Abstract
Purpose
Type 2 diabetes is a common comorbidity among breast cancer survivors. Our aim was to assess the association between diabetes and quality of life (QOL) in newly diagnosed early-stage (0-IIA) breast cancer patients over a 2-year follow-up.
Methods
We used data from a longitudinal study of 549 breast cancer patients, aged ≥ 40 years. During four telephone interviews administered 4-6 weeks and 6, 12, and 24 months after definitive surgical treatment, we measured QOL using the Functional Assessment of Cancer Therapy-Breast (FACT-B) scale; higher scores indicate better QOL. Repeated-measures analysis of variance was used to test the change over time in total FACT-B and each of the five subscales (physical, social, emotional and functional well-being, and breast cancer concerns), comparing patients with and without diabetes at baseline.
Results
After adjusting for covariates (age, race, body mass index, education, marital status, cancer staging, and surgical side effects), patients with (vs. without) diabetes reported lower QOL over time on the total FACT-B (least-squares mean [standard error]: 106.2 [2.1] vs. 112.0 [1.1]; p = 0.0038) and on physical, social, emotional and functional well-being subscales (each p < 0.05). Over the 2-year follow-up, QOL improved significantly for the emotional well-being (p < 0.0001) and breast cancer concern subscales (p = 0.0282) among patients without diabetes, but not among patients with diabetes.
Conclusion
Early-stage breast cancer patients with diabetes may need additional care considerations to improve QOL.
Keywords: Breast cancer, Diabetes, Cohort study, Quality of life, Functional Assessment of Cancer Therapy-Breast (FACT-B), Surgical side effects
Introduction
Breast cancer is the most common female cancer in the United States [1]. In addition, up to 16%-20% of women have diabetes at the time of breast cancer diagnosis [2, 3], which may adversely affect breast cancer outcomes [4-6]. The impact of diabetes on breast cancer-related outcomes may be either through the metabolic effect of hyper-insulinemia and insulin resistance on cancer progression, or through the effect of diabetes burden on general health and subsequent cancer management [4]. However, despite the multi-dimensional impact of diabetes on breast cancer outcomes, including survival and quality-of-life (QOL) outcomes, less attention has been given to the potential impact of diabetes on QOL over the first few years after diagnosis.
Recent advances in breast cancer diagnosis and treatment have significantly improved long-term survival in patients with breast cancer, and thus, QOL has emerged as an important breast cancer outcome. About 60% of breast cancer cases are currently diagnosed at an early stage, where the cancer is localized (limited to breast tissue) and has a 5-year relative survival rate of 98.6% [7]. Shortly after diagnosis and treatment, patients with early-stage breast cancer tend to report lower QOL than women without a history of breast cancer [8]. Over time, however, QOL of breast cancer survivors improves [9] and reaches levels comparable to those of women without breast cancer [10, 11].
People with diabetes generally face daily diabetes management challenges, which can negatively affect their QOL [12]. Factors such as female gender, number of diabetes complications and number of comorbidities have been found to be negatively associated with QOL [13]. Cross-sectional data have shown that having both diabetes and any cancer is associated with worse QOL than having either diabetes or cancer alone [14]. However, it is unclear whether pre-existing diabetes would affect anticipated improvements in QOL over time in newly diagnosed, early-stage breast cancer patients following surgery and early in the recovery process.
With increasing prevalence of breast cancer survivors with diabetes, understanding the impact of diabetes on change in QOL among newly diagnosed breast cancer patients could have clinical implications for improving breast cancer care and QOL over time. This issue is especially important among early-stage breast cancer patients, who generally have a good prognosis and are living longer as a result of improvements in early detection and treatment [15, 16]. We hypothesized that diabetes would be associated with poorer QOL outcomes in newly diagnosed early-stage breast cancer patients over a 2-year period.
Methods
Participants
This study involved a secondary analysis of data collected during a longitudinal QOL study of women with and without early-stage breast cancer [17]. Briefly, patients with newly diagnosed ductal carcinoma in situ (DCIS, stage 0) and early-stage (stage I or IIA) breast cancer were prospectively identified and recruited between October 2003 and June 2007 from the Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine and from Saint Louis University School of Medicine. Inclusion criteria were: age ≥ 40 years (as many early-stage breast cancers are diagnosed during screening mammography [18], which was recommended for this age group at the time [19]), no prior history of breast cancer, completed definitive surgical treatment, no prior neoadjuvant chemotherapy, English speaking, and no cognitive impairment for women ≥ 65 years, based on the Orientation-Memory-Concentration Test [20].
Following Institutional Review Board approval at both institutions, participants completed four computer-assisted telephone interviews, conducted by trained interviewers, at four to six weeks (Time1), six months (Time2), one year (Time3), and two years (Time4) following their definitive surgery.
Measures
Quality of life outcomes
We measured QOL with the Functional Assessment of Cancer Therapy-Breast (FACT-B) scale, a reliable and validated instrument that is sensitive to change [21]. The FACT-B questionnaire uses a five-point response scale ranging from 0-4; total scores range from 0-144, with higher scores indicating better QOL. The total FACT-B score includes scores for five subscales, including physical well-being (7 items), social well-being (7 items), emotional well-being (6 items), functional well-being (7 items), and breast cancer concerns (9 items). A minimally important difference for the total FACT-B score is 7-8 points [22].
Covariates
Pre-existing diabetes was determined at baseline (Time1) using the Katz interview [23] based on the Charlson Comorbidity Index [24]. Diabetes severity was categorized as complicated or uncomplicated, depending upon whether or not their diabetes caused kidney/eye problems. The Katz interview [23] also was used to measure the number and severity of other comorbid conditions: myocardial infarction, diabetes, congestive heart failure, peripheral vascular disease, cerebrovascular disease, hemiplegia, chronic pulmonary disease, ulcer disease, renal disease, rheumatologic disease, dementia, liver disease, and other cancers. We computed a weighted index of comorbidity based on the presence and severity of all of these other conditions affirmed by participants (excluding diabetes).
In addition, we used a reliable and validated 8-item, self-report measure of the severity of surgical side effects in the past month, including limited arm mobility/frozen shoulder, tightness/tenderness in chest wall, tightness/tenderness/discomfort in the breast, arm weakness, lymphedema/swelling of the arm, swelling of the chest/breast/axilla, numbness/tingling or pins and needles, and tightness/pulling/stretching in the arm/axilla [25-27]. Responses ranged from 1 (not at all) to 5 (very much); with higher mean scores indicating more severe surgical side effects.
Depressed mood at baseline was measured using the validated 20 items Center for Epidemiologic Studies Depression (CES-D) Scale scores were dichotomized for analysis (≥ 16 [elevated depressed mood] vs. < 16 [not depressed]) [28]. Clinical data obtained from the medical record included patient's receipt of adjuvant radiotherapy, chemotherapy, and hormone therapy at any time over the 2-year study, type of definitive surgery (breast-conserving or mastectomy), and pathological cancer stage (ductal carcinoma in situ [DCIS], stage I, or stage IIA). Demographic data included self-reported age, race, education, and marital status. Body-mass index (BMI), was calculated from self-reported weight and height (kg/m2).
Statistical analyses
Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). Two-tailed p values < 0.05 were considered significant. We compared characteristics of the sample at baseline between women with breast cancer without diabetes and those with breast cancer and diabetes, using chi-square tests and analysis of variance (ANOVA) as appropriate. The PROC MIXED procedure was used for the repeated-measures analysis of variance (RM-ANOVA) to test change in QOL scores on the total FACT-B and each of the five subscales for the main effects of group (with vs. without diabetes) and time (Time1 to Time4) and their interaction. When the group by time interaction was significant, multiple pairwise comparisons were tested using Scheffé's post-hoc test. Models were controlled for covariates of QOL at Time1; we report least-squares means (LSM) and standard errors (SE) for the adjusted analyses. Moreover, in an exploratory analysis using one-way ANOVA, we compared QOL scores on the total FACT-B, separately at Time1 and Time4, between three groups of patients: without diabetes, with complicated diabetes, and with uncomplicated diabetes.
Results
A total of 772 breast cancer patients were eligible for study participation, of whom 549 (71%) enrolled in the study. Among participants, 435 (79%) were white and 114 (21%) were non-white, including 104 African Americans and 10 of other racial/ethnic groups. Significant racial and age differences were observed between the 549 participants and 223 non-participants, where participants were more likely than non-participants to be white (79% vs. 64%, p < 0.001) and younger (mean age 58 vs. 61 years, p = 0.01). Participants and non-participants did not, however, differ significantly by marital status (married vs. not married, p = 0.07), pathologic cancer stage (DCIS vs. stage I vs. stage IIA, p = 0.84) or type of surgery (breast-conserving surgery vs. mastectomy, p = 0.10). Study retention remained high during 2-year follow-up with 514 (94%) breast cancer patients completing all four interviews.
At baseline (Time1), 62 patients (11%) reported having diabetes (Table 1); there were no new cases of diabetes reported during the follow-up interviews. The most common diabetes treatment was use of oral agents (68%). Among women with diabetes, 9 (15%) reported that diabetes caused problems with their kidneys or eyes. Patients with diabetes were on average older, more likely to be non-white, and had higher BMI and comorbidity index scores than patients without diabetes; but comorbidity was low, on average in both groups. Patients with vs. without diabetes did not differ significantly in terms of the severity of surgical side effects at baseline. However, at Time4, patients with diabetes reported more severe surgical side effects than patients without diabetes; mean [SE]: 1.6 [0.09] vs. 1.4 [0.02], p = 0.0109.
Table 1.
Baseline demographics, clinical characteristics, and QOL in individuals with breast cancer by history of diabetes
| With diabetes ( n = 62) | Without diabetes (n = 487) | p | |
|---|---|---|---|
| Demographic | |||
| Age, years, mean (SE) | 62 (1.3) | 58 (0.5) | 0.0039 |
| Race, n (%) | 0.0178 | ||
| White | 42 (68%) | 393 (81%) | |
| Non-White | 20 (32%) | 94 (19%) | |
| Education, n (%) | 0.3937 | ||
| < 12 years | 6 (10%) | 37 (8%) | |
| GED / high school graduate | 18 (29%) | 110 (23%) | |
| > 12 years | 38 (61%) | 340 (70%) | |
| Marital status, n (%) | 0.4016 | ||
| Married | 32 (52%) | 298 (62%) | |
| Divorced/ Separated | 10 (16%) | 72 (15%) | |
| Widowed | 10 (16%) | 56 (12%) | |
| Never married | 10 (16%) | 55 (11%) | |
| Clinical | |||
| Diabetes treatment, n (%) | |||
| None (diet) | 8 (13%) | - | |
| Oral agents | 42 (68%) | - | |
| Insulin | 12 (19%) | - | |
| Diabetes complication, n (%) | |||
| None | 50 (81%) | - | |
| Kidney and/or eyes problems | 9 (15%) | - | |
| Don't know | 3 (5%) | - | |
| BMI, kg/m2, mean (SE) | 34 (0.9) | 28 (0.3) | < 0.0001 |
| Elevated depressed mood, yesa, n (%) | 12 (19%) | 81 (17%) | 0.5904 |
| Surgical-side-effects severity, mean (SE) | 1.7 (0.10) | 1.7 (0.03) | 0.9225 |
| Comorbidity index, mean (SE) | 0.7 (0.15) | 0.4 (0.03) | 0.0074 |
| Cancer stage, n (%) | 0.3975 | ||
| DCIS | 25 (40%) | 159 (33%) | |
| Stage I | 27 (44%) | 255 (52%) | |
| Stage IIA | 10 (16%) | 73 (15%) | |
| Type of surgery, n (%) | 0.6120 | ||
| Breast-conserving | 42 (68%) | 314 (64%) | |
| Mastectomy | 20 (32%) | 173 (36%) | |
| Radiation therapy, yesb, n (%) | 39 (63%) | 311 (64%) | 0.8826 |
| Chemotherapy, yesb, n (%) | 15 (24%) | 121 (25%) | 0.9108 |
| Hormone therapy, yesb, n (%) | 37 (60%) | 307 (64%) | 0.5371 |
| Quality of Life | |||
| FACT-B (range), mean (SE) | |||
| Total score (0-144) | 110.7 (3.0) | 115.9 (0.9) | 0.0506 |
| Physical well-being | 22.3 (0.7) | 23.5 (0.2) | 0.0761 |
| Social well-being | 21.6 (0.8) | 23.4 (0.2) | 0.0046 |
| Emotional well-being | 20.7 (0.5) | 20.8 (0.2) | 0.7638 |
| Functional well-being | 19.2 (0.9) | 20.3 (0.3) | 0.1762 |
| Breast cancer concerns | 26.9 (0.9) | 27.8 (0.2) | 0.2180 |
Center for Epidemiologic Studies Depression (CES-D) Scale scores ≥ 16 (compared with < 16)
Data represent the frequencies over the 2-year follow-up (Time1-Time4)
Quality of life at baseline
At Time1, patients with and without diabetes did not differ significantly on the total FACT-B scores (Table 1). Of the five subscales, only the social well-being subscale score was lower for patients with diabetes (difference = 1.8, p = 0.0046). However, QOL did not differ significantly between the two groups for the emotional (p = 0.7638), physical (p = 0.0761) or functional (p = 0.1762) well-being subscales or for the breast cancer concerns (p = 0.218) subscale.
In exploratory analysis at Time1, patients with complicated diabetes reported lower total FACT-B scores than patients without diabetes (mean [SE]: 102.3 [6.5] vs. 115.9 [0.9]; p = 0.0395). Among only those patients with diabetes at Time1, total FACT-B scores did not differ significantly between patients with complicated vs. uncomplicated diabetes (102.3 [6.5] vs. 111.3 [2.8]; p = 0.2069). In addition, at Time4, the differences between the three groups remained similar to those of Time1; i.e., lower total FACT-B scores reported by patients with complicated diabetes than patients without diabetes (107.2 [5.6] vs. 121.5 [0.9]; p = 0.0118), and total FACT-B scores did not differ significantly between patients with complicated vs. uncomplicated diabetes (107.2 [5.6] vs. 115.8 [2.6]; p = 0.1641).
Quality of life over time
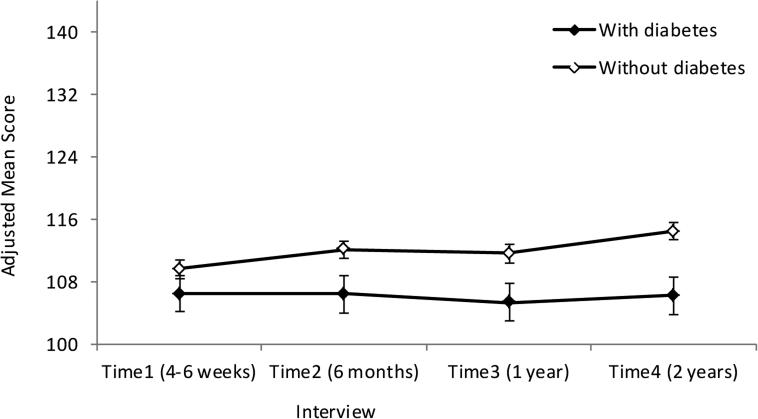
We adjusted for age, race, BMI, education, marital status, cancer stage, and surgical-side-effects severity at Time1, in the multivariable RM-ANOVA models. Although comorbidities were low in this sample, patients with diabetes had higher comorbidity scores (Table 1); thus we did not control for other comorbid conditions in our statistical models, because they were potentially due to diabetes and could produce over-adjustment bias [29]. After adjusting for covariates, the main effect of diabetes on the total FACT-B and the physical, social, emotional and functional well-being subscales was significant; patients with (vs. without) diabetes reported significantly lower QOL for total FACT-B and each of these four subscales (Table 2, Figure 1). Among the covariates in the multivariable model, older age (p = 0.0001), lower BMI (p = 0.0028), higher level of education (p = 0.0003), being married (p < 0.0001), earlier stage of disease (p = 0.0037), and less severe surgical side effects (p < 0.0001) were each associated with higher total FACT-B scores.
Table 2.
Least-squares means (LSM) from repeated-measures analysis of variance for total FACT-B, and each of the five subscales among early-stage breast cancer patients (N = 540)
| Total FACT-B |
Physical well-being |
Social well-being |
Emotional well-being |
Functional well-being |
Breast cancer concerns |
||
|---|---|---|---|---|---|---|---|
| Effects | LSM (SE) | LSM (SE) | LSM (SE) | LSM (SE) | LSM (SE) | LSM (SE) | |
| Diabetes | |||||||
| Yes | 106.2 (2.1) | 22.1 (0.5) | 20.7 (0.5) | 20.0 (0.4) | 17.3 (0.6) | 26.1 (0.6) | |
| No | 112.0 (1.1) | 23.2 (0.2) | 22.0 (0.3) | 20.8 (0.2) | 18.9 (0.3) | 27.2 (0.3) | |
| P value | 0.0038 | 0.0158 | 0.0152 | 0.0473 | 0.0058 | 0.0676 | |
| Time | |||||||
| Time1 | 108.1 (1.4) | 22.3 (0.3) | 21.3 (0.4) | 20.1 (0.3) | 18.0 (0.4) | 26.4 (0.4) | |
| Time2 | 109.3 (1.4) | 22.2 (0.3) | 21.1 (0.4) | 20.7 (0.3) | 18.7 (0.4) | 26.8 (0.4) | |
| Time3 | 108.5 (1.4) | 23.2 (0.4) | 21.3 (0.4) | 20.6 (0.3) | 16.5 (0.4) | 27.0 (0.4) | |
| Time4 | 110.4 (1.4) | 23.0 (0.4) | 21.6 (0.4) | 20.3 (0.3) | 19.2 (0.4) | 26.4 (0.4) | |
| P value | 0.1024 | 0.0016 | 0.4912 | 0.0123 | <.0001 | 0.1754 | |
| Diabetes × Time | |||||||
| With diabetes × | Time1 | 106.5 (2.3) | 22.0 (0.6) | 20.7 (0.6) | 19.9 (0.5) | 17.7 (0.7) | 26.2 (0.7) |
| Time2 | 106.5 (2.4) | 21.4 (0.6) | 20.4 (0.6) | 20.4 (0.5) | 17.8 (0.7) | 26.5 (0.7) | |
| Time3 | 105.4 (2.4) | 22.8 (0.6) | 20.7 (0.6) | 20.2 (0.5) | 15.5 (0.7) | 26.3 (0.7) | |
| Time4 | 106.3 (2.4) | 22.4 (0.6) | 21.0 (0.6) | 19.5 (0.5) | 18.1 (0.7) | 25.4 (0.7) | |
| Without diabetes × | Time1 | 109.7 (1.2) | 22.6 (0.3) | 21.9 (0.3) | 20.2 (0.2)** | 18.3 (0.3) | 26.6 (0.3)* |
| Time2 | 112.2 (1.2) | 23.0 (0.3) | 21.8 (0.3) | 20.9 (0.2) | 19.5 (0.3) | 27.1 (0.3) | |
| Time3 | 111.7 (1.2) | 23.7 (0.3) | 22.0 (0.3) | 21.0 (0.2) | 17.4 (0.4) | 27.6 (0.3) | |
| Time4 | 114.5 (1.2) | 23.5 (0.3) | 22.1 (0.3) | 21.1 (0.2)** | 20.4 (0.4) | 27.4 (0.3)* | |
| P value | 0.0882 | 0.5301 | 0.9771 | 0.0142 | 0.0748 | 0.0401 |
Abbreviations: FACT-B, Functional Assessment of Cancer Therapy-Breast; LSM, least-squares means; SE, standard error.
Covariates: age, race, body mass index, education, marital status, cancer staging, and surgical-side-effects severity.
Scheffé post-hoc comparison between Time1 and Time4 among patients without diabetes, p = 0.0282.
Scheffé post-hoc comparison between Time1 and Time4 among patients without diabetes, p < 0.0001.
Figure 1.
Adjusted mean scores of total FACT-B over time among women with breast cancer, by history of diabetes.
Error bars represent standard errors.
There also was a significant main effect of time for change in scores on physical (p = 0.0016), emotional (p = 0.0123) and functional (p < 0.0001) well-being subscales. The group by time interaction was significant only for emotional well-being (p = 0.0142) and breast cancer concerns (p = 0.0401) subscales. Post-hoc multiple pairwise comparisons showed that, from Time1 to Time4, QOL improved significantly for the emotional well-being subscale (LSM [SE] at Time1: 20.2 [0.2] and at Time4: 21.1 [0.2]; Scheffé's post-hoc test p < 0.0001) and breast cancer concerns subscale (LSM [SE] at Time1: 26.6 [0.3] and at Time4: 27.4 [0.3]; Scheffé's post-hoc test p = 0.0282) among patients without diabetes but not among patients with diabetes.
Discussion
This study examined the impact of pre-existing diabetes on change in QOL among newly diagnosed early-stage breast cancer patients. At baseline, 11% of our sample reported having diabetes, which is comparable to the general population [30], and no patients developed diabetes over the 2-year follow-up. Our findings showed that patients with pre-existing diabetes reported worse QOL on average over this time compared with patients without diabetes, and that QOL improved among patients without pre-existing diabetes, but remained unchanged among those with diabetes.
In our exploratory analysis of differences between patients with diabetes complications and patients without diabetes, we observed a large, 13.6-point (at Time1) and 14.3-point (at Time4), difference in total FACT-B scores, which are larger than a minimally clinically important difference of 7-8 points [22]. Among only patients with diabetes, there was a 9.0-point and 8.6-point difference, at Time1 and Time4, respectively, between patients with and without diabetes complications, also considered to be clinically important, although the difference was not statistically significant (likely due to the small number of patients with complications). In addition, although the group-by-time interaction was not statistically significant (Table 2), the 8.2-point difference in LSM of total FACT-B scores at Time4 between patients with and without diabetes, also was large [22]. Thus, the lack of improvement in QOL among patients with diabetes might be largely explained by the impact of diabetes complications.
Another possible explanation for our finding might be that diabetes results in a less favorable recovery [29, 30], which affects QOL. In our study, there were no significant differences between patients with and without diabetes at Time1 in terms of type of definitive surgical treatment, severity of surgical side effects, or receipt of adjuvant radiotherapy, chemotherapy and/or hormone therapy. We previously found in this cohort that, although surgical-side-effects severity declined over the first six months after definitive surgical treatment, regardless of type of surgery, patients who had mastectomy continued to endure more severe surgical side effects over the remaining 18 months of follow-up compared with patients who had breast-conserving surgery [27]. Compared with breast-conserving surgery, mastectomy generally is associated with greater morbidity and risk of surgical site infections [33-35], which delays healing, and with more severe surgical side effects [27]. These findings are consistent with studies showing a higher rate of surgical site infection [36] and post-surgical arm difficulties [37] in breast cancer patients with diabetes than in those without diabetes. Moreover, greater surgical-side-effects severity in this cohort was found to be correlated with greater body image and sexual functioning problems; all three of these variables negatively impact psychosocial aspects of QOL, like depressed mood and anxiety [27, 28]. To our knowledge, the impact of diabetes on long-term breast cancer-related surgical-side-effects severity and QOL outcomes has not been reported.
It is not surprising that having diabetes was associated with a lower QOL, as diabetes can be associated with other health conditions that can potentially have a negative impact on QOL [12]. In addition, it is reasonable to believe that the general domains of the FACT-B questionnaire (i.e., the four non-breast-cancer-specific subscales) also reflect the impact of non-cancer health conditions, such as diabetes, on QOL, because even though some items on the FACT-B specifically address breast cancer concerns, most items on the questionnaire ask about “my illness” and not “my cancer” specifically. Our results showed that having diabetes was associated with having greater comorbidity (Table 1) and a lack of improvement in QOL during the recovery from breast cancer treatment over the 2-year follow-up. Further study is warranted to examine the extent to which diabetes, potential complications of diabetes, and other comorbidities may offset the improvement in cancer-related QOL seen in patients without diabetes.
Strengths of our study include the prospective design with longitudinal assessments, the inclusion of patients from different demographic background, and very high rate (94%) of completion of four interviews over two years. Our study also has some limitations. First, in our analysis, diabetes was self-reported. Self-reported diabetes has a high specificity (99.7%) and low sensitivity (66.0%) [38]. Thus, false negative reports of diabetes may have resulted in misclassification of those patients with diabetes, and therefore, underestimated the between-groups differences suggesting that even larger differences are present. Second, we only examined cross-sectional differences at Time1 and Time4 between three groups of patients, grouped by diabetes and diabetes complications, because of the small sample of patients reporting diabetes complications. Third, patients who did not participate were older and more likely to be non-white than those who participated in this study, and most non-white participants were African American. Although the representation of racial/ethnic groups in our sample reflects their representation in the St. Louis area population, the non-response bias and lower representation of participants from other racial/ethnic groups may limit the generalizability of our results.
In conclusion, pre-existing diabetes was associated with a lower QOL among women with early-stage breast cancer at each interview. Over the 2-year follow-up, QOL improved in breast cancer patients without diabetes, but did not change in patients with diabetes. At baseline, patients with vs. without diabetes did not differ in terms of surgical side effects. However, after the 2-year follow-up, patients with diabetes reported worse surgical side effects, which has implications for treatment and follow-up care. Given that diabetes is a major health problem, and many women with breast cancer have diabetes, understanding the impact of pre-existing diabetes on breast cancer management is of great importance, with potential clinical implications for patients with early-stage and more advanced disease. To improve recovery from breast cancer treatment and aspects of QOL, patients with diabetes, and especially patients with diabetes complications, may need additional care considerations in terms of their diabetes management or to find other ways to foster improvement in their QOL during the early recovery period to close the gap in QOL between breast cancer patients with and without diabetes—a gap that increased over the 2-year follow-up in this patient cohort.
Acknowledgments
The authors thank the study participants, interviewers, the Siteman Cancer Center's Health Behavior, Communication and Outreach Core for data management, and the many physicians and nurses who helped us recruit their patients for this study at Washington University School of Medicine and at Saint Louis University School of Medicine.
Grant Support: The National Cancer Institute and Breast Cancer Stamp Fund (R01 CA102777; PI: D.B. Jeffe) and the National Cancer Institute Cancer Center Support Grant (P30 CA091842; PI: T. Eberlein) to the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri.
Footnotes
Compliance with Ethical Standards:
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA: Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Yancik R, Wesley MN, Ries LG, et al. EFfect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–92. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 3.Srokowski TP, Fang S, Hortobagyi GN, Giordano SH. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27:2170–2176. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol. 2005;6:103–111. doi: 10.1016/S1470-2045(05)01736-5. [DOI] [PubMed] [Google Scholar]
- 5.Peairs KS, Barone BB, Snyder CF, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011;29:40–46. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute [22 May 2014];Surviellance, Epidemiology, and End Results Program: SEER Stat Fact Sheets: Breast Cancer. seer.cancer.gov/statfacts/html/breast.html.
- 8.Kissane DW, Clarke DM, Ikin J, et al. Psychological morbidity and quality of life in Australian women with early-stage breast cancer: a cross-sectional survey. Med J Australia. 1998;169:192–196. doi: 10.5694/j.1326-5377.1998.tb140220.x. [DOI] [PubMed] [Google Scholar]
- 9.King M, Kenny P, Shiell A, Hall J, Boyages J. Quality of life three months and one year after first treatment for early stage breast cancer: influence of treatment and patient characteristics. Qual Life Res. 2000;9:789–800. doi: 10.1023/a:1008936830764. [DOI] [PubMed] [Google Scholar]
- 10.Jeffe DB, Pérez M, Liu Y, et al. Quality of life over time in women diagnosed with ductal carcinoma in situ, early-stage invasive breast cancer, and age-matched controls. Breast Cancer Res Treat. 2012;134:379–391. doi: 10.1007/s10549-012-2048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorval M, Maunsell E, Deschenes L, Brisson J, Masse B. Long-term quality of life after breast cancer: comparison of 8-year survivors with population controls. J Clin Oncol. 1998;16:487–494. doi: 10.1200/JCO.1998.16.2.487. [DOI] [PubMed] [Google Scholar]
- 12.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes-Metab Res. 1999;15:205–218. doi: 10.1002/(sici)1520-7560(199905/06)15:3<205::aid-dmrr29>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Glasgow RE, Ruggiero L, Eakin EG, Dryfoos J, Chobanian L. Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes Care. 1997;20:562–567. doi: 10.2337/diacare.20.4.562. [DOI] [PubMed] [Google Scholar]
- 14.Bowker SL, Pohar SL, Johnson JA. A cross-sectional study of health-related quality of life deficits in individuals with comorbid diabetes and cancer. Health Qual Life Out. 2006;4:17. doi: 10.1186/1477-7525-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.President's Cancer Panel, US Department of Health and Human Services, National Institutes of Health, National Cancer Institute [29 May 2014];Living beyond cancer: finding a new balance. http://deainfo.nci.nih.gov/advisory/pcp/annualReports/pcp03-04rpt/Survivorship.pdf.
- 16.National Cancer Institute . Estimated US cancer prevalence counts: who are our cancer survivors in the US? National Cancer Institute; Bethesda, MD: 2009. [May 29, 2014]. http://cancercontrol.cancer.gov/ocs/prevalence/index.html. [Google Scholar]
- 17.Bailey EH, Pérez M, Aft RL, et al. Impact of multiple caregiving roles on elevated depressed mood in early-stage breast cancer patients and same-age controls. Breast Cancer Res Treat. 2010;121:709–718. doi: 10.1007/s10549-009-0645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 19.Leitch AM, Dodd GD, Costanza M, et al. American Cancer Society Guidelines for the early detection of breast cancer: update 1997. CA Cancer J Clin. 1997;47:150–153. doi: 10.3322/canjclin.47.3.150. [DOI] [PubMed] [Google Scholar]
- 20.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 21.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 22.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57:898–910. doi: 10.1016/j.jclinepi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Pérez M, Schootman M, Aft RL, Gillanders WE, Ellis MJ, Jeffe DB. A longitudinal study of factors associated with perceived risk of recurrence in women with ductal carcinoma in situ and early-stage invasive breast cancer. Breast Cancer Research and Treatment. 2010;124:835–844. doi: 10.1007/s10549-010-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins KK, Liu Y, Schootman M, Aft R, Yan Y, Dean G, Eilers M, Jeffe DB. Effects of breast cancer surgery and surgical side effects on body image over time. Breast Cancer Res Treat. 2011;126:167–176. doi: 10.1007/s10549-010-1077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez M, Liu Y, Schootman M, Aft RL, Schechtman KB, Gillanders WE, Jeffe DB. Changes in sexual problems over time in women with and without early-stage breast cancer. Menopause. 2010;17:924–937. doi: 10.1097/gme.0b013e3181d5dd26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 29.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) [October 2, 2015];National Diabetes Fact Sheet. Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
- 31.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, et al. Less aggressive treatment and worse overall survival in cancer patients with diabetes: A large population based analysis. Int J Cancer. 2007;120:1986–1992. doi: 10.1002/ijc.22532. [DOI] [PubMed] [Google Scholar]
- 32.Srokowski TP, Fang S, Hortobagyi GN, et al. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27:2170–2176. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen M, Lefta M, Dietz J, Brandt KE, Aft R, Matthews P, Mayfield J, Fraser VJ. Risk factors for surgical site infection after major breast operation. J Am Coll Surg. 2008;207:326–335. doi: 10.1016/j.jamcollsurg.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen MA, Chu-Ongsakul S, Brandt KE, Dietz JR, Mayfield J, Fraser VJ. Hospital-associated costs due to surgical site infection after breast surgery. Arch Surg. 2008;143:53–60. doi: 10.1001/archsurg.2007.11. [DOI] [PubMed] [Google Scholar]
- 35.El-Tamer MB, Ward BM, Schifftner T, Neumayer L, Khuri S, Henderson W. Morbidity and mortality following breast cancer surgery in women. Ann Surg. 2007;245:665–671. doi: 10.1097/01.sla.0000245833.48399.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis GB, Peric M, Chan LS, Wong AK, Sener SF. Identifying risk factors for surgical site infections in mastectomy patients using the National Surgical Quality Improvement Program database. Am J Surg. 2013;205:194–199. doi: 10.1016/j.amjsurg.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Hölzel D. Axilla surgery severely affects quality of life: results of a 5-year prospective study in breast cancer patients. Breast Cancer Res Treat. 2003;79:47–57. doi: 10.1023/a:1023330206021. [DOI] [PubMed] [Google Scholar]
- 38.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]