect (0.617 fold ± 0.080) (Figure 2e).
These data together demonstrate that decreased NF-κB activity through plasmid, pharmacological inhibition, or triptolide treatment downregulates Epithelial-Mesenchymal Transition genes and decreases invasiveness.
Inhibition of EMT in PDAC decreases invasion potential and metastatic spread
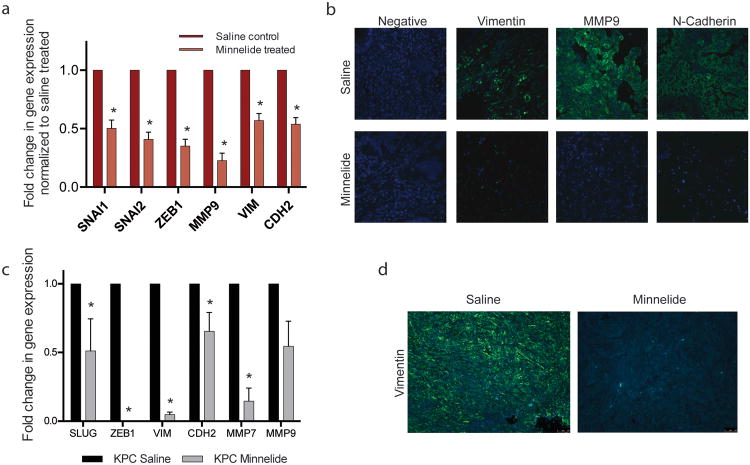
Early gene expression changes with Minnelide treatment in a human tumor xenograft model were assessed at 7 days after start of treatment. EMT associated genes, upregulated during this transition were decreased significantly with Minnelide treatment. EMT inducing transcription factors: SNAI1 (0.503 ± 0.070 fold), SNAI2 (0.408 ± 0.061 fold), and ZEB1 (0.350 ± 0.060 fold) were significantly down regulated. MMP9, VIM, and CDH2 decreased to 0.227 ± 0.063, 0.569 ±0.061, and 0.539 ± 0.056 of saline control, respectively (Figure 3a).
Figure 3.
Inhibition of NF-κB in PDAC decreases EMT related marker expression in vivo: Minnelide treatment in patient derived xenografts decreased (a) EMT gene expression and (b) mesenchymal marker expression as well as in KPC tumors (c) and (d), respectively. Each bar is representative of three or more independent experiments; error bars are represented in SEM; and the asterisk (*) indicates a p value < 0.05.
In addition to the downregulation of EMT associated genes with Minnelide treatment, this effect was also seen at the protein level. Tumor sections from saline or Minnelide treated tumors demonstrated a downregulation of vimentin, MMP9, and N-cadherin via immunofluorescence (Figure 3b).
Inhibition of NF-κB through Minnelide treatment in vivo is also demonstrated in the transgenic, spontaneous KPC murine model. EMT related transcription factor gene expression upon Minnelide treatment decreased in SNAI2 (0.511 ± 0.234 fold), and ZEB1 (2.075e-5 ± 6.853e-6 fold) as compared to saline tumors. Mesenchymal markers VIM, CDH2, MMP7, and MMP9 decreased to 0.048 ± 0.018, 0.655 ± 0.137, 0.146 ±0.096, and 0.545 ± 0.183 of saline tumor control, respectively (Figure 3c). Vimentin expression is also markedly decreased by Minnelide treatment as seen via immunofluorescence (Figure 3d).
Triptolide also significantly decreased in vitro migration (Supplementary figure 1a) and invasion (Supplementary Figure 1b) in KPC cell line, as well as, invasion in established human pancreatic ductal adenocarcinoma cell lines. Invasion as compared to untreated control decreased by 97.7% and 97.4% in MIA PaCa-2 and SUIT-2 derived S2013, respectively (Supplementary Figure 1c).
Based on these data we concluded that Minnelide inhibits the Epithelial-Mesenchymal Transition, limiting cellular motility and invasiveness. We have previously demonstrated that in vivo Minnelide treatment markedly decreases metastasis to distant sites in a murine model of pancreatic cancer 25, these results demonstrate that this is likely due to NF-κB inhibition decreasing EMT and cellular invasiveness.
Inhibition of NF-κB inhibits Epithelial-Mesenchymal Transition
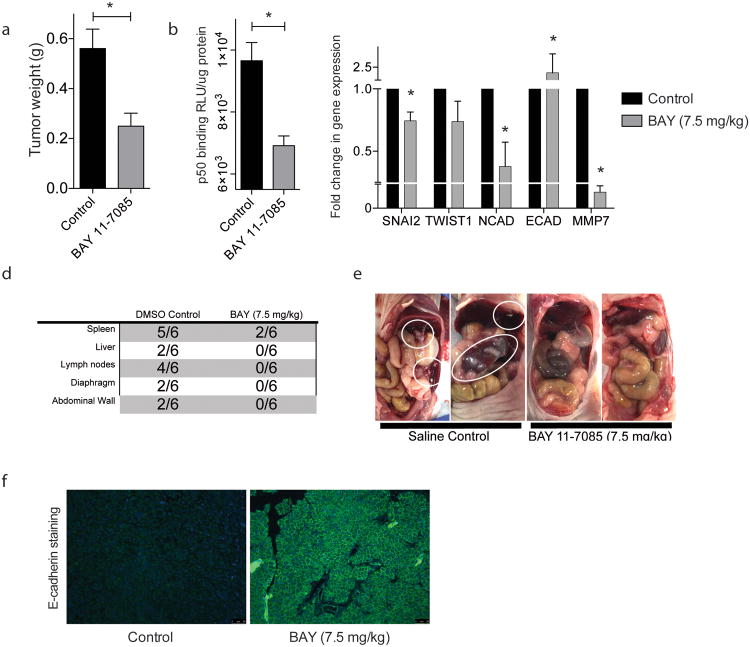
To determine whether the EMT inhibition and invasion which was seen in vitro translates in vivo, we used BAY 11-7085 treatment via intraperitoneal injection to inhibit NF-κB signaling in an orthotopic mouse model of pancreatic cancer. Treatment for 3 weeks resulted in a decrease in primary tumor weight (0.561g ± 0.078 in the control group versus 0.250g ± 0.052 in the BAY 11-7085 treatment group) (Figure 4a). Tumor NF-κB activity was determined by p50 binding ELISA, in which BAY 11-7085 treated tumors displayed decreased p50 binding (9657 RLU/μg protein ± 586.6) as compared to DMSO control (7248 RLU/μg protein ± 538.3) (Figure 4b). BAY 11-7085 treated tumors also exhibited decreased gene expression of EMT transcription factors (SNAI2 0.745 fold ± 0.070 and TWIST1 0.737 fold ± 0.164) and mesenchymal marker (N-Cadherin 0.377 fold ± 0.196); increased E-cadherin expression (2.392 fold ± 0.265); and decreased MMP7 gene expression (0.002 fold ± 0.001) (Figure 4c).
Figure 4.
Inhibition of NF-κB inhibits EMT and metastasis in vivo: BAY 11-7085 treatment in a orthotopic MIA PaCa-2 model decreases (a) tumor weight; (b) tumor NF-κB activity; (c) tumor EMT gene expression; and (d) (e) metastasis to distant sites. Each bar is representative of three or more independent experiments; error bars are represented in SEM; and the asterisk (*) indicates a p value < 0.05.
When observing the spread of disease in these mice, after 3 weeks there was metastasis to several distant sites within the DMSO control group, with a lower extent of metastasis in the BAY 11-7085 group (Figure 4d). Representative images of the abdominal cavity are shown in Figure 4e, with metastatic lesions circled in white. Additionally, these tumors demonstrate an increased E-cadherin staining at the cell membrane, indicating an increase in cell-cell adhesion (figure 4f).
Together, these data demonstrate that invasion in vitro and metastasis in vivo are mediated by the NF-κB signaling and inhibition of this this pathway inhibits epithelial-mesenchymal transition.
Inhibition of neurotrophic signaling decreases neural invasion in pancreatic cancer
As described in Figure 1, NF-κB inhibition decreases neurotrophin gene expression in pancreatic cancer cells. Using triptolide as a NF-κB inhibitor, we wanted to study the effect of NF-κB inhibition on tumor-neural cross talk and invasion of both tumor and neural cells.
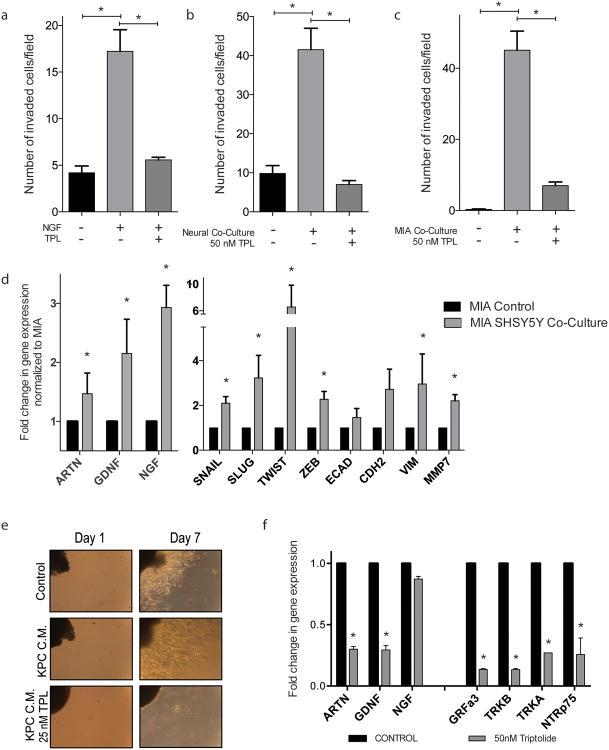
Triptolide treatment decreased MIA PaCa-2 NGF stimulated in vitro invasion (Figure 5a), in which in vitro invasion increased from 4.180 (± 0.737) invaded cells to 17.21 (± 2.32) invaded cells upon NGF stimulation. Treatment with triptolide inhibited NGF stimulation and invasion decreased to 5.571 (± 0.286) invaded cells, similar to control. Inhibition of in vitro invasion was also observed upon co-culture with neuroblastoma cell line, SHSY-5Y (Figure 5b). MIA PaCa-2 cells invaded significantly more when neural cells were cultured at the bottom of the well (41.50 ± 5.50 invaded cells, compared to control (9.857 ± 2.00)), which was inhibited by triptolide treatment (7.00 ± 1.00). Conversely, when the invasiveness of SHSY-5Y cells were tested with MIA PaCa-2 cells cultured at the bottom of the well, their increased invasiveness (45.07 ± 5.36 invaded cells, compared to control (0.286 invaded cells ± 1.43)), again, inhibited by triptolide treatment (7.00 invaded cells ± 1.00) (Figure 5c).
Figure 5.
Inhibition of neurotrophic signaling decreases neural invasion in pancreatic cancer cells: triptolide treatment inhibits increased MIA PaCa-2 invasion mediated by (a) NGF stimulation and (b) neural co-culture; triptolide also inhibits SHSY-5Y invasion stimulated by MIA PaCa-2 co-culture; (d) SHSY-5Y co-culture increases MIA PaCa-2 neurotrophin and EMT related gene expression; (e) conditioned medium from KPC cells increased neurite outgrowth from murine DRG and triptolide treatment decreases this outgrowth; (f) triptolide treatment decreased neurotrophin and neurotrophin receptor gene expression in cultured murine DRGs. Each bar is representative of three or more independent experiments; error bars are represented in SEM; and the asterisk (*) indicates a p value < 0.05.
Co-culture of SHSY-5Y cells increased gene expression of neurotrophins and EMT markers in MIA PaCa-2 cells (Figure 5d). Upon stimulation by neural cells, increased gene expression in artemin (1.470 fold ± 0.349), glial derived neurotropic factor (GDNF) (2.153 fold ± 0.579), and neural growth factor (NGF) (2.930 fold ± 0.378). This also increased EMT-associated gene expression: SNAI1 (2.098 fold ± 0.295), SLUG (3.229 fold ± 1.005), TWIST1 (6.575 fold ± 3.264), ZEB1 (2.278 fold ± 0.347), CDH2 (2.722 fold ± 0.901), VIM (2.961 fold ± 1.338), and MMP7 (2.206 fold ± 0.279).
As an ex vivo method, murine dorsal root ganglia (DRGs) were assayed for outgrowth when stimulated with conditioned cancer cell medium. Conditioned medium from KPC cells (KPC C.M.) increased outgrowth length of DRGs, as compared to control medium. Conditioned KPC medium with a low concentration of triptolide inhibited outgrowth completely (Figure 5e). Triptolide treatment of DRGs decreased gene expression of neurotrophins and neurotrophin receptors (Figure 5f): ARTN (0.300 fold ± 0.022), GDNF (0.294 fold ± 0.035), NGF (0.873 fold ± 0.022), GRFa3 (0.135 fold ± 0.006), TRKB (0.133 fold ± 0.009), TRKA (0.271 fold ± 0.000), NTRp75 (0.257 fold ± 0.135).
Inhibition of neurotrophic signaling in vivo decreases neural invasion
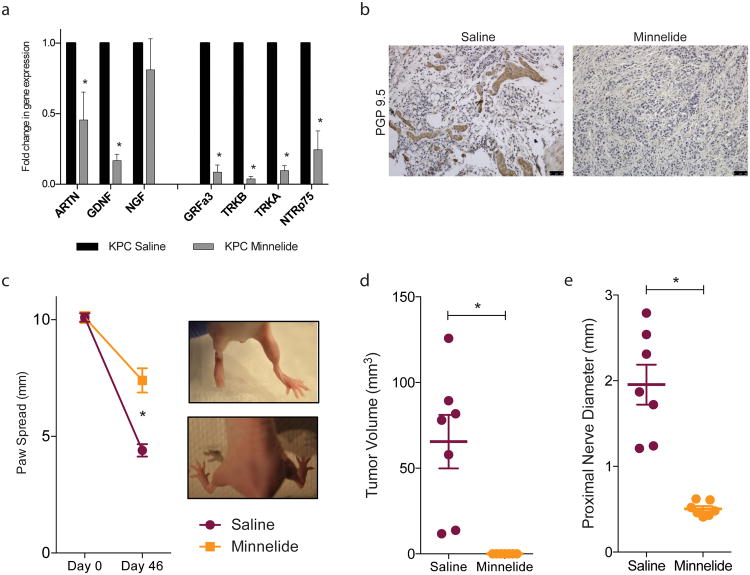
Using the spontaneous pancreatic cancer murine model, KPC (LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre transgenic), mice were treated with Minnelide, a pro-drug, water soluble formulation of triptolide. Minnelide treatment decreased expression of neurotrophins ARTN (0.456 fold ± 0.197) and GDNF (0.167 fold ± 0.045) and neurotrophin receptors GRFa3 (0.085 fold ± 0.051), TRKB (0.038 fold ±0.018), TRKA (0.097 fold ± 0.035), NTRp75 (0.244 fold ± 0.133) (Figure 6a). PGP 9.5 staining of the tumors revealed that the thickness and density of intratumoral nerves was decreased (Figure 6b).
Figure 6.
Inhibition of neurotrophic signaling in vivo decreases neural invasion: Minnelide (a) decreased neurotrophin and neurotrophin receptor gene expression in KPC tumors and (b) nerve size and density. In a sciatic nerve invasion model Minnelide prevented nerve destruction as measured by (c) increased paw spread as compared to saline control; (d) decreased primary tumor volume in the sciatic nerve and (e) decreased invasion through the nerve as determined by nerve diameter proximal to the primary tumor site. Each bar is representative of three or more independent experiments; error bars are represented in SEM; and the asterisk (*) indicates a p value < 0.05.
Next, using an in vivo assay of invasion, MIA PaCa-2 cells were injected into the sciatic nerve of athymic mice. Treatment with Minnelide inhibited invasion of cells through the nerve, preserving nerve function. Nerve function was determined by paw spread (Figure 6c), where at day 0 both saline and Minnelide groups had a paw spread of 10.10 ± 0.18 and 0.23, respectively. By day 46, paw spread decreased to 4.40 ± 0.27 and Minnelide only decreased to 7.40 ± 0.52. To examine the extent of neural invasion, tumor volume and proximal nerve diameter were measured. Tumor volume decreased from saline control (65.49 mm3 ± 15.62) to 0.0 mm3 ± 0.0 in Minnelide treatment group (Figure 6d). To determine the invasion within the nerve, the diameter of the nerve 2 cm from the primary tumor site was measured which demonstrated a significant decrease from 1.21 mm ± 0.23 in saline control to 0.50 mm ± 0.03 in Minnelide treatment group (Figure 6e).
Taken together, these date indicate that Minnelide treatment in vivo decreases neurotrophin signaling; nerve density and size; and neural invasion.
Discussion
The pancreatic tumor microenvironment contains several cellular components, which support tumor growth and progression. The interaction between cancer cells and intratumoral nerves has been studied in pancreatic cancer for several years, upon the observation that up to 100% of patients exhibit perineural invasion. Cancer cells have been known to upregulate receptors for growth factors not normally found on epithelial cells to utilize those factors secreted by other cell types in the tumor microenvironment. Pancreatic cancer cells upregulate many neural related receptors (NGFR, TRKA/B) and also secrete these neurotrophic factors themselves, providing growth factors for nerves and the capacity to benefit from the neurotrophins secreted from intratumoral nerves.
Pancreatic cancer neurotrophin stimulation by NGF has been shown to increase invasion in pancreatic cancer 11, 12. In this study, we show that NF-κB is an essential pathway for the regulation of invasion and metastasis in pancreatic cancer and the inhibition of this pathway leads to decreased EMT related marker expression.
Although, recent evidence has come out that induction of the epithelial-mesenchymal transition program isn't required for invasion and metastasis 26. In this study, we do demonstrate that many of these mesenchymal markers are upregulated upon the stimulation by neural cells, leading to increased invasiveness. We also see that the inhibition of NF-κB signaling either by triptolide/Minnelide treatment or pharmacological inhibition of NF-κB (BAY 11-7085) decrease in vitro invasion and in vivo metastasis; decreasing EMT transcription factions, mesenchymal markers, and increasing E-Cadherin at cellular junctions.
We also show in this study that Minnelide treatment decreases tumor-nerve crosstalk through the downregulation of both neurotrophins and neurotrophin receptors within neural, as well as, cancer cells. We also demonstrate that neural outgrowth and cancer cell invasion within the nerve is inhibited by treatment with Minnelide.
Neural invasion in pancreatic cancer has been shown especially relevant in pancreatic cancer, revealing an important, but less studied mechanism of metastasis. Perineural invasion is characterized as the invasion of tumor cells into, around, and through the nerve and has been hypothesized to be responsible for local tumor recurrence upon curative resection. We demonstrate in this study that Minnelide treatment is effective in limiting neural growth within the tumor and tumor growth within the nerve. Our group has also previously shown that Minnelide is capable of preventing tumor recurrence, even upon discontinuation of therapy, indicating that this therapy may be effective in eliminating primary tumor burden, as well as, either preventing neural invasion or treating any cancer cells that have already invaded the nerves. Minnelide is currently undergoing phase I clinical trials at several sites in the United States.
Several studies have described an increase in nerve size and density within pancreatic ductal adenocarcinoma 7, 8. Similarly, in this study, we saw a decrease in intratumoral neuronal size and density with the treatment of Minnelide.
In conclusion, this study demonstrates the importance of NF-κB activity in the induction of epithelial-mesenchymal transition, invasion, and metastasis as well as in neural invasion in pancreatic cancer.
Supplementary Material
Acknowledgments
Financial support: This study was funded by NIH grants R01-CA170946 and R01-CA124723 (to AKS); NIH grant R01-CA184274 (to SB); Katherine and Robert Goodale foundation support (to AKS), Minneamrita Therapeutics LLC (to AKS) and Alice Nomura was supported by T32 DA007097-32.
Footnotes
Conflict of interest disclosure statement: University of Minnesota has a patent for Minnelide, which has been licensed to Minneamrita Therapeutics, LLC. AKS is the co-founder and the Chief Scientific Officer of this company. Dr. Banerjee is a consultant with Minneamrita Therapeutics LLC and this relationship is managed by University of Miami. The other authors have nothing to disclose.
References
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1-2):349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liebig C, Ayala G, Wilks JA, et al. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115(15):3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 5.Liu B, Lu KY. Neural invasion in pancreatic carcinoma. Hepatobiliary & pancreatic diseases international : HBPD INT. 2002;1(3):469–476. [PubMed] [Google Scholar]
- 6.Pour PM, Bell RH, Batra SK. Neural invasion in the staging of pancreatic cancer. Pancreas. 2003;26(4):322–325. doi: 10.1097/00006676-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Ceyhan GO, Bergmann F, Kadihasanoglu M, et al. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136(1):177–186 e171. doi: 10.1053/j.gastro.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Demir IE, Ceyhan GO, Rauch U, et al. The microenvironment in chronic pancreatitis and pancreatic cancer induces neuronal plasticity. Neurogastroenterol Motil. 2010;22(4):480–490. e112–483. doi: 10.1111/j.1365-2982.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- 9.Ceyhan GO, Giese NA, Erkan M, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244(2):274–281. doi: 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada Y, Eibl G, Duffy JP, et al. Glial cell-derived neurotrophic factor upregulates the expression and activation of matrix metalloproteinase-9 in human pancreatic cancer. Surgery. 2003;134(2):293–299. doi: 10.1067/msy.2003.239. [DOI] [PubMed] [Google Scholar]
- 11.Okada Y, Eibl G, Guha S, et al. Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis. 2004;21(4):285–292. doi: 10.1023/b:clin.0000046131.24625.54. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Z, Kleeff J, Kayed H, et al. Nerve growth factor and enhancement of proliferation, invasion, and tumorigenicity of pancreatic cancer cells. Mol Carcinog. 2002;35(3):138–147. doi: 10.1002/mc.10083. [DOI] [PubMed] [Google Scholar]
- 13.Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, et al. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. The Journal of biological chemistry. 2003;278(24):21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 14.Maier HJ, Schmidt-Strassburger U, Huber MA, et al. NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer letters. 2010;295(2):214–228. doi: 10.1016/j.canlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Fujioka S, Sclabas GM, Schmidt C, et al. Inhibition of constitutive NF-kappa B activity by I kappa B alpha M suppresses tumorigenesis. Oncogene. 2003;22(9):1365–1370. doi: 10.1038/sj.onc.1206323. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Abbruzzese JL, Evans DB, et al. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 1999;5(1):119–127. [PubMed] [Google Scholar]
- 17.Alsaied OA, Sangwan V, Banerjee S, et al. Sorafenib and triptolide as combination therapy for hepatocellular carcinoma. Surgery. 2014;156(2):270–279. doi: 10.1016/j.surg.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee S, Sangwan V, McGinn O, et al. Triptolide-induced cell death in pancreatic cancer is mediated by O-GlcNAc modification of transcription factor Sp1. The Journal of biological chemistry. 2013;288(47):33927–33938. doi: 10.1074/jbc.M113.500983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rousalova I, Banerjee S, Sangwan V, et al. Minnelide: a novel therapeutic that promotes apoptosis in non-small cell lung carcinoma in vivo. PLoS One. 2013;8(10):e77411. doi: 10.1371/journal.pone.0077411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee S, Nomura A, Sangwan V, et al. Minnelide reduces CD133+ tumors initiating “stem-like” cells in a syngenic murine model of pancreatic ductal adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-13-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichert M, Takano S, Heeg S, et al. Isolation, culture and genetic manipulation of mouse pancreatic ductal cells. Nat Protoc. 2013;8(7):1354–1365. doi: 10.1038/nprot.2013.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chua HL, Bhat-Nakshatri P, Clare SE, et al. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26(5):711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 23.Huber MA, Azoitei N, Baumann B, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114(4):569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maier HJ, Schmidt-Strassburger U, Huber MA, et al. NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer letters. 2010;295(2):214–228. doi: 10.1016/j.canlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Chugh R, Sangwan V, Patil SP, et al. A preclinical evaluation of minnelide as a therapeutic agent against pancreatic cancer. Science translational medicine. 2012;4(156):156ra139. doi: 10.1126/scitranslmed.3004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.