Abstract
Some animals, including songbirds, vocalize at high rates when alone or in large groups. In songbirds, vocal behavior in these contexts is important for song learning and group cohesion. It is not obviously targeted at any particular individual and is described as “undirected.” Studies suggest a role for dopamine in undirected song. The neuropeptide neurotensin can enhance dopaminergic signaling upon binding to the neurotensin receptor 1 (NTR1) and is found in regions where dopamine can influence song, including the ventral tegmental area (VTA), septum, and the song-control nucleus Area X. To begin to test the hypothesis that neurotensin and dopamine in these regions interact to influence undirected song, we used quantitative real time PCR to relate undirected singing to mRNA expression of neurotensin, NTR1, tyrosine hydroxylase (a synthetic enzyme for dopamine), and D1 and D2 receptors in male European starlings. Tyrosine hydroxylase and neurotensin expression in VTA, and neurotensin and D1 expression in Area X, positively correlated with song. Neurotensin markers also correlated positively with dopamine markers in VTA. Given the role of VTA projections to Area X in song learning, these results suggest that interactions between neurotensin and dopamine in these regions may contribute to vocal learning. In septum, NTR1 expression positively correlated with song, and neurotensin and dopamine markers were correlated, suggesting that neurotensin in this region may influence dopaminergic transmission to facilitate undirected vocalizations. Overall, these findings implicate interactions between neurotensin and dopamine in affiliative communication.
Keywords: dopamine, neurotensin, vocal communication, motivation, social behavior, songbird
Introduction
Many species communicate with vocalizations that are specific to particular situations, such as courtship or territorial defense [Bradbury and Vehrencamp, 2011]. The neural circuitry underlying the learning and production of vocal signals is well-known for some species, such as songbirds [Zeigler and Marler, 2008], but it is less clear how the brain regulates vocalizations across contexts [Riters, 2012]. Neuropeptides may mediate behaviors in appropriate social contexts by modifying neurotransmitter activity in highly conserved brain regions that, across vertebrates, are involved in motivation and social behavior [Goodson, 2005; Newman, 1999; O'Connell and Hofmann, 2011]. However, relatively little is known about the function, adaptive value, or neural control of vocalizations used for purposes other than attracting mates or repelling intruders.
Some animals, including songbirds, vocalize at high rates spontaneously when alone or in large groups. In songbirds, this type of communication does not appear to be targeted at any particular individual even when produced in flocks, and it has been referred to as “undirected” in studies in zebra finches and more recently in European starlings [Dunn and Zann, 1996a; Immelmann, 1962; Kelm-Nelson and Riters, 2013; Riters, 2012]. The exact functions of undirected song may differ across species, but this type of song is generally thought to be important for flock maintenance [Hausberger et al., 1995] and song learning or practice [Eens, 1997; Kao et al., 2005]. Data also suggest that male undirected song may be attended to by females and used in future mating decisions [Dunn and Zann, 1996b; Holveck and Riebel, 2007, 2009] and that the production of undirected song may be rewarding [Riters and Stevenson, 2012; Riters et al., 2014].
In addition to being involved in motivation, reward, and learning [Berridge and Kringelbach, 2015; Björklund and Dunnett, 2007; Schultz, 2013], dopaminergic projections from the ventral tegmental area (VTA) to striatal regions also have a role in birdsong [Kubikova and Košťál, 2010; Riters, 2012; Simonyan et al., 2012]. In zebra finches, electrophysiological recordings show that neural activity in VTA increases when a male sings undirected song [Yanagihara and Hessler, 2006]. VTA sends dense dopaminergic projections to Area X [Lewis et al., 1981], a song-control nucleus involved in song learning [Scharff and Nottebohm, 1991; Sohrabji et al., 1990] and altering structural features of song [Leblois and Perkel, 2012; Leblois et al., 2010]. Immediate early gene (IEG) activity in Area X is greater during undirected song than directed song [Hara et al., 2007; Jarvis et al., 1998], and lesions of dopaminergic projections from VTA to Area X reduce IEG activity in Area X during undirected song [Hara et al., 2007]. Dopamine (DA) levels in Area X are higher during directed singing than undirected singing, but inhibition of DA reuptake removes this context difference, which may indicate that DA reuptake during undirected singing is greater [Sasaki et al., 2006]. DA in Area X affects acoustic features of undirected song, as use of a neurotoxin to reduce dopaminergic input to Area X decreases variability in undirected song [Miller et al., 2015]. IEG expression in Area X neurons containing D1 receptors increases when male zebra finches sing undirected song, but decreases in neurons containing D2 receptors [Kubikova et al., 2010]. In starlings, however, D2 receptors have not previously been linked to undirected song [Heimovics et al., 2009]. The lateral septum (LS) is another site in which DA may influence undirected song. Immunolabeling for the IEGs ZENK and cFOS in LS positively correlates with undirected song [Heimovics and Riters, 2006, 2007], and autoradiography for D1-like DA receptors in LS relates positively to undirected song in male starlings [Heimovics et al., 2009].
Neuropeptides are proposed to modify neurotransmitter activity to regulate behaviors in appropriate social contexts [O'Connell and Hofmann, 2011]. Neurotensin (NT) is a candidate neuropeptide to modulate undirected song because it strongly interacts with dopaminergic signaling [Binder et al., 2001] and is implicated in social behavior [Driessen et al., 2014; Gammie et al., 2009; Merullo et al., 2015b; Merullo et al., 2015a] and learning [Azmi et al., 2006; Feifel et al., 2009; Keiser et al., 2014; Laszlo et al., 2010; Tirado-Santiago et al., 2006; Xiao et al., 2014]. NT and the NT1 receptor (NTR1; the only known avian NT receptor [Numao et al., 2011]) co-localize with DA neurons in the ventral tegmental area (VTA) and are also found in LS and Area X [Atoji et al., 1996; Binder et al., 2001; Merullo et al., 2015b; Merullo et al., 2015a; Xie et al., 2010]. Upon binding to NTR1, NT can modulate dopaminergic transmission in two main ways: either by depolarizing DA neurons or reducing the affinity of inhibitory D2 receptors, both of which can result in an overall increase of dopaminergic release [Binder et al., 2001; Ferraro et al., 2016; Lu et al., 2009; St-Gelais et al., 2006]. Interactions between NT and DA may contribute to the process of incorporating new features into vocal repertoires during undirected song, since DA plays a role in song learning [Kubikova and Košťál, 2010; Simonyan et al., 2012] and dopaminergic projections from VTA to Area X are hypothesized to be especially critical for song acquisition [Fee and Goldberg, 2011].
The role of DA in undirected song and song learning, combined with the functional relationships between NT and DA, suggest that NT may interact with dopaminergic signaling to influence undirected song. To begin to test this hypothesis, we observed adult male starlings singing undirected song and used quantitative real time PCR (qPCR) to measure mRNA expression of tyrosine hydroxylase (TH; a precursor synthetic enzyme to DA), D1 receptors, D2 receptors, NT and NTR1. We focused on VTA, Area X, and LS because they are rich in NT and NTR1, and DA in these regions has been associated previously with undirected song.
Materials and Methods
Animals
This study used 21 male European starlings that had been caught during 2010–2014 in Madison, WI with baited fly-in traps and brought to the University of Wisconsin-Madison. Birds were housed indoors in stainless steel cages (91 cm × 47 cm × 47 cm) in single sex groups of 5. Food and water were provided ad libitum. All procedures and protocols followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Housing Conditions
Birds were housed indoors on a photoperiod of 18 h light:6 h dark for at least 6 weeks. Under this condition, starlings become photorefractory, a state in which they have regressed gonads and are not in reproductive condition [Dawson et al., 2001]. This mimics the early fall condition, when starlings sing high rates of undirected song that are important for learning [Böhner et al., 1990]. Males were randomly assigned to indoor aviaries (3.5 m × 2.25 m × 2 m) containing 4 individuals with 4 perches, a nest box, a water bath, and ad libitum food and water. Birds habituated to the aviaries for at least 2 weeks before behavioral testing began.
Behavioral Observations
A single experimenter seated behind a one-way mirror observed 4 birds simultaneously for 20 min and recorded behavior. Starling complete song consists of a distinct sequence of 4 vocal segments: introductory whistles, complex phrases, click-series, and high frequency phrases [Eens, 1997; Eens et al., 1989]. The observer recorded the number of complete songs, total time singing (s), incomplete songs (vocalized elements of the song sequence that do not comprise a complete song), and calls (non-song vocalizations). Displacements of other individuals were counted when a bird flew to within 5 cm of another bird and the approached bird immediately flew away. Other behaviors observed were bouts of feeding, drinking, and preening, with bouts separated by at least 2 s. Each of these behaviors is readily quantifiable in real time by an observer watching four starlings because starlings generally do not constantly change locations and an individual bird does not produce multiple behaviors simultaneously. These methods are common in studies of starling behavior and have been used previously to quantify behavior in flocks of this size [e.g. Eens et al., 1990; Merullo et al., 2015b; Merullo et al., 2015a].
We observed birds singing three times for 20 min. First we observed birds as part of a separate study not reported here (Day 1), then a second time (Day 2) five to 26 days later (mean = 11.29 days, median = 9.00, sd ± 6.47), and finally a third time (Day 3) approximately two days later (mean = 2.52, median = 2.00, sd ± 1.33). Starlings typically sing consistently across observation days [e.g., Riters et al., 2014], but in the present study the number of songs was not correlated across days. Birds sang most on Day 2 with a substantial drop in song production on Day 3 (mean / median / sd of time singing (s): Day 1 = 265.4 / 209.0 / 224.29; Day 2 = 282.7 / 318.0 / 189.34; Day 3 = 143.4 / 109.0 / 145.31). We therefore decided to use Day 2 for analysis because we consider the day of maximal singing nearest to brain collection to be most representative of a bird’s individual propensity to sing. Furthermore, for each individual bird, the number of songs on Day 2 was consistent with the number of songs on Day 1 and Day 3 with the exception of only four birds; one sang at high rates on Day 1, two sang at high rates on Day 2 only, and one sang at high rates on Day 3. Without these points, there are positive correlations between the number of songs on Day 2 and the number of songs on Day 1 (r = 0.53. p = 0.028) and Day 3 (r = 0.67, p = 0.0032). Thus, we consider that the consistency in singing behavior observed for 17 of 21 birds indicates persistent individual differences in the propensity to sing, and we focused on the day when birds sang most in order to collect a representative sample of general singing activity.
Tissue Collection and Tissue Preparation
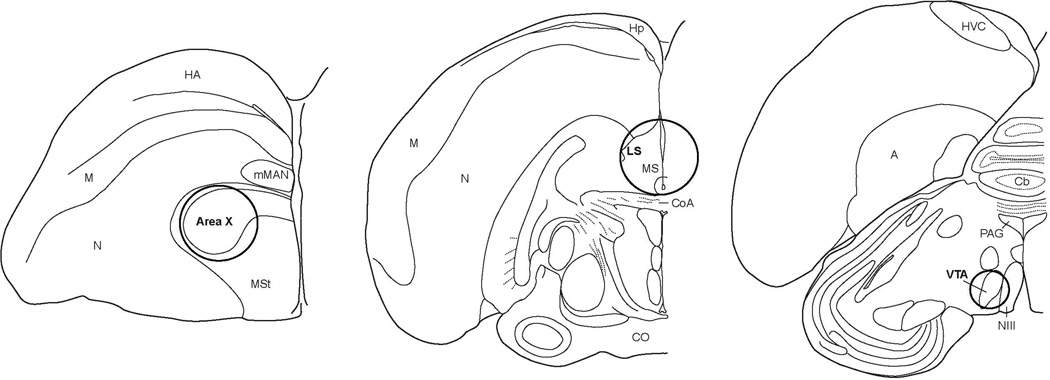
Animals were sacrificed within a week of the observation day (mean = 2.52 days, median = 2.00, sd ± 1.33). Brains (n = 21) were collected by rapid decapitation, fresh frozen in isopentane (Catalog No. 277258; Sigma-Aldrich, St. Louis, MO) chilled by dry ice for at least 30 s, and then stored at −80 °C. Brains were sectioned coronally at 200 µm on a cryostat (−14 °C) and mounted onto slides. Punches of samples were taken from target brain regions (in Area X, 4 punches of 2 mm diameter; in LS, 1 punch of 2 mm diameter; in VTA, 2 punches of 1 mm diameter; Fig. 1) using Fine Science Tools sample corers (Catalog Nos. 18035-01 and 18035-02; Foster City, CA). Due to technical limitations, tissue punches centered on LS also contained medial septum so we refer to this region as septum (see Fig. 1), and tissues punches centered on VTA also contained small amounts of surrounding area (see Fig. 1). Punches were then transferred to centrifuge tubes on dry ice and stored at −80 °C.
Figure 1.
Approximate sizes and locations for tissue punches in Area X (2 mm diameter), LS (2 mm diameter), and VTA (1 mm diameter). Abbreviations: A, arcopallium; Cb, cerebellum; CoA, anterior commissure; CO, optic chiasm; HA, hyperpallium accessorium; Hp, hippocampus; HVC, letter-based proper name; M, mesopallium; mMAN, medial magnocellular nucleus of the anterior nidopallium; MS, medial septum; MSt, medial striatum; N, nidopallium; NIII, third cranial nerve; LS, lateral septum; PAG, periaqueductal gray; VTA, ventral tegmental area.
RNA was extracted with the Bio-Rad Aurum Total RNA Fatty and Fibrous Tissue Kit (Catalog No. 732–6830; Bio-Rad, Hercules, CA) following the manufacturer’s instructions. RNA concentration was measured and integrity confirmed with NanoDrop (Thermoscientific, Wilmington, DE). RNA was converted to single-stranded cDNA with the Invitrogen SuperScript III First-Strand Synthesis System (Catalog No. 18080-051; Life Technologies, Carlsbad, CA) following the manufacturer’s instructions. RNA starting amounts for synthesis were 100 ng. cDNA pooled from tissue punches collected in surrounding regions was used for standards.
qPCR
Primers were designed and screened with NCBI Primer-Blast using either the zebra finch (Taeniopygia guttata) or chicken (Gallus gallus) genome. Primer quality was examined on NetPrimer (Premier Biosoft) for secondary structures. Details of the primers are in Table 1. Sanger sequencing of both the forward and reverse primers was done at the University of Wisconsin Biotechnology Center for all genes reported (Table 2). All sequences match the specified targets using NCBI BLAST. The D1 receptor primer specifically targets the D1A receptor subtype [Yamamoto et al., 2015], which is highly expressed in Area X and septum of zebra finches [Kubikova et al., 2010]; the D2 receptor primer targets the D2 receptor subtype of the D2 family (which also includes D3 and D4 receptors) [Yamamoto et al., 2015], which is expressed in VTA, Area X, and septum [Kubikova et al., 2010].
Table 1.
Information for primers used. GAPDH and HMBS were reference genes.
| Gene | Accession Number | Direction | Sequence | Ta (°C) | Product (bp) |
|---|---|---|---|---|---|
| TH | XM_002198931.2 | Forward | GCCATGCTGAACCTCTTCTT | 60 | 298 |
| Reverse | GATGGTGGCACTTGTCCAAT | ||||
| D1 | NM_001243833.1 | Forward | ACGAGAGGAAAATGACCCCC | 58 | 112 |
| Reverse | GTTGTAGCCTTGTGCCAGTT | ||||
| D2 | XM_002191611.2 | Forward | TACCAGTCCCCCTGAGAAAG | 58 | 96 |
| Reverse | GTAGAGTTGTTGCCCCGATT | ||||
| NT | NM_001245684.1 | Forward | TGCTCAGATTCAGAAGAGGA | 58 | 169 |
| Reverse | CTACCTCTACTGTTTCCCCC | ||||
| NTR1 | NM_001245982.1 | Forward | ATTGCCTTCGTGGTCTGTTG | 58 | 295 |
| Reverse | AAGTGTGGTTGCTGGAGATG | ||||
| GAPDH | NM_204305.1 | Forward | AGCAATGCTTCCTGCACTAC | 57.5 | 121 |
| Reverse | CTGTCTTCTGTGTGGCTGTG | ||||
| HMBS | XM_002187761.1 | Forward | ACCTGCCAACTTCTCTTCCT | 58 | 140 |
| Reverse | GGTTCCAATCACACTCTTTTCA |
Table 2.
Product sequencing of primers. GAPDH and HMBS were reference genes.
| Gene | Species for primers | Sequence |
|---|---|---|
| TH | Taeniopygia guttata | CTTCCCACTGGTCCCGCGGTCCTTAAGGTGTTTGAGACATTTG AAGCTAAAATTCACCACCTGGAGACGAGGCTTAGCCGAAAG CCCCGTGAAGGGACTGCTGAACTGGAATACTTTGTGCGCTGT GAAGTCCACAGCTCAGACCTCAATACTTTCATTAGCTCCATC AAGAGAGTGGCAGAAGATGTGAGGACTACTAAGGAGGACAA ATTTCACTGGTTTCCCAGAAAGATCTGTGAATTGGACAAGTG CCACCATCA |
| D1 | Taeniopygia guttata | GAGGAANACACGGGACAAAGGTCCACGGCCACGCCTGGATC ATGGATGAAGGGCTGCCTTGGGGGGTCATTTTCCCTCTT |
| D2 | Taeniopygia guttata | GGGGNTNNTTGGTGCTGTGGGNTTTGAAGGGCCGCTTTCTCA GGGGGACCTGGATAA |
| NT | Taeniopygia guttata | GCTCGATCGAGAGGAATGAAAGCATTAGAGGCAGATTTATTG ACCAATATGTACACATCAAAGATTAACAGAGCAAAACTTCCT TACTGGAAAATGACCCTGCTA |
| NTR1 | Gallus gallus | TCCCCTCCAGGCCACTGGGAACAGATTTCCTTTTACAACTTCT ACCACTACCTTTTTACATGGCTGACAAACGTCCTCTTCTACGG TCAGCATCAGCAATCAACCCCATCCTCTACAACCTGGTGTCG GCAAACTTCCGCCAGATCTTCCTCTCCACGCTCACCATCNTGT GCCTGCCATGGAGGAAGAAGAAGAAACGACTGGCCTTCACC AGGAAATCCAACAGCATCTCCAGCAACCACACTTA |
| GAPDH | Gallus gallus | AGGGCTGGCAGGTATCCATGACACTTTGGCATCGTGGAGGGT CTCATGACCACTGGTCCATGCCATCACAGCCACACAGAAGAC AGAT |
| HMBS | Taeniopygia guttata | TCGCAAAGGGAAACCCACCTTGNATGCTGTTGTCTTTCATCCC AAAAACTGTGGAAAAACACTGAGCCTCCTTCCTGAAAAGAGT GTGATTGGAACNAA |
The BioRad CFX96 Touch Real-Time PCR Detection System (Catalog No. 185–5195; Bio-Rad, Hercules, CA) was used to run qPCR. Samples were mixed with Sso Fast Evagreen Supermix (Catalog No. 172–5204; Bio-Rad, Hercules, CA), nuclease free H2O, and forward and reverse primers for the gene of interest (prepared by the University of Wisconsin, Biotechnology center) as per manufacturer’s instructions. Samples were run along with 5 amplification standards in a 1:10 dilution series, beginning with 500 ng/µl, and a non-template control containing nucleotide free water instead of cDNA. Samples and standards were run in triplicate. Runs had an initiation step at 95 °C for 30s, followed by 40 cycles of 95 °C for 5 s, followed by a 30 s annealing phase based on the annealing temperature for the primer, and a 30 s elongation phase at 72 °C. Plates went through a melt curve from 65 °C to 88 °C, at 0.5 °C for each 5 s step. Plates were read at each elongation and melt curve step. Runs were only used if the reaction efficiencies as assayed by the standard amplification curve were between 90–110% and had an R2 greater than 0.990. A melt curve with a single peak verified the specificity of the PCR products.
Relative gene expression for NT, NTR1, TH, D1, and D2 was quantified as a normalized ratio to two reference genes (glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and hydroxymethylbilane synthase (HMBS)). Mean Ct values (the cycle number at which a sample crossed the amplification threshold of 200 RFU) were transformed for each sample through the Pfaffl Method [Cordes et al., 2015; Pfaffl, 2001]. The geometric mean of the Ct values for the two reference genes for each region was used to transform the Ct values for each gene to a normalized ratio, resulting in a relative expression value for each gene of interest. Expression levels for some genes in some regions were so low that they were unquantifiable for these numbers of individuals: in VTA 1 for TH, 6 for D1, 5 for D2, and 7 for NTR1; in Area X 17 for TH; in septum 5 for TH, 1 for D2, and 1 for NTR1. Due to technical problems with tissue, gene expression in VTA could not be quantified for 2 individuals.
Statistics
Linear correlations were performed between each behavior and gene expression in Area X, VTA, and septum. To account for multiple comparisons between regions, the Holm-Bonferroni test was employed post-hoc; 3 regions were analyzed, so the thresholds for alpha in respective order of significance were p < 0.017, p < 0.025, and p < 0.05. Correlations were also analyzed in each region for expression of NT and TH, D1, or D2, as well as expression of NTR1 and TH, D1, or D2. The Holm-Bonferroni test was used to correct for each of these 6 correlations per region post-hoc, so the thresholds for alpha in respective order of significance were p < 0.0083, p < 0.01, p < 0.0125, p < 0.017, p < 0.025, and p < 0.05. Assumptions were not violated for any of the correlations as shown by residual plots. Outlier qPCR values were removed from analyses if they were greater than 2 standard deviations from the mean and were significantly influential such that their inclusion or removal entirely drove or masked a correlation. Specific instances are detailed in the Results; as seen from descriptive statistics, these high qPCR values appear to be abnormal and may be a result of technical errors such as contamination. Analyses to control for group were performed and no contributing effects were seen. Statistics were conducted in R v3.1.1 [R Core Team, 2014].
Results
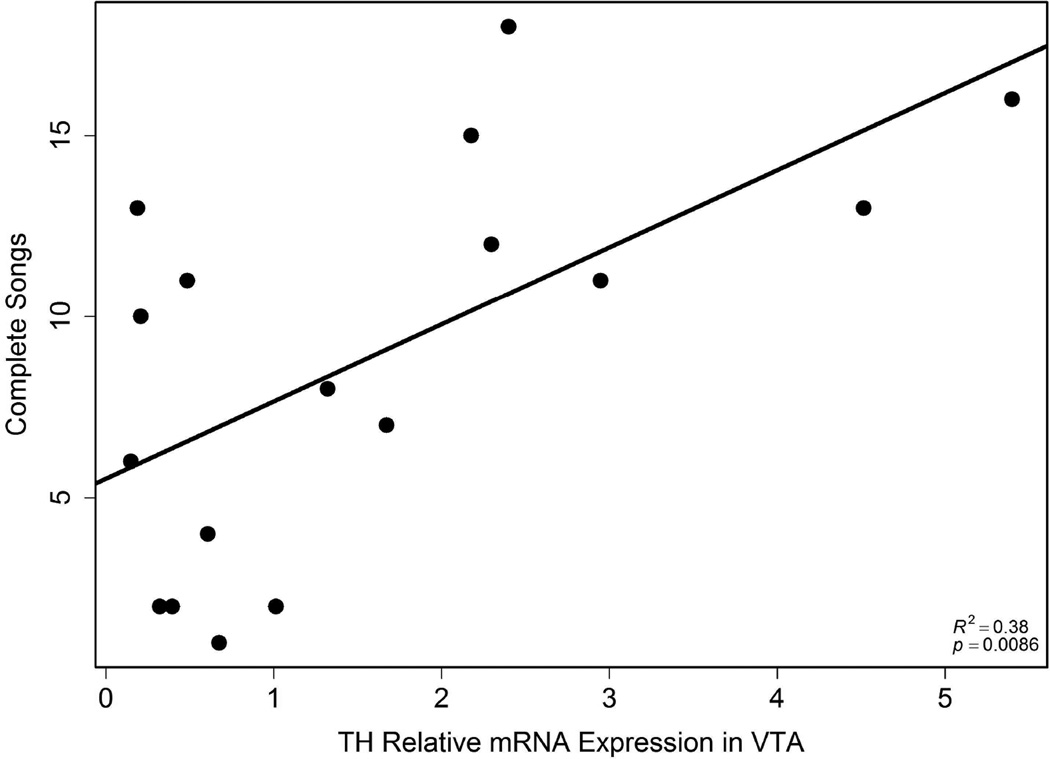
TH expression in VTA positively correlated with song
TH mRNA expression in VTA positively related to the number of complete songs (r2 = 0.38, p = 0.0086; Fig. 2). One outlier was excluded from this analysis (relative expression value = 9.61; average = 2.02 ± 2.42) and although its inclusion did not affect the significance of the correlation, its removal improved the fit; therefore, n = 17. TH mRNA expression in Area X or septum did not relate significantly to singing.
Figure 2.
Correlation between the total number of complete songs and TH expression in VTA (n = 17). Each point represents data from a single individual.
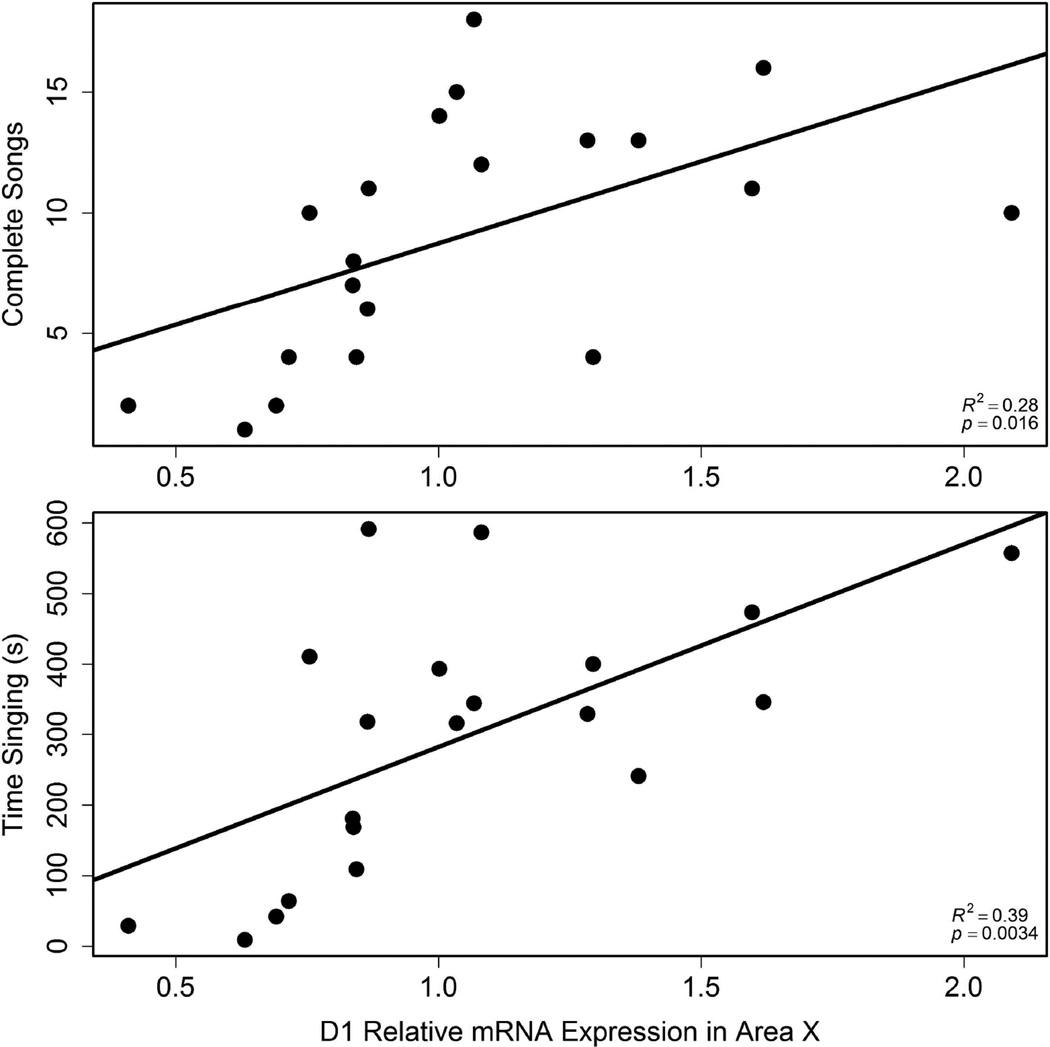
D1 expression in Area X positively correlated with song
D1 mRNA expression in Area X positively related to the number of complete songs (r2 = 0.28, p = 0.016) and time singing (r2 = 0.39, p = 0.0034; Fig. 3). One outlier was removed from these analyses (relative expression value = 2.43; average = 1.11 ± 0.49), so n = 20. D1 expression in VTA or septum did not correlate significantly with singing, and D2 mRNA expression in any brain region did not correlate significantly with song measures.
Figure 3.
Correlations between D1 expression in Area X and the total number of complete songs (top; n = 20) and time singing (bottom; n = 20). Each point represents data from a single individual.
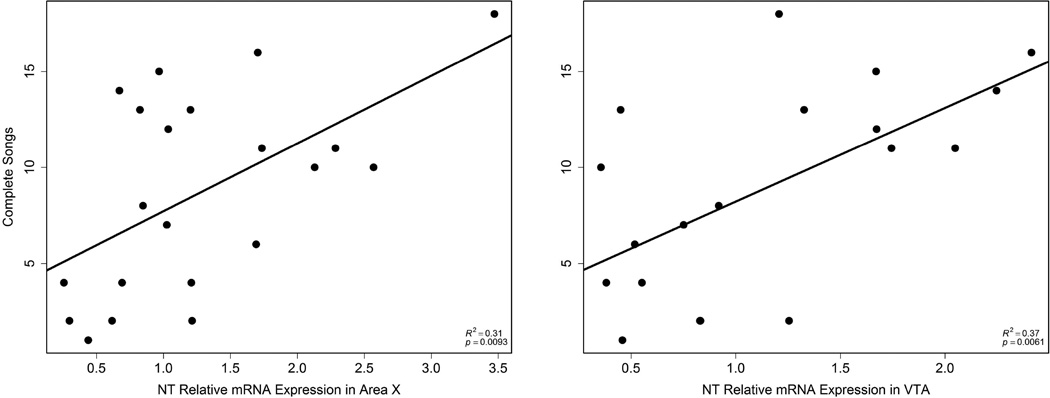
NT expression in Area X and VTA positively correlated with song
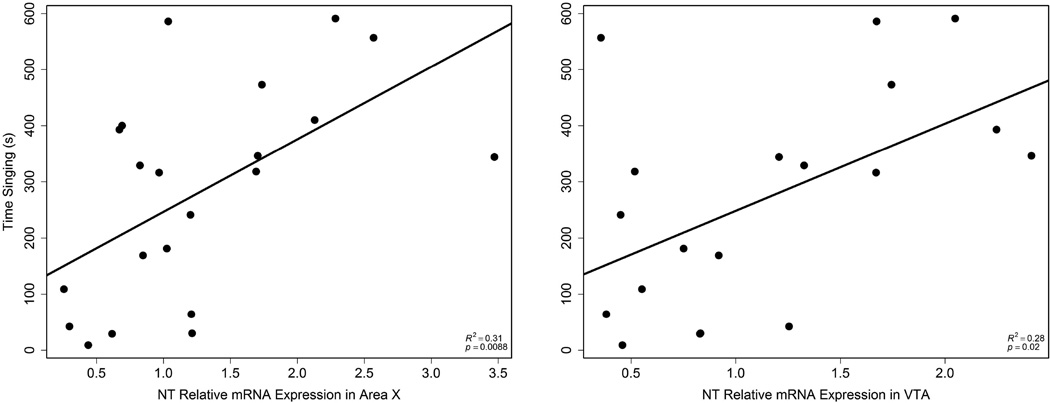
NT mRNA expression in Area X also positively correlated with the number of complete songs (r2 = 0.31, p = 0.0093; Fig. 4) and time singing (r2 = 0.31, p = 0.0088; Fig. 5); n = 21. NT mRNA expression in VTA positively correlated with the number of complete songs (r2 = 0.37, p = 0.0061; Fig. 4) and time singing (r2 = 0.28, p = 0.020; Fig. 5); n = 19. There were no significant relationships between NT expression in septum and singing behavior.
Figure 4.
Correlations between the total number of complete songs and NT expression in Area X (left; n = 21) and VTA (right; n = 19). Each point represents data from a single individual.
Figure 5.
Correlations between time singing and NT expression in Area X (left; n = 21) and VTA (right; n = 19). Each point represents data from a single individual.
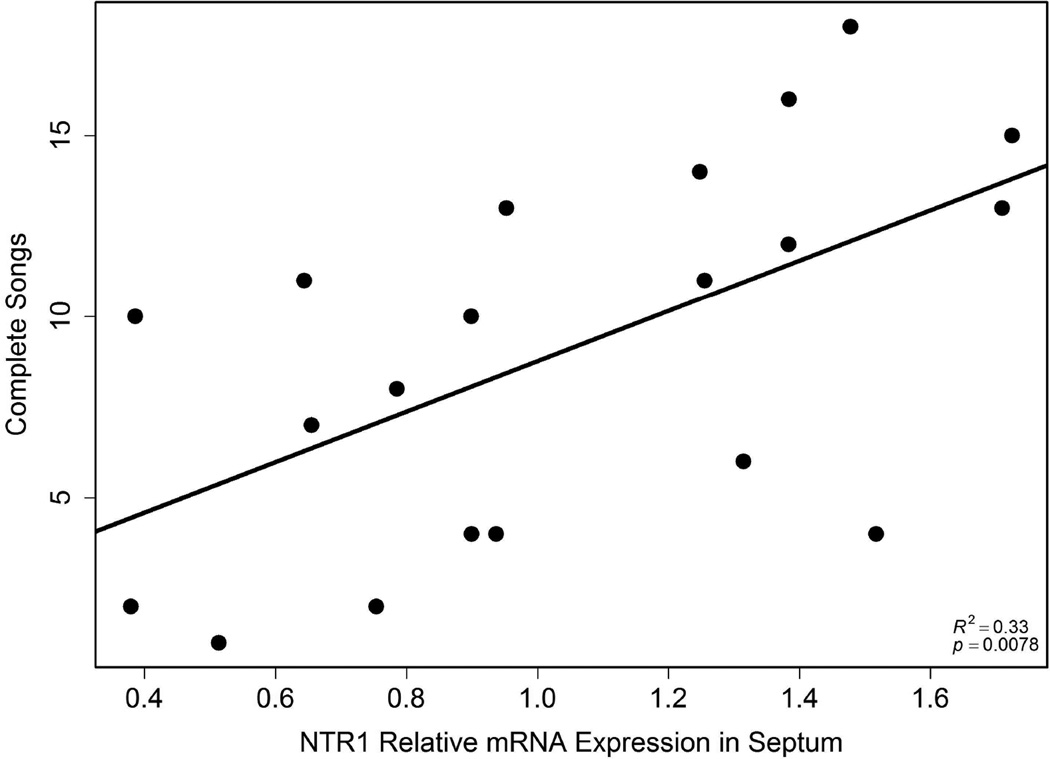
NTR1 expression in septum positively correlated with song
NTR1 expression in septum positively correlated with the number of complete songs (r2 = 0.33, p = 0.0078; Fig. 6); n = 20. NTR1 expression in Area X and VTA did not relate significantly to song.
Figure 6.
Correlation between the total number of complete songs and NTR1 expression in septum (n = 20). Each point represents data from a single individual.
Other behaviors
Gene expression in any brain regions for the genes mentioned above did not relate significantly to calling, displacements, preening, feeding, or drinking.
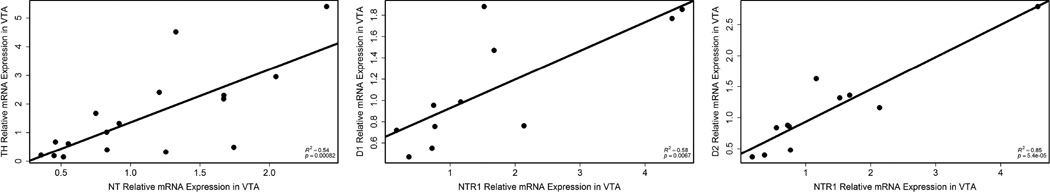
Correlations between NT and DA expression markers
In VTA, NT expression correlated with TH expression (r2 = 0.54, p = 0.00082; n = 17; Fig. 7). One outlier was removed because its value for TH expression was 3 standard deviations beyond the mean (relative expression value = 9.61; average = 2.02 ± 2.42). Also in VTA, NTR1 expression correlated with both D1 (r2 = 0.58, p = 0.0067; n = 11; Fig. 7) and D2 (r2 = 0.85, p = 5.4e-05; n = 11; Fig. 7) expression. One outlier was removed from the analysis with D2 because its expression value was 2 standard deviations beyond the mean (relative expression value = 6.43; average = 1.42 ± 1.58).
Figure 7.
Correlations in VTA between NT expression and TH expression (left; n = 17), NTR1 expression and D1 expression (middle; n = 11), and NTR1 expression and D2 expression (right; n = 11). Each point represents data from a single individual.
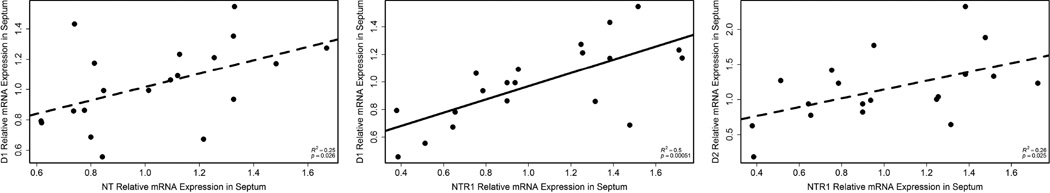
In septum, NTR1 expression correlated with D1 expression (r2 = 0.50, p = 0.00051; n = 20; Fig. 8); one outlier was removed (relative expression value = 2.80; average = 1.12 ± 0.48). Two additional correlations were seen in septum, although their statistical significance did not surpass the threshold of the Holm-Bonferroni correction. NT expression in septum related to D1 expression (r2 = 0.25, p = 0.026; n = 20; Fig. 8) and NTR1 expression in septum related to D2 expression (r2 = 0.26, p = 0.025; n = 19; Fig. 8). In Area X, there were no significant correlations between expression of NT or NTR1 and TH, D1, or D2.
Figure 8.
Correlations in septum between NT expression and D1 expression (left; n = 20), NTR1 expression and D1 expression (middle; n = 20), and NTR1 and D2 expression (right; n = 19). A dashed line indicates that the statistical significance of the correlation did not surpass the threshold of a post-hoc test corrected for 6 comparisons. Each point represents data from a single individual.
Discussion
This study examined neural gene expression associated with a form of undirected communication that is important for vocal learning [Adret-Hausberger et al., 1990; Chaiken et al., 1994] and group maintenance [Eens, 1997; Hausberger et al., 1995] in adult starlings. The data highlight relationships between DA- and NT-related genes in Area X and VTA and undirected song. Correlations between NT and DA expression markers within these regions are consistent with the hypothesis that these systems interact to facilitate undirected song. This study additionally indicates that NTR1 expression in the septum relates to the production of undirected song. Based on past studies showing that DA can modify birdsong and NT interacts with DA (reviewed in the introduction), we interpret our findings to suggest a causal role for interactions between DA and NT in undirected song; however, because all results are correlational, it is also possible that expression is influenced by singing or that another factor is responsible for both singing and gene expression. No correlations among any of the genes in any region were seen for calling, displacements, feeding, drinking, or preening, suggesting that our findings were specific to song rather than general motor behaviors.
Song and gene expression in VTA
Both TH and NT mRNA expression in VTA positively correlated with undirected singing. TH expression also positively correlated with NT expression in VTA. High TH in VTA could lead to DA production and release into projection regions, and binding of NT to receptors on dopaminergic neurons in VTA could enhance DA release [Lu et al., 2009; Mercuri et al., 1993]. Dopaminergic projections from VTA to Area X are hypothesized to be important for song learning [Fee and Goldberg, 2011], so it is possible that NT could act on TH-positive neurons in VTA to enhance DA release into Area X to facilitate song modification. We also found that NTR1 expression in VTA correlated positively with both D1 and D2 receptor expression. In VTA, NTR1 is found on dopaminergic neurons that can contain D2 receptors and on non-dopaminergic neurons that can contain D1 and D2 receptors [Binder et al., 2001]. These correlations suggest that coordinated patterns of DA- and NT-related gene expression in VTA may fine tune DA release and undirected singing behavior. The final locations and functional interactions of the protein products of these genes must now be examined.
Song and gene expression in Area X
Area X contains high densities of D1 receptors [Casto and Ball, 1994; Heimovics et al., 2009] and receives a major dopaminergic projection from VTA [Lewis et al., 1981]. The finding that undirected singing positively correlated with D1 receptor expression in Area X may indicate that these D1 receptors are a target of DA synthesized and released from VTA. This idea is supported by multiple past studies. DA and D1 receptor expression levels in Area X peak when male zebra finches are actively learning songs, i.e., singing undirected song [Harding et al., 1998; Kubikova et al., 2010]. In Area X of adult zebra finches, IEG expression in neurons containing D1A receptors increases during undirected singing [Kubikova et al., 2010] and relates to song variability [Bosikova et al., 2012]. Although [Bosikova et al., 2012] did not find a correlation between D1 receptor expression and the number of songs, [Bosikova et al., 2012] differs from the present study in that it examined zebra finches (a different species), it used in situ hybridization (a less quantitative method than qPCR), and animals were housed in isolation (a different social condition), all of which could have contributed to the differences in results. Activation of D1 receptors in Area X can alter spiny neuron excitability [Ding et al., 2003], facilitate long term potentiation [Ding and Perkel, 2004], and influence song variability [Leblois and Perkel, 2012; Leblois et al., 2010]. These findings have led to strong proposals for the roles of DA and D1 receptors in Area X in song learning [Fee and Goldberg, 2011; Kubikova and Košťál, 2010; Simonyan et al., 2012].
The positive correlation between undirected singing and NT expression in Area X suggests that NT may act in this region to modify the learning, maintenance, and variability of song. A previous study also found a positive correlation between NT expression in Area X and sexually-motivated song in starlings [Merullo et al., 2015b]. Given that song variability differs between directed and undirected song [Sossinka and Böhner, 1980], and that DA in Area X can alter song variability within distinct contexts, it could be that NT modifies DA activity in Area X to adjust song variability context-appropriately. Future studies are needed to examine the causal relationship between song production, NT, and DA in Area X.
Song and NTR1 expression in septum
NTR1 expression in septum positively correlated with the production of undirected song, as well as with D1 and D2 expression. This is the first instance in which NTR1 has been associated with singing behavior, although LS has previously been implicated in undirected song production [Heimovics and Riters, 2006, 2007]. In male starlings, concentrations of the DA metabolite DOPAC and D1 receptor densities in LS positively correlate with undirected song [Heimovics et al., 2009; Heimovics et al., 2011], suggesting that DA activity in LS may stimulate this type of vocalization. NT neurons in VTA project to LS [Binder et al., 2001], and NTR1 activation in septum could depolarize DA neurons or reduce dopaminergic inhibition, as reported in other brain regions [Binder et al., 2001; Lu et al., 2009; Mercuri et al., 1993], and thereby facilitate song. In both rodents and birds, NT neurons are also found in sub-regions of LS that receive dopaminergic projections from VTA [Atoji et al., 1996; Binder et al., 2001; Merullo et al., 2015b; Merullo et al., 2015a; Xie et al., 2010]. The observed correlations in septum between expression of NT and D2, and expression of NTR1 with D1 and D2, support the possibility that NT and DA in LS may interact to facilitate undirected song.
D2 receptor expression
In the present study we did not find any associations between undirected singing and D2 receptor expression in VTA, Area X, or LS, agreeing with a previous experiment in male starlings that found no correlations between the production of undirected song and D2 receptors in these regions as measured using autoradiography [Heimovics et al., 2009]. Although D2 expression did not correlate with singing behavior, we did find positive correlations between D2 expression and NTR1 expression in both VTA and septum. Upon binding to NTR1, NT can modulate dopaminergic transmission by reducing the affinity of inhibitory D2 receptors, which can result in an increase in dopaminergic activity [Binder et al., 2001; Ferraro et al., 2016; St-Gelais et al., 2006]. This suggests that interactions between NT and DA activity at the D2 receptor may occur in VTA and septum to modify dopaminergic signaling. Research is now needed to understand these relationships and the extent to which they influence song.
Technical Limitations
Since the methods in this study measured mRNA expression, the final location of the protein product is not known, and upregulated gene expression in a particular region could indicate either that the translated protein remains there or is transported down axons that terminate in another area. For example, it is unknown if the upregulated NTR1 mRNA production in septum reflects receptors that remain there or are transferred down axons to another area, and likewise for DA- and NT-related gene expression in VTA and Area X. Subsequent work is needed to identify the sites of action for the genes described here. Furthermore, all the reported results are correlational, and it is a non-mutually exclusive possibility that singing behavior alters expression of the observed genes. Studies manipulating the activity of these genes and receptors are needed to determine the directionality of these relationships.
Conclusion
The present data implicate both the DA and NT systems in undirected song and provide further support for the hypothesis that these two systems interact to modulate vocal communication. The DA and NT systems may be involved in regulating undirected song differently compared to female-directed song, or they could function similarly across contexts. Future work should investigate the causal role of these neurochemicals in song production and song learning, as well as seasonal or context-specific differences of these measures.
Acknowledgments
This material is based upon work supported by the National Institute of Mental Health (R01 MH080225 to LVR) and by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1256259. The authors thank Dr. Melissa A. Cordes for invaluable technical assistance; Dr. Allison H. Hahn, Dr. Changjiu Zhao, and Jeremy A. Spool for thoughtful manuscript comments; and Chris Elliott, Kate Skogen, and Jeffrey Alexander for exemplary animal care.
References
- Adret-Hausberger M, Güttinger H-R, Merkel FW. Individual life history and song repertoire changes in a colony of starlings (Sturnus vulgaris) Ethology. 1990;84:265–280. [Google Scholar]
- Atoji Y, Shibata N, Yamamoto Y, Suzuki Y. Distribution of neurotensin-containing neurons in the central nervous system of the pigeon and the chicken. J Comp Neurol. 1996;375:187–211. doi: 10.1002/(SICI)1096-9861(19961111)375:2<187::AID-CNE2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Azmi N, Norman C, Spicer CH, Bennett GW. Effects of a neurotensin analogue (PD149163) and antagonist (SR142948A) on the scopolamine-induced deficits in a novel object discrimination task. Behav Pharmacol. 2006;17:357–362. doi: 10.1097/01.fbp.0000224382.63744.20. [DOI] [PubMed] [Google Scholar]
- Berridge Kent C, Kringelbach Morten L. Pleasure systems in the brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacol Rev. 2001;53:453–486. [PubMed] [Google Scholar]
- Björklund A, Dunnett SB. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Böhner J, Chaiken ML, Ball GF, Marler P. Song acquisition in photosensitive and photorefractory male European starlings. Horm Behav. 1990;24:582–594. doi: 10.1016/0018-506x(90)90043-w. [DOI] [PubMed] [Google Scholar]
- Bosikova E, Kostal L, Cvikova M, Bilcik B, Niederova-Kubikova L. Song-related dopamine receptor regulation in Area X of zebra finch male. Gen Physiol Biophys. 2012;31:291–298. doi: 10.4149/gpb_2012_034. [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of Animal Communication. Sunderland, Mass: Sinauer Associates; 2011. [Google Scholar]
- Casto JM, Ball GF. Characterization and localization of D1 dopamine receptors in the sexually dimorphic vocal control nucleus, Area X, the basal ganglia of European starlings. J Neurobiol. 1994;25:767–780. doi: 10.1002/neu.480250703. [DOI] [PubMed] [Google Scholar]
- Chaiken M, Böhner J, Marler P. Repertoire turnover and the timing of song acquisition in European starlings. Behaviour. 1994;128:25–39. [Google Scholar]
- Cordes MA, Stevenson SA, Driessen TM, Eisinger BE, Riters LV. Sexually-motivated song is predicted by androgen-and opioid-related gene expression in the medial preoptic nucleus of male European starlings (Sturnus vulgaris) Behav Brain Res. 2015;278:12–20. doi: 10.1016/j.bbr.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Ding L, Perkel DJ, Farries MA. Presynaptic depression of glutamatergic synaptic transmission by D1-like dopamine receptor activation in the avian basal ganglia. J Neurosci. 2003;23:6086–6095. doi: 10.1523/JNEUROSCI.23-14-06086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Perkel DJ. Long-term potentiation in an avian basal ganglia nucleus essential for vocal learning. J Neurosci. 2004;24:488–494. doi: 10.1523/JNEUROSCI.4358-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen TM, Zhao C, Whittlinger A, Williams H, Gammie SC. Endogenous CNS expression of neurotensin and neurotensin receptors is altered during the postpartum period in outbred mice. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0083098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AM, Zann RA. Undirected song in wild zebra finch flocks: Contexts and effects of mate removal. Ethology. 1996a;102:529–539. [Google Scholar]
- Dunn AM, Zann RA. Undirected song encourages the breeding female zebra finch to remain in the nest. Ethology. 1996b;102:540–548. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Temporal and sequential organization of song bouts in the starling. Ardea. 1989;77:6. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. On the function of singing and wing-waving in the European Starling Sturnus vulgaris. Bird Study. 1990;37:48–52. [Google Scholar]
- Eens M. Understanding the complex song of the European starling: An integrated ethological approach. In: Slater PJB, Rosenblatt JS, editors. Adv Stud Behav. Vol. 26. Academic Press; 1997. pp. 355–434. [Google Scholar]
- Fee MS, Goldberg JH. A hypothesis for basal ganglia-dependent reinforcement learning in the songbird. Neuroscience. 2011;198:152–170. doi: 10.1016/j.neuroscience.2011.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Mexal S, Melendez G, Liu PY, Goldenberg JR, Shilling PD. The brattleboro rat displays a natural deficit in social discrimination that is restored by clozapine and a neurotensin analog. Neuropsychopharmacology. 2009;34:2011–2018. doi: 10.1038/npp.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Tiozzo Fasiolo L, Beggiato S, Borelli AC, Pomierny-Chamiolo L, Frankowska M, Antonelli T, Tomasini MC, Fuxe K, Filip M. Neurotensin: A role in substance use disorder? J Psychopharamcol. 2016;30:112–127. doi: 10.1177/0269881115622240. [DOI] [PubMed] [Google Scholar]
- Gammie SC, D'Anna KL, Gerstein H, Stevenson SA. Neurotensin inversely modulates maternal aggression. Neuroscience. 2009;158:1215–1223. doi: 10.1016/j.neuroscience.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara E, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur J Neurosci. 2007;25:3406–3416. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CF, Barclay SR, Waterman SA. Changes in catecholamine levels and turnover rates in hypothalamic, vocal control, and auditory nuclei in male zebra finches during development. J Neurobiol. 1998;34:329–346. [PubMed] [Google Scholar]
- Hausberger M, Richard-Yris M-A, Henry L, Lepage L, Schmidt I. Song sharing reflects the social organization in a captive group of European starlings (Sturnus vulgaris) J Comp Psychol. 1995;109:222–241. [Google Scholar]
- Heimovics SA, Riters LV. Breeding-context-dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris) Horm Behav. 2006;50:726–735. doi: 10.1016/j.yhbeh.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. ZENK labeling within social behavior brain regions reveals breeding context-dependent patterns of neural activity associated with song in male European starlings (Sturnus vulgaris) Behav Brain Res. 2007;176:333–343. doi: 10.1016/j.bbr.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Cornil CA, Ball GF, Riters LV. D1-like dopamine receptor density in nuclei involved in social behavior correlates with song in a context-dependent fashion in male European starlings. Neuroscience. 2009;159:962–973. doi: 10.1016/j.neuroscience.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Salvante KG, Sockman KW, Riters LV. Individual differences in the motivation to communicate relate to levels of midbrain and striatal catecholamine markers in male European starlings. Horm Behav. 2011;60:529–539. doi: 10.1016/j.yhbeh.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holveck M-J, Riebel K. Preferred songs predict preferred males: Consistency and repeatability of zebra finch females across three test contexts. Anim Behav. 2007;74:297–309. [Google Scholar]
- Holveck M-J, Riebel K. Low-quality females prefer low-quality males when choosing a mate. Proc R Soc B. 2009;283:1–8. doi: 10.1098/rspb.2009.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immelmann K. Beiträge zu einer vergleichenden Biologie australischer Prachtfinken (Spermestidae) Zool Jahrb Abt Anat Ontog Tiere. 1962;90:1–196. [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: Context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Keiser AA, Matazel KS, Esser MK, Feifel D, Prus AJ. Systemic administration of the neurotensin NTS(1)-receptor agonist PD149163 improves performance on a memory task in naturally deficient male brown Norway rats. Exp Clin Psychopharm. 2014;22:541–547. doi: 10.1037/a0037912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Riters LV. Curvilinear relationships between mu-opioid receptor labeling and undirected song in male European starlings (Sturnus vulgaris) Brain Res. 2013;1527:29–39. doi: 10.1016/j.brainres.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubikova L, Wada K, Jarvis ED. Dopamine receptors in a songbird brain. J Comp Neurol. 2010;518:741–769. doi: 10.1002/cne.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubikova Ľ, Košťál Ľ. Dopaminergic system in birdsong learning and maintenance. J Chem Neuroanat. 2010;39:112–123. doi: 10.1016/j.jchemneu.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo K, Toth K, Kertes E, Peczely L, Ollmann T, Lenard L. Effects of neurotensin in amygdaloid spatial learning mechanisms. Behav Brain Res. 2010;210:280–283. doi: 10.1016/j.bbr.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Leblois A, Wendel BJ, Perkel DJ. Striatal dopamine modulates basal ganglia output and regulates social context-dependent behavioral variability through D1 receptors. J Neurosci. 2010;30:5730–5743. doi: 10.1523/JNEUROSCI.5974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Perkel DJ. Striatal dopamine modulates song spectral but not temporal features through D1 receptors. Eur J Neurosci. 2012;35:1771–1781. doi: 10.1111/j.1460-9568.2012.08095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to Area X in the zebra finch. J Comp Neurol. 1981;196:347–354. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- Lu B, Su Y, Das S, Wang H, Wang Y, Liu J, Ren D. Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature. 2009;457:741–744. doi: 10.1038/nature07579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri NB, Stratta F, Calabresi P, Bernardi G. Neurotensin induces an inward current in rat mesencephalic dopaminergic neurons. Neurosci Lett. 1993;153:192–196. doi: 10.1016/0304-3940(93)90320-k. [DOI] [PubMed] [Google Scholar]
- Merullo DP, Cordes MA, Stevenson SA, Riters LV. Neurotensin immunolabeling relates to sexually-motivated song and other social behaviors in male European starlings (Sturnus vulgaris) Behav Brain Res. 2015a;282:133–143. doi: 10.1016/j.bbr.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merullo DP, Cordes MA, DeVries MS, Stevenson SA, Riters LV. Neurotensin neural mRNA expression correlates with vocal communication and other highly-motivated social behaviors in male European starlings. Physiol Behav. 2015b;151:155–161. doi: 10.1016/j.physbeh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JE, Hafzalla GW, Burkett ZD, Fox CM, White SA. Reduced vocal variability in a zebra finch model of dopamine depletion: Implications for Parkinson disease. Physiol Rep. 2015;3 doi: 10.14814/phy2.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Ann NY Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Numao M, Sudo H, Yamamoto I, Nakao N, Kaiya H, Miyazato M, Tsushima N, Tanaka M. Molecular characterization of structure and tissue distribution of chicken neurotensin receptor. Gen Comp Endocrinol. 2011;171:33–38. doi: 10.1016/j.ygcen.2010.12.021. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J Comp Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. URL http://www.R-project.org/ [Google Scholar]
- Riters LV. The role of motivation and reward neural systems in vocal communication in songbirds. Front Neuroendocrinol. 2012;33:194–209. doi: 10.1016/j.yfrne.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Stevenson SA. Reward and vocal production: Song-associated place preference in songbirds. Physiol Behav. 2012;106:87–94. doi: 10.1016/j.physbeh.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Stevenson SA, DeVries MS, Cordes MA. Reward associated with singing behavior correlates with opioid-related gene expression in the medial preoptic nucleus in male European starlings. PLoS ONE. 2014;9:e115285. doi: 10.1371/journal.pone.0115285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singingregulated dopamine. J Neurosci. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Horwitz B, Jarvis ED. Dopamine regulation of human speech and bird song: A critical review. Brain Lang. 2012;122:142–150. doi: 10.1016/j.bandl.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Sossinka R, Böhner J. Song types in the zebra finch Poephila guttata castanotis. Z Tierpsychol. 1980;53:123–132. [Google Scholar]
- St-Gelais F, Jomphe C, Trudeau LE. The role of neurotensin in central nervous system pathophysiology: What is the evidence? J Psychiatry Neurosci. 2006;31:229–245. [PMC free article] [PubMed] [Google Scholar]
- Tirado-Santiago G, Lazaro-Munoz G, Rodriguez-Gonzalez V, Maldonado-Vlaar CS. Microinfusions of neurotensin antagonist SR 48692 within the nucleus accumbens core impair spatial learning in rats. Behav Neurosci. 2006;120:1093–1102. doi: 10.1037/0735-7044.120.5.1093. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Cilz NI, Kurada L, Hu B, Yang C, Wada E, Combs CK, Porter JE, Lesage F, Lei S. Activation of neurotensin receptor 1 facilitates neuronal excitability and spatial learning and memory in the entorhinal cortex: Beneficial actions in an Alzheimer's disease model. J Neurosci. 2014;34:7027–7042. doi: 10.1523/JNEUROSCI.0408-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, London SE, Southey BR, Annangudi SP, Amare A, Rodriguez-Zas SL, Clayton DF, Sweedler JV. The zebra finch neuropeptidome: Prediction, detection and expression. BMC Biol. 2010;8:28. doi: 10.1186/1741-7007-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Fontaine R, Pasqualini C, Vernier P. Classification of Dopamine Receptor Genes in Vertebrates: Nine Subtypes in Osteichthyes. Brain Behav Evol. 2015;86:164–175. doi: 10.1159/000441550. [DOI] [PubMed] [Google Scholar]
- Yanagihara S, Hessler NA. Modulation of singing-related activity in the songbird ventral tegmental area by social context. Eur J Neurosci. 2006;24:3619–3627. doi: 10.1111/j.1460-9568.2006.05228.x. [DOI] [PubMed] [Google Scholar]
- Zeigler HP, Marler P. Neuroscience of Birdsong. Cambridge, UK: Cambridge University Press; 2008. [Google Scholar]