Abstract
The dopamine hypothesis of schizophrenia is supported by a large number of imaging studies that have identified an increase in dopamine binding at the D2 receptor selectively in the striatum. Here we review a decade of work using a regionally restricted and temporally regulated transgenic mouse model to investigate the behavioral, molecular, electrophysiological, and anatomical consequences of selective D2 receptor upregulation in the striatum. These studies have identified new and potentially important biomarkers at the circuit and molecular level that can now be explored in patients with schizophrenia. They provide an example of how animal models and their detailed level of neurobiological analysis allow a deepening of our understanding of the relationship between neuronal circuit function and symptoms of schizophrenia, and as a consequence generate new hypotheses that are testable in patients.
Keywords: Schizophrenia, Dopamine, D2 Receptor, mouse model, striatal circuit function, Negative Symptoms
Classifications: Animal Models, Basic Neuroscience, Behavioral science, Schizophrenia/Psychosis, Electrophysiology
What is known about abnormalities in the dopamine system in schizophrenia
The dopamine hypothesis of schizophrenia originally formulated by van Rossum in the 1960s postulated that “overstimulation of dopamine receptors could be part of the etiology” of schizophrenia (1). The hypothesis was supported by Philip Seeman and Solomon Snyder, and their colleagues, seminal findings in the 1970s that there is an inverse relationship between the therapeutic dose of antipsychotic medication and its binding affinity for dopamine receptors. These findings imply that efficacy of antipsychotic medication is directly proportional to the degree to which the medication engages dopamine receptors (2)(3).
In the last 20 years human imaging studies have consistently found alterations in the striatal dopamine system. At the presynaptic level increased striatal uptake of 18F-fluorodopa (or L-β-11C-DOPA) and increased amphetamine-induced dopamine release have been measured in patients in over 10 independent studies (4). Changes in presynaptic dopamine are thus among the most reliably replicated findings observed in patients with schizophrenia. Alterations in presynaptic dopamine appear to occur early on in the disease process as they are observed in prodromal subjects that are at high risk for conversion (5)(6). Surprisingly, high resolution imaging revealed that the largest effect size of these abnormalities is not in the limbic striatum, as has been postulated for many years, but rather in the associative striatum (4; 7). This finding has significant implications for relating dopamine dysfunction to specific symptoms of the disease because the limbic and associative areas of the striatum are involved in anatomically and functionally distinct cortico-striatal circuits. The limbic striatum receives input from the ventromedial prefrontal cortex (vmPFC) and this connection is involved in affective-emotional processing. The associative striatum receives dense input from the dorso-lateral prefrontal cortex (dlPFC) and is important for cognition (8). It is currently unknown how differential changes in dopamine function occur in different compartments of the striatum. Potential factors include presynaptic changes that are pathway specific or cellular changes that affect dopamine release locally within the striatum.
At the postsynaptic level, imaging studies have identified an increased density of D2Rs in the striatum. In 1998 Marc Laruelle performed a meta-analysis of 13 different imaging studies and calculated a 12% increase in striatal D2R density in drug-naïve or drug-free patients (9). However, a more recent meta-analysis questions whether the increase in D2R density is a result of the disorder or whether it is induced by antipsychotic treatment (4). Dopamine depletion experiments have additionally reported increased basal occupancy of striatal D2Rs in drug-free patients that not only correlates with positive symptoms but predicts their response to antipsychotic medication, thus suggesting a tight relationship between D2R occupancy in the striatum and psychosis (10; 11). In contrast to the positive correlation between increased striatal dopamine and positive symptoms, the severity of negative symptoms has been correlated with low dopamine activity in the ventral striatum (7). This finding suggests that while dopamine tone is consistently found to be increased at the level of the whole striatum, subregional analyses may be highly informative about specific symptoms of the disease.
In comparison to the increase in dopamine that has been identified at the level of the whole striatum in patients with schizophrenia, a decrease in dopamine in the cortex has long been proposed as a potential mediator of the cognitive symptoms of schizophrenia. A series of early, pioneering functional imaging studies determined that when patients performed a working memory task, no significant increase in PFC activity could be observed (12–14). This contrasted with healthy controls in which the same task increased cerebral blood flow in the PFC (12–14). The third study of this series found that the degree of dlPFC activation in patients performing a working memory task was inversely correlated with the concentration of dopamine metabolites in the cerebrospinal fluid (14). More direct evidence of a decrease in cortical dopamine comes from a recent imaging study using a newly developed high affinity PET tracer showing that amphetamine-induced dopamine release is lower in the cortex of patients compared to healthy controls (15). Because dopamine plays a critical role in several prefrontal cortical dependent cognitive functions, including working memory, associative learning, cognitive flexibility and attention (16), this finding suggests that a sub-optimal level of cortical dopamine release could be contributing to some of the cognitive deficits observed in patients with schizophrenia.
Using D2R overexpressing mice to explore the dopamine hypothesis of schizophrenia
In addition to imaging studies supporting the dopamine hypothesis, 70–80% of patients respond to dopamine D2 receptor antagonists (reviewed in (17)), further implicating D2 receptor overstimulation in the pathophysiology of schizophrenia for a majority, if not all, patients. Animal models provide a powerful tool in which invasive and terminal procedures can be used to probe possible pathophysiological mechanisms that contribute to dysregulation of the dopamine system and lead to symptoms of the disease.
To mimic the increase in density and occupancy of striatal D2Rs in patients, we previously generated mice in which D2Rs are selectively overexpressed in the striatum beginning in late embryonic development (18). Transgene expression is not only spatially restricted but also temporally controlled by using an artificial promoter system, the tetracycline transactivator system (for detailed explanation of this transgenic system, see (19). This Bi-transgenic system allows for switching off the D2R transgene by supplementing the mice’s diet with the tetracycline analogue, doxycycline. In situ hybridization determined that transgene expression is restricted to striatal projection neurons with no expression in cholinergic interneurons and dopaminergic midbrain neurons, (18) (20). An Ex-vivo ligand-binding assay further determined that fortuitously, the level of increase in receptor expression is comparable to that measured in patients, around 15% (Fig 1D). Single cell PCR methods determined that 33% of striatal output neurons express the transgene. Striatal projection neurons are divided into two pathways, striatopallidal and striatonigral neurons, 40% and 26% of which, respectively, express the transgene (21).
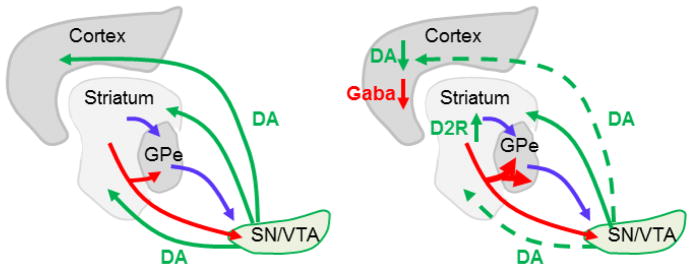
Figure 1. Circuit abnormalities induced by striatal D2R upregulation.
Left: Cortico-striatal circuitry in wild-type mice. The striatum is the input area of the basal ganglia receiving projections from cortex and thalamus. Via two functional opposing pathways, the direct (red) and indirect (blue) pathways it projects to the substantia nigra (SN) and ventral tegmental area (VTA) output nuclei of the basal ganglia. Dopaminergic neurons in the SN and VTA modulate activity in cortex, dorsal and ventral striatum.
Right: Upregulation of D2Rs in the striatum led to several changes in this circuitry: 1) It enhanced the density of direct pathway collaterals in the GPe thereby affecting the functional balance between both pathways. 2) It decreases activity in dopaminergic neurons projecting to the cortex and ventral striatum but not to the dorsal striatum. 3) It decreases GABA transmission in the cortex.
Despite its limitations that are inherent to any rodent model studying human disorders, D2R-OE mice have been very informative for schizophrenia research. Here, we will summarize the findings that have been made over the last 10 years using D2R-OE mice, with an emphasis on understanding how striatal D2Rs regulate circuit function and behaviors that are relevant for schizophrenia.
Cognitive deficits in schizophrenia can be modeled in rodents
Patients with schizophrenia may display positive, negative and cognitive symptoms. Although the positive symptoms are the most characteristic symptoms of the disorder it is the degree of the cognitive and negative symptoms that predicts long-term prognosis (22–24). Cognitive symptoms predate the onset of psychosis, range in severity from moderate to severe and some of the cognitive deficits can be studied in rodents.
Over the last few decades, cognitive neuroscientists have generated a wealth of literature on the specificity of cognitive dysfunctions that occur in schizophrenia including attention (25), working memory (26) and executive function (27). Detailed examinations have identified the component sub-processes involved in these cognitive deficits. For example, in the case of working memory, evidence suggests that the maintenance of information is not critically disrupted in schizophrenia. Instead, the ability to manipulate information within working memory is more severely affected (28; 29) Working memory capacity (the maximum amount of information that can be held) is also significantly reduced (30). Because experimental psychologists have developed cognitive assays for rodents that selectively measure specific cognitive domains, we have been able to apply behavioural assays to D2R-OE mice that measure cognitive domains that are specifically relevant to schizophrenia (25–27).
Table 1 summarizes cognitive tests performed on D2R-OE mice, the homologous tasks used for patients, and the reported outcomes in each case. D2R-OE mice showed deficits in conditional associative learning, decreased temporal precision and a deficit in the acquisition of a spatial working memory tasks whereas performance in a spatial reference memory was unimpaired (18). The nature of the acquisition deficit in the spatial working memory task is unclear and may not represent a deficit in memory (the retention and recall of information), given that maintenance of information in working memory appeared normal in a two choice visual discrimination task. D2R-OE mice performed poorly in the conditional associative learning task due to cognitive interference from the previous trial (31), therefore, it is possible that also in the delayed non-match to sample T-maze working memory task the observed deficit was due to interference from the previous trial.
Table 1.
Cognitive Domains Affected in D2R-OE Mice and Patients With Schizophrenia
| Cognitive Domain | Test(s) applied in Humans | Outcome in patients with Schizophrenia (references) | Test applied in mice | Outcome in D2R-OE mice (reference) |
|---|---|---|---|---|
| Conditional Associative Learning | Visual conditional associative learning tests | Deficit in learning the task (32) and (33) | Auditory conditional associative learning tests | Deficit in learning the task. (31) |
| Inhibitory control | Visual Go/NoGo learning task | Reduced Response accuracy due to omitted Go trials (34) | Auditory Go/NoGo Task | Reduced response accuracy due to interference from prior trial (31) |
| Reversal Learning | Visual discrimination task | Deficit in simple discrimination and reversal trials (35) | Odor/texture discrimination task | Deficit in reversal trials-(36) |
| Time perception | Auditory temporal bisection procedure | Decreased temporal precision (37) | Auditory temporal bisection procedure | Decreased temporal precision (38; 39) |
| Time production | Self-paced time production task | Decreased temporal precision (40) | Peak Interval timing procedure | Decreased temporal precision (20) |
| Spatial Working Memory | oculomotor and haptic delayed-response tasks | Deficits in performance at 2 time delays. (41) | Non match to sample T-maze task | Deficit in acquisition (18) |
| Maintenance of information in Working memory | A visual, color based retention task. | Normal-(30) | Two choice visual discrimination task | Normal (42) |
| Problem Solving/Executive Function | Spatial mazes (Selected by MATRICS) | Poor performance (43) | Puzzle Box digging task | Impaired problem solving (44) |
D2R-OE mice do not show a deficit in PPI (18), a clinical sign that is prevalent in several disorders, including schizophrenia (45; 46) and can be improved by some antipsychotic medications (47). That D2R-OE mice do not show PPI deficits may suggest that the model recreates an altered pathology that is downstream of enhanced pre-synaptic dopamine release. For a more detailed discussion of modeling cognitive endophenotypes of schizophrenia in mice, see (48).
Because we used the Bi-transgenic system, we were able to investigate the temporal relationship between striatal D2R overexpression and the cognitive phenotypes. We found that switching off the transgene once the mice had reached adulthood does not ameliorate the deficit in conditional associative learning or the deficit in acquiring the spatial working memory task (18; 31), and resulted in only partial improvement in timing (20; 38). These findings suggest that an increase in striatal D2R density or occupancy may not only be important for positive symptoms but could also be linked to cognitive symptoms. It is surprising, since D2R antagonists do not reverse the cognitive deficits in patients (49). One possibility is that antipsychotic medications are ineffective at treating cognitive symptoms because they are given too late. Diagnosis typically occurs around the second decade of life, this may be long after alterations in D2 receptors has resulted in persistent brain abnormalities that can no longer be reversed by treatment with D2R blockers. Future studies must investigate the potential benefits as well as the risks of targeting D2Rs early on in individuals at high risk for schizophrenia
Negative symptoms of schizophrenia can be modeled in rodents
Negative symptoms of schizophrenia include deficits in motivation, emotional responsiveness, socialization and speech. Understanding the underlying pathophysiology of negative symptoms is a high priority because negative symptoms like cognitive deficits are highly correlated with functional outcomes. In particular, amotivation/apathy has been shown to strongly predict psychosocial functioning (24). The D2R-OE mouse model displays deficits in motivation. In the course of extensive cognitive testing, we identified a significant difference in the rate of responding for reinforcement (typically lever pressing) of D2R-OE mice compared to their control littermates. To determine if this decrease in response vigor was related to a deficit in motor abilities, sensitivity to fatigue, or an indifference to the food reinforcers being earned in the tasks, we performed a series of studies which led to the conclusion that D2R-OE mice are not impacted in any of those ways (20; 50; 51) Instead the mice, like patients, express a deficit in incentive motivation.
There are a large number of component behavioral processes involved in motivated behavior. These include evaluation and encoding of information about current, as well as future positive and negative consequences. Such consequences include both benefits (meeting physiological and psychological needs) and costs (effort expenditure, time, discomfort etc). All such information must be valued and counter-valued (in a cost-benefit analysis), and is subject to typical learning factors(52). Determining which of these processes are disrupted in schizophrenia has been the focus of much recent research (53). Many studies suggest that the motivational deficit in patients with schizophrenia is not due to an inability to experience pleasure in the moment as hedonic reaction appears intact (54). Instead, the motivation deficit represents a reduced capacity for anticipating future pleasure resulting from goal-directed action. This diminished anticipation appears to be a consequence of an inability to accurately represent the expected reward values of actions (55; 56) and also impairment in the allocation of effort in the pursuit of reward (57; 58).
A number of rodent paradigms that measure aspects of amotivation and apathy related behaviours have been developed (59–61) and the application of such assays has revealed a striking similarity between the incentive motivation phenotype in D2R-OE mice and patients. D2R-OE mice exhibit normal hedonic reactions to appetitive reinforcers and show a reduced sensitivity to the value of future outcomes (50). D2R-OE mice also show a reduced allocation of greater effort for more highly preferred rewards, compared to their control littermates (50). Although the assays used in mice and patients to measure components of motivation differ in the sensory modalities and rewards used, the findings are convergent, suggesting that an acute increase in striatal D2 receptors may underlie the deficit in anticipatory motivation in patients (table 2).
Table 2.
Motivational Deficits in D2R-OE Mice and Patients With Schizophrenia
| Component of motivation | Test(s) applied in Humans | Outcome in patients with Schizophrenia (references) | Test applied in mice (Reference for test validation) | Outcome in D2R-OE mice (references) |
|---|---|---|---|---|
| Hedonic Reaction | Experience sampling and self-report measure of anticipatory and consummatory pleasure. Measurement of hedonic response to sucrose. |
A deficit in anticipatory but not consummatory pleasure (54). Normal hedonic reaction to sucrose solutions across a range of concentrations (62). |
Sucrose preference (63) and positive affective orofacial reactions to sucrose (64) | Normal sucrose preference and normal orofacial “liking” reactions to sucrose (50). |
| Persistence in effort expenditure | Progressive Ratio schedule of reinforcement for money | Deficit in performance (65), No group deficit but performance correlates with negative symptoms (66) | Progressive Ratio schedule of food reinforcement (67) | Deficit over several different ratios (20)(51) |
| Allocation of Effort for rewards | effort-based decision-making for money (EEfRT) | Less optimal decision making (a bias for easier to obtain but smaller rewards) (58)(68) | effort-related choice procedure (69) | Diminished effort allocation for preferred rewards (50) |
| Reward value based decision-making | Probabilistic decision-making task | Impaired value-based decision-making (70) | Concurrent choice of operant schedules (71) | reduced sensitivity to the value of future outcomes (50) |
Not only does the specific type of motivational deficit appear to be highly similar in the D2R-OE mice and patients, in both the mice and patients, systemic pharmacological blockade of D2Rs with antipsychotic medication haloperidol, does not improve motivation (51). Because removing the excess transgenic D2 receptors by switching off the transgene did improve motivation, it suggests that the mouse model provides a tool for investigating novel treatment strategies that are downstream of striatal D2 receptor as opposed to D2Rs in other areas of the brain. Indeed, the model has been used to identify a 5-HT2C receptor ligand (SB242084) that enhances goal directed action in both D2R-OE and wild-type mice (38; 51; 72). Earlier studies have suggested that this drug may increase impulsivity in some conditions (73; 74), however using a novel behavioural assay, we determined that SB242084 treatment can enhance goal-directed efficiency, even when sustained responses are required to obtain rewards (72).
Not all cognitive and negative symptoms are readily separable in patients, or disease models
There has been much debate over whether some of the negative symptoms of schizophrenia, such as decreased motivation, should really be considered a cognitive symptom, since they are due to deficits in information processing (75). It is also the case that in some tests of cognition a lack of motivation may account for a proportion of the poor performance (76).
In fact, it is the interactions between motivational and cognitive deficits that is implicated in producing functional impairments in patients (77). Because this interaction is not well understood at either the behavioral or neural level, we developed a procedure for mice in which a cognitive measure, sustained attention, is modulated by a motivationally relevant signal that predicts reward probability on a trial-by-trial basis (42). We found that whereas in control mice attention was modulated by reward-related cues, in D2R-OE mice this modulation was absent. These results indicate that deficits in motivation impair the ability to use reward-related cues to recruit attention. In a separate study, we employed a cognitive timing task that allowed us to detect changes in cognitive performance that are not influenced by general activity or arousal factors such as the speed or persistence of responding. This approach allowed us to manipulate motivation and measure the impact on cognitive performance. We found that manipulating motivation genetically, pharmacologically and psychologically (by increasing reward value), all resulted in enhanced temporal cognition (38). Together, if generalized to patients with schizophrenia these results suggest that addressing motivational impairments in patients could be critical to achieving substantive cognitive and functional gains, as recently proposed (78).
Negative symptoms of schizophrenia include deficits in social interaction that can also be modeled in rodents
Another core negative symptom is deficits in social behaviors that tend to emerge prior to the full blown onset of the disease. Specifically, by the time of adolescence, social withdrawal becomes a fairly sensitive, if not specific, marker of schizophrenia (79). To determine if the increase in striatal dopamine observed in patients with schizophrenia might play a role in the emergence of deficits in social interaction, we carried out a multimodal characterization of social behavior at different development time points (juvenile, adolescent and adult) in control and D2R-OE mice (80). This characterization included measures of passive and active physical interactions as well as ultrasonic vocalizations. D2R-OE mice show a reduction in social interaction that emerges in adolescence and becomes more pronounced in adulthood. These results suggest that striatal dopamine dysfunction plays an important role in the development of social behavior that may be relevant to schizophrenia.
Neuronal mechanism by which striatal D2R upregulation impair cognitive behaviors
The finding that up-regulation of D2Rs in the striatum would lead to acquisition deficits in spatial working memory and conditional associative learning tasks was unexpected at the time because acquisition and performance of these tasks is critically dependent on the prefrontal cortex (18; 31). Subsequently, studies in humans have shown correlations between striatal dopamine function and prefrontal activity during working memory performance (81–83). D2R-OE mice are therefore useful tools to investigate mechanisms that underlie functional interactions between striatum and cortex. In this context we observed that D2R up-regulation in the striatum led to a decrease in dopamine turnover and an increase in D1R sensitivity in the prefrontal cortex (18) (Figure 1). Disrupted cortical D1R activation may be responsible for the cognitive phenotype in D2R-OE mice as a tight relationship has been described between D1R activation in the cortex and cognition, especially working memory (84; 85), More recently, we have discovered a decrease in burst firing of dopaminergic neurons of the ventral tegmental area (VTA) in D2R-OE mice that cannot be reversed by normalizing striatal D2R expression (86). A decrease in the burst firing of meso-cortical VTA neurons is expected to reduce phasic dopamine release in the cortex. Therefore, it is possible that D2R upregulation in the ventral striatum, via polysynaptic projections to the VTA, alters cortical dopamine turnover, leading to deficits in PFC dependent functions.
The observation that both, cognitive deficits and VTA burst firing are not reversed by switching off the transgene in the adult animal again suggests that antipsychotic medication may not ameliorate cognitive deficits because they are given too late. Increased striatal D2R activity during development may have altered VTA function in a persistent, irreversible way. Our observation, if extrapolated to humans, stresses the importance of early diagnosis of the disorder and suggests that cognitive outcomes may be significantly improved with early pharmacological, as well as psychosocial interventions, a notion that has been proposed before (87).
Interestingly, the decrease in dopamine neuron burst firing in the VTA of D2R-OE mice was associated with a decrease in mRNA expression of NMDA receptor subunits NR1 and NR2B in VTA dopamine neurons (86). Because NMDA receptors are important for burst firing, downregulation of NR1/NR2B may indeed underlie the observed changes in firing patterns (88). This would suggest that pharmacological enhancement of NMDA receptor function could be an effective therapeutic strategy for enhancing cognition.(89). Indeed, enhancing NMDA receptor function has been attempted by inhibiting the Glycine transporter (90). Phase III trials of this drug did however not remediate motivational (or cognitive) deficits in patients, suggesting that a different pharmacological approach, that is either cell or receptor type specific may be required. The finding that striatal D2R upregulation affects cortical dopamine function may have implications with regard to the etiology of the disorder. The long prevailing hypothesis has been that a presumed cortical hypofunction comes first and leads to the increase in striatal dopamine release and D2R occupancy observed in schizophrenia. Alterations in striatal dopamine and cortical activity seem to coexist in patients or subjects with high risk for schizophrenia (5; 91) though see (92). However, it is unknown which area is affected first in patients, the cortex or the subcortical dopamine system. Animal models have shown that cortical hypofunction can lead to a hyperactive dopamine system (93–95). The D2R-OE mice suggest that the opposite directionality is also possible where an increased in striatal D2Rs leads to changes in cortical dopamine function.
Further indications for altered prefrontal function in D2R-OE mice comes from an electrophysiological study that showed that D2R-OE mice display decreased GABAergic transmission in the cortex. This decrease in GABAergic transmission was reversed when the transgene was switched off, suggesting that it could be related to the motivational deficits of D2R-OE mice rather than their cognitive impairments (96).
Neuronal mechanism by which D2R upregulation alters striatal circuit function
As discussed, the cognitive deficits in D2R-OE mice are due to irreversible developmental changes whereas the deficit in motivation is largely rescued by switching off the D2R transgene (20; 39; 50). Strikingly, tonic firing of dopamine VTA neurons is reduced in D2R-OE mice and unlike the decrease in burst firing, the reduction in tonic firing was rescued after switching off the D2R transgene (86). Although the dopamine neurons with altered firing rates were not anatomically traced, based on location within the VTA, they are likely to include neurons that project to the NAc. Therefore, a decrease in tonic VTA firing may change ambient dopamine concentrations in the NAc. The coexisting phenotypes of decreased motivation and reduced tonic DA firing in D2R-OE mice is consistent with the observations that local dopamine levels in the NAc modulate incentive motivation, with dopamine depletion decreasing motivation (97; 98) and increased dopamine enhancing motivation (99).
In addition to identifying changes in the activity of dopamine neurons in D2R-OE mice, we have also identified changes in striatal circuitry downstream of dopamine neuron functions. We measured the physiological properties of striatal projection neurons in D2R-OE and control mice. We found that excitability of striatal projections neurons was enhanced in D2R-OE mice, due to a down-regulation in the expression of inward-rectifying potassium (Kir2) channels (21). Remarkably, this downregulation in Kir function had a dramatic impact on the morphology of the striatal output pathways, and as a consequence, on the function of those pathways (21).
Striatal projection neurons are organized into the direct and indirect projection pathways. The direct pathway predominantly expresses D1Rs and projects monosynaptically to the basal ganglia output nuclei, the internal segment of the globus pallidus (GPi) and the substantia nigra pars reticulata (SNr). In contrast, the indirect pathway predominantly expresses D2Rs and modulates GPi/SNr output through a polysynaptic circuit via the external segment of the globus pallidus (GPe) (100). In the classical model of basal ganglia circuitry both pathways are functionally opposing with regard to thalamo-cortical activation (101). The direct “Go” pathway dis-inhibits thalamo-cortical activity whereas this is inhibited by the indirect “NoGo” pathway. Although classical descriptions of these pathways suggest that they are anatomically segregated, this is not accurate. Several tracing studies have shown that within the dorsal striatum, the majority of direct pathway neurons that project to the midbrain also project to the GPe via axon collaterals (102–105). Since these collaterals “bridge” the direct with the indirect pathway we recently termed them bridging collaterals (106). In the ventral striatum there may be even less segregation as the functional connectivity between direct pathway and the “indirect” pallidum (here: ventral pallidum) is even stronger than it is in the dorsal striatum (107).
Surprisingly, the axon collaterals, which bridge to the GPe are extremely plastic in the adult animal (106). Moreover, their density is regulated by D2R levels in the adult animal via regulation of Kir2 channel function (106). Genetic up-regulation of striatal D2Rs (as in D2R-OE mice) enhances the density of bridging collaterals (Figure 1) whereas genetic down-regulation leads to a gene dosage-dependent decrease in their density (106). As a consequence, the functional balance between the two pathways is altered leading to an inefficient direct pathway as can be observed after optogenetic stimulation of the direct pathway. Stimulation of the direct pathway in D2R-OE mice does not result in the same level of locomotor activation as in control animals (106; 108). Switching off the D2R transgene as well as chronic treatment with haloperidol for 2 weeks reversed the anatomical changes as well as the changes in excitability, Kir2 function and direct pathway inefficiency (106).
Behavioural consequences of altered striatal circuit function in D2R-OE mice
Although we are currently lacking the much needed tools to reliably measure behavioural correlates of positive symptoms in mice, it is possible that the physiological and anatomical abnormalities in striatal circuitry observed in D2R-OE mice are relevant to the positive symptoms of schizophrenia. We hypothesize this because, as previously mentioned, striatal D2R occupancy in the striatum and amphetamine-induced dopamine release correlate with positive symptom severity and predict antipsychotic treatment response (10; 11), indicating a tight relationship between striatal dopamine and psychosis. Full efficacy with antipsychotic medication is achieved after days to weeks of treatment (109), a duration that is comparable with haloperidol-induced retraction of bridging collaterals in mice (106). It is therefore possible that drug-naïve patients that exhibit an increase in density and occupancy of striatal D2Rs also have an increase in bridging collaterals in direct pathway striatal neurons, which contribute to the generation of positive symptoms which are relieved after chronic treatment with antipsychotic medication, treatment which normalizes bridging collateral density. Future longitudinal imaging studies of patients before and after treatment will allow for testing this hypothesis.
In addition to these changes in the anatomy and functioning of the striatal output pathways it is likely that cortico-striatal plasticity may be altered in D2R-OE mice e.g. by alterations in retrograde endocannabinoid signaling or other mechanisms (110). Such alterations may well contribute to the described behavioral deficits. The physiological and anatomical alterations observed in D2R-OE mice are summarized in table 3.
Table 3.
Physiological and Anatomical Alterations in D2R-OE Mice
| Brain Region | With D2R transgene ON | After D2R transgene switched OFF | Ref. |
|---|---|---|---|
| STRIATUM | 15% increase in D2R membrane binding | Normalized | (18) |
| Reduced dopamine stimulated adenylate cyclase activity | Not tested | (18) | |
| Enhanced excitability and decreased dendritic arborization of projection neurons | Normalized | (21) | |
| Decreased glucose metabolism in vivo | Normalized | (18) | |
| Decreased striatal volume | Partially reversed | (21) | |
| GLOBUS PALLIDUM | Enhanced density of direct pathway collaterals (bridging collaterals) | Normalized | (106) |
| Enhanced inhibition of pallidal activity after striatonigral stimulation in vivo | Not tested | (106) | |
| VTA | Decreased tonic firing of VTA dopamine neurons in vivo | Normalized | (86) |
| Decreased phasic firing of VTA dopamine neurons in vivo | Not reversed | (86) | |
| Decreased expression of NMDA receptor subunits NR1/NR2B in VTA dopamine neurons | Not tested | (86) | |
| CORTEX | Increased D1R sensitivity in mPFC In Vivo | Reduced sensitivity (opposite phenotype) | (18) |
| Increased glucose metabolism in vivo (motor and sensory cortices) | Normalized | (18) | |
| Increased excitatory transmission in mPFC slices | Normalized | (96) | |
| Reduced inhibitory transmission in mPFC slices | Normalized | (96) | |
| Reduced D2R sensitivity in mPFC slices | Not tested | (96) |
Conclusions
In summary, by modelling a single, well replicated pathophysiological abnormality observed in patients with schizophrenia in the laboratory mouse, we have been able to expand our understanding of how striatal D2 receptors impact striatal circuity and behavioral abnormalities suffered by patients. We have identified new and potentially critical biological markers that were not considered a priori, and must now be evaluated in patients. Given the serious nature of the disease and the longstanding need to discover safer and more effective treatments for patients, the use of animal models such as the one described here provide valuable tools for understanding the biological basis of schizophrenia.
Acknowledgments
Our research is supported by the National Institute of Mental Health Silvio O. Conte Center for Schizophrenia Research (1P50MH086404 to EHS and CK), NIMH RO1MH093672 to CK, and the Lieber Center for Schizophrenia Research (EHS). We are grateful to Sarah Canetta for helpful comments on this manuscript.
Footnotes
Disclosures:
Dr. Kellendonk has received research support from Forest Laboratories. Dr. Simpson has no biomedical financial interests or potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Baumeister AA, Francis JL. Historical development of the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2002;11:265–277. doi: 10.1076/jhin.11.3.265.10391. [DOI] [PubMed] [Google Scholar]
- 2.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 3.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 4.Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 6.Egerton A, Chaddock CA, Winton-Brown TT, Bloomfield MA, Bhattacharyya S, Allen P, et al. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry. 2013;74:106–112. doi: 10.1016/j.biopsych.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 8.Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q J Nucl Med. 1998;42:211–221. [PubMed] [Google Scholar]
- 10.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naïve schizophrenic subjects. Biol Psychiatry. 2009;65:1091–1093. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 13.Berman KF, Zec RF, Weinberger DR. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. II. Role of neuroleptic treatment, attention, and mental effort. Arch Gen Psychiatry. 1986;43:126–135. doi: 10.1001/archpsyc.1986.01800020032005. [DOI] [PubMed] [Google Scholar]
- 14.Weinberger DR, Berman KF, Illowsky BP. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. III. A new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry. 1988;45:609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- 15.Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA psychiatry. 2015;72:316–324. doi: 10.1001/jamapsychiatry.2014.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranganath A, Jacob SN. Doping the Mind: Dopaminergic Modulation of Prefrontal Cortical Cognition. Neuroscientist. 2015 doi: 10.1177/1073858415602850. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Remington G, Mulsant BH, Rajji TK, Uchida H, Graff-Guerrero A, Mamo DC. Treatment resistant schizophrenia and response to antipsychotics: a review. Schizophr Res. 2011;133:54–62. doi: 10.1016/j.schres.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Morozov A, Kellendonk C, Simpson E, Tronche F. Using conditional mutagenesis to study the brain. Biol Psychiatry. 2003;54:1125–1133. doi: 10.1016/s0006-3223(03)00467-0. [DOI] [PubMed] [Google Scholar]
- 20.Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27:7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cazorla M, Shegda M, Ramesh B, Harrison NL, Kellendonk C. Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels. J Neurosci. 2012;32:2398–2409. doi: 10.1523/JNEUROSCI.6056-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 23.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 24.Fervaha G, Foussias G, Agid O, Remington G. Amotivation and functional outcomes in early schizophrenia. Psychiatry Res. 2013;210:665–668. doi: 10.1016/j.psychres.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barch DM, Smith E. The cognitive neuroscience of working memory: relevance to CNTRICS and schizophrenia. Biol Psychiatry. 2008;64:11–17. doi: 10.1016/j.biopsych.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biol Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Glahn DC, Nuechterlein KH, Cannon TD. Maintenance and manipulation of information in schizophrenia: further evidence for impairment in the central executive component of working memory. Schizophr Res. 2004;68:173–187. doi: 10.1016/S0920-9964(03)00150-6. [DOI] [PubMed] [Google Scholar]
- 29.Horan WP, Braff DL, Nuechterlein KH, Sugar CA, Cadenhead KS, Calkins ME, et al. Verbal working memory impairments in individuals with schizophrenia and their first-degree relatives: findings from the Consortium on the Genetics of Schizophrenia. Schizophr Res. 2008;103:218–228. doi: 10.1016/j.schres.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gold JM, Hahn B, Zhang WW, Robinson BM, Kappenman ES, Beck VM, Luck SJ. Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Arch Gen Psychiatry. 2010;67:570–577. doi: 10.1001/archgenpsychiatry.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bach ME, Simpson EH, Kahn L, Marshall JJ, Kandel ER, Kellendonk C. Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. Proc Natl Acad Sci U S A. 2008;105:16027–16032. doi: 10.1073/pnas.0807746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemali D, Maj M, Galderisi S, Monteleone P, Mucci A. Conditional associative learning in drug-free schizophrenic patients. Neuropsychobiology. 1987;17:30–34. doi: 10.1159/000118337. [DOI] [PubMed] [Google Scholar]
- 33.Gold JM, Bish JA, Iannone VN, Hobart MP, Queern CA, Buchanan RW. Effects of contextual processing on visual conditional associative learning in schizophrenia. Biol Psychiatry. 2000;48:406–414. doi: 10.1016/s0006-3223(00)00930-6. [DOI] [PubMed] [Google Scholar]
- 34.Ford JM, Gray M, Whitfield SL, Turken AU, Glover G, Faustman WO, Mathalon DH. Acquiring and inhibiting prepotent responses in schizophrenia: event-related brain potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 2004;61:119–129. doi: 10.1001/archpsyc.61.2.119. [DOI] [PubMed] [Google Scholar]
- 35.Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, Joyce EM. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning.[see comment] Neuron. 2006;49:603–15. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 37.Carroll CA, Boggs J, O’Donnell BF, Shekhar A, Hetrick WP. Temporal processing dysfunction in schizophrenia. Brain Cogn. 2008;67:150–161. doi: 10.1016/j.bandc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avlar B, Kahn JB, Jensen G, Kandel ER, Simpson EH, Balsam PD. Improving temporal cognition by enhancing motivation. Behav Neurosci. 2015;129:576–588. doi: 10.1037/bne0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward RD, Kellendonk C, Simpson EH, Lipatova O, Drew MR, Fairhurst S, et al. Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits. Behav Neurosci. 2009;123:720–730. doi: 10.1037/a0016503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cogn. 2009;71:345–353. doi: 10.1016/j.bandc.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 42.Ward RD, Winiger V, Higa KK, Kahn JB, Kandel ER, Balsam PD, Simpson EH. The impact of motivation on cognitive performance in an animal model of the negative and cognitive symptoms of schizophrenia. Behav Neurosci. 2015;129:292–299. doi: 10.1037/bne0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holmén A, Juuhl-Langseth M, Thormodsen R, Sundet K, Melle I, Rund BR. Executive function tests in early-onset psychosis: which one to choose? Scand J Psychol. 2012;53:200–205. doi: 10.1111/j.1467-9450.2012.00940.x. [DOI] [PubMed] [Google Scholar]
- 44.Ben Abdallah NM, Fuss J, Trusel M, Galsworthy MJ, Bobsin K, Colacicco G, et al. The puzzle box as a simple and efficient behavioral test for exploring impairments of general cognition and executive functions in mouse models of schizophrenia. Exp Neurol. 2011;227:42–52. doi: 10.1016/j.expneurol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 46.Kohl S, Heekeren K, Klosterkötter J, Kuhn J. Prepulse inhibition in psychiatric disorders--apart from schizophrenia. J Psychiatr Res. 2013;47:445–452. doi: 10.1016/j.jpsychires.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Wynn JK, Green MF, Sprock J, Light GA, Widmark C, Reist C, et al. Effects of olanzapine, risperidone and haloperidol on prepulse inhibition in schizophrenia patients: a double-blind, randomized controlled trial. Schizophr Res. 2007;95:134–142. doi: 10.1016/j.schres.2007.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci. 2009;32:347–358. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry. 2012;17:1206–1227. doi: 10.1038/mp.2012.47. [DOI] [PubMed] [Google Scholar]
- 50.Ward RD, Simpson EH, Richards VL, Deo G, Taylor K, Glendinning JI, et al. Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology. 2012;37:1699–1707. doi: 10.1038/npp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simpson EH, Kellendonk C, Ward RD, Richards V, Lipatova O, Fairhurst S, et al. Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia. Biol Psychiatry. 2011;69:928–935. doi: 10.1016/j.biopsych.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simpson EH, Balsam PD. The Behavioral Neuroscience of Motivation: An Overview of Concepts, Measures, and Translational Applications. Current topics in behavioral neurosciences. 2015 doi: 10.1007/7854_2015_402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reddy LF, Horan WP, Green MF. Motivational Deficits and Negative Symptoms in Schizophrenia: Concepts and Assessments. Current topics in behavioral neurosciences. 2015 doi: 10.1007/7854_2015_379. [DOI] [PubMed] [Google Scholar]
- 54.Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013;74:130–136. doi: 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gold JM, Waltz JA, Frank MJ. Effort Cost Computation in Schizophrenia: A Commentary on the Recent Literature. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barch DM, Treadway MT, Schoen N. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol. 2014;123:387–397. doi: 10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Treadway MT, Peterman JS, Zald DH, Park S. Impaired effort allocation in patients with schizophrenia. Schizophr Res. 2015;161:382–385. doi: 10.1016/j.schres.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young JW, Markou A. Translational Rodent Paradigms to Investigate Neuromechanisms Underlying Behaviors Relevant to Amotivation and Altered Reward Processing in Schizophrenia. Schizophr Bull. 2015;41:1024–1034. doi: 10.1093/schbul/sbv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simpson EH, Waltz JA, Kellendonk C, Balsam PD. Schizophrenia in translation: dissecting motivation in schizophrenia and rodents. Schizophr Bull. 2012;38:1111–1117. doi: 10.1093/schbul/sbs114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ward RD, Simpson EH, Kandel ER, Balsam PD. Modeling motivational deficits in mouse models of schizophrenia: behavior analysis as a guide for neuroscience. Behav Processes. 2011;87:149–156. doi: 10.1016/j.beproc.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13:303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- 63.Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 2002;27:461–474. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- 64.Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 65.Wolf DH, Satterthwaite TD, Kantrowitz JJ, Katchmar N, Vandekar L, Elliott MA, Ruparel K. Amotivation in schizophrenia: integrated assessment with behavioral, clinical, and imaging measures. Schizophr Bull. 2014;40:1328–1337. doi: 10.1093/schbul/sbu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strauss GP, Whearty KM, Morra LF, Sullivan SK, Ossenfort KL, Frost KH. Avolition in schizophrenia is associated with reduced willingness to expend effort for reward on a Progressive Ratio task. Schizophr Res. 2016;170:198–204. doi: 10.1016/j.schres.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 68.McCarthy JM, Treadway MT, Bennett ME, Blanchard JJ. Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophr Res. 2016 doi: 10.1016/j.schres.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- 70.Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol Psychiatry. 2008;64:62–69. doi: 10.1016/j.biopsych.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herrnstein RJ. Relative and absolute strength of response as a function of frequency of reinforcement. J Exp Anal Behav. 1961;4:267–272. doi: 10.1901/jeab.1961.4-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bailey MR, Williamson C, Mezias C, Winiger V, Silver R, Balsam PD, Simpson EH. The effects of pharmacological modulation of the serotonin 2C receptor on goal-directed behavior in mice. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-4135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- 74.Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- 75.Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40(Suppl 2):S107–S116. doi: 10.1093/schbul/sbt197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fervaha G, Zakzanis KK, Foussias G, Graff-Guerrero A, Agid O, Remington G. Motivational deficits and cognitive test performance in schizophrenia. JAMA psychiatry. 2014;71:1058–1065. doi: 10.1001/jamapsychiatry.2014.1105. [DOI] [PubMed] [Google Scholar]
- 77.Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012;69:1216–1224. doi: 10.1001/archgenpsychiatry.2012.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saperstein AM, Medalia A. The Role of Motivation in Cognitive Remediation for People with Schizophrenia. Current topics in behavioral neurosciences. 2015 doi: 10.1007/7854_2015_373. [DOI] [PubMed] [Google Scholar]
- 79.Tarbox SI, Pogue-Geile MF. Development of social functioning in preschizophrenia children and adolescents: a systematic review. Psychol Bull. 2008;134:561–583. doi: 10.1037/0033-2909.34.4.561. [DOI] [PubMed] [Google Scholar]
- 80.Kabitzke PA, Simpson EH, Kandel ER, Balsam PD. Social behavior in a genetic model of dopamine dysfunction at different neurodevelopmental time points. Genes Brain Behav. 2015;14:503–515. doi: 10.1111/gbb.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bertolino A, Taurisano P, Pisciotta NM, Blasi G, Fazio L, Romano R, et al. Genetically determined measures of striatal D2 signaling predict prefrontal activity during working memory performance. PLoS ONE. 2010;5:e9348. doi: 10.1371/journal.pone.0009348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Landau SM, Lal R, O’Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb Cortex. 2009;19:445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Braskie MN, Landau SM, Wilcox CE, Taylor SD, O’Neil JP, Baker SL, et al. Correlations of striatal dopamine synthesis with default network deactivations during working memory in younger adults. Hum Brain Mapp. 2011;32:947–961. doi: 10.1002/hbm.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krabbe S, Duda J, Schiemann J, Poetschke C, Schneider G, Kandel ER, et al. Increased dopamine D2 receptor activity in the striatum alters the firing pattern of dopamine neurons in the ventral tegmental area. Proc Natl Acad Sci U S A. 2015;112:E1498–E1506. doi: 10.1073/pnas.1500450112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lieberman JA, Dixon LB, Goldman HH. Early detection and intervention in schizophrenia: a new therapeutic model. JAMA. 2013;310:689–690. doi: 10.1001/jama.2013.8804. [DOI] [PubMed] [Google Scholar]
- 88.Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Javitt DC. Treatment of negative and cognitive symptoms. Curr Psychiatry Rep. 1999;1:25–30. doi: 10.1007/s11920-999-0007-z. [DOI] [PubMed] [Google Scholar]
- 90.Umbricht D, Alberati D, Martin-Facklam M, Borroni E, Youssef EA, Ostland M, et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA psychiatry. 2014;71:637–646. doi: 10.1001/jamapsychiatry.2014.163. [DOI] [PubMed] [Google Scholar]
- 91.Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 92.Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16:67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- 93.Pycock CJ, Carter CJ, Kerwin RW. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on neurotransmitter systems in subcortical sites in the rat. J Neurochem. 1980;34:91–99. doi: 10.1111/j.1471-4159.1980.tb04625.x. [DOI] [PubMed] [Google Scholar]
- 94.Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J Neurosci. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim IH, Rossi MA, Aryal DK, Racz B, Kim N, Uezu A, et al. Spine pruning drives antipsychotic-sensitive locomotion via circuit control of striatal dopamine. Nat Neurosci. 2015;18:883–891. doi: 10.1038/nn.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li YC, Kellendonk C, Simpson EH, Kandel ER, Gao WJ. D2 receptor overexpression in the striatum leads to a deficit in inhibitory transmission and dopamine sensitivity in mouse prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:12107–12112. doi: 10.1073/pnas.1109718108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mingote S, Weber SM, Ishiwari K, Correa M, Salamone JD. Ratio and time requirements on operant schedules: effort-related effects of nucleus accumbens dopamine depletions. Eur J Neurosci. 2005;21:1749–1757. doi: 10.1111/j.1460-9568.2005.03972.x. [DOI] [PubMed] [Google Scholar]
- 98.Nunes EJ, Randall PA, Podurgiel S, Correa M, Salamone JD. Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: effects of drugs acting on dopamine, adenosine, and muscarinic acetylcholine receptors. Neurosci Biobehav Rev. 2013;37:2015–2025. doi: 10.1016/j.neubiorev.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 99.Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 100.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 102.Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci. 1990;10:3421–3438. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lévesque M, Parent A. The striatofugal fiber system in primates: a reevaluation of its organization based on single-axon tracing studies. Proc Natl Acad Sci U S A. 2005;102:11888–11893. doi: 10.1073/pnas.0502710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, Kaneko T. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur J Neurosci. 2011;33:668–677. doi: 10.1111/j.1460-9568.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- 105.Wu Y, Richard S, Parent A. The organization of the striatal output system: a single-cell juxtacellular labeling study in the rat. Neurosci Res. 2000;38:49–62. doi: 10.1016/s0168-0102(00)00140-1. [DOI] [PubMed] [Google Scholar]
- 106.Cazorla M, de Carvalho FD, Chohan MO, Shegda M, Chuhma N, Rayport S, et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81:153–164. doi: 10.1016/j.neuron.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–1232. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sherwood M, Thornton AE, Honer WG. A meta-analysis of profile and time-course of symptom change in acute schizophrenia treated with atypical antipsychotics. Int J Neuropsychopharmacol. 2006;9:357–366. doi: 10.1017/S1461145705005961. [DOI] [PubMed] [Google Scholar]
- 110.Mathur BN, Lovinger DM. Endocannabinoid-dopamine interactions in striatal synaptic plasticity. Frontiers in pharmacology. 2012;3:66. doi: 10.3389/fphar.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]