Abstract
Purpose
To evaluate the safety and potential efficacy of gevokizumab, an anti-interleukin 1β (IL-1β) monoclonal antibody, in the treatment of active, non-infectious, non-necrotizing, anterior scleritis.
Design
Phase 1/2, open label, non-randomized, prospective, single-arm, pilot trial
Methods
Eight patients with active, non-infectious, non-necrotizing, anterior scleritis with a scleral inflammatory grade of +1 to +3 in at least one eye were enrolled. In one patient, both eyes were enrolled, for a total of nine eyes (four eyes with +1, one eye with +2, and four eyes with +3). Patients received one subcutaneous injection of 60 mg gevokizumab at baseline and then every four weeks for 12 weeks. Complete physical and ocular examinations were performed at each visit. The primary outcome was at least a 2-step reduction or reduction to grade 0 in scleral inflammation on a 0 to +4 scale according to a standardized photographic scleritis grading system by 16 weeks in the study eye compared to baseline. Secondary outcomes included changes in visual acuity, intraocular pressure, and trends in scleral grading. Participants who met the primary outcome were eligible to continue in the study for up to 52 weeks and received additional gevokizumab injections every four weeks until week 36 followed by two safety visits at weeks 40 and 52.
Results
Seven eyes from seven patients met the primary outcome within a median time of two weeks following the first gevokizumab injection. No definitive changes in visual acuity or IOP were identified. There were no serious adverse events related to the study drug. A total of 43 adverse effects were reported with 93% described as mild, 95% as non-ocular, and only 14% deemed possibly caused by the investigational treatment.
Conclusions
The results of this small study suggest that blockage of IL-1β using gevokizumab may be beneficial in treating active, non-infectious, anterior scleritis and that gevokizumab is well-tolerated. Larger randomized trials are warranted to assess the true efficacy of gevokizumab in the treatment of non-necrotizing anterior scleritis.
Introduction
Non-infectious anterior scleritis, a potentially vision-threatening condition which may be necrotizing or non-necrotizing, is often associated with systemic inflammatory diseases, such rheumatoid arthritis, spondyloarthropathies, and granulomatosis with polyangiitis.1 Non-necrotizing anterior uveitis is categorized as nodular or diffuse depending on the extent and morphology of the scleral lesions. While mild cases of non-necrotizing non-infectious anterior scleritis may be treated with oral non-steroidal anti-inflammatory drugs (NSAIDs), cases of more severe non-necrotizing or any necrotizing non-infectious scleritis require systemic corticosteroids and often steroid-sparing immunomodulatory therapy (IMT), including antimetabolites (e.g. azathioprine, methotrexate, mycophenolate),2–5 T cell inhibitors (e.g. cyclosporine A),6 cytotoxic agents (e.g. cyclophosphamide),6 or biologic agents (e.g. adalimumab, infliximab, rituximab).7–11
Several inflammatory cell types, including T and B lymphocytes, and cytokines, including TNF-α, have been implicated in the pathogenesis of non-infectious scleritis,12 and targeting these cells and cytokines with the above mentioned medications has proven beneficial in treating this disease in small to medium-sized cohorts. Interleukin 1β (IL-1β) is another inflammatory cytokine implicated in the immunopathogenesis of non-infectious scleritis. Patients with diffuse anterior scleritis were found to have elevated levels of IL-1β in their serum compared to healthy controls,13 and blockade of the IL-1 receptor with anakinra has been reported to improve anterior scleritis associated with rheumatoid arthritis (RA).14 In the current study, we sought to investigate the safety and potential efficacy of gevokizumab, an anti-IL-1β monoclonal antibody, in the treatment of active, non-infectious anterior scleritis.
Methods
This was a phase I/II nonrandomized, prospective, single-center study that evaluated subcutaneous gevokizumab (XOMA, Berkeley, CA) as a treatment for active, non-infectious, non-necrotizing anterior scleritis. The study protocol was reviewed and approved by the Institutional Review Board of the National Institutes of Health, a HIPAA-compliant institution, and all procedures conformed to the tenets of the Declaration of Helsinki (Clinical Trials registration: NCT01835132; NEI protocol ID: 13-EI-0102). Informed consent was obtained from all participants at the time of enrollment. Patients received one subcutaneous injection of 60 mg gevokizumab at baseline and then every four weeks for 12 weeks. Participants could continue taking ≤ 20 mg/day prednisone or equivalent during the study. All other immunosuppressive medications had to be stopped upon receiving the first injection of gevokizumab. Participants who met the primary outcome measure, as defined below, and did not develop a severe scleritis complication (e.g., corneal complications) that caused vision loss > 20 ETDRS letters, were eligible to continue in the first extension phase of the study. In this extension phase, participants received six additional gevokizumab injections every four weeks until week 36 followed by two safety visits at weeks 40 and 52. After 52 weeks, patients were eligible for a second extension phase in which they would receive gevokizumab injections at weeks 52, 54, 58, 62 and then as needed for flares of scleritis at the Investigator’s discretion until week 110. However, the implementation of second extension was delayed with an average of 10.2 months (range 7.5–14) interruption between the last injection under the first extension phase and the reintroduction of treatment under the second extension phase.
Inclusions and exclusion criteria
Inclusion criteria included age ≥18 years and a diagnosis of active, non-infectious, non-necrotizing anterior scleritis. The study eye was required to have ≥1+ scleritis in at least one quadrant based on a standardized grading system, the NEI Scleritis Grading Scale,15 and visual acuity of 20/640 or better. If both eyes met the criteria for the study eye, both eyes were analyzed and evaluated. Participants on systemic anti-inflammatory therapy (including corticosteroids) must not have had a dose escalation in any of their immunosuppressive treatments within the last four weeks prior to enrollment. Participants must not have received another systemic biologic immunosuppressive agent (e.g. infliximab, daclizumab, etanercept, adalimumab, or anakinra) within the last three months or rituximab or an alkylating agent (e.g. cyclophosphamide) within the last 12 months prior to enrollment. Exclusion criteria included any active infection requiring treatment, a history of tuberculosis (TB), human immunodeficiency virus (HIV), Hepatitis B or C, or a history of cancer (other than a non-melanoma skin cancer or carcinoma in situ of the cervix) diagnosed within the last five years. Also, eyes with periocular or intravitreal steroid injection within the last six weeks prior to enrollment, dexamethasone intravitreal implant (Ozurdex) within the last six months prior to enrollment, fluocinolone intravitreal implant (Retisert) within the last three years prior to enrollment, or intraocular surgery of any kind in the last four weeks prior to enrollment were excluded.
Ophthalmic and medical evaluations
At all visits, participants underwent a complete physical and ocular examination that included visual acuity assessment using the standardized Early Treatment Diabetic Retinopathy Study (ETDRS) refraction protocol, vital signs, concomitant medications assessment, adverse event assessment, intraocular pressure, slit-lamp examination, dilated fundus examination, standardized scleral photographs, complete blood count with differential, basic metabolic panel, urine pregnancy testing in female participants, and urinalysis.
Primary, secondary, and safety outcomes
The primary outcome was at least a 2-step reduction or reduction to grade 0 in scleral inflammation in the study eye (or eyes, if both eyes meet study eye criteria) in at least one quadrant according to a standardized photographic scleritis grading system developed at NEI,15 on or before the week 16 visit as compared to baseline. Two separate investigators evaluated the primary outcome, which was based on scores from the clinical exam and not scleral photographs of the participant. If there was a difference in the grading between the two investigators, then the difference was discussed between the investigators and an opinion was reached by consensus. The secondary outcomes included mean and median change in visual acuity via ETDRS at all follow-up visits, changes in intraocular pressure, and trends in scleral grading. Safety outcomes included the number and severity of systemic and ocular toxicities and adverse events and the proportion of participants with loss of ≥ 15 ETDRS letters at any follow-up visit.
Study drug administration
All participants received four subcutaneous injections of 60 mg gevokizumab administered at baseline and weeks 4, 8 and 12. At week 16, participants were assessed for eligibility in the extension phase of the study. Participants were eligible for the extension phase if they did not develop a severe scleritis complication (e.g., corneal complications) that caused vision loss > 20 ETDRS letters during the first 16 weeks of the study, if they showed a 2-step reduction or reduction to grade 0 in scleral inflammation in the study eye (or eyes, if both eyes meet study eye criteria) on or before week 16 (primary outcome), and if they elected to participate in the extension phase of the study. Six of eight participants continued into the extension phase, and the remaining two participants did not meet the study primary outcome. No additional injections were given outside the scheduled injections.
Results
Nine eyes from eight patients (patient 2 had both eyes enrolled) with non-infectious anterior scleral inflammation ranging from +1 to +3 were enrolled (four patients with +1, one patient with +2, and four patients with +3). The average age was 59.9 years (range 34–81), and there were four female and four male patients (Table 1). All patients had previously been treated with systemic therapy (details listed in Table 1). Patient 8 had scleral inflammation recalcitrant to several immunosuppressive agents, including infliximab, adalimumab and rituximab. Two patients had identifiable systemic disease associations (patient 2 had RA and patient 8 had systemic lupus erythematosis and Sjogren’s syndrome).
Table 1.
Demographics, treatment history, and systemic disease associations in patients with autoimmune anterior uveitis treated with gevokizumab.
| Pt | Demographics | Prior systemic treatments | Systemic associations |
Concomitant Steroids |
|---|---|---|---|---|
| 1 | 62 year-old white man |
Oral corticosteroids, mycophenolate mofetil (off both at the time of enrollment) |
None | None |
| 2 | 50 year-old black woman |
Oral corticosteroids (off prior to enrollment), methotrexate (last dose 15mg one month prior to enrollment) |
Rheumatoid arthritis |
Topical prednisolone acetate BID |
| 3 | 34 year-old white woman |
Intravenous corticosteroids (off prior to enrollment) |
None | none |
| 4 | 75 year-old black man |
Indomethacin (50mg/day stopped ~4 months prior to enrollment) |
None | Topical loteprednol TID |
| 5 | 65 year-old white man |
Ibuprofen (off prior to enrollment), methotrexate (last dose 10mg five days prior to enrollment), mycophenolate mofetil (1250mg BID stopped upon enrollment), infliximab (off prior to enrollment) |
None | Topical loteprednol BID |
| 6 | 61 year-old black man |
Intravenous corticosteroids (off prior to enrollment) |
None | None |
| 7 | 81 year-old white man |
Indomethacin (50mg TID stopped upon enrollment) |
None | none |
| 8 | 51 year-old white woman |
Oral corticosteroids (prednisone 7.5mg/day), adalimumab (off for 3 months prior to enrollment), cyclosporine (off prior to enrollment), azathioprine (off prior to enrollment), methotrexate (off prior to enrollment), mycophenolate mofetil (off prior to enrollment), rituximab (off prior to enrollment) |
Systemic lupus erythematosus, Sjogren’s syndrome |
Oral prednisone (max dose 7.5mg/day), Topical loteprednol QID |
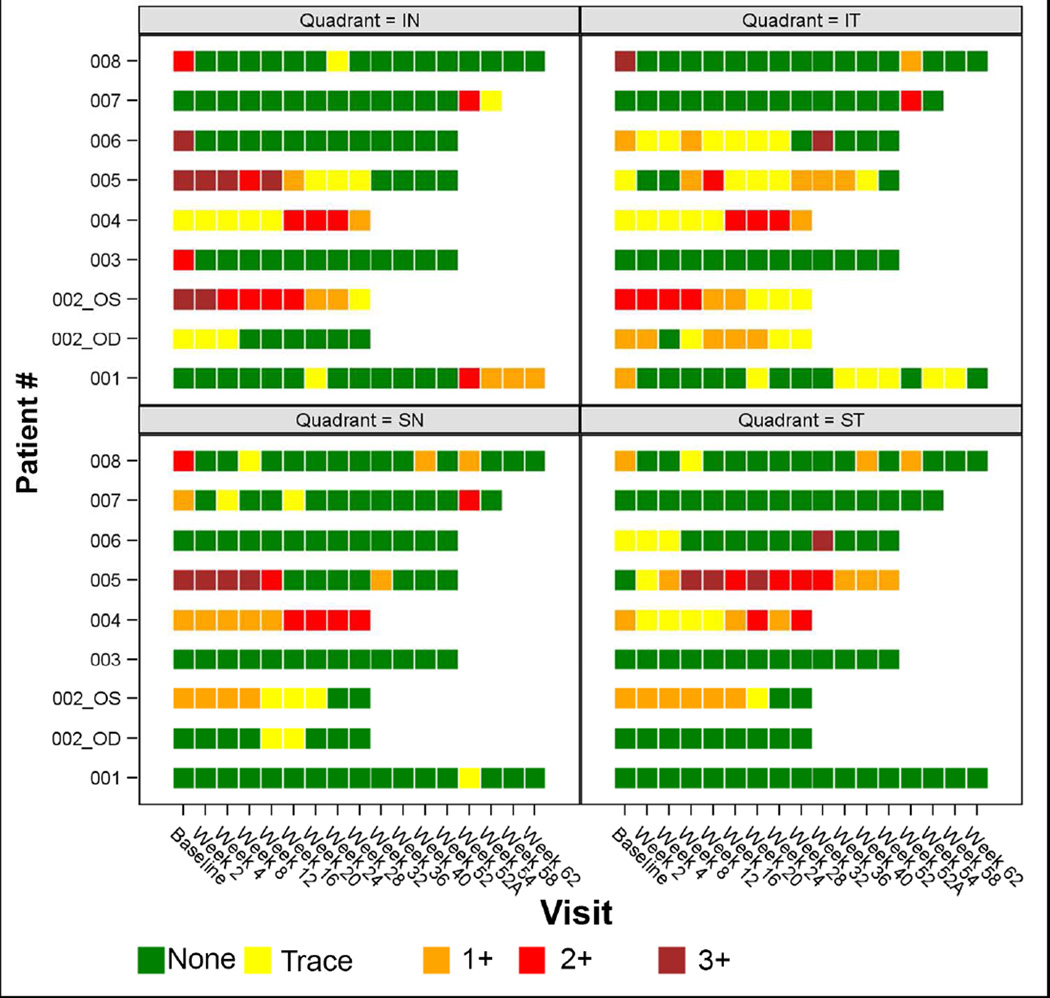
Seven eyes from seven patients met the primary outcome within 16 weeks (median 2 weeks) following the first gevokizumab injection (Fig. 1 & 2). Among the seven eyes that met the primary outcome, there were three eyes with +3, one eye with +2, and three eyes with +1 scleral inflammation at baseline. Most patients maintained the initially achieved positive response, although some patients experienced new activity in previously uninvolved quadrants (patients 2 & 5).
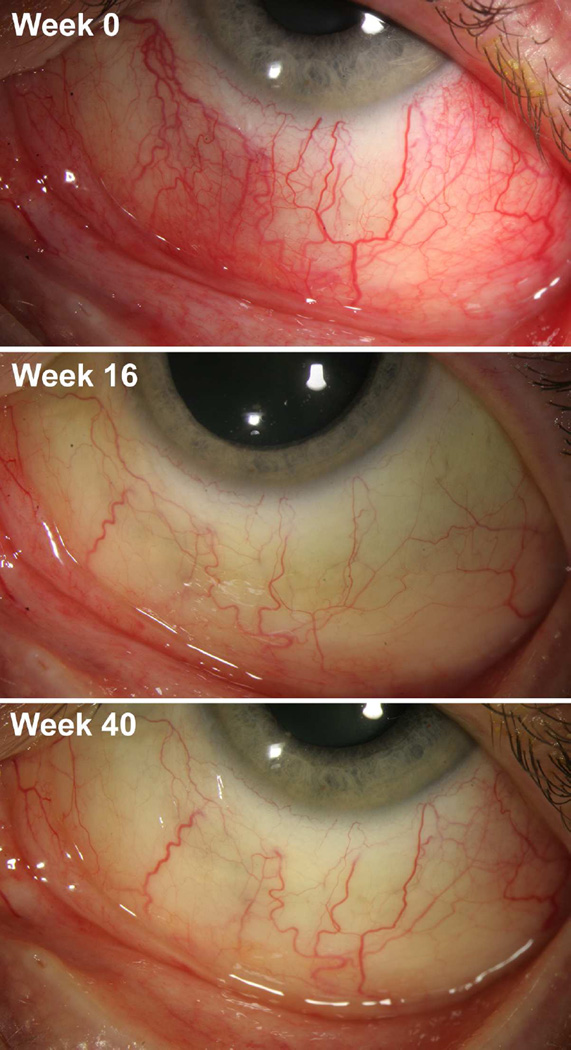
Figure 1.
Scleral photographs of patient 1. Scleral photographs of the inferior left eye were taken after administration of topical phenylephrine 10% at the indicated times in the study. Week 0 was the baseline image before gevokizumab treatment, week 16 was the primary end point after four monthly gevokizumab injections, and week 40 was four weeks following the last gevokizumab injection of the first extension phase.
Figure 2.
Graphical representation of clinical scleral grades for all patients. Each scleral quadrant is represented separately. Green blocks represent an inflammatory score of 0, yellow blocks represent trace (0.5+), red blocks represent 2+, and maroon blocks represent 3+. Visit week 52A designates enrollment in the second extension phase, and was different for the three patients (1, 7 & 8) averaging 6.2 months after their week 52 visit.
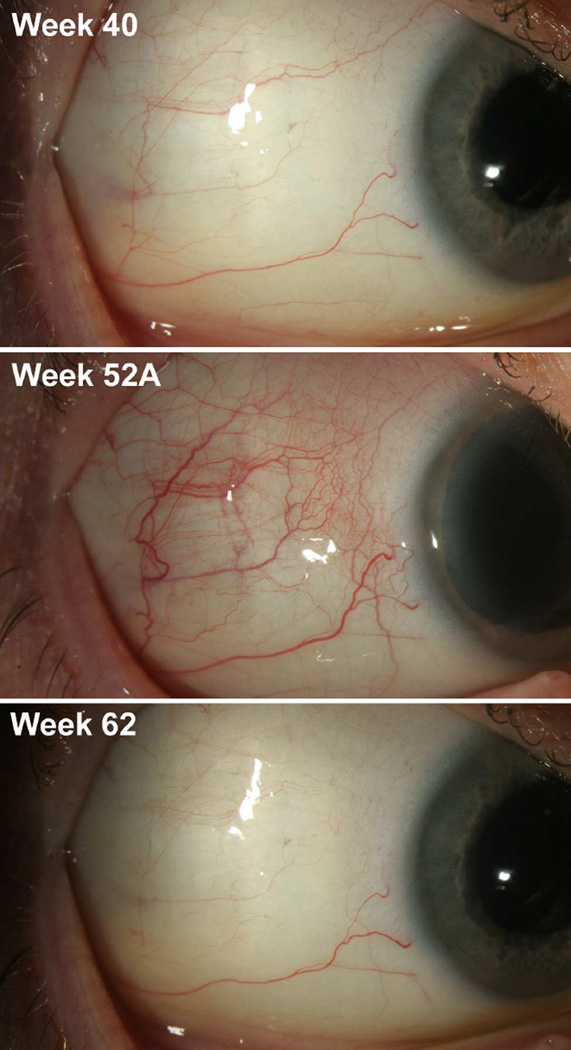
Six patients who met the primary outcome at week 16 were continued in the first extension phase of the study (patients 1, 3, 5, 6, 7 & 8). While the right eye of patient 2 met the primary outcome, the more severely affected left eye did not substantially improve with treatment; therefore, she was not continued in the extension phase to allow for alternative therapy. Patient 4 (one study eye) failed to meet the primary outcome by week 16. In general, eyes that responded by week 16 maintained their response through week 52 (Fig. 2). Three patients (patients 1, 7 & 8) continued into the second extension phase of the study. The last treatment of the first extension phase occurred on week 36 with no treatments administered on weeks 40 or 52, which were safety visits. Implementation of the second extension phase was delayed, and there was an average of 10.2 months (range 7.5–14) interruption between the last treatment under the first extension phase and the reintroduction of treatment under the second extension phase. The second extension phase was planned for re-induction injections (0, 2, 6 & 10 weeks) followed by pro renata (PRN) injections with study visits occurring every 8 weeks after the re-induction. At the start of the second extension phase, all three patients had recurrent scleral inflammation (grades 1+−2+). All three patients experienced a reduction to grade 0 in at least one quadrant after a single gevokizumab injection during the second extension phase (Fig. 2 & 3). All patients received 4 treatments as part of the “re-induction” in the second extension phase; however, the trial was discontinued early by the company due to the company’s decision to cease development activities in ophthalmology.
Figure 3.
Scleral photographs of patient 8. Scleral photographs of the temporal right eye were taken after administration of topical phenylephrine 10% at the indicated times in the study. Week 40 is four weeks following the last gevokizumab injection of the first extension phase. Week 52A represents the enrollment date of the second extension phase, which in this patient occurred 7.5 months after her last gevokizumab injection as part of the first extension phase. Week 62 represents the last time point for gevokizumab injection after receiving three injections at weeks 52A, 54, and 58.
No definitive changes in visual acuity or IOP were identified. There were no serious adverse events related to the study drug. However, one patient (patient 5) who met the primary outcome and continued in the first extension phase experienced progressive corneal thinning in the absence of clinical corneal or scleral inflammation. A total of 43 adverse effects were reported with 93% described as mild, 95% as non-ocular, and 14% as possibly caused by the investigational treatment (Table 2). Adverse effects deemed to be probably caused by the study medication included mild injection site discomfort (one patient), hepatic enzyme elevation (one patient), irritable bowel syndrome (three patients), and hypotension (one patient).
Table 2.
Summary of adverse events in patients with autoimmune anterior uveitis treated with gevokizumab.
| N (%) | |
|---|---|
| Severity | |
| Mild | 40 (93%) |
| Moderate | 2 (5%) |
| Severe | 1 (2%) |
| Eye | |
| Non-ocular | 41 (95%) |
| OD | 1 (2%) |
| OS | 1 (2%) |
| Outcome | |
| Resolved | 37 (86%) |
| Resolved with sequelae | 2 (5%) |
| Resolved by convention | 4 (9%) |
|
Reasonable possibility that the investigational product caused the event |
|
| No | 37 (86%) |
| Yes | 6 (14%) |
Ocular side effects included eye redness and stye.
Systemic adverse events with reasonable possibility of causal relationship with treatment included injection site achiness, elevated liver enzymes, irritable bowel syndrome, and hypotension.
Resolved: The adverse event resolved while the participant was still in the study and the date of resolution is known.
Resolved with sequelae: The adverse event resolved while the participant was still in the study, but the participant retained pathological conditions resulting from the reported adverse event.
Resolved by convention: The adverse event was considered resolved as a result of not being able to follow the adverse event to its actual resolution.
Discussion
Non-infectious anterior scleritis is a potentially blinding condition that is often associated with systemic inflammatory disease.1 Systemic immunosuppression in the form of corticosteroids or steroid-sparing IMT is often required in severe cases of scleral inflammation. Here, we report the results of a prospective, non-controlled, phase I/II clinical trial of gevokizumab, an anti-IL-1β monoclonal antibody, in the treatment of active, non-infectious anterior scleritis.
IL-1β is a proinflammatory cytokine secreted by innate immune cells, such as monocytes, macrophages, dendritic cells, and neutrophils,16 and macrophages and neutrophils have been identified in histopathologic specimens from eyes with scleritis.12 IL-1β binds to IL-1 receptor type 1 (IL-1R1) on target cells, which leads to activation of intracellular inflammatory signaling pathways. IL-1β signaling results in increased production of matrix metalloproteinases (MMPs),16 which mediate tissue destruction, such as that seen in scleritis.12 IL-1β has been reported to be elevated in many inflammatory diseases, including juvenile idiopathic arthritis (JIA), RA, Behçet disease, and non-infectious scleritis.13, 16 Results from two randomized trials demonstrated the efficacy of anti-IL-1β antibody therapy in patients with JIA,17 and several reports have suggested efficacy in Behçet disease, including Behçet-associated uveitis.18, 19 In these previous studies, different anti-IL-1β agents were used; namely canakinumab was used for the treatment of systemic JIA,17 anakinra or canakinumab was used for the treatment of Behçet disease,18 and gevokizumab for the treatment of Behçet uveitis.19 Furthermore, a recent prospective non-controlled trial also concluded that systemic gevokizumab (administered either intravenously or subcutaneously) was well tolerated and rapidly controlled acute exacerbations of uveitis associated with Behçet disease.20 Evidence for targeting this cytokine as a therapeutic strategy in the treatment of non-infectious scleritis is limited with a single report of two patients treated with anakinra, an IL-1R antagonist, suggesting improvement in scleral inflammation.14
Here, we report clinically meaningful improvements in scleritis scores in seven of nine eyes from seven of eight patients treated with monthly subcutaneous injections of the anti-IL-1β monoclonal antibody gevokizumab. Responses to gevokizumab were sustained in most patients through 52 weeks with ongoing monthly gevokizumab treatments; however, some patients experienced new activity in previously uninvolved quadrants (patients 2 & 5) and therefore were considered clinical failures despite meeting the definition of success for the study. While the two eyes from two participants that did not meet the primary outcome began the study with severe 3+ scleral inflammation, two other participants began the study with severe 3+ scleral inflammation and responded to gevokizumab treatment, including patient 8 whose scleritis was recalcitrant to several immunosuppressive agents, suggesting that the more severe cases were not necessarily refractory to treatment with subcutaneous gevokizumab. However, the small number of participants precludes identification of any particular pattern of treatment responses. Three patients continued into the second extension phase after not receiving gevokizumab treatment for an average of 10.2 months and all had recurrent scleral inflammation that was reduced to grade 0 in at least one quadrant following a single repeat injection of gevokizumab, thus demonstrating a positive challenge-dechallenge-rechallenge response. Unfortunately, the second extension phase was limited to three patients who received four additional injections due to the company’s decision to cease development activities in ophthalmology.
No serious adverse events have been reported in relation to gevokizumab use.21 Of note, no serious infections have been reported. The most common reported side effects include upper respiratory tract infection, headache, and dizziness. In the current study, no serious adverse events were reported and only 14% of 43 reported adverse events were considered possibly caused by gevokizumab. These included mild injection site discomfort, hepatic enzyme elevation, irritable bowel syndrome, and hypotension.
As a phase I/II trial, the major limitations of this study include the small number of patients and non-controlled, non-masked design. Nonetheless, the study medication appeared to be well tolerated by all participants without any serious adverse events related to the study drug, and there was a 78% (7/9 eyes) response rate. The results of this small study suggest that larger randomized trials are warranted to assess efficacy and safety of gevokizumab in the treatment of non-infectious anterior scleritis.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by the Intramural Research Program of the NIH, NEI. The study medication was provided by XOMA Corporation (Berkeley, CA).
Other Acknowledgements: None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: American Uveitis Society Spring Meeting; Seattle, WA; April 30, 2016
Financial Disclosures: No conflicting relationship exists for any author.
References
- 1.Rachitskaya A, Mandelcorn ED, Albini TA. An update on the cause and treatment of scleritis. Curr Opin Ophthalmol. 2010;21(6):463–467. doi: 10.1097/ICU.0b013e32833f1060. [DOI] [PubMed] [Google Scholar]
- 2.Gangaputra S, Newcomb CW, Liesegang TL, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009;116(11):2188–2198. e1. doi: 10.1016/j.ophtha.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasadhika S, Kempen JH, Newcomb CW, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol. 2009;148(4):500–509. e2. doi: 10.1016/j.ajo.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen HN, Suhler EB, Al-Khatib SQ, Djalilian AR, Nussenblatt RB, Buggage RR. Mycophenolate mofetil for the treatment of scleritis. Ophthalmology. 2003;110(9):1750–1755. doi: 10.1016/S0161-6420(03)00570-0. [DOI] [PubMed] [Google Scholar]
- 5.Thorne JE, Jabs DA, Qazi FA, Nguyen QD, Kempen JH, Dunn JP. Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology. 2005;112(8):1472–1477. doi: 10.1016/j.ophtha.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Kacmaz RO, Kempen JH, Newcomb C, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117(3):576–584. doi: 10.1016/j.ophtha.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan S, Kamal A, Thompson RN, Estrach C, Moots RJ. Rituximab for treatment of scleritis associated with rheumatoid arthritis. Br J Ophthalmol. 2009;93(7):984–985. doi: 10.1136/bjo.2008.147157. [DOI] [PubMed] [Google Scholar]
- 8.Kurz PA, Suhler EB, Choi D, Rosenbaum JT. Rituximab for treatment of ocular inflammatory disease: a series of four cases. Br J Ophthalmol. 2009;93(4):546–548. doi: 10.1136/bjo.2007.133173. [DOI] [PubMed] [Google Scholar]
- 9.Sen HN, Sangave A, Hammel K, Levy-Clarke G, Nussenblatt RB. Infliximab for the treatment of active scleritis. Can J Ophthalmol. 2009;44(3):e9–e12. doi: 10.3129/i09-061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suhler EB, Lim LL, Beardsley RM, et al. Rituximab therapy for refractory scleritis: results of a phase I/II dose-ranging, randomized, clinical trial. Ophthalmology. 2014;121(10):1885–1891. doi: 10.1016/j.ophtha.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 11.Restrepo JP, Molina MP. Successful treatment of severe nodular scleritis with adalimumab. Clin Rheumatol. 2010;29(5):559–561. doi: 10.1007/s10067-009-1368-8. [DOI] [PubMed] [Google Scholar]
- 12.Wakefield D, Di Girolamo N, Thurau S, Wildner G, McCluskey P. Scleritis: Immunopathogenesis and molecular basis for therapy. Prog Retin Eye Res. 2013:3544–3562. doi: 10.1016/j.preteyeres.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Palexas GN, Puren A, Savage N, Welsh NH. Serum interleukin (IL-1 beta) in patients with diffuse scleritis. Scand J Immunol Suppl. 1992:11171–11172. doi: 10.1111/j.1365-3083.1992.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 14.Botsios C, Sfriso P, Ostuni PA, Todesco S, Punzi L. Efficacy of the IL-1 receptor antagonist, anakinra, for the treatment of diffuse anterior scleritis in rheumatoid arthritis. Report of two cases. Rheumatology (Oxford) 2007;46(6):1042–1043. doi: 10.1093/rheumatology/kem052. [DOI] [PubMed] [Google Scholar]
- 15.Sen HN, Sangave AA, Goldstein DA, et al. A standardized grading system for scleritis. Ophthalmology. 2011;118(4):768–771. doi: 10.1016/j.ophtha.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schett G, Dayer JM, Manger B. Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol. 2016;12(1):14–24. doi: 10.1038/nrrheum.2016.166. [DOI] [PubMed] [Google Scholar]
- 17.Ruperto N, Brunner HI, Quartier P, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2396–2406. doi: 10.1056/NEJMoa1205099. [DOI] [PubMed] [Google Scholar]
- 18.Emmi G, Talarico R, Lopalco G, et al. Efficacy and safety profile of anti-interleukin-1 treatment in Behcet's disease: a multicenter retrospective study. Clin Rheumatol. 2015 doi: 10.1007/s10067-015-3004-0. [DOI] [PubMed] [Google Scholar]
- 19.Gul A, Tugal-Tutkun I, Dinarello CA, et al. Interleukin-1beta-regulating antibody XOMA 052 (gevokizumab) in the treatment of acute exacerbations of resistant uveitis of Behcet's disease: an open-label pilot study. Ann Rheum Dis. 2012;71(4):563–566. doi: 10.1136/annrheumdis-2011-155143. [DOI] [PubMed] [Google Scholar]
- 20.Tugal-Tutkun IM, Kadayifcilar SM, Khairallah MM, et al. Safety and Efficacy of Gevokizumab in Patients with Behcet's Disease Uveitis: Results of an Exploratory Phase 2 Study. Ocular immunology and inflammation. 2016:1–9. doi: 10.3109/09273948.2015.1092558. [DOI] [PubMed] [Google Scholar]
- 21.Rubin P, Cafaro DP, Kramer S, Zayed H, editors. Gevokizumab (XOMA 052) Investigator's Brochure (version 9) Berkeley, CA: 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.