Abstract
Purpose
Diabetes and obesity are associated with inflammasome-mediated low-grade, chronic inflammation that may induce pancreatic beta-cell dysfunction and apoptosis. We examined the effects of Roux-en-Y gastric bypass (RYGB) surgery on NOD-like receptor family, pyrin domain containing-3 (NLRP3) inflammasome-related genes from pancreatic islets of Zucker diabetic fatty rats.
Materials and Methods
Islets were collected from Zucker diabetic fatty sham control and RYGB, 30 days after surgery. We assessed expression of genes that regulate glucose metabolism and the NLRP3 inflammasome (NLRP3, caspase-1, IL-1β, IL-18, apoptosis-associated speck-like protein), IL-6, and monocyte chemoattractant protein-1.
Results
Gene expression for NLRP3 (p < 0.02), IL-1β (p < 0.04), and IL-6 (p < 0.01) was reduced by RYGB and positively correlated with change in body weight. IL-1β positively correlated with glucose AUC response.
Conclusion
Suppression of the NLRP3 inflammasome in pancreatic islets may contribute to improved glycemic control after RYGB.
Keywords: Inflammation, Diabetes, Cellular regulation, Insulin resistance, Beta-cells, Cytokine
Introduction
Bariatric surgery is more effective than medical therapy in controlling type 2 diabetes (T2D) in obese patients [1]. Obesity and T2D are both characterized by chronic, low-grade inflammation [2] and the reversal of this inflammation may contribute to surgery-induced diabetes remission. The inflammasome is a multiprotein oligomer that plays a key role in activation of inflammatory pathways involved in the development of insulin resistance [3] and the pathogenesis of T2D [4, 5]. We have previously shown that bariatric surgery reverses inflammation in visceral adipose tissue from obese, non-diabetic rats, by suppressing activation of the NOD-like receptor family, pyrin domain containing-3 (NLRP3) inflammasome [6]. NLRP3 is a pattern recognition receptor that forms a multiprotein inflammasome complex and initiates the inflammatory response [4, 5]. Activation of NLRP3 induces the recruitment and the autocatalytic activation of the cystein protease caspase-1 that leads to the formation of an inflammasome complex mediated by apoptosis-associated speck-like protein (ASC) [5]. The formation of the NLRP3 inflammasome and activation of caspase-1 triggers IL-1β and IL-18 processing and subsequent secretion as active cytokines [5, 7]. Importantly, NLRP3-deficient mice fed a high-fat diet are more insulin sensitive than HF diet-fed wild-type mice [4], while calorie restriction in mice and patients with type 2 diabetes leads to reduced IL-1β and NLRP3 messenger RNA (mRNA) in adipose tissue, and these are associated with decreased inflammation and improved insulin sensitivity [4]. However, it is not known whether bariatric surgery can reduce inflammasome activation in pancreatic islets in T2D.
We investigated the effects of RYGB surgery on NLRP3 inflammasome in pancreatic islets in the obese Zucker diabetic fatty (ZDF) rat model of T2D and hypothesized that RYGB would reduce the inflammatory profile in pancreatic islets determined by downregulation of NLRP3 inflammasome gene expression.
Materials and Methods
Animal Care
The protocol was approved and performed in compliance with the Cleveland Clinic Institutional Animal Care and Use Committee (IACUC). Thirty-two 8–10-week-old ZDF rats (fa/fa) (Charles River Laboratories, Wilmington, MA) were evaluated. Animals were provided an ad libitum diabetogenic diet (Purina 5008, 56.4 % carbohydrate, Research Diets, New Brunswick, NJ) for 6–8 weeks. At age 21–22 weeks, the animals were randomized into a control sham-operated (N = 16) or RYGB (N = 16) surgery group.
Surgical Intervention
All animals were fasted overnight (16 h). Ceftriaxone 75 mg/kg was administered intramuscularly for prophylaxis; an isoflurane gas chamber was used for anesthesia. We used the gastric bypass model as previously described [8] and developed by Meguid et al. [9]. The rats were maintained on an ad libitum liquid diet with Boost (Nestle, Buffalo Grove, IL) for up to 7 days after surgery. Thereafter, they were fed a Purina 5008 diet ad libitum.
Islet Isolation
All rats were euthanized on postoperative day 30 and islets were isolated as previously described [8]. The pancreas was inflated with collagenase, excised, and incubated at 37 °C for 30–35 min. Islets were separated from acinar tissue after washes with 10 % fetal bovine serum in the Roswell Park Memorial Institute medium (Gibco, Carlsbad, CA). Islets were picked under a dissecting microscope.
Glucose Tolerance Test
A fasting oral glucose tolerance test (OGTT) was performed preoperatively and on day 28 post-surgery using 3.0 g/kg glucose solution (70 % dextrose).
Total RNA Extraction
Total RNA was extracted using commercial solutions of guadinium thiocynate-phenol, Trizol (Life Technologies, Beverly, MA). Isolated RNA was aliquoted and stored at −80 °C.
cDNA Synthesis
One microgram of complementary DNA (cDNA) was prepared from total RNA by reverse transcription reaction using an iScript cDNA synthesis kit (BioRad, Hercules, CA) using a PX2 Thermal Cycler (Thermo Scientific), and samples were stored at −80 °C.
qRT-PCR Primer Pairs
Primer pairs for specific target genes were obtained from the Primer Bank database (pga.mgh.harvard.edu/primerbank/) for rodent and checked for specificity to genes of interest (Table 1). Gene-specific primers for qRT-PCR analysis included the following: MCP-1—monocyte chemoattractant protein-1, NLRP3—NOD-like receptor family, pyrin domain containing 3, CASP1—caspase 1, IL18—interleukin 18, IL1β—interleukin 1-beta, IL6—interleukin 6, ASC—apoptosis-associated speck-like protein containing a caspase recruitment domain, GAPDH—glyceraldehyde-3-phosphate dehydrogenase.
Table 1.
Gene-specific primers for qRT-PCR analysis
| Gene | Primer sequence | Amplicon size (nt) | GeneBank accession # | |
|---|---|---|---|---|
| MCP-1 | Forward | TAGCATCCACGTGCTGTCTC | 94 | NM_031530.1 |
| Reverse | CAGCCGACTCATTGGGATCA | |||
| NLRP3 | Forward | TTCCCAGACCCTCATGTTGC | 306 | NM_001191642.1 |
| Reverse | CAGGGCATTGTCACTGAGGT | |||
| CASP1 | Forward | GCCGTGGAGAGAAACAAGGA | 319 | NM_012762.2 |
| Reverse | ACCCTTTCAGTGGTTGGCAT | |||
| IL18 | Forward | GACCGAACAGCCAACGAATC | 84 | NM_019165.1 |
| Reverse | TAGGGTCACAGCCAGTCCTC | |||
| IL1β | Forward | GAGTCTGCACAGTTCCCCAA | 88 | NM_031512.2 |
| Reverse | TGTCCCGACCATTGCTGTTT | |||
| IL6 | Forward | GCAAGAGACTTCCAGCCAGT | 143 | NM_012589.2 |
| Reverse | CCTCCGACTTGTGAAGTGGT | |||
| ASC | Forward | GGACAGTACCAGGCAGTTCG | 140 | NM_172322.1 |
| Reverse | GTCACCAAGTAGGGCTGTGT | |||
| GAPDH | Forward | TCAAGAAGGTGGTGAAGCAG | 111 | NG_028301.2 |
| Reverse | AGGTGGAAGAATGGGAGTTG |
Semi-quantitative RT-PCR Analysis
Determination of relative mRNA expression was performed in duplicate on an MX3000P qPCR system (Agilent Technologies/Stratagene, Santa Clara, CA) using 10 ng of cDNA. Sample normalization was done using the rat GAPDH gene as an internal standard. The relative changes in mRNA abundance were calculated using the comparative ΔΔCt method.
Statistical Analysis
Repeated measure ANOVA analysis was used to determine differences in body weight and the glucose AUC response during OGTT at pre- and post-surgery intervention. Gene expression was measured in duplicate. Statistical differences in gene expression between the sham and RYGB groups were analyzed using t test statistics. Correlations between gene expression and bodyweight or OGTT were determined using the Pearson correlation analysis. Data are expressed as mean ± SE, the threshold of significance was set at p < 0.05. The software used for the statistical calculations was the Microsoft Excel. Correlations between changes in each gene expression and changes in body weight or OGTT were determined using Pearson correlation analysis functions in the MS Excel.
Results
Body Weight and Glycemic Control
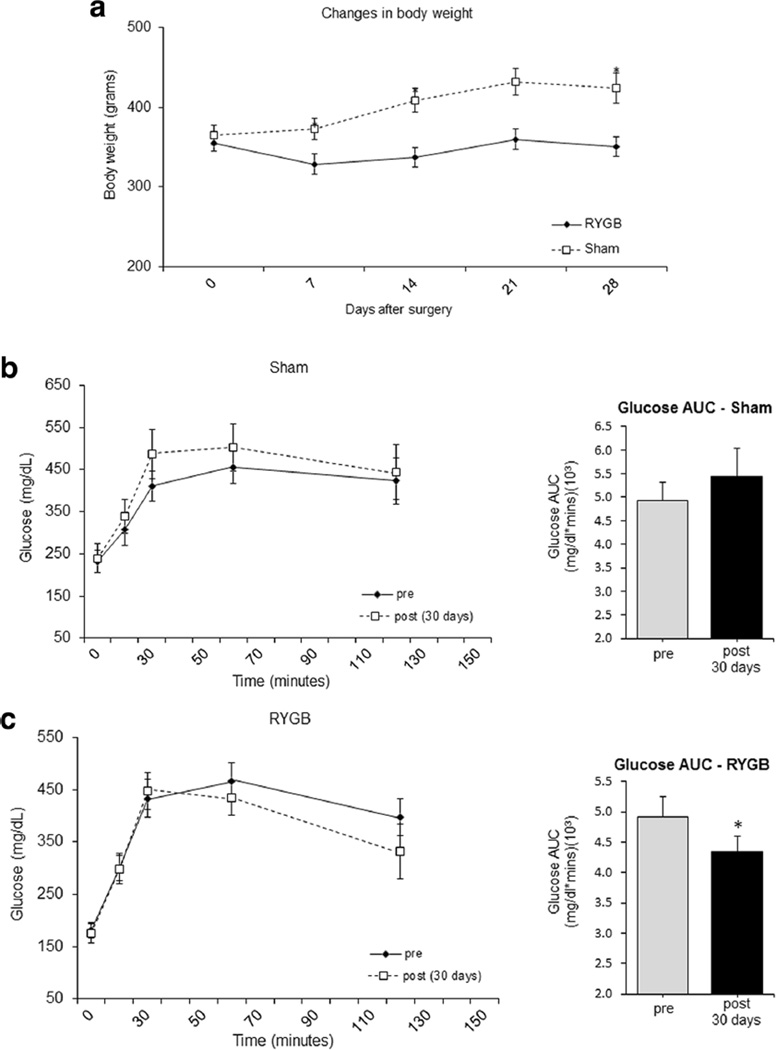
Baseline body weight was 365 ± 13 and 355 ± 13 g for control and RYGB, respectively. The sham group showed a 16 % weight gain over the 30 days of the study, while the RYGB animals lost −1 %of body weight (Fig. 1). Repeated measures ANOVA revealed a difference in body weight between groups across time (p < 0.0001) and an interaction effect (time × group) between the sham and RYGB groups (p < 0.0001). RYGB surgery improved glycemic control compared to sham (Fig. 1). The decrease in the OGTT AUC for the RYGB-treated animals was significant (p < 0.02), but this did not happen in the sham group (p < 0.18).
Fig. 1.
RYGB surgery protects against weight gain (a) and facilitates glycemic control as measured by OGTT AUC (b) in ZDF rats fed with a diabetogenic diet for 30 days. Records of body weight for the sham control vs. RYGB animals (a). Plasma Glucose response (left) and glucose AUC (right) (b, c) during OGTT for sham (b) and RYGB (c), before (gray) and after (black) surgery. Data are mean ± SE. The asterisk denotes a significant difference between sham control vs. RYGB, P < 0.05
Gene Expression in Pancreatic Islets
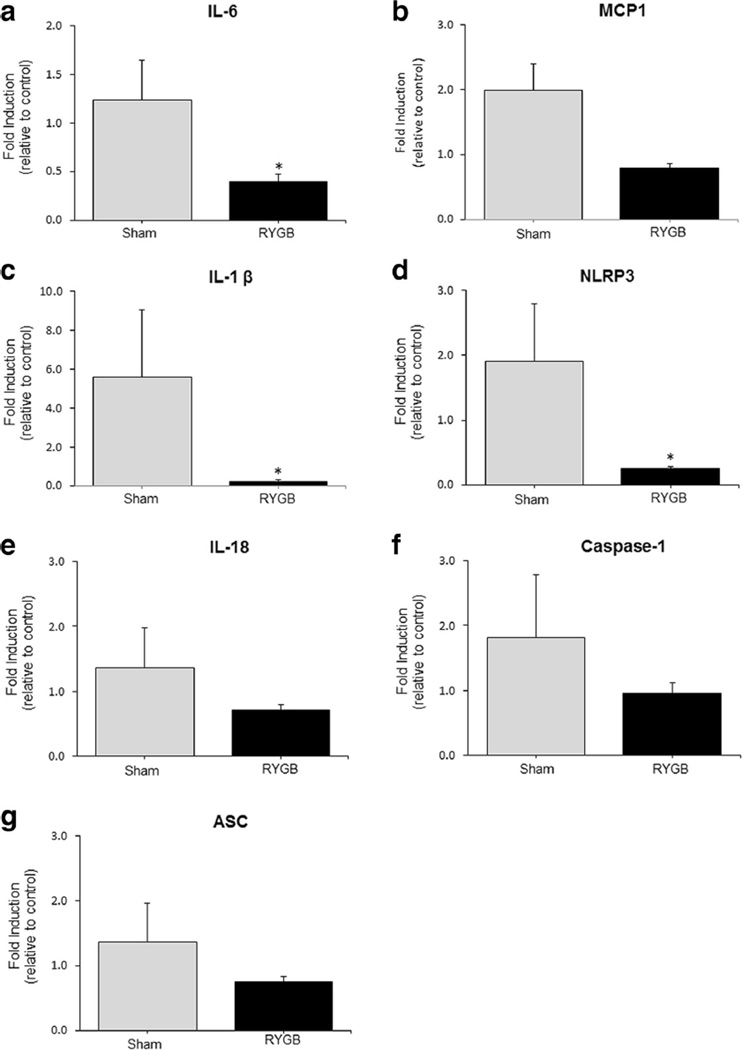
There was a significant decrease in IL-6 (p < 0.01), IL-1β (p < 0.04), and NLRP3 (p < 0.02) mRNA expression in islets obtained from the RYGB animals compared to sham (Fig. 2).
Fig. 2.
The expression of IL-6 (a), MCP-1 (b), IL-1β (c), NLRP3 (d), IL-18 (e), caspase-1 (f), and ASC (g) genes in pancreatic islets from sham and RYGB groups. Sham (N = 16) and RYGB (N = 16). Data are mean ± SE. The asterisk denotes a significant difference between sham control vs. RYGB
Correlation Analysis
In islets (Table 2), IL-1β gene expression was significantly correlated with changes in body weight (r = 0.88, p = 0.01) and glucose AUC (r = 0.54, p = 0.04). IL-6 and NLRP3 gene expression were significantly correlated with changes in body weight (r = 0.48, p = 0.03 and r = 0.71, p = 0.004, respectively), but not with glucose AUC. For MCP1, IL-18, ASC, and caspase-1, there was no correlation with changes in either body weight or glucose AUC.
Table 2.
Correlation analysis between gene expression level vs. changes in body weight (left) and absolute changes of insulin resistance (right) in pancreatic islets beta-cells
| Gene expression | Δ body weight (grams) | Δ glucose AUC (mg/dl*min) | ||
|---|---|---|---|---|
| r | p value | r | p value | |
| IL-6 | 0.71 | 0.002* | 0.62 | 0.06 |
| MCP1 | 0.11 | 0.37 | 0.33 | 0.19 |
| IL-1β | 0.58 | 0.01* | 0.54 | 0.04* |
| NLRP3 | 0.71 | 0.004* | 0.41 | 0.09 |
| IL-18 | 0.22 | 0.26 | 0.35 | 0.15 |
| ASC | 0.12 | 0.34 | 0.37 | 0.14 |
| CASP1 | 0.20 | 0.26 | 0.46 | 0.09 |
Threshold of significance set at p < 0.05
Discussion
The precise mechanism for improved glycemic control in patients with T2D after bariatric surgery is not known. A reduction in chronic low grade inflammation has the potential to greatly attenuate insulin resistance and represents a potentially powerful mechanism for reestablishing glycemic status in these patients. We have previously shown that bariatric surgery improves glucose tolerance in obese rats and this effect is mediated through the NLRP3 inflammasome [6]. However, the effect was observed in adipose tissue and the animals were obese and insulin resistant, but did not have diabetes per se. We can now report that the NLRP3 inflammasome and several of its attending components are significantly downregulated in pancreatic islets isolated from obese diabetic rats (ZDF) after RYGB surgery. These data provide novel insight into the metabolic effects of surgery in a key diabetes regulating organ.
There is evidence that at the cellular level inflammasome activation of IL-1β may play an important role in the pathogenesis of T2D [4]. Here, we see that NLRP3 in islets, which was highly expressed in sham, was significantly reduced in the RYGB animals. We recognize that the evidence is indirect, but there is the suggestion that the NLRP3 inflammasome is implicated in the pathogenesis of T2D through islet inflammation. Furthermore, NLRP3 correlated with changes in body weight in the two groups. RYGB surgery also decreased IL-1β gene expression in the pancreatic islets of diabetic rats, and there was a significant correlation between islet IL-1β expression with change in body weight and glucose tolerance. We acknowledge that these are primarily correlative data and thus we cannot attribute causality to the reduced NLRP3 and IL-1β expression. However, the data raise the distinct possibility that RYGB reduces pancreatic islet inflammation, reduces apoptosis, and increases islet viability. This improved metabolic milieu may then facilitate improved glucose homeostasis and may provide a cellular explanation for the reversal of diabetes that is seen in obese patients after bariatric surgery.
It has also been shown that beta-cell-specific overexpression of IL-6 promotes islet inflammation, but this appears to be insufficient to induce overt diabetes [10]. We found that islet IL-6 was highly expressed in sham and significantly decreased in RYGB and that it correlated positively with changes in body weight. Expression of all other inflammasome components (caspase-1, ASC, IL-18, and MCP-1) in the islet decreased in the RYGB animals, but these changes were not statistically significant and did not correlate with body weight or glucose tolerance. These findings are in agreement with previous data [10] and suggest that IL-6 and NLRP3 are not always interdependent in the pathogenesis of T2D. Furthermore, the caspase-1-ASC protein complex activation may be independent of macrophage infiltration and the effects of RYGB on the NLRP3 complex are not exerted through infiltrating macrophages alone. In summary, our data obtained in islets from the ZDF animal model are in line with previous findings of the effects of RYGB surgery on the expression of these genes in adipose tissue from the obese and insulin resistant SD model. The data suggest that RYGB surgery may reduce pancreatic islet inflammation by suppressing gene expression of the components of the NLRP3 inflammasome, therefore reducing the chronic inflammatory state associated with obesity and the underlying pathophysiology of insulin resistance.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11695-016-2373-z) contains supplementary material, which is available to authorized users.
A.O.M. and J.P.K. developed the NLRP3 hypothesis. A.O.M. was primarily responsible for the sample and statistical analysis. A.O.M., A.M., O.D., and H.H. performed the data collection. A.O.M. and J.P.K. wrote the manuscript and all authors contributed to the data interpretation and editing the manuscript. J.P.K. takes the responsibility for the data integrity.
Compliance with Ethical Standards
Ethical Consent For this type of study, formal consent is not required.
Statement of Informed Consent Does not apply.
Ethical Approval There were no human participants in this study.
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Conflict of Interest The authors declare that they have no competing interests.
References
- 1.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370(21):2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou R, Tardivel A, Thorens B, et al. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 4.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pétrilli V, Dostert C, Muruve DA, et al. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19(6):615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Mocanu AO, Mulya A, Huang H, et al. Effect of Roux-en-Y gastric bypass on the NLRP3 inflammasome in adipose tissue from obese rats. PLoS ONE. 2015;10(10):e0139764. doi: 10.1371/journal.pone.0139764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J Leukoc Biol. 2008;83(1):13–30. doi: 10.1189/jlb.0607402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gatmaitan P, Huang H, Talarico J, et al. Pancreatic islet isolation after gastric bypass in a rat model: technique and initial results for a promising research tool. Surg Obes Relat Dis. 2010;6(5):532–537. doi: 10.1016/j.soard.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Meguid MM, Ramos EJ, Suzuki S, et al. A surgical rat model of human Roux-en-Y gastric bypass. J Gastrointest Surg. 2004;8(5):621–630. doi: 10.1016/j.gassur.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good the, bad, or the indifferent? Diabetes. 2005;54(Suppl 2):S114–S124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]