Abstract
As well recognized, serotonergic (5-HT) fibers distribute widely throughout the forebrain, including the amygdala. Although a few reports have examined the 5-HT innervation of select nuclei of the amygdala in the rat, no previous report has described overall 5-HT projections to the amygdala in the rat. Using immunostaining for the serotonin transporter, SERT, we describe the complete pattern of distribution of 5-HT fibers to the amygdala (proper) and to the extended amygdala in the rat. Based on its ontogenetic origins, the amygdalar was subdivided into two major parts: pallial and subpallial components, with the pallial component further divided into superficial and deep nuclei (Olucha-Bordonau et al., 2015). SERT+ fibers were shown to distribute moderately to densely to the deep and cortical pallial nuclei, but, by contrast, lightly to the subpallial nuclei. Specifically, (1) of the deep pallial nuclei, the lateral, basolateral and basomedial nuclei contained a very dense concentration of 5-HT fibers; (2) of the cortical pallial nuclei, the anterior cortical and amygdala-cortical transition zone, rostrally, and the posteromedial and posterolateral nuclei, caudally, contained a moderate concentration of 5-HT fibers; and (3) of the subpallial nuclei, the anterior nuclei and the rostral part of the medial (Me) nuclei contained a moderate concentration of 5-HT fibers, whereas caudal regions of Me as well as the central nuclei and the intercalated nuclei contained a sparse/light concentration of 5-HT fibers. Regarding the extended amygdala (primarily the bed nucleus of stria terminalis, BST), on the whole, the BST contained moderate numbers of 5-HT fibers, spread fairly uniformly throughout BST. The findings were discussed with respect to a critical serotonergic influence on the amygdala, particularly on the basal complex, and extended amygdala in the control of states of fear and anxiety.
Keywords: fear, anxiety, stress, basolateral complex of amygdala, central nucleus of amygdala, pallial amygdala, subpallial amygdala, bed nucleus of stria terminalis, 5-HT1A receptors, 5-HT2C receptors
Graphical Abstact
Transverse section mid-rostrocaudally through the amygdala showing the pattern of distribution of serotonin-immunoreactive (SERT-positive) fibers to nuclei of the amygdala. As depicted, SERT+ fibers terminate heavily in the anterior and posterior basolateral nucleus of amygdala (BLAa and BLAp), which contrasts with the sparse distribution to the central nucleus of amygdala, dorsomedial to BLA.
The neurochemical and neuroanatomical substrates for affective behavior have been well studied. At the forefront, both serotonin, chemically, and the amygdala, anatomically, are key substrates for emotional behavior (LeDoux, 2000, 2003; Shinnick-Gallagher et al., 2003; Whalen and Phelps, 2009; Asan et al., 2013; Bauer, 2015). The amygdala (and extended amygdala) has been shown to be critically involved in affective behaviors including fear, stress, anxiety and reward, and is central to the processing of emotionally relevant information (LeDoux, 2000, 2003; Shinnick-Gallagher et al., 2003; Phelps, 2006; Whalen and Phelps, 2009; Duvarci and Pare, 2014; Olucha-Bordonau et al., 2015). Serotonin also serves a well-documented role in affective processes (Lowry et al., 2005, 2008; Hayes and Greenshaw, 2011; Asan et al., 2013; Bauer, 2015). Dysfunction of either system has been associated with a host of psychiatric disorders including depression, chronic anxiety, and post-traumatic stress disorder (PTSD) (Walker et al., 2003; Kalia, 2005; Nemeroff et al., 2006; Shin et al., 2006; Holmes, 2008; Shin and Liberzon, 2010; Hale et al., 2012; Fox and Lowry, 2013)
While earlier studies in the rat, using various techniques, identified 5-HT fibers within select nuclei of the amygdala, no previous report has comprehensively examined the overall distribution of 5-HT fibers to the amygdala, and the 5-HT innervation of the ‘extended amygdala’ has been largely unexplored (Parent et al., 1981; Steinbush, 1981). Previous studies in the rat have mainly focused on 5-HT input to the central and basolateral (BLA) nuclei of the amygdala, showing that 5-HT fibers distribute densely to BLA and by comparison lightly to Ce (Asan et al., 2005; Muller et al., 2007; Smith and Porrino, 2008). Interestingly, this is essentially the reverse of the pattern shown for (non-human) primates wherein Ce is densely populated, BLA relatively lightly so, with 5-HT axons (Sadikot and Parent, 1990; Freedman and Shi, 2001; Bauman and Amaral, 2005; O’Rourke and Fudge, 2006; Smith and Porrino, 2008).
As both the amygdala and serotonergic systems critically participate in several affective functions, notably fear and anxiety, it would be important to determine the precise pattern of termination of 5-HT fibers to the amygdala and BST -- as they influence these functions. Using immunohistochemical procedures for the detection of the serotonin transporter protein (SERT), we examined the distribution of 5-HT fibers to the amygdala (proper) and to BST, with attention to differential innervation of subnuclei. In brief, we showed serotonergic fibers distribute strongly, but heterogeneously, throughout the amygdala and BST. Overall, 5-HT fibers terminate densely in the deep and cortical pallial nuclei, particularly pronounced to the basolateral complex. By contrast, the central and medial nuclei of the subpallial amygdala receive a modest serotonergic innervation. These differences in the pattern and density of 5-HT afferents to the amygdala would appear to reflect a differential serotonergic influence on discrete regions of the amygdala in various affective behaviors.
MATERIALS AND METHODS
Ten (5 male, 5 female) naïve Sprague Dawley rats (Harlan, Indianapolis, IN) weighing 275–300 g on arrival were housed in pairs on a 12:12 light cycle for 7 days, during which food and water were given ad libitum. These experiments were approved by the Florida Atlantic University Institutional Animal Care and Use Committee and conform to all federal regulations and National Institutes of Health guidelines for the care and use of laboratory animals. Rats were deeply anaesthetized with an intraperitoneal injection of sodium pentobarbital (Nembutal, 75 mg/kg). Rats were perfused transcardially with 30–50 ml of ice cold heparinized 0.1 M phosphate buffer saline (PBS) followed by 200–300 ml of chilled 4% paraformaldehyde in 0.1 M phosphate buffer (PB) at a pH = 7.4. The brains were removed and postfixed for 24–48 hrs in 4% paraformaldehyde in 0.1M PB. Brains were then placed in a 30% sucrose solution for another 48 hours. Following sucrose cryoprotection, 50 μm sections were cut on a freezing sliding microtome. Sections were collected in a six well plate using 0.1 M PB as a storage solution, so that every sixth section was represented throughout the brain for each series of sections. Sections were stored in 0.1 M PB at 4°C until the tissue was prepared for immunohistochemistry.
SERT immunohistochemistry
Immunohistochemical analysis to detect serotonergic axons was done with an antiserum to SERT using an avidin biotin protein complex protocol as was described previously (Vertes et al., 2010; Linley et al., 2013). Free-floating sections were treated with 1% sodium borohydride (NaBH4) in 0.1 M PB to remove excess aldehydes. Following copious 0.1 M PB washes, sections were stored for one hour in 0.5% bovine serum albumin (BSA) in 0.1 M tris buffered saline (TBS; pH = 7.6) containing 0.25% Triton X-100. Sections were then incubated in the primary polyclonal antibody, rabbit anti-SERT (ImmunoStar, cat. # 24330, RRID: AB_572209; Hudson, WI). The SERT antibody was raised to a synthetic peptide corresponding to amino acids 579–599 of rat SERT coupled to keyhole limpet hemocyanin (KLH). The primary antibody was placed in a diluent of 0.1% BSA TBS containing 0.25% Triton X-100 at a concentration of 1:5,000 at room temperature for 24–48 hours. Following further washes, sections were placed in a secondary antibody, biotinylated goat anti-rabbit immunoglobulin (Vector Laboratories, cat. # BA-1000, RRID: AB_2313606, Burlingame, CA) in diluent at a 1:500 concentration for two hours. This was followed by another series of PB washes. Sections were then incubated for two hours in a tertiary antibody: biotinylated horse anti-goat immunoglobulin (Vector Laboratories, cat. # BA9500, RRID: AB_2313580, Burlingame, CA) in diluent at a 1:500 concentration. After washing the tissue in 0.1M PB, sections were incubated for one hour in an avidin biotin complex (ABC) using the VECTASTAIN Elite ABC-Peroxidase kit (Vector Laboratories, cat. # PK-7100, RRID: AB_2336827, Burlingame, CA) in a diluent at a 1:200 concentration. Following final 0.1 M PB washes, serotonin fibers expressing the serotonin transporter protein were visualized with the chromogen: 0.022% 3,3′ diaminobenzidine (DAB) (Aldrich, Milwaukee, WI) and 0.003% hydrogen peroxide in TBS for approximately 4–6 minutes. Sections were stored in 0.1M PB at 4°C until mounted onto chrome-alum gelatin-coated slides, dehydrated using graded methanols and coverslipped with Permount. The specificity of the primary and secondary antibodies have been verified in previous studies (Vertes et al., 2010; Linley et al., 2013). Using the present antiserum to SERT, immunostained sections through the pons and mesencephalon displayed identical patterns of cell and fiber SERT+ labeling as shown previously for these regions of the upper brainstem (Sur et al., 1996; Vertes and Crane, 1997; Yamamoto et al., 1998). Additionally, sections reacted without the primary or secondary antibodies did not show immunoreactivity (data are not shown).
Nissl staining
To reference the cytoarchitectonic borders of nuclei of the amygdala and BST, sections throughout the amygdala and extended amygdala were visualized using a cresyl violet stain. Briefly, sections were mounted on chrome-alum–coated slides, rinsed 2 × 10 min in xylene, rehydrated in graded methanols, and immersed in a 0.1% cresyl violet (Acros Organics, Thermo Fisher Scientific, Waltham, MA) in an acetic acid buffer for 15–20 minutes. Slides were then rinsed briefly with deionized water, dehydrated in graded methanols (to 100% methanol), cleared with xylene, and coverslipped with Permount.
Photomicroscopy
Lightfield photomicrographs at 100× magnification were taken for visualization of SERT+ fibers throughout the rostrocaudal extent of the amygdala (proper) and the extended amygdala. Photomicrographs were captured using a Q Imaging (QICAM) camera mounted onto a Nikon Eclipse E600 microscope. Digital images were captured and reconstructed using Nikon Elements and then imported into Adobe Illustrator and Photoshop CC 2014 (Mountain View, CA) to adjust brightness and contrast and to outline borders of nuclei of the amygdala/BST. Representative Nissl stained sections throughout the extent of the amygdala/BST were captured and imported into Adobe Illustrator to map the subdivisions of nuclear groups.
A note on nomenclature for the amygdala and extended amygdala
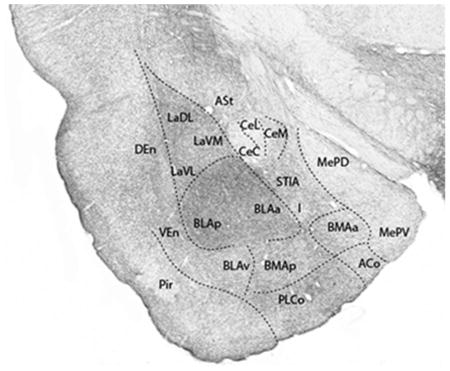
The nomenclature and nuclear demarcations used for nuclei of the amygdala and extended amygdala are those of Olucha-Bordonau et al. (2015), as follows. The amygdaloid complex has recently been subdivided according to its ontogenetic origin into two major divisions: pallial and subpallial components, with the pallial component further divided into superficial and deep nuclei (Medina et al., 2004; García-López et al., 2008; Martínez-García et al., 2008; Bupesh et al., 2011; Hertel et al., 2012; Olucha-Bordonau et al., 2015). This demarcation is analogous to a cortical/subcortical differentiation -- with the cortical component showing a distinct lamination, and with a few exceptions the subcortical sector exhibiting no layering. Further, the pallial amygdala, like the cortex, contains a greater proportion of glutamatergic to GABAergic cells whereas the subpallial amygdala, like subcortical telencephalic structures, contains significantly more GABAergic relative to glutamatergic cells. The superficial division of the pallial amygdala (or the cortical pallial amygdala) consists of the anterior (ACo), posterolateral (PLCo) and posteromedial (PMCo) cortical nuclei as well as transition zones between the ACo and piriform cortex; that is, rostrally, the cortical-amygdala transition zone (CxA) and caudally the amygdalo-piriform transition area (APir). The deep (or nuclear) division of the pallial amygdala consists of the oft-referred to ‘basolateral complex’ of the amygdala comprised of the lateral (La), basolateral (BLA) and basomedial (BMA) nuclei -- as well as the amygdalo-hippocampal area (AHi). The subpallial division of the amygdala primarily consists of the medial (Me) and central (Ce) and anterior nuclei, and the intercalated cell masses.
The subpallial region of the ‘extended amygdala’ (EA) consists of two components: (1) the bed nucleus of the stria terminalis (BST) which includes the intra-amygdala area (STIA), the supracapuslar EA (STS) and the BST (proper) divisions; and (2) the sublenticular extended amygdala (SLEA) composed of the sublenticular part of the substantia innominata (SI) and the interstitial nucleus of the posterior limb of the anterior commissure (IPAC), lying dorsolateral to the anterior amygdaloid area. (Olucha-Bordonau et al., 2015). The septal sector of BST (or BST proper) surrounds the anterior commissure and is composed of several subnuclei -- with those of the anterior division associated with the central nucleus of amygdala and those of the posterior division associated with the medial nucleus of amygdala (Dong et al., 2001; Alheid, 2003; Olucha-Bordonau et al., 2015).
RESULTS
We describe the pattern of distribution of serotonergic fibers to the amygdala (proper) as well as that to the extended amygdala (or bed nucleus of the stria terminalis complex), as illustrated by a representative case (case 14). The findings of non-illustrated cases directly correspond to those of the illustrated case.
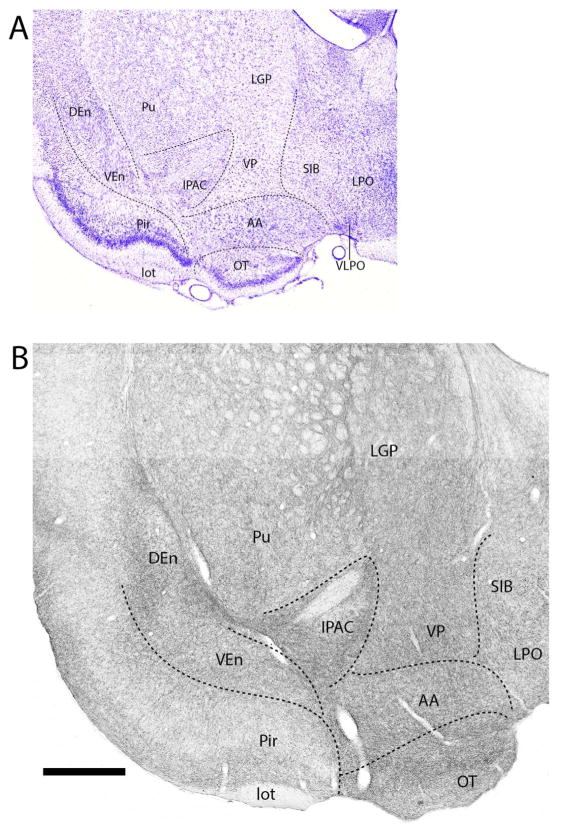
Amygdala
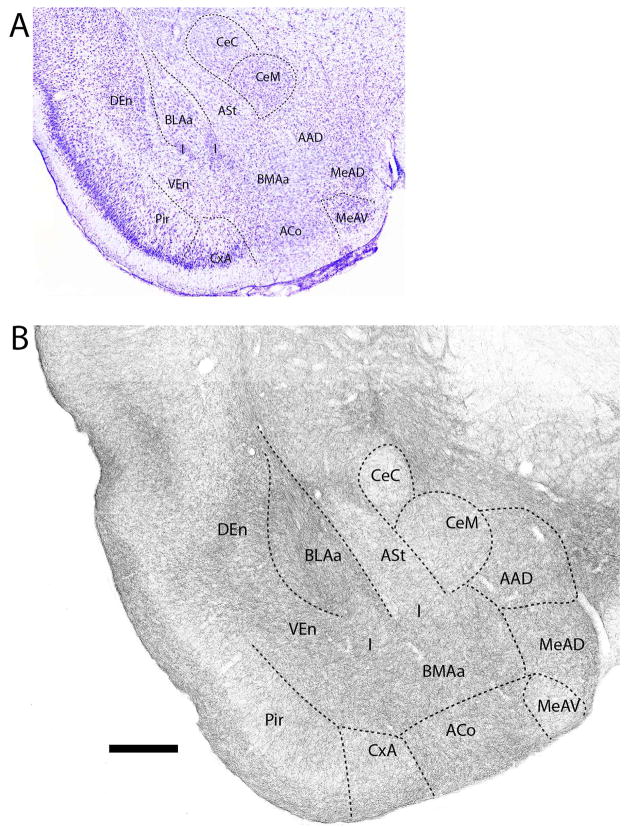
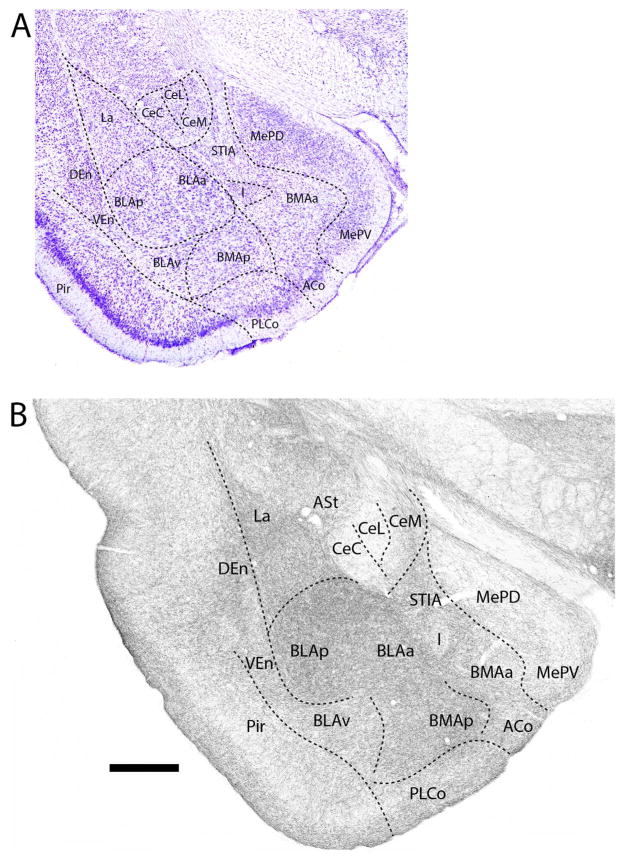
Figures 1–11 depict the pattern of distribution of 5-HT fibers rostrocaudally throughout the amygdala, and consist of a series of Nissl-stained sections (Figs. 1A–11A) showing the locations of nuclei of the amygdala and corresponding SERT-immunostained sections (Figs. 1B–11B) depicting patterns of labeling at these levels of the amygdala. Figure 1B shows the pattern of distribution 5-HT fibers at a caudal level of the extended amygdala (EA) as it merges with the amygdala proper. As depicted (Fig. 1B), the interstitial nucleus of the posterior limb of the anterior commissure (IPAC), surrounding the anterior commissure (ac), was moderately labeled. This contrasts with considerably lighter labeling of IPAC at a more anterior level (see Fig. 14). At the rostral pole of the amygdala (Fig. 2B), the anterior dorsal (AAD) and anterior ventral (AAV) nuclei as well as the rostral extent of the basomedial nucleus (BMA) were moderately to densely labeled. SERT+ fibers spread moderately throughout cortical pallial nuclei, distributing non-homogenously to the anterior cortical nucleus (ACo) and the cortical amygdala transition zone (CxA), but evenly within the nucleus of the lateral olfactory tract (LOT). Regarding ACo and CxA, labeling was dense in superficial layers (layer 1) and tapered in deeper layers. This same pattern continued laterally from CxA to the piriform cortex. With the emergence of the central nucleus (Ce) at this level (Fig. 2B), the capsular division of Ce (CeC) was lightly labeled; the medial division (CeM) moderately labeled.
Figure 1.
A: Low magnification Nissl-stained transverse section at the transition between the amygdala (proper) and the extended amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the amygdala at the same level – or to sublenticular region of the amygdala. See list for abbreviations. Scale bar for A = 1.2 mm; for B = 500 μm.
Figure 11.
A: Low magnification Nissl-stained transverse section through the posterior amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the amygdala at the same level. Note the dense labeling of the posterior part of the basomedial and basolateral nuclei. See list for abbreviations. Scale bar for A = 750 μm; for B = 500 μm.
Figure 14.
A: Low magnification Nissl-stained transverse section through the posterior part of the extended amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the extended amygdala at the same level. See list for abbreviations. Scale bar for A = 375 μm; for B = 250 μm.
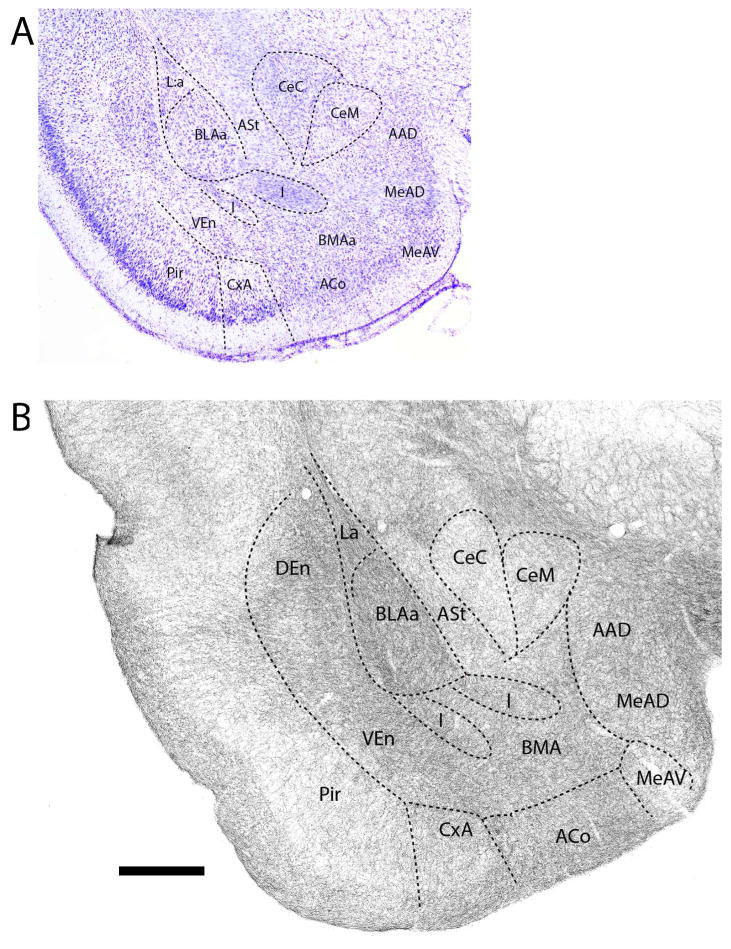
Figure 2.
A: Low magnification Nissl-stained transverse section through the anterior amygdala showing the locations of nuclei at the same level. B: Pattern of the distribution of 5-HT fibers to nuclei of the amygdala at this level (B). See list for abbreviations. Scale bar for A = 1.3 mm; for B = 500 μm.
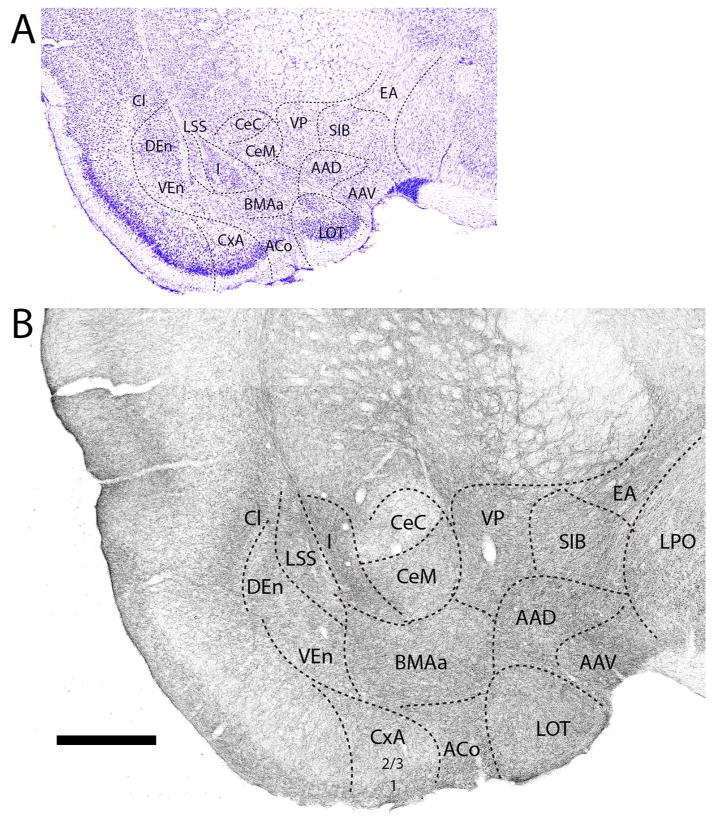
Further caudally in the anterior amygdala (Fig. 3B), 5-HT fibers distributed heavily to the anterior basolateral (BLAa) and anterior basomedial (BMAa) nuclei, and significantly but less densely to AAD and to the anterodorsal part of the medial nucleus (MeAD), lying ventral to AAD. By contrast, labeling was less pronounced (or moderate) in CeM and in the amygdalostriatal transition area (ASt), lateral to CeC. The pattern of labeling of ACo and CxA was similar to that of rostral levels, with a decrease in density from superficial to deep layers, and was stronger in ACo and CxA than in the medially adjacent bed nucleus of the accessory olfactory tract (BAOT). As seen rostrally, the CeC was lightly labeled, whereas the intercalated nuclei (I) were lightly to moderately labeled.
Figure 3.
A: Low magnification Nissl-stained transverse section through the anterior amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the amygdala at the same level. See list for abbreviations. Scale bar for A = 800 μm; for B = 500 μm.
Progressing posteriorly (Fig. 4B), marked differences began to emerge in the relative density of labeling across nuclei of the amygdala. More precisely, the very dense labeling of the lateral nucleus (La) and the anterior (BLAa) and posterior (BLAp) divisions of the basolateral nucleus (BLA), strongly contrasted with the light labeling of the medially adjacent central nucleus and ASt. This trend would continue further caudally. The ventral basolateral nucleus (BLAv) and BMAa contained slightly fewer labeled fibers than did BLA, whereas AAD, the dorsal (MeAD) and ventral (MeAV) medial nuclei and the cortical nuclei (CxA, ACo, and BAOT) were less densely labeled than the basal groups. As rostrally, layer 1 of cortical areas ACo and CxA was heavily labeled; layers 2/3 moderately labeled.
Figure 4.
A: Low magnification Nissl-stained transverse section through the anterior amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the amygdala at the same level. Note the dense labeling of nuclei of the basolateral complex (La, BMA, BLA) and the light labeling of divisions of the central nucleus (Ce). See list for abbreviations. Scale bar for A = 1 mm; for B = 500 μm.
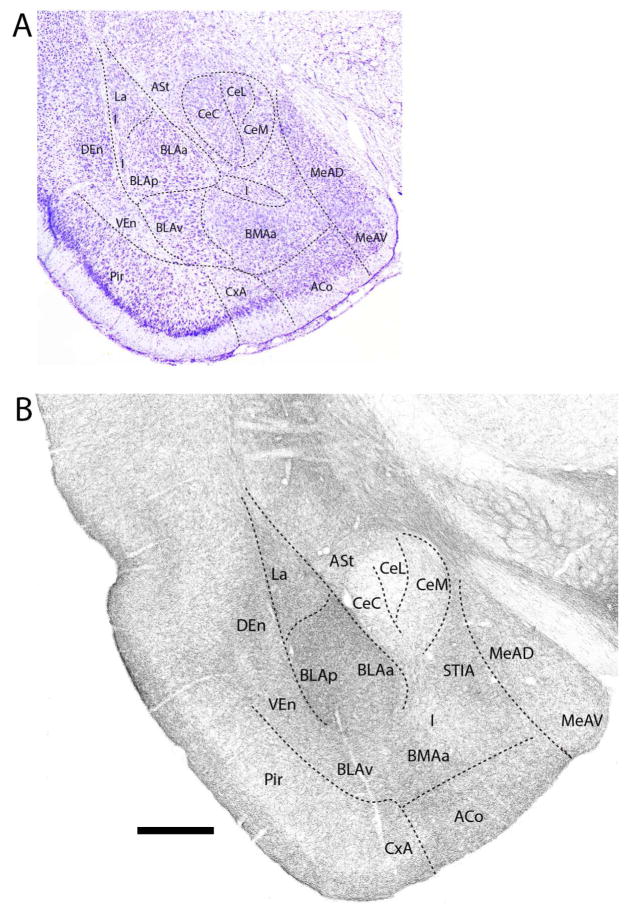
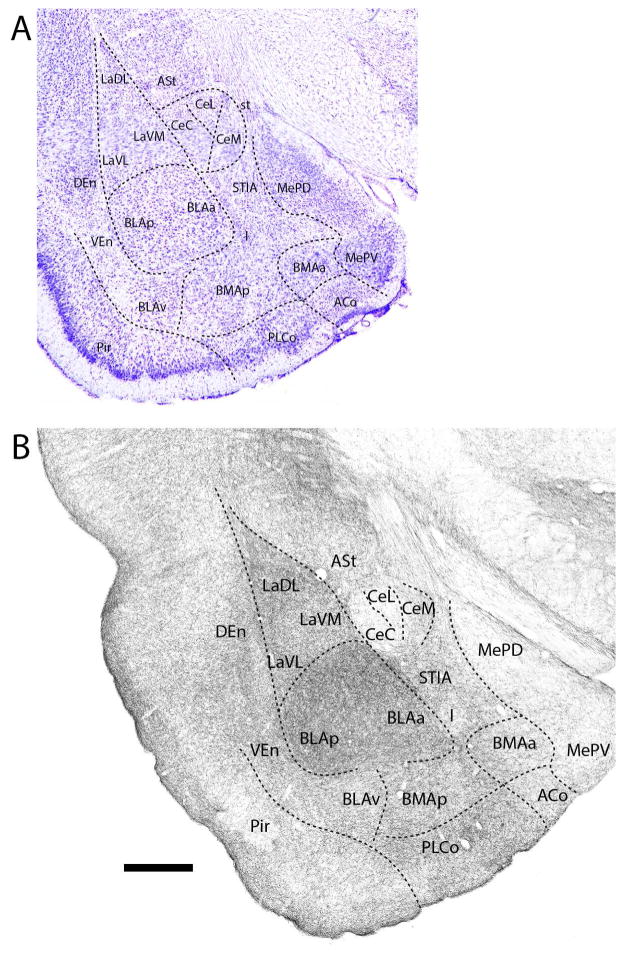
At the mid-rostrocaudal amygdala (Fig. 5B), the basal group continued to be strongly labeled which, as rostrally, differed significantly from the sparse to light labeling of Ce. Labeling was slightly heavier in CeM than in CeC or in the lateral division of Ce (CeL). Of the basal group, labeling was densest in BLA (BLAa and BLAp) followed by La, BMAa and BLAv which were similarly labeled. With the exception of the intercalated group which was lightly labeled, remaining nuclei at this level were moderately labeled. This includes the ASt, MeAD, MeAV, ACo, CxA, and the intra-amygdalar component of BST (STIA). As rostrally, labeling gradually weakened from superficial to deep layers of ACo and CxA.
Figure 5.
A: Low magnification Nissl-stained transverse section through the mid-portion of the amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the amygdala at the same level. Note the dense labeling of nuclei of the basolateral complex (La, BMA, BLA), particularly BLA, and the sparse labeling of divisions of the central nucleus (Ce). See list for abbreviations. Scale bar for A = 1 mm; for B = 500 μm.
Further caudally (Fig. 6B), labeled fibers were densely concentrated in La and bordering regions of BLAa and BLAp, but tapered slightly in ventral parts of the basal complex; that is, in the anterior (BMAa) and posterior (BMAp) divisions of BMA and in BLAv. All divisions of the Ce were sparsely labeled, with a few more fibers in CeM than in the other divisions of Ce. The ASt component of Ce was moderately labeled. Labeling thinned from that of anterior levels in MeAD and MeAV such at this level both divisions of Me were lightly labeled. The ACo and the posterolateral cortical nucleus (PLCo) (which now emerged), as well as the STIA were moderately labeled; the intercalated group lightly labeled.
Figure 6.
A: Low magnification Nissl-stained transverse section through the mid-portion of the amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the amygdala at the same level. Note the dense labeling of nuclei of the basolateral complex (La, BMA, BLA), particularly BLA, and the sparse labeling of divisions of the central nucleus (Ce). See list for abbreviations. Scale bar for A = 1 mm; for B = 500 μm.
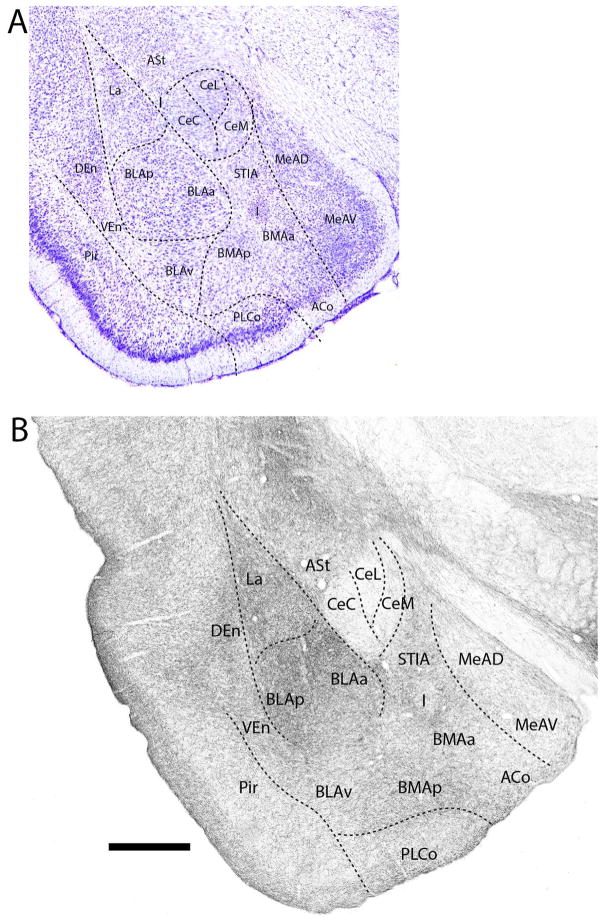
At a mid-posterior level (Fig. 7B), labeled fibers distributed moderately to densely to both superficial (cortical) and deep pallial nuclei; that is, to ACo, PLCo, La, BLAa, BLAp, BLAv, BMAa and BMAp. Labeling was heaviest in La, the anterior and posterior basolateral nuclei, and BMAp. By contrast with the dense labeling of pallial structures, the major subpallial nuclei were lightly labeled. These included the posterodorsal (MePD) and posteroventral (MePV) divisions of Me, CeL, CeC, and the intercalated group. As rostrally, although light, labeling was more pronounced in CeM than in CeL or CeC. Other subpallial nuclei, ASt and STIA, were moderately labeled.
Figure 7.
A: Low magnification Nissl-stained transverse section through the mid-portion of the amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the amygdala at the same level. Note the dense labeling of nuclei of the basolateral complex (La, BMA, BLA), particularly BLA, and the sparse to light labeling of divisions of the central (Ce) and medial nucleus (Me). See list for abbreviations. Scale bar for A = 835 μm; for B = 500 μm.
Proceeding caudally (Fig. 8B), labeled fibers continued to densely innervate pallial structures, particularly BLA. Labeling was very pronounced in BLAa and BLAp as well in the dorsolateral part of La (LaDL). By comparison, the ventrolateral (LaVL) and ventromedial (LaVM) divisions of La were moderately labeled, as were BLAv, BMAp and PLCo. For subpallial structures, ASt and STIA were moderately labeled, whereas MePD and MePV, all divisions of Ce, and the intercalated nuclei were lightly labeled.
Figure 8.
A: Low magnification Nissl-stained transverse section through the posterior amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the amygdala at the same level. Note the dense labeling of nuclei of the basolateral complex (La, BMA, BLA), particularly BLA, and the sparse to light labeling of divisions of the central (Ce) and medial nuclei (Me). See list for abbreviations. Scale bar for A = 700 μm; for B = 500 μm.
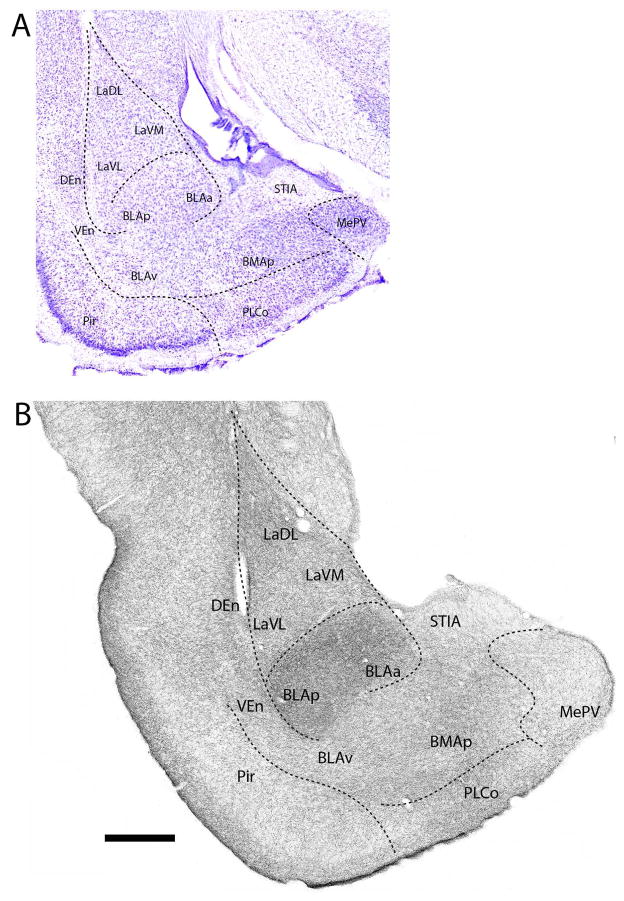
Further posteriorly (Fig. 9B), labeling remained dense in the basal complex, heaviest in BLAa and BLAp, slightly less so in LaDL, and strong but somewhat weaker still in BLAv, BMAp and medial (LaVM) and lateral (LaVL) divisions of La. As with the anterior cortical nuclei, labeling was denser in superficial than deep layers of PLCo. Subpallial structures, including STIA, MePD and MePV, were lightly to moderately labeled. Similar to the central and medial nuclei (see above), labeling gradual thinned in STIA from rostral to caudal levels.
Figure 9.
A: Low magnification Nissl-stained transverse section through the posterior amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the amygdala at the same level. Note the dense labeling of nuclei of the basolateral complex (La, BMA, BLA), particularly BLA, and the light labeling of the medial nucleus. See list for abbreviations. Scale bar for A = 670 μm; for B = 500 μm.
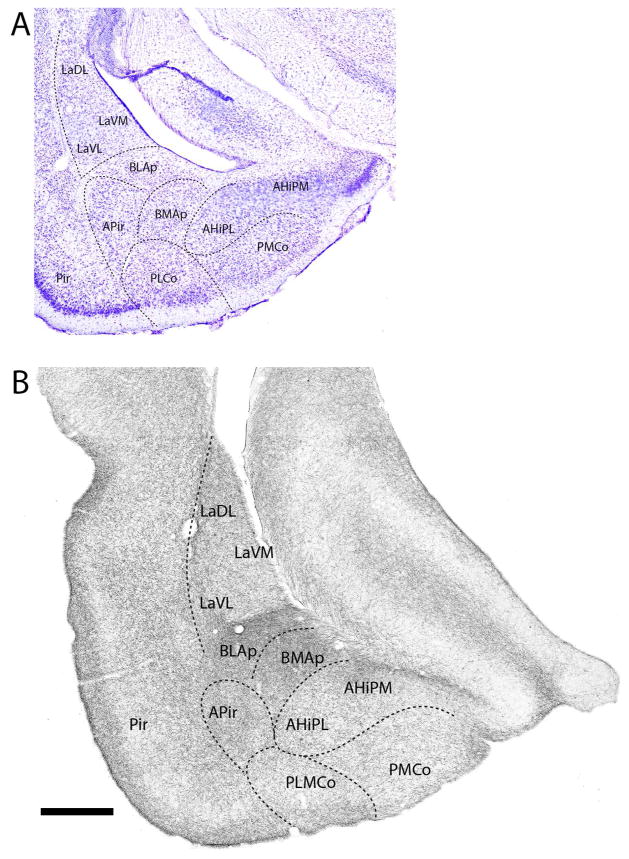
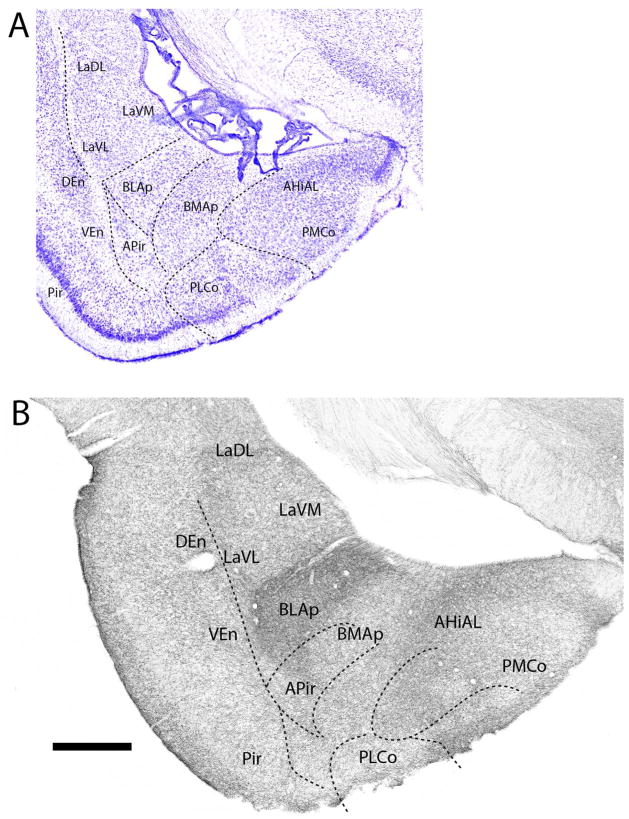
At the posterior amygdala (Fig. 10B), BLAp was densely (or massively) labeled -- clearly differentiating it from surrounding structures. Labeling was also pronounced in other deep pallial structures including La (LaDL, LaVM, LaVL), BMAp, and within the anterolateral amygdalohippocampal transition area (AHiAL). Of cortical pallial structures, the posteromedial cortical nucleus (PMCo), the PLCo, and the amygdalopiriform transition area (APir) were moderately to densely labeled. Unlike the laminar differences in labeling of PMCo and PLCo, labeled fibers spread quite uniformly throughout APir.
Figure 10.
A: Low magnification Nissl-stained transverse section through the posterior amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the amygdala at the same level. Note the dense labeling of the posterior part of the basolateral nucleus, setting it apart from surrounding structures. See list for abbreviations. Scale bar for A = 1 mm; for B = 500 μm.
At the caudal aspect of the amygdala (Fig. 11B), deep and cortical pallial nuclei contained moderate to dense concentrations of labeled fibers, with labeling heaviest in medial aspects of BLAp and BMAp. While less pronounced, La was also strongly labeled with a dorsoventral gradient such that LaDL was more heavily labeled than either LaVM or LaVL. The medial (AHiPM) and lateral (AHiPL) nuclei of the amygdalohippocampal transition area were moderately labeled; AHiPM slightly more heavily than AHiPL. PMCo and APir, contained moderate numbers of labeled fibers, with labeling heaviest in layer 1 of PMCo.
Bed nucleus of the stria terminalis (BST)
As indicated (see Introduction), the subpallial extended amygdala consists of two components, BST and the sublenticular extended amygdala (SLEA). The BST contains three subdivisions, the main one being BST proper. The BST (proper), which at rostral levels straddles the anterior commissure (ac), is composed of several subnuclei which, as will be described, contain differing concentrations of 5-HT fibers. Figures 12–15 depict the pattern of distribution of 5-HT fibers rostrocaudally throughout BST, and consist of a series of Nissl-stained sections (Figs. 12A–15A) showing the locations of nuclei of BST and corresponding SERT immuno-stained sections (Figs. 12B–15B) depicting the patterns of labeling at these levels of BST.
Figure 12.
A: Low magnification Nissl-stained transverse section through the anterior part of the extended amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the extended amygdala at the same level. See list for abbreviations. Scale bar for A = 380 μm; for B = 250 μm.
Figure 15.
A: Low magnification Nissl-stained transverse section through the posterior part of the extended amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the extended amygdala at the same level. See list for abbreviations. Scale bar for A = 275 μm; B = 250 μm.
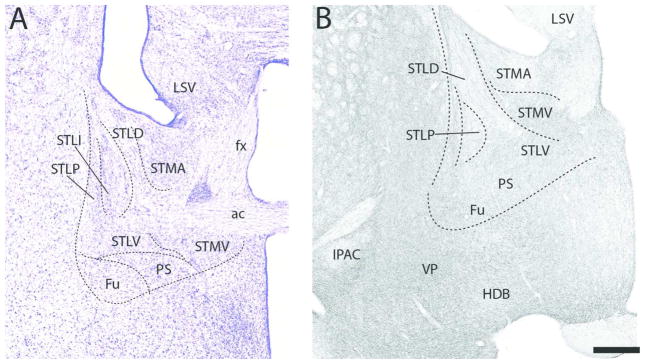
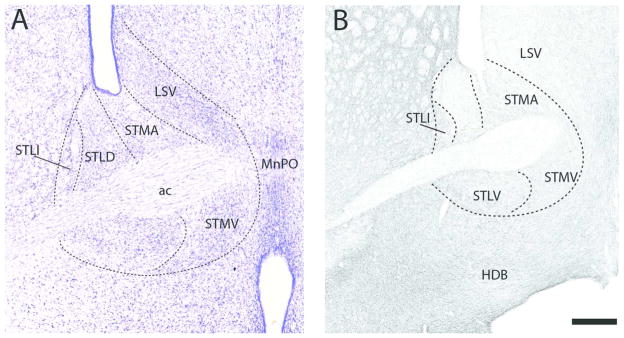
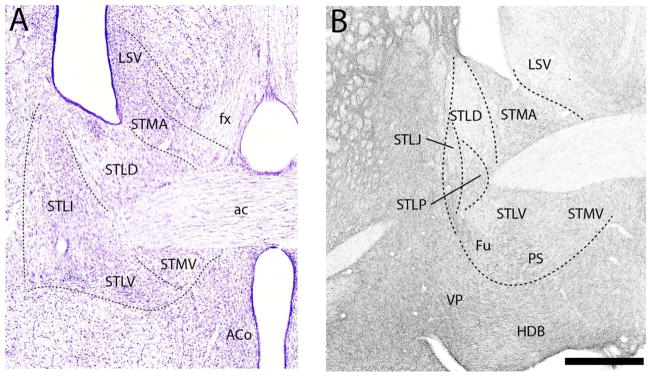
At the rostral pole of BST (Fig. 12B), labeled fibers spread quite homogenously throughout BST distributing moderately to three subnuclei, the anterior medial (STMA), the lateral intermediate (STLI) and the lateral ventral (STLV) nuclei, and sparsely to the lateral dorsal nucleus (STLD). A similar pattern was observed further caudally (Fig. 13B). Specifically, with exception of STLD which, as rostrally, was sparsely labeled, each of the subnuclei surrounding the anterior commissure were moderately labeled. The dorsal subnuclei, including STMA, STLI, and lateral posterior (STLP) nuclei, were slightly more densely labeled than the ventral group which included the medial ventral nucleus (STMV), fusiform nucleus (Fu) and the parastrial nucleus (PS).
Figure 13.
A: Low magnification Nissl-stained transverse section through the anterior part of the extended amygdala showing the locations of nuclei at this level. B: Pattern of distribution of 5-HT fibers to nuclei of the extended amygdala at the same level. See list for abbreviations. Scale bar for A = 360 μm; for B = 250 μm.
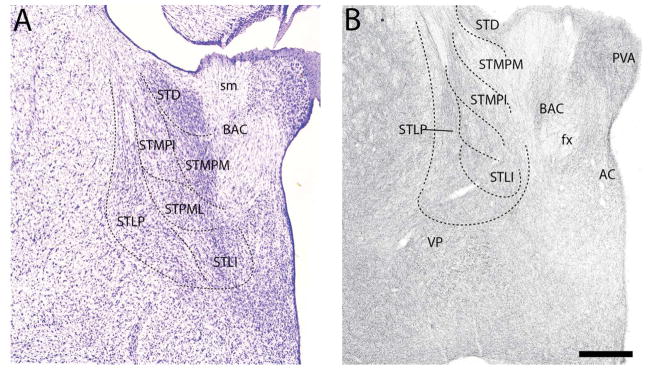
The pattern of labeling further caudally in BST (Figs. 14B, 15B) largely mirrored that at rostral levels. As shown (Fig. 14B), the STLD was lightly labeled, whereas neighboring regions, medially and ventrally, were moderately labeled. They included STMA, STMV, STLV, STLP, STLJ, PS, and Fu. Of these nuclei, labeling was heaviest in FU and lightest in STMA. At the caudal extent of BST (Fig. 15B) labeling was stronger ventrally/ventrolaterally than dorsomedially. The medal part of the medial posterior nucleus (STMPM) was lightly labeled, whereas adjacent nuclei dorsally and ventrolaterally were moderately labeled. This included the dorsal subgroup (STD), the intermediate (STMPI) and lateral parts (STMPL) of the medial posterior nucleus, the STLP, the lateral intermediate nucleus (STLI) and the bed nucleus of the anterior commissure (BAC).
DISCUSSION
The present report describes the pattern of distribution of serotonergic (5-HT) fibers to the amygdala (proper) and to the extended amygdala – or the bed nucleus of the stria terminalis complex (BST).
Amygdala
Overall, serotonergic fibers distribute densely but differentially throughout nuclei of the amygdala. SERT+ fibers heavily innervate most of the deep and cortical pallial nuclei of amygdala which contrasts with minimal innervation of subpallial nuclei. Of the deep pallial structures, the nuclei of the basal complex, consisting of the lateral, basolateral and basomedial nuclei (and their subregions) contained the densest concentration of 5-HT fibers, particularly posterior aspects of the basolateral complex. In general, cortical pallial structures contained fewer SERT+ fibers than the deep pallial nuclei.
Of the subpallial amygdalar nuclei, there is a rostrocaudal gradient, whereby SERT+ fibers distribute more heavily to anterior than to posterior divisions of subpallial structures. 5-HT fibers terminate moderately in the anterior dorsal and anterior ventral nuclei of the anterior amygdala, in the amygdalostriatal transition area and in the anterodorsal and anteroventral parts of the medial nucleus; lightly in the posterodorsal and posteroventral sectors of Me, and in the intercalated nuclei, and generally sparsely in the central nucleus.
Bed nucleus of the stria terminalis (BST)
The subpallial extended amygdala consists of two components: (1) BST including the intra-amygdalar (STIA) and BST (proper) divisions; and (2) the sublenticular extended amygdala consisting of SI and IPAC (Olucha-Bordonau et al., 2015). The BST proper contained a moderate collection of SERT+ fibers, which on the whole was of lesser magnitude than those to the amygdala. With the exception of a light innervation of lateral dorsal division of BST (STLD) and the medial posterior nucleus (STMPM), 5-HT fibers terminate moderately within structures of BST. These included: STMA, STMV, STLI, STLV, STLP, Fu and PS, rostrally, and the STD, STMPI, STMPL, STLI, and the lateral STLP, caudally. The anterior pole of IPAC contained moderate numbers of 5-HT fibers, as did the STIA, lying ventral to Ce and co-extensive with it.
Comparison with previous examinations of serotonergic innervation of the amygdala
As discussed, no previous report has described the overall pattern of distribution of 5-HT fibers to the amygdala (or to BST) in the rat. Nonetheless, some studies have examined the 5-HT innervation of select nuclei of the amygdala, concentrating on the basal group and the central nucleus (Sur et al., 1996; Commons et al., 2003; Muller et al., 2007; Smith and Porrino, 2008; Bonn et al., 2013). With minor differences among reports, the common finding was that 5-HT fibers distribute densely to the basolateral nucleus, strongly but less heavily to the lateral nucleus, and sparsely to the central nucleus. The 5-HT innervation of the basomedial and medial nuclei was intermediate to that of BLA/La and Ce. The present results are generally consistent with these findings with the exception that we observed: (1) denser labeling of the posterior than the anterior BLA; (2) stronger labeling of the medial than lateral or capsular divisions of Ce; and (3) light labeling of the posterodorsal and posteroventral divisions of the medial nucleus.
Correspondence between 5-HT innervation of the amygdala/BST and midbrain raphe projections to the amygdala/BST
The serotonergic input to the forebrain originates almost entirely from midbrain raphe nuclei; namely, the dorsal (DR) and median raphe (MR) nuclei and the B9 group (Vertes and Martin, 1988; Vertes, 1991; Vertes and Crane, 1997; Vertes et al., 1999; Morin and Meyer-Bernstein, 1999; Vertes and Linley, 2007, 2008; Muzerelle et al., 2015). Early reports in the rat (Vertes, 1991; Vertes et. al., 1999) and hamster (Morin and Meyer-Bernstein, 1999), using anterograde tracers, described pronounced DR projections (mainly from the rostral DR) to the amygdala -- with minor projections from MR. In accord with present findings, the DR was shown to strongly target the lateral, basolateral and cortical nuclei of amygdala and lightly the medial nucleus (Vertes, 1991; Morin and Meyer-Bernstein, 1999; Muller et al., 2007; Bonn et al., 2013). Unlike, the present demonstration of sparse 5-HT labeling of the central nucleus, Bienkowski and Rinaman (2013) described moderate DR-Ce projections. Some of the DR input to Ce, however, undoubtedly originated from non-serotonergic DR neurons.
Similar to the amygdala (proper), midbrain raphe projections to BST predominately arise from DR (or the rostral DR) and minimally from MR (Vertes, 1991; Vertes et al., 1999; Morin and Meyer-Bernstein, 1999; Vertes and Linley, 2008; Muzerelle et al., 2015). Consistent with present findings, DR fibers were shown to distribute moderately, and quite uniformly, throughout BST, with a preference for the anterior ventrolateral sector of BST – which includes the fusiform, parastrial, and medial and lateral ventral nuclei of BST (Vertes, 1991; Morin and Meyer-Bernstein, 1999; Vertes and Linley, 2008; Bienkowski and Rinaman, 2013; Muzerelle et al., 2015).
Functional role of serotonergic input to the amygdala -- with a focus on the basolateral nucleus
As discussed, both the amygdala and the serotonergic system serve direct, and likely complementary, roles in emotional behavior, prominently including fear and anxiety (Davis, 2000). For instance, SSRIs have been described as the “gold standard” in the treatment of anxiety disorders (Inoue et al., 2011; Burghardt and Bauer, 2013), possibly in large part involving their actions on the amygdala.
The detailed circuitry of the amygdala responsible for the acquisition and expression of fear/anxiety has been well described (see Duvarci and Pare, 2014; Herry and Johansen, 2014; Tovote et al. 2015). The primary cell groups of the amygdala involved in fear/anxiety are the basal group (La, BLA and BMA), the central nucleus (or CeL and CeM) and the intercalated nuclei. For the most part, information flows unidirectionally from La to BLA to CeL and then to CeM, such that La is the principal afferent node and CeM the output site in fear/anxiety. In general, associations between neutral sensory stimuli and aversive events (tone-foot shock pairings) are made in La, transferred with modifications through BLA/BMA and the intercalated nuclei to CeL and then to CeM to affect behavior. Accordingly, the BLA (and BMA) is pivotally positioned to modify information transferred from La to CeM. This coupled with the present demonstration that the BLA receives a dense 5-HT input, suggests that BLA may be a primary (amygdalar) target for 5-HT actions on fear/anxiety (Amano et al., 2011). Consistent with this, serotonin has been shown to exert significant modulatory effects at the BLA in anxiety-like behaviors (Asan et al., 2013; Burghardt and Bauer, 2013; Bauer, 2015).
Anxiety-producing stimuli/conditions release serotonin to the amygdala/BLA (Hale et al., 2012; Fox and Lowry, 2013) which is initially (or acutely) anxiogenic, but with time (or chronically) becomes anxiolytic (Burghardt et al., 2004; Burghardt and Bauer, 2013). In a parallel manner, various serotonin reuptake inhibitors (SSRIs) initially elicit fear/anxiety-like behaviors (Burghardt et al., 2004, 2007; Grillion et al., 2007; Ravinder et al., 2011), but with long term use become anxiolytic (Zhang et al., 2000; Li et al., 2001; Burghardt et al., 2004; Hashimoto et al., 2009; Deschaux et al., 2011; Inoue et al., 2011).
While the effects of SSRIs on fear/anxiety are not limited to the amygdala or BLA, the BLA appears to be a principal site for the early anxiogenic and later anxiolytic effects of SSRIs (Inoue et al. 2004, 2011; Kitaichi et al. 2014). In addition, the acute anxiogenic actions of SSRIs reportedly involve 5-HT2C receptors (mainly of BLA), whereas the chronic anxiolytic effects involve 5-HT1A receptors of BLA (Burghardt et al., 2007; Christianson et al., 2010; Vicente and Zangrossi, 2012, 2014; de Andrade Strauss et al., 2013). For instance, regarding 5-HT2C receptors, Vicente and Zangrossi (2012) showed that: (1) intra-BLA injections of the 5-HT2C receptor agonist, MK-212, and the 5-HT2C receptor antagonist, SB-242084, enhanced or supressed anxiety-like behaviors, respectively, on the elevated T-maze; and (2) SB-242084 blocked the (acute) anxiogenic effect elicited by the systemic administration of the antidepressants, imipramine or fluoxetine. Vicente and Zangrossi (2014) subsequently confirmed that MK-212 injected into BLA was anxiogenic, and further showed that anxiogenic actions were attenuated with chronic treatment with imipramine or fluoxetine. Taken together the findings support the view that the acute anxiogenic, as well as the chronic anxiolytic, effects of antidepressants are, in part, mediated through 5-HT2C receptors of BLA. Specifically, a 5-HT2C mediated activation of BLA neurons is anxiogenic, whereas the gradual (chronic) desensitization of 5-HT2C receptors of these cells contributes to anxiolysis.
By contrast with the anxiogenic effects of 5-HT2C receptors, several studies have shown that 5-HT1A receptors produce anxiolytic actions at the BLA (Zangrossi et al., 1999; Li et al., 2012; de Andrade Strauss et al., 2013; Vicente and Zangrossi, 2014). For example, de Andrade Strauss et al. (2013) showed that BLA injections of the 5HT1A receptor agonist, 8-OH-DPAT, suppressed anxiety-like behavior on three tests of anxiety – with effects blocked by the prior administration of the 5-HT1A antagonist, WAY-100635. Moreover, Vicente and Zangrossi (2014) reported that the anxiogenic actions produced by intra-BLA injections of the 5-HT2C receptor agonist, MK-212, were blocked with chronic antidepressant administration, but were re-instated with intra-BLA injections of the 5-HT1A antagonist, WAY-100635. Accordingly, the authors (Vincente and Zangrossi, 2014) concluded that “both a reduction in 5-HT2C-R- and a facilitation of 5-HT1A-R mediated neurotransmission in the BLA are involved in the anxiolytic effect of antidepressant drugs”. Consistent with this, Li et al. (2012), using recombinant adenovirus-induced alterations in 5-HT receptor expression in the amygdala, demonstrated that decreases in 5-HT1A, or increases in the 5-HT2C, receptor expression produced anxiogenic actions on two tests of anxiety-like behavior in mice.
While the precise mechanism(s) whereby 5-HT1A and 5-HT2C receptors of BLA cells affect anxiety-like states remains to be fully determined, the process understandably involves interneurons and principal cells. With some exceptions (see below) increases in BLA pyramidal cell activity appears to be anxiogenic, decreases anxiolytic. With respect to 5-HT1A receptors, an early report in anesthetized rats (Stein et al., 2000) showed that the 5-HT1A agonist, 8-OH-DPAT, suppressed the activity of most pyramidal cells (PCs) of BLA. In accord with this, Cheng et al. (1998) had shown that serotonin, acting via 5-HT1A receptors, suppressed the activity of principal BLA cells and reduced a depolarization-evoked influx of calcium into these cells.
Whereas the effects of serotonin on 5-HT1A-receptor-containing cells of BLA are fairly straightforward, specific 5-HT actions on 5-HT2C receptor cells of BLA are not fully resolved. For instance, Rainnie (1999) demonstrated in the slice preparation that the application of 5-HT or 5-HT2 agonists, to BLA activated GABAergic interneurons -- with the consequent inhibition of pyramidal cells. While this finding appears inconsistent with a proposed role for 5-HT2C receptors in anxiogenesis (i.e., the activation, not the suppression, of principal BLA activity is thought to be anxiogenic) it should be noted that: (1) a non-specific 5-HT2 agonist was used (α-methyl-5-HT); results may differ with a more specific 5-HT2c agonist; and (2) high concentrations or the prolonged application of 5-HT suppressed the excitatory action of 5-HT on BLA interneurons -- and hence their inhibitory effect on PCs. Further, in contrast to these results, Stein et al., (2000) showed, in intact rats, that the 5-HT2C agonist, DOI, increased the activity of virtually all principal BLA neurons, while Reznikov et al. (2008) reported that restraint stress produced elevated levels of c-fos expression in the vast majority of PCs of BLA. Taken together, the foregoing findings would indicate that 5-HT2/2C receptor cell activation excites both interneurons and principal cells of BLA, but as noted by Campbell and Merchant (2003), the likely net effect would be an “augmentation of BLA output which could underlie the anxiogenic responses of 5-HT2C agonists at behaviourally relevant doses.”
Whereas a reduction of principal cell activity of BLA is generally thought to contribute to anxiolysis, Tye et al. (2011) recently identified a subset of BLA cells whose activation suppressed anxiety-like behavior in mice. Specifically, using optogenetic techniques, they demonstrated that stimulation of a select population of BLA cells projecting to CeL produced anxiolytic actions, presumably via a BLA-induced activation of GABAergic CeL cells and the consequent inhibition of CeM output neurons. Interestingly, however, the overall (or non-specific) activation of BLA neurons was found to be anxiogenic which is consistent with previously-discussed reports (Vicente and Zangrossi, 2012; Zangrossi and Graeff, 2014).
Finally, based on the pronounced 5-HT input to the basal nuclei of the amygdala, we focused on the role of 5-HT afferents to the BLA complex in fear/anxiety, but it is certainly well recognized that the amygdala participates in wide range of emotional behaviors, many of which have been linked to particular subnuclei. For instance, Ferguson et al. (2001) showed that oxytocin acts on the medial nucleus of the amygdala to facilitate social recognition in mice. Possibly directly related to this, it has recently been found that selective serotonin reuptake inhibitors (SSRIs) taken during the second or third trimester, for the treatment for depression, increases the risk for autism spectrum disorders (Boukhris et al., 2015). Although we showed 5-HT fibers distribute moderately to the medial nucleus, it is nevertheless possible that a SSRI-induced developmental disruption (or reorganization) of serotonergic projections to the medial nucleus could contribute to the social deficits associated with autism spectrum disorders.
Functional role of serotonergic input to the BST
Similar to the amygdala proper, the BST also plays a prominent role in affective behaviors, prominently stress and anxiety (Davis et al., 2010). In general, serotonin exerts anxiolytic effects at the BST (Levita et al., 2004; Gomes et al., 2011, 2012). This partly involves a 5-HT-mediated suppression of excitatory glutamatergic and corticotrophin releasing factor (CRF) inputs to the BST as well as on intrinsic CRF-mediated excitation at BST. For instance, CRF infused into the anterolateral BST elicits anxiety-like behaviors including increased startle and reduced exploratory behavior in the elevated plus maze (Lee and Davis, 1997; Sahuque et al., 2006).
Rainnie and colleagues (Hammack et al. 2009; Daniel and Rainnie, 2015) recently proposed a model for the interactive roles of the DR and BST in stress/anxiety. Specifically, stressors produce an increase in the release of CRF to both the DR and BST which initially is anxiogenic but with time becomes anxiolytic. In effect, at low doses CRF binds to high affinity CRF1 receptors of the DR to inhibit the firing of 5-HT DR cells, whereas at higher doses CRF also binds to CRF2 receptors to increase the discharge of DR neurons – and thereby releases 5-HT to the BST in the suppression of anxiety (Hammack et al., 2003).
Three types of neurons have been identified in the lateral BST of rodents: type I–III cells. Type I and III neurons discharge at regular rates, with the threshold to activation higher for type III than type I cells, whereas type II cells display a bursting pattern of discharge which has been associated with the release of various peptides from the cells (Egli and Winder, 2003; Hammack et al., 2007). Type I neurons constitute 29%, type II neurons 55%, and type III neurons 16% of the population of lateral BST neurons (Hammack et al., 2007). The BST contains a rich array of 5-HT receptors which differentially populate the three cell types – and thus determine their roles in stress/anxiety (Daniel and Rainnie, 2015). The main 5-HT receptor types are 5-HT1A, 5-HT1B, 5-HT2C, and 5-HT7 receptors – and most abundantly 5-HT1A receptors. The 5-HT1A receptor is expressed on all three types of BST cells (Hazra et al., 2012). Since 5-HT1A receptor activation hyperpolarizes neurons, the predominant (or net) effect of 5-HT applied to BST is a hyperpolarization of BST neurons (Guo et al., 2009; Hazra et al., 2012) – and anxiolytic actions at BST (Levita et al., 2004; Gomes et al., 2011, 2012). In contrast to 5-HT1A receptors, 5-HT depolarizes 5-HT2C and 5-HT7 receptor-containing neurons of the BST. 5-HT2C receptors are almost entirely located on type III cells, which are mainly CRF neurons, and reportedly form a feed forward loop with the DR in the elicitation of anxiety-like behaviors. Specifically, a DR release of 5-HT to the BST activates type III cells which, in turn, releases CRF to the DR, drives DR neurons, and thus maintains the cycle. However, the release of 5-HT to BST also activates 5-HT7 receptor containing BST neurons, present on type I and type II neurons which suppress the activity of type III CRF neurons to dampen anxiety-like states. In sum, the actions of 5-HT at the BST are complex with ultimate effects on stress/anxiety dependent on the relative recruitment of various types of 5-HT receptors -- favoring 5-HT1A receptors and anxiolysis.
Acknowledgments
Grant sponsor: NIMH, grant number: MH099590
Abbreviations
- 5-HT
serotonin
- AAA
anterior amygdaloid area
- AAD
anterior amygdaloid area, dorsal part
- AAV
anterior amygdaloid area, ventral part
- ac
anterior commissure
- AC
nucleus accumbens
- ACo
anterior cortical nucleus of amygdala
- AHi
amygdalo-hippocampal area
- AHiAL
amygdalohippocampal transition area, anterolateral part
- AHiPL
amygdalohippocampal transition area, posterolateral part
- AHiPM
amygdalohippocampal transition area, posteromedial division
- APir
amygdalopiriform transition area
- ASt
amygdalostriatal transition area
- BAC
bed nucleus of anterior commissure
- BAOT
bed nucleus of accessory olfactory tract
- BLA
basolateral amygdala
- BLAa
basolateral nucleus of amygdala, anterior part
- BLAp
basolateral nucleus of amygdala, posterior part
- BLAv
basolateral nucleus of amygdala, ventral part
- BMA
basomedial amygdala
- BMAa
basomedial nucleus of amygdala, anterior part
- BMAp
basomedial nucleus of amygdala, posterior part
- BST
bed nucleus of the stria terminalis
- Ce
central nucleus of amygdala
- CeC
central nucleus of amygdala, capsular division
- CeL
central nucleus of amygdala, lateral division
- CeM
central nucleus of amygdala, medial division
- CRF
corticotrophin releasing factor
- CxA
cortical amygdala transition zone
- DEn
dorsal endopiriform nucleus
- DR
dorsal raphe nucleus
- EA
extended amygdala
- Fu
fusiform nucleus of BST
- fx
fornix
- HDB
nucleus of horizontal limb of diagonal band
- I
intercalated nuclei of amygdala
- IPAC
interstitial nucleus of posterior limb of anterior commissure
- La
lateral nucleus of amygdala
- LaDL
lateral nucleus of amygdala, dorsolateral part
- LaVL
lateral nucleus of amygdala, ventrolateral part
- LaVM
lateral nucleus of amygdala, ventromedial part
- LGP
globus pallidus, lateral division
- lot
lateral olfactory tract
- LOT
nucleus of lateral olfactory tract
- LPO
lateral preoptic area
- LSS
lateral stripe of striatum
- LSV
lateral septal nucleus, ventral part
- Me
medial nucleus of amygdala
- MeAD
medial nucleus of amygdala, anterodorsal part
- MeAV
medial nucleus of amygdala, anteroventral part
- MePD
medial nucleus of amygdala, posterodorsal part
- MePV
medial nucleus of amygdala, posteroventral part
- mPFC
medial prefrontal cortex
- MR
median raphe nucleus
- OT
olfactory tubercle
- Pir
piriform cortex
- PLCo
posterolateral cortical nucleus of amygdala
- PMCo
posteromedial cortical nucleus of amygdala
- PS
parastrial nucleus of BST
- Pu
putamen
- PVA
paraventricular nucleus of thalamus, anterior part
- SERT
serotonin transporter protein
- SI
substantia innominata
- SIB
substantia innominata, basal division
- SLN
supralemniscal nucleus
- sm
stria medullaris
- SO
supraoptic nucleus
- SLEA
sublenticular extended amygdala
- STD
dorsal division of BST
- STIA
intra-amygdala division of BST
- STLD
lateral dorsal division of BST
- STLI
lateral intermediate division of BST
- STLJ
lateral juxtacapsular division of BST
- STLP
lateral posterior division of BST
- STLV
lateral ventral division of BST
- STMA
medial anterior division of BST
- STMPI
medial posterior intermediate division of BST
- STPML
medial posterior lateral division of BST
- STMPM
medial posterior medial division of BST
- STMV
medial ventral division of BST
- STS
supracapsular extended amygdala
- VEn
ventral endopiriform nucleus
- VP
ventral pallidum
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ROLE OF THE AUTHORS
SBL collected the data, SBL and FO-B served major roles in the preparation of the figures, and RPV served a major role in writing the manuscript. All authors, however, were involved in all phases of the planning and execution of the research.
LITERATURE CITED
- Alheid GF. Extended amygdala and basal forebrain. Ann NY Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Amano T, Duvarci S, Popa D, Paré D. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci. 2011;31:15481–15489. doi: 10.1523/JNEUROSCI.3410-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch KP, Schmitt A. The corticotropin-releasing factor (CRF)-system and monoaminergic afferents in the central amygdala: investigations in different mouse strains and comparison with the rat. Neuroscience. 2005;131:953–967. doi: 10.1016/j.neuroscience.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Asan E, Steinke M, Lesch KP. Serotonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histochem Cell Biol. 2013;139:785–813. doi: 10.1007/s00418-013-1081-1. [DOI] [PubMed] [Google Scholar]
- Bauer EP. Serotonin in fear conditioning processes. Behav Brain Res. 2015;277:68–77. doi: 10.1016/j.bbr.2014.07.028. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Amaral DG. The distribution of serotonergic fibers in the macaque monkey amygdala: an immunohistochemical study using antisera to 5-hydroxytryptamine. Neuroscience. 2005;136:193–203. doi: 10.1016/j.neuroscience.2005.07.040. [DOI] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Common and distinct neural inputs to the medial central nucleus of the amygdala and anterior ventrolateral bed nucleus of stria terminalis in rats. Brain Struct Funct. 2013;218:187–208. doi: 10.1007/s00429-012-0393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn M, Schmitt A, Lesch KP, Van Bockstaele EJ, Asan E. Serotonergic innervation and serotonin receptor expression of NPY-producing neurons in the rat lateral and basolateral amygdaloid nuclei. Brain Struct Funct. 2013;218:421–435. doi: 10.1007/s00429-012-0406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhris T, Sheehy O, Mottron L, Bérard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr. 2016;170:117–124. doi: 10.1001/jamapediatrics.2015.3356. [DOI] [PubMed] [Google Scholar]
- Bupesh M, Abellan A, Medina L. Genetic and experimental evidence supports the continuum of the central extended amygdala and a mutiple embryonic origin of its principal neurons. J Comp Neurol. 2011;519:3507–3531. doi: 10.1002/cne.22719. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Bauer EP. Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: implications for underlying fear circuits. Neuroscience. 2013;247:253–272. doi: 10.1016/j.neuroscience.2013.05.050. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Bush DE, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol Psychiatry. 2007;62:1111–1118. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Cheng LL, Wang SJ, Gean PW. Serotonin depresses excitatory synaptic transmission and depolarization-evoked Ca2+ influx in rat basolateral amygdala via 5-HT1A receptors. Eur J Neurosci. 1998;10:2163–2172. doi: 10.1046/j.1460-9568.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry. 2010;67:339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Daniel SE, Rainnie DG. Stress modulation of opposing circuits in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.178. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The amygdala. Vol. 2. Oxford, UK: Oxford University Press; 2000. pp. 213–287. [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade Strauss CV, Vicente MA, Zangrossi H. Activation of 5-HT1A receptors in the rat basolateral amygdala induces both anxiolytic and antipanic-like effects. Behav Brain Res. 2013;246:103–110. doi: 10.1016/j.bbr.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Deschaux O, Spennato G, Moreau JL, Garcia R. Chronic treatment with fluoxetine prevents the return of extinguished auditory-cued conditioned fear. Psychopharmacology (Berl) 2011;215:231–237. doi: 10.1007/s00213-010-2134-y. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Winder DG. Dorsal and ventral distribution of excitable and synaptic properties of neurons of the bed nucleus of the stria terminalis. J Neurophysiol. 2003;90:405–414. doi: 10.1152/jn.00228.2003. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JH, Lowry CA. Corticotropin-releasing factor-related peptides, serotonergic systems, and emotional behavior. Front Neurosci. 2013;7:169. doi: 10.3389/fnins.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LJ, Shi C. Monoaminergic innervation of the macaque extended amygdala. Neuroscience. 2001;104:1067–1084. doi: 10.1016/s0306-4522(01)00157-9. [DOI] [PubMed] [Google Scholar]
- García-López M, Abellán A, Legaz I, Rubenstein JL, Puelles L, Medina L. Histogenetic compartments of the mouse centromedial and extended amygdala based on gene expression patterns during development. J Comp Neurol. 2008;506:46–74. doi: 10.1002/cne.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Resstel LB, Guimarães FS. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology (Berl) 2011;213:465–473. doi: 10.1007/s00213-010-2036-z. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Reis DG, Alves FH, Corrêa FM, Guimarães FS, Resstel LB. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT1A receptors. J Psychopharmacol 2012. 2012;26:104–113. doi: 10.1177/0269881110389095. [DOI] [PubMed] [Google Scholar]
- Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology. 2007;32:225–231. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- Guo JD, Hammack SE, Hazra R, Levita L, Rainnie DG. Bi-directional modulation of bed nucleus of stria terminalis neurons by 5-HT: molecular expression and functional properties of excitatory 5-HT receptor subtypes. Neuroscience. 2009;164:1776–1793. doi: 10.1016/j.neuroscience.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Shekhar A, Lowry CA. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol Neurobiol. 2012;32:695–708. doi: 10.1007/s10571-012-9827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Mania I, Rainnie DG. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J Neurophysiol. 2007;98:638–656. doi: 10.1152/jn.00382.2007. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1309–1320. doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Inoue T, Muraki I, Koyama T. Effects of acute citalopram on the expression of conditioned freezing in naive versus chronic citalopram-treated rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:113–117. doi: 10.1016/j.pnpbp.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Greenshaw AJ. 5-HT receptors and reward-related behaviour: a review. Neurosci Biobehav Rev. 2011;35:1419–1449. doi: 10.1016/j.neubiorev.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Hazra R, Guo JD, Dabrowska J, Rainnie DG. Differential distribution of serotonin receptor subtypes in BNST(ALG) neurons: modulation by unpredictable shock stress. Neuroscience. 2012;225:9–21. doi: 10.1016/j.neuroscience.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci. 2014;17:1644–1654. doi: 10.1038/nn.3869. [DOI] [PubMed] [Google Scholar]
- Hertel N, Redies C, Medina L. Cadherin expression delineates the divisions of the postnatal and adult mouse amygdala. J Comp Neurol. 2012;520:3982–4012. doi: 10.1002/cne.23140. [DOI] [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Li XB, Abekawa T, Kitaichi Y, Izumi T, Nakagawa S, Koyama T. Selective serotonin reuptake inhibitor reduces conditioned fear through its effect in the amygdala. Eur J Pharmacol. 2004;497:311–316. doi: 10.1016/j.ejphar.2004.06.061. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kitaichi Y, Koyama T. SSRIs and conditioned fear. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1810–1819. doi: 10.1016/j.pnpbp.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Kalia M. Neurobiological basis of depression: an update. Metabolism. 2005;54(5 Suppl 1):24–27. doi: 10.1016/j.metabol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Kitaichi Y, Inoue T, Nakagawa S, Omiya Y, Song N, An Y, Chen C, Kusumi I, Koyama T. Local infusion of citalopram into the basolateral amygdala decreased conditioned fear of rats through increasing extracellular serotonin levels. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:216–222. doi: 10.1016/j.pnpbp.2014.05.018. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hammack SE, Mania I, Li XY, Davis M, Rainnie DG. 5-hydroxytryptamine1A-like receptor activation in the bed nucleus of the stria terminalis: electrophysiological and behavioral studies. Neuroscience. 2004;128:583–596. doi: 10.1016/j.neuroscience.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Li Q, Luo T, Jiang X, Wang J. Anxiolytic effects of 5-HT1A receptors and anxiogenic effects of 5-HT2C receptors in the amygdala of mice. Neuropharmacology. 2012;62:474–484. doi: 10.1016/j.neuropharm.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XB, Inoue T, Hashimoto S, Koyama T. Effect of chronic administration of flesinoxan and fluvoxamine on freezing behavior induced by conditioned fear. Eur J Pharmacol. 2001;425:43–50. doi: 10.1016/s0014-2999(01)01159-1. [DOI] [PubMed] [Google Scholar]
- Linley SB, Hoover WB, Vertes RP. Pattern of distribution of serotonergic fibers to the orbitomedial and insular cortex in the rat. J Chem Neuroanat. 2013;48–49:29–45. doi: 10.1016/j.jchemneu.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann NY Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Martínez-García F, Novejarque A, Lanuza E. Two interconnected functional systems in the amygdala of amniote vertebrates. Brain Res Bull. 2008;75:206–213. doi: 10.1016/j.brainresbull.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Medina L, Legaz I, Gonzalez G, De Castro F, Rubenstein JL, Puelles L. Expression of Dbx1, neurogenin 2, semaphoring 5A, cadherin 8, and Emx1 distinguish ventral and lateral pallial histogenetic divisions in the developing mouse claustroamygdaloid complex. J Comp Neurol. 2004;474:504–523. doi: 10.1002/cne.20141. [DOI] [PubMed] [Google Scholar]
- Morin LP, Meyer-Bernstein EL. The ascending serotonergic system in the hamster: comparison with projections of the dorsal and median raphe nuclei. Neuroscience. 1999;91:81–105. doi: 10.1016/s0306-4522(98)00585-5. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;505:314–335. doi: 10.1002/cne.21486. [DOI] [PubMed] [Google Scholar]
- Muzerelle A, Scotto-Lomassese S, Bernard JF, Soiza-Reilly M, Gaspar P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5–B9) to the forebrain and brainstem. Brain Struct Funct. 2015 doi: 10.1007/s00429-014-0924-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Olucha-Bordonau FE, Fortes-Marco L, Otero-García M, Lanuza E, Martínez-García F. Amgydala: Structure and Function. In: Paxinos G, editor. The rat nervous system. 4. San Diego: Academic Press; 2015. pp. 441–490. [Google Scholar]
- O’Rourke H, Fudge JL. Distribution of serotonin transporter labeled fibers in amygdaloid subregions: implications for mood disorders. Biol Psychiatry. 2006;60:479–490. doi: 10.1016/j.biopsych.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Descarries L, Beaudet A. Organization of ascending serotonin systems in the adult rat brain. A radioautographic study after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1981;6:115–138. doi: 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Ravinder S, Pillai AG, Chattarji S. Cellular correlates of enhanced anxiety caused by acute treatment with the selective serotonin reuptake inhibitor fluoxetine in rats. Front Behav Neurosci. 2011;5:88. doi: 10.3389/fnbeh.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov LR, Reagan LP, Fadel JR. Activation of phenotypically distinct neuronal subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress. J Comp Neurol. 2008;508:458–472. doi: 10.1002/cne.21687. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A. The monoaminergic innervation of the amygdala in the squirrel monkey: an immunohistochemical study. Neuroscience. 1990;36:431–447. doi: 10.1016/0306-4522(90)90439-b. [DOI] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, Mcgeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology (Berl) 2006;186:122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann NY Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick-Gallagher P, McKernan MG, Xie J, Zinebi F. L-type voltage-gated calcium channels are involved in the in vivo and in vitro expression of fear conditioning. Ann NY Acad Sci. 2003;985:135–149. doi: 10.1111/j.1749-6632.2003.tb07078.x. [DOI] [PubMed] [Google Scholar]
- Smith HR, Porrino LJ. The comparative distributions of the monoamine transporters in the rodent, monkey, and human amygdala. Brain Struct Funct. 2008;213:73–91. doi: 10.1007/s00429-008-0176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Davidowa H, Albrecht D. 5-HT(1A) receptor-mediated inhibition and 5-HT(2) as well as 5-HT(3) receptor-mediated excitation in different subdivisions of the rat amygdala. Synapse. 2000;38:328–337. doi: 10.1002/1098-2396(20001201)38:3<328::AID-SYN12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Sur C, Betz H, Schloss P. Immunocytochemical detection of the serotonin transporter in rat brain. Neuroscience. 1996;73:217–231. doi: 10.1016/0306-4522(96)00030-9. [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. A PHA L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Martin GF. Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J Comp Neurol. 1988;275:511–541. doi: 10.1002/cne.902750404. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM. Distribution, quantification, and morphological characteristics of serotonin-immunoreactive cells of the supralemniscal nucleus (B9) and pontomesencephalic reticular formation in the rat. J Comp Neurol. 1997;378:411–424. doi: 10.1002/(sici)1096-9861(19970217)378:3<411::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB. Comparisons of projections of the dorsal and median raphe nuclei, with some functional considerations. In: Takai K, editor. Interdisciplinary conference on tryptophan and related substances: chemistry, biology, and medicine. Oxford, UK: Elsevier; 2007. pp. 98–120. International Congress Series, 1304. [Google Scholar]
- Vertes RP, Linley SB. Efferent and afferent connections of the dorsal and median raphe nuclei in the rat. In: Monti JM, Pandi-Perumal SR, Jacobs BL, Nutt D, editors. Serotonin and sleep: molecular, functional and clinical aspects. Basel, Switzerland: Birkhauser-Verlag; 2008. pp. 69–102. [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Pattern of distribution of serotonergic fibers to the thalamus of the rat. Brain Struct Funct. 2010;215:1–28. doi: 10.1007/s00429-010-0249-x. [DOI] [PubMed] [Google Scholar]
- Vicente MA, Zangrossi H. Serotonin-2C receptors in the basolateral nucleus of the amygdala mediate the anxiogenic effect of acute imipramine and fluoxetine administration. Int J Neuropsychopharmacol. 2012;15:389–400. doi: 10.1017/S1461145711000873. [DOI] [PubMed] [Google Scholar]
- Vicente MA, Zangrossi H. Involvement of 5-HT2C and 5-HT1A receptors of the basolateral nucleus of the amygdala in the anxiolytic effect of chronic antidepressant treatment. Neuropharmacology. 2014;79:127–135. doi: 10.1016/j.neuropharm.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Phelps EA. The human amygdala. New York: Guilford Press; 2009. [Google Scholar]
- Yamamoto H, Fujimiya M, Shirai Y, Nakashita M, Oyasu M, Saito N. Immunohistochemical localization of serotonin transporter in normal and colchicine treated rat brain. Neurosci Res. 1998;32:305–312. doi: 10.1016/s0168-0102(98)00097-2. [DOI] [PubMed] [Google Scholar]
- Zangrossi H, Graeff FG. Serotonin in anxiety and panic: contributions of the elevated T-maze. Neurosci Biobehav Rev. 2014;3:397–406. doi: 10.1016/j.neubiorev.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Zangrossi H, Viana MB, Graeff FG. Anxiolytic effect of intra-amygdala injection of midazolam and 8-hydroxy-2-(di-n-propylamino)tetralin in the elevated T-maze. Eur J Pharmacol. 1999;369:267–270. doi: 10.1016/s0014-2999(99)00075-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Raap DK, Garcia F, Serres F, Ma Q, Battaglia G, Van de Kar LD. Long-term fluoxetine produces behavioral anxiolytic effects without inhibiting neuroendocrine responses to conditioned stress in rats. Brain Res. 2000;855:58–66. doi: 10.1016/s0006-8993(99)02289-1. [DOI] [PubMed] [Google Scholar]