Abstract
Organoids represent both a potentially powerful tool for the study cell-cell interactions within tissue-like environments, and a platform for tissue regenerative approaches. The development of lung tissue-like organoids from human adult-derived cells has not previously been reported. Here we combined human adult primary bronchial epithelial cells, lung fibroblasts, and lung microvascular endothelial cells in supportive 3D culture conditions to generate airway organoids. We demonstrate that randomly-seeded mixed cell populations undergo rapid condensation and self-organization into discrete epithelial and endothelial structures that are mechanically robust and stable during long term culture. After condensation airway organoids generate invasive multicellular tubular structures that recapitulate limited aspects of branching morphogenesis, and require actomyosin-mediated force generation and YAP/TAZ activation. Despite the proximal source of primary epithelium used in the airway organoids, discrete areas of both proximal and distal epithelial markers were observed over time in culture, demonstrating remarkable epithelial plasticity within the context of organoid cultures. Airway organoids also exhibited complex multicellular responses to a prototypical fibrogenic stimulus (TGF-β1) in culture, and limited capacity to undergo continued maturation and engraftment after ectopic implantation under the murine kidney capsule. These results demonstrate that the airway organoid system developed here represents a novel tool for the study of disease-relevant cell-cell interactions, and establishes this platform as a first step toward cell-based therapy for chronic lung diseases based on de novo engineering of implantable airway tissues.
Keywords: 3D culture, Self-organization, de novo lung regeneration, YAP, Organoid implantation, Pulmonary fibrosis modeling
1. Introduction
Respiratory diseases such as chronic obstructive pulmonary disease and pulmonary fibrosis represent a large and growing public health burden [1, 2], are associated with substantial morbidity and mortality, and currently lack curative therapies. At their end-stage, such diseases require lung transplantation for therapy, but the supply of donor organs is extremely limited, and lung transplant outcomes remain suboptimal [3]. Regenerative approaches offer potential long-term hope for addressing both the epidemic of chronic lung diseases, and the shortage of donor organs, but critical hurdles remain to be overcome [4]. While recent studies have made great progress delineating the mechanisms of lung development and developing methods to drive iPS cells toward mature lung lineages [5–7], relatively less progress has been made in designing strategies by which these advances might be translated into tissue repair, and ultimately advanced toward human studies. Current approaches to engraft dissociated cells in the lung show promise, but have thus far been limited to the setting of severe infections [8] or radiation-induced preconditioning [9]. A major alternative emphasis has been on the generation of decellularized and recellularized lung scaffolds as an engineered organ replacement [10–13]. Relatively less attention has been devoted to the de novo generation of complex three-dimensional lung-like tissues in culture suitable for eventual translational applications. A potential additional benefit from developing such complex engineered lung tissues is for disease modeling. Chronic lung diseases are distinguished by specific tissue remodeling processes and complex cell-cell interactions that are not easily recapitulated in typical cell culture systems. Therefore, we sought to develop an airway organoid culture system combining multiple lung cell types as both a step toward eventual regenerative approaches, and as a system to study disease-relevant cell-cell interactions and complex tissue remodeling processes.
To generate highly organized 3D tissues that mimic organ structure and function, tissue engineers have attempted to recapitulate the in vivo organogenesis process by manipulating critical aspects of the cell culture environment. During embryonic development, the lungs and other internal organs first emerge as organ buds composed of epithelial and mesenchymal progenitors. Through repeated rounds of outgrowth and branching primitive organ buds grow into mature organs [14]. The reciprocal epithelial-mesenchymal interactions critical to organogenesis during embryonic development can be recapitulated in three dimensional co-culture systems to guide formation of similar tissue-like structures in vitro [15]. Recently, complex structures termed organoids [16] have been generated for brain [17], liver [18], pancreas, and lung [19] using combinations of induced pluripotent stem cells, inductive soluble factors, and supportive three dimensional culture conditions. Alternatively, resident progenitor cells from adult tissues can be cultured in supportive 3D systems, and can also generate organoids. Typical examples include LGR5+ cells from intestine and liver [20], and in the field of lung biology, the generation of tracheospheres [21] and alveospheres [22, 23] from airway and alveolar epithelial progenitors. While organoids have shown promise in transplantation models in the colon [24] and liver [25], similar advances have not been reported using adult-derived lung progenitors. Similarly, although organoids have potential for disease modeling and drug screening, tractable human lung cell-based organoid systems have not been reported.
Here we combined adult human primary bronchial epithelial cells, lung fibroblasts, and lung microvascular endothelial cells in 3D culture conditions to generate airway organoids. By combining epithelial differentiation conditions with a multicellular aggregation culture system, we generated self-assembling bioengineered airway organoids that are amenable to ectopic transplantation and study of cell-cell interactions crucial to tissue biology. This system represents a novel tool for studying disease-relevant cellular and molecular function, and an important step toward cell-based therapy for chronic lung diseases based on de novo engineered airway tissues.
2. Material and methods
2.1 2D cell culture and labeling
Human bronchial epithelial cells (NHBE, 8 cell lines used) were purchased from Lonza and cultured in bronchial epithelial growth medium (BEGM, Lonza) with 1% Antibiotic-Antimycotic. Human microvascular lung endothelial cells (HMVEC-L, 3 cell lines used) were also purchased from Lonza and cultured in Endothelial Cell Growth Medium (EBM-2MV, Lonza) with 1% Antibiotic-Antimycotic. Human lung fibroblasts (generously provided by Carol Feghali-Bostwick) previously isolated by explant culture from donor lungs rejected for transplantation under a protocol approved by the University of Pittsburgh Institutional Review Board, were culture in DMEM with 10% FBS, 1% Antibiotic-Antimycotic. The cells were regularly maintained in humidified 5% CO2 at 37 °C. For cell labeling, cells were incubated with pre-warmed CellTracker™ Working Solution (1µM from 1000× DMSO dissolved stock solution) for 30 mintues.
2.2 3D cell culture and generation of human airway organoids
Culture plates were coated with 40% Matrigel (Corning) combined with PneumaCult-ALI Maintenance Medium (Stemcell Technologies). The former is an extracellular matrix preparation derived from a gelatinous protein mixture secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells, composed of approximately 60% laminin, 30% collagen IV, and 8% entactin. Entactin is a bridging molecule that interacts with laminin and collagen IV, and contributes to structural organization. Although Matrigel has inherent long term limitations for clinical use, it is a valuable proof of concept matrix system for supporting organoid morphogenesis [26]. PneumaCult-ALI is a media formulation optimized to induce primary human bronchial epithelial cell mucociliary differentiation under air-liquid interface (ALI) culture conditions. After 45 minutes incubation at 37°C for gelation of the thick Matrigel layer, a single cell suspension including NHBE, HMVEC-L and HLF cells was combined with 5% Matrigel and PneumaCult-ALI Maintenance Medium, and was seeded on top of the Matrigel layer. The ratio of each type of cell was human NHBE:HMVEC-L:HLF = 10:7:2 following a previously described approach for generation of liver organoids [18]. The cells were fed with 5% Matrigel in PneumaCult- ALI Maintenance Medium every other day and observed by light microscopy. Cells labelled with Fluorescent CellTracker™ were observed in a Cytation 5 Cell Imaging Multi-Mode Reader for 24 hour time-lapse fluorescence microscopy to visualize organoid compaction and formation.
2.3 Organoid actomyosin and YAP analysis
Day 2 organoids were used for treatment. ON-TARGETplus Human YAP1 (10413) siRNA SMARTpool (30 pmol, Dharmacon) in Opti-Mem medium (Life Technologies) was transfected using Lipofectamine RNAi Max (Life Technologies) followed by incubation for 24 hours at 37 °C, with media than changed to the normal differentiation medium for another 48 hours. Non-muscle myosin inhibitor blebbistatin (10µmol, Sigma, B0560), F-actin inhibitor cytochalasin D (10µmol, Sigma, C8273) and Rho kinase inhibitor Y-27632 (100µmol, Stemcell Technologies, 72304) were used to treat day 2 organoids for 72 hours. Photos were taking by Nikon TMS Microscope or Cytation 5 Cell Imaging system. Buds or invasive tubular structures were counted manually and average diameters of organoids were counted in 3 organoids per group using ImageJ.
2.4 Atomic force microscopy
Organoids were embedded in O.C.T (Optimal Cutting Temperature, n°4583) and stored at −80°C. 10 µm thickness organoid slices were cut by cryosection (Leica) at −21°C and mounted on poly-L-lysine coated glass slides. To avoid drying, tissue slices were maintained in PBS. Measurements were performed using a BioScope Catalyst AFM (Bruker) mounted on an inverted microscope equipped with epifluorescence (Olympus) using a spherical tip (Novascan) with a radius of 2.5 µm and a spring constant of ~98 pN/nm. Force curves were acquired with MIRO 2.0 (NanoScope 9.1, Bruker) at room temperature in PBS. The indentation was estimated at ~250 nm for an applied force of ~24 nN. The preparation of organoid slice sample and the AFM measurements were performed on the same day. Force curves were analyzed by NanoScope Analysis (Bruker). The extend curve was fitted to determine the Young’s modulus using the Hertz model assuming Poisson’s ratio of 0.4 [27].
2.5 Gene expression analysis (RNA isolation, cDNA synthesis, RT-PCR)
Total RNA was isolated with RNeasy Plus Mini kit and cDNA was synthesized with SuperScript™ IV Reverse Transcriptase. Gene expression levels were quantified by qRT-PCR on the Lightcycler 96 Real-Time PCR System (Roche) according to the manufacturer’s instruction. qRT-PCR was performed by incubating at 95°C for 10 min and then cycling 40 times at 95°C for 10 s, 60°C for 10s, 72°C for 10s. Ct values within each experiment were normalized against GAPDH. Primers are listed in Supplemental Table 1.
2.6 ELISA for MUC5AC
Total protein was collected from organoids and Human MUC5AC ELISA Quantitation Kit (LifeSpan BioSciences) was used to assess protein expression of MUC5AC according to the manufacturer’s instructions. One measurement was made per single organoid.
2.7 Tissue processing, histological tissue assessment, immunostaining and confocal microscopy
Organoids were collected and embedding in O.C.T. 10 µm cryosections were placed on Poly-L-Lysine Coated Slides (Fisher Scientific) for immunostaining or standard histological staining with the BBC Histo·Perfect H&E Staining kit. For immunostaining, tissues were fixed in 4% paraformaldehyde for 20 minutes and permeabilized using 0.25% Triton X-100 for 15 minutes. Antigen retrieval was applied with PH=6.0 citrate buffer when necessary. Organoids were treated with 5% goat serum in 1% BSA/PBS (block solution) for 1 hour. Following two additional PBS washes, samples were incubated overnight with primary antibodies at 4°C. Specimens were washed twice in PBS and incubated with the corresponding secondary antibodies at 1:500 dilution for 1 hour (Alexa Fluor488, 546, 555; Invitrogen). Samples were mounted with mounting media containing nuclear counterstain DAPI (Vector Lab). Images were acquired using the LSM780 inverted laser scanning confocal microscope (Carl Zeiss). Images were processed using ZEN microscope and imaging software, Photoshop or ImageJ. Cell quantification of immune-positive cells and DAPI was performed using the Cytation 5 Cell Imaging Reader software GEN 2.09 or ImageJ using the cell counter plugin. Antibodies are listed in Supplemental Table 2.
2.9 Statistical Analysis
Data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software). The significance of the differences between two mean values was determined using an unpaired t test. P values less than 0.05 were accepted as significant.
3. Results
3.1 Development of an airway organoid from adult lung cells
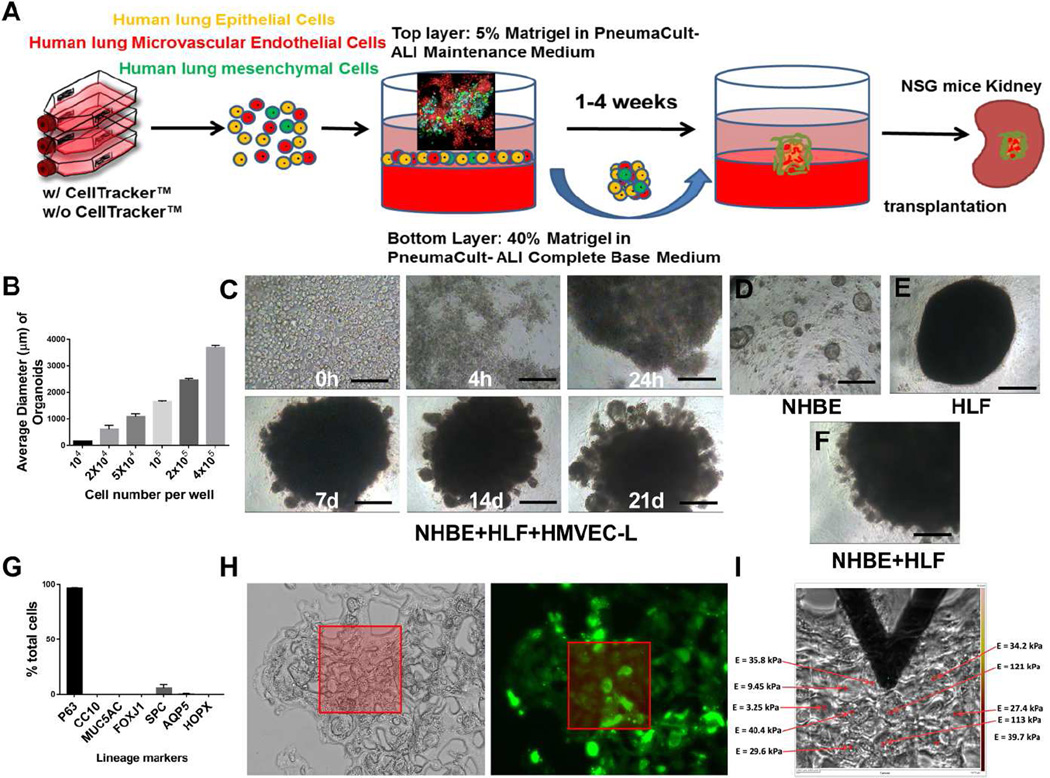
To develop airway organoids from adult lung cells we combined methods optimized for growth and differentiation of human airway epithelial cells [21] with mesenchymal cell-guided condensation on a supportive Matrigel layer [28]. Specifically, we combined adult primary human bronchial epithelial cells (NHBE) at a ratio of 10:7:2 with adult primary human microvascular lung endothelial cells (HMVEC-L) and adult primary human lung fibroblasts (HLF) atop a 3D Matrigel-coating in each well of a 96 well culture plate (Fig 1A). The cells rapidly condensed atop the Matrigel layer and formed 3D cell aggregates within approximately 24 hours, continuing to condense up to 72 hours. When seeding fewer than 1×105 epithelial cells per well, multiple spheroids formed in each well, sometimes with large variance in organoid size, and with increasing organoid numbers and smaller organoids observed as the epithelial cell density was decreased (Fig 1B and S1). For seeding densities of 1×105 epithelial cells and higher, a single organoid formed in each well, with consistent formation noted across multiple NHBE donor lines (Fig S1). In order to minimize variation in organoid formation associated with low seeding density, and potential complications introduced by diffusion limitations associated with high seeding densities and larger organoids, we chose to focus all subsequent studies on organoids formed from 1 × 105 adult primary human bronchial epithelial cells (total cell number of ~1.9×105 cells/well). At this seeding density, multicellular aggregates remained stable in overall size after 3 days, with diameters in the range of ~1000–2000 µm (Fig 1C). To identify the critical cell types for this dynamic and directed condensation process, we examined single cell type behaviors and various combinations of the three cell lineages (Fig 1D–F). Omitting fibroblasts from the co-culture led to a failure in cell aggregate condensation, though smaller spherical colonies did ultimately appear (Fig 1D), recapitulating previous descriptions of tracheosphere formation [21]. In contrast, omission of endothelial cells did not prevent organoid formation in the 3D culture system. Fibroblasts alone (Fig 1E) or in combination (Fig 1F) with either or both other cell types were sufficient for aggregation, confirming that the presence of fibroblasts is critical for the self-condensation process. These observations are consistent with previous reports that fibroblast-dependent paracrine support through the activation of FGF and BMP pathways and fibroblast-dependent cytoskeletal contractions are essential for cell aggregate condensation [28].
Figure 1.
Airway organoid formation from adult human lung cells. Schematic overview of methods for forming and growing airway organoids in vitro and transplanting into NSG mice in vivo (A). Early formation of a cell aggregate visualized at day 3 by confocal imaging using Cell Tracker labeled cells (Green = HLF, Red = NHBE, Cyan = HMVEC-L) is shown in the inset. Correlation between the number of NHBE cells input and organoid sizes (B). Light microscopy images show the self-condensation of mixed NHBE, HMVEC-L and HLF cells and formation of airway organoids over time (C) NHBE cells alone form small spheres in 3D culture at 21days (D). HLFs only in 3D culture at 21 days (E). Mixed NHBE and HLF 3D culture at 21 days (F). Epithelial lineage marker quantification for NHBE cells (mean ± SEM from 4 individuals) in monolayer culture demonstrate a dominant basal cell (P63+) phenotype prior to organoid culture (G). The highly heterogeneous mechanical properties of airway organoids (day 7) were measured by AFM (I) and correlated with light microscopy and fluorescence images (Green = HLF, H). Values in graph represent mean ± SEM; n=4 (B, G). Scale bars, 200µm (C–F).
Because of our ultimate interest in regenerative and disease-modeling applications, we continued to focus on three cell co-cultures that include epithelial, endothelial and mesenchymal resident populations of the mature lung in all subsequent work. We termed our organ bud-like 3D structures “airway organoids” because the epithelial cell type used originated in human bronchial airways. Comparison of epithelial markers expressed in primary NHBE cells in monolayer culture confirmed the dominant proximal basal phenotype (P63+) of this starting cell population (Fig 1G and Fig S2). As a preliminary evaluation of the self-organizing capability and mechanical stability of airway organoids, we labelled the cells individually with different CellTracker™ probes in 2D culture conditions before seeding them randomly on Matrigel-coated plates for organoid culture. Fluorescence time-lapse images were then obtained, allowing us to visualize self-organization of endothelial cell (Cyan) and fibroblast (Green) clusters among comparatively homogenously distributed NHBE cells (Red) (Fig. 1A). Using the approach detailed above, successful organoids formed (diameter larger than 500µm) at a rate of ~80% (64/80), and airway organoids were manipulable with spatulas or forceps, suggestion potential for surgical handling and implantation. Atomic force microscopy (AFM) micro-indentation was performed to evaluate the local elastic modulus within airway organoids (expressed as Young’s modulus) guided by light and fluorescence microscopy (Fig 1H). The relatively high modulus observed within the aggregates was consistent with our manual manipulation observations, and suggested that nascent airway organoids form physically robust aggregates through a mechanically dynamic process that includes considerable mechanical heterogeneity during the process of condensation and self-organization (Fig 1I).
3.2 Epithelial, mesenchymal, and endothelial cells re-organize within the airway organoid
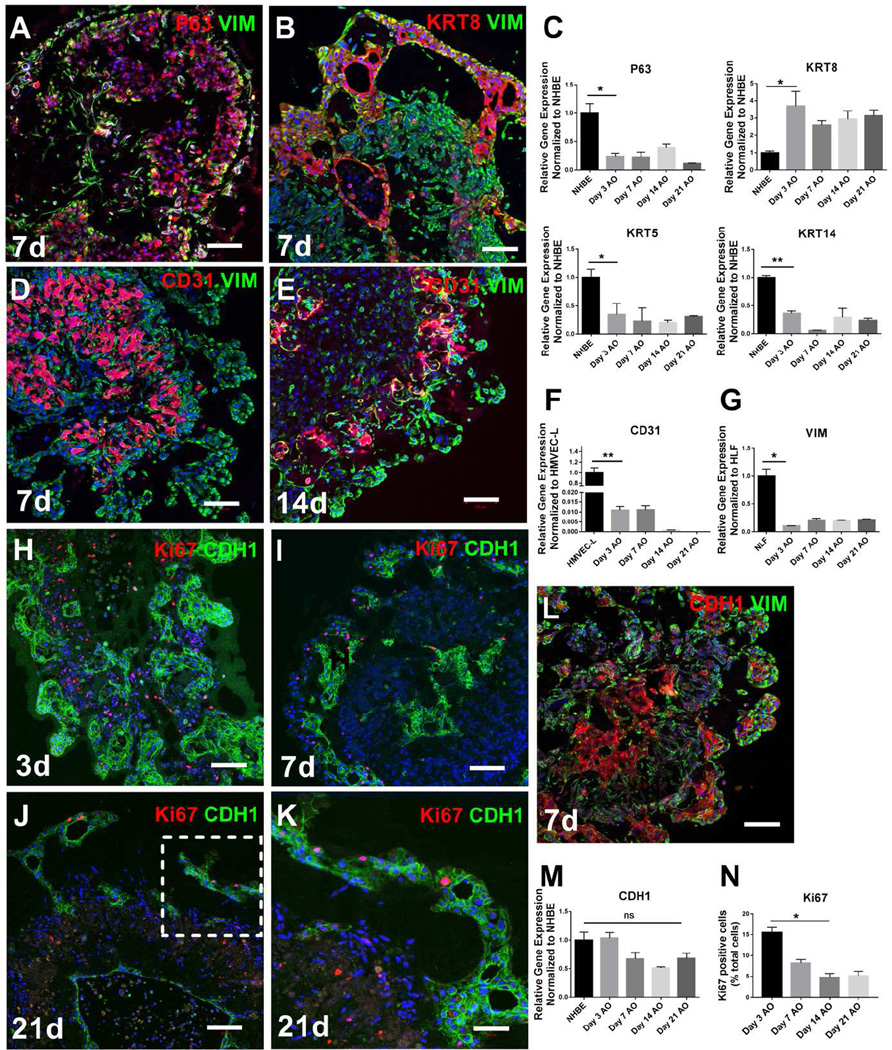
To further study the self-organization of lung epithelium, endothelium and mesenchyme within airway organoids, we performed immunostaining and confocal microscopy at various time points after cell seeding. At early time points, we found that airway organoids possessed broadly distributed mesenchymal cells, identified by vimentin immunostaining, and clusters of epithelial cells positive for E-Cadherin (CDH1), airway epithelial basal marker P63 [5], and luminal marker KRT8 [21] (day 7, Fig 2A, 2B, 2L). Epithelial cell clusters self-assembled into tubular structures of varying shape and size. The expression of basal airway stem cell [5] transcripts (P63+, KRT5+, KRT14+) diminished in 3D airway organoid culture compared with 2D cultures of NHBE cells (Fig 2C), consistent with a transition away from the predominant basal stem cell phenotype expressed by these primary cells in 2D culture. A gradual basal to luminal shift in epithelial phenotype was confirmed by the increased observation of the luminal airway epithelial marker (KRT8+) in the airway organoids (Fig 2C).
Figure 2.
Self-organization of cells within airway organoids. P63 (red, A), KRT8 (red, B) and E-cadherin (CDH1, red, L and green, H, I, J, K) positive epithelial cells clustered together and were also visualized in close contact with vimentin positive mesenchymal cells in airway organoids at day 7 (A, B, L). CD31 positive cells also clustered together with close interactions with vimentin positive (green) cells in the airway at day 7 (D) and day 14 (E). Relative gene expression of P63, KRT5, KRT14, KRT8, vimentin, CD31 and CDH1 in the airway organoids at different time points was compared to NHBE (C, M), HMVEC-L (F) or HLF (G) cells in traditional 2D monocultures. Proliferation was assessed by ki67 (red) in the airway organoids at various time points (H, I, J, K) and quantified (N). Values in graphs represent mean ± SEM; n=3, *P < 0.05; **P < 0.01; t-test. Scale bars, 100µm (A–J), 50µm (K).
Similar to the observed epithelial self-organization, CD31+ endothelial cells were also visualized to form cell clusters along with tube like-structures in airway organoids (Fig 2D, 2E). These endothelial structures were closely associated with fibroblasts, but not epithelial cells. CD31+ endothelial cells decreased dramatically in abundance over time (Fig 2D, 2E) and after 14 days CD31+ cells were rarely observed. This finding was confirmed by the gradual loss of CD31 transcripts over time in airway organoid co-cultures (Fig 2F). Such a finding was not surprising given our use of culture media optimized for NHBE cells. These findings thus demonstrate the capacity of human microvascular endothelial cells to self-organize in airway organoid culture, but clearly indicate the need to optimize long term culture to promote endothelial 3D stabilization and maturation of vascular structures.
Proliferations in the airway organoids, assessed and quantified by Ki67 expression, was most abundant at the earliest time point analyzed (day 3, Figure 2H) and decreased over time (Fig 2H–K, N), consistent with the relative stability in airway organoid size over time. The vast majority of Ki67 expression was not in cells expressing epithelial lineage markers (CDH1+), but rather in cells expressing the mesenchymal lineage (VIM+, CD31−), suggesting that only limited epithelial proliferation and modest fibroblast proliferation was present during long-term culture of airway organoids. The apparent low cell turn over in airway organoids mimics adult lung and airway homeostasis [29], but also suggests that more proliferative progenitor populations, or more stimulative culture conditions, may be required to enhance organoid growth. Taken together the results documented in Figure 2 demonstrate that the airway organoids formed from adult human bronchial epithelial, fibroblast and endothelial cell populations undergo rapid self-organization over time into highly dense epithelial-lined structures surrounded by mesenchyme, with a gradual loss of vascular endothelium.
3.3 Proximal airway epithelial differentiation in airway organoids
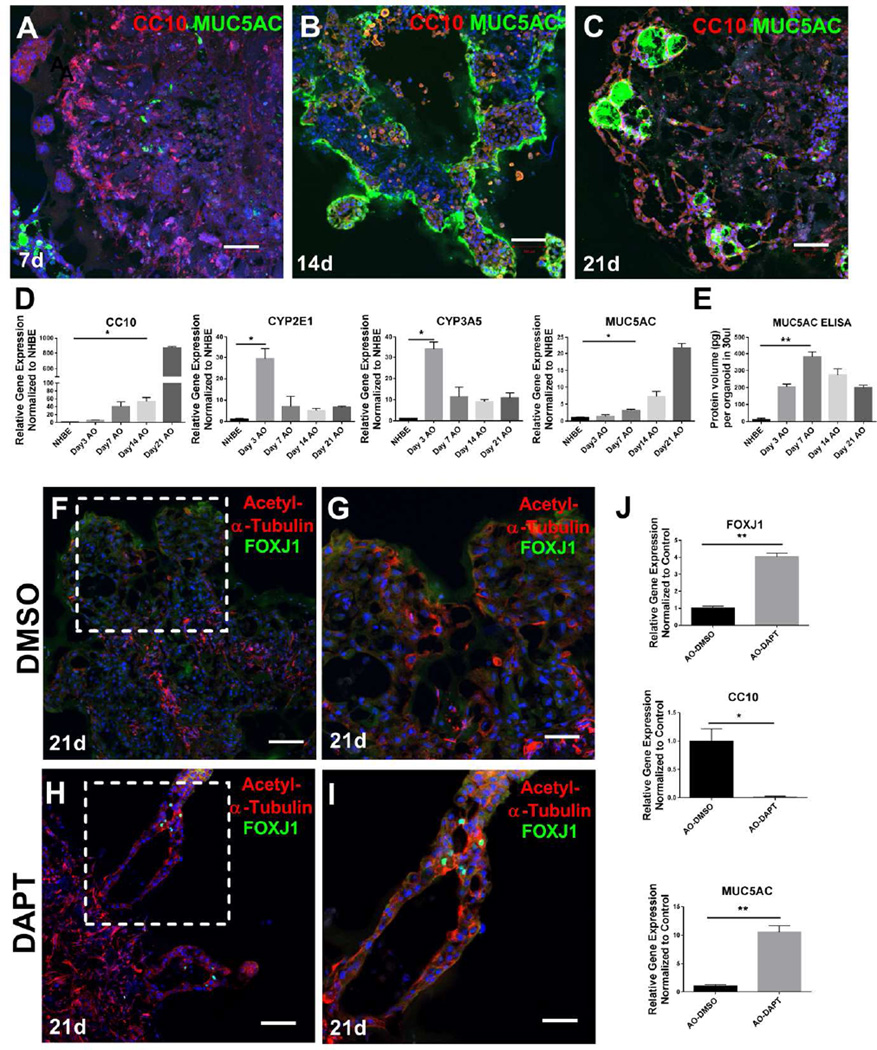
NHBE cells cultured under defined conditions at air-liquid interface are capable of undergoing maturation and differentiation to a pseudo-stratified, mucociliary epithelium [30]. To evaluate the capacity of NHBE cells to undergo such differentiation in 3D airway organoid culture, we analyzed epithelial protein and transcript markers by immunostaining and quantitative PCR respectively. Airway organoids demonstrated gradual increases in expression of the key secretory product of airway club cells, the protein uteroglobin [31], denoted SCGB1A1 or Club Cell-Specific 10 kD Protein (CC10, Fig. 3A–C). Club cells are responsible for detoxifying harmful substances inhaled into the lungs, including engulfment of airborne toxins and breakdown via their cytochrome P-450 enzymes [32]. Consistent with this function, we found that mRNA expression of cytochrome P-450 enzymes increased significantly in the 3D culture (Fig 3D). Secretory club cells are mitotically active and also act as progenitors for epithelial repair, as they are able to differentiate into ciliated cells or non-ciliated cells to regenerate the bronchiolar epithelium [31]. CC10 protein (Fig 3A–C) and transcript levels (Fig 3D) increased dramatically over time in the airway organoid 3D culture compared with NHBE 2D culture (Fig 3D).
Figure 3.
Proximal airway epithelial differentiation in airway organoids. Immunostaining of airway markers CC10 (red) and MUC5AC (green) at day 7, day 14, and day 21 (A–C). Relative gene expression of CC10, CYP2E1, CYP3A5 and MUC5AC in the airway organoids at different time points as compared to NHBE 2D cell culture (D), n=3. ELISA measurements of human MUC5AC production per organoid at each time point, n=5 (E). Airway organoids were stained with ciliated markers acetylated alpha-tubulin (red) and FOXJ1 (green) with low and high power magnification in the airway organoids under control (F, G) and DAPT treatment conditions (H, I). Comparison of relative gene expression of FOXJ1, CC10 and MUC5AC in the airway organoids between control and DAPT treated conditions (J), n=3. Values in graphs represent mean ± SEM; *P < 0.05; **P < 0.01; t-test. Scale bars, 100µm (A, B, C, F, H), 50µm (G, I).
Goblet cells are specialized secretory cells found in the respiratory epithelium of the trachea, bronchi, and larger bronchioles, and are responsible for mucus secretion and maintenance [33]. Goblet cells are denoted by secretory vesicles containing the highly charged glycoprotein MUC5AC. We observed increasing organization of MUC5AC+ immunostaining inside apparent lumens within airway organoids over time (Fig 3A–3C), consistent with epithelial organization and goblet cell differentiation. Transcript levels for MUC5AC were also significantly increased in prolonged airway organoid culture, consistent with epithelial secretory differentiation over time in airway organoids (Fig 3D). Moreover, robust MUC5AC protein expression was detected by ELISA, peaking at day 7 but maintained out to day 21 in airway organoids (Fig 3E).
In contrast to abundant signs of secretory differentiation, comparatively few cells expressed the ciliated cell marker [34] FOXJ1 (Fig 3F, 3G), suggesting that the 3D culture environment employed here does not adequately promote terminal differentiation of all airway epithelial cell types. Recent work has highlighted a prominent role for Notch signaling in controlling airway epithelial differentiation [35]. Notch signaling inhibition with DAPT treatment increased the mRNA expression level of FOXJ1 (Fig 3J) as well as FOXJ1 immunostaining (Fig 3H, 3I). FOXJ1+ cells were accompanied by intense and polarized co-staining for acetylated-alpha tubulin (Fig 3H–I), consistent with mature ciliogenesis [36]. DAPT treatment also dramatically reduced transcript levels of CC10 while increasing transcript levels of MUC5AC (Fig 3J). In combination, these data demonstrate that the current airway organoid culture conditions support gradual club and goblet cell epithelial differentiation over time, and that the cells remain capable of responding to additional cues to undergo enhanced ciliated cell formation, recapitulating many of the maturation and differentiation processes that play out in gold-standard ALI culture of NHBE cells.
3.4 Expression of distal lung epithelial lineage markers in airway organoids
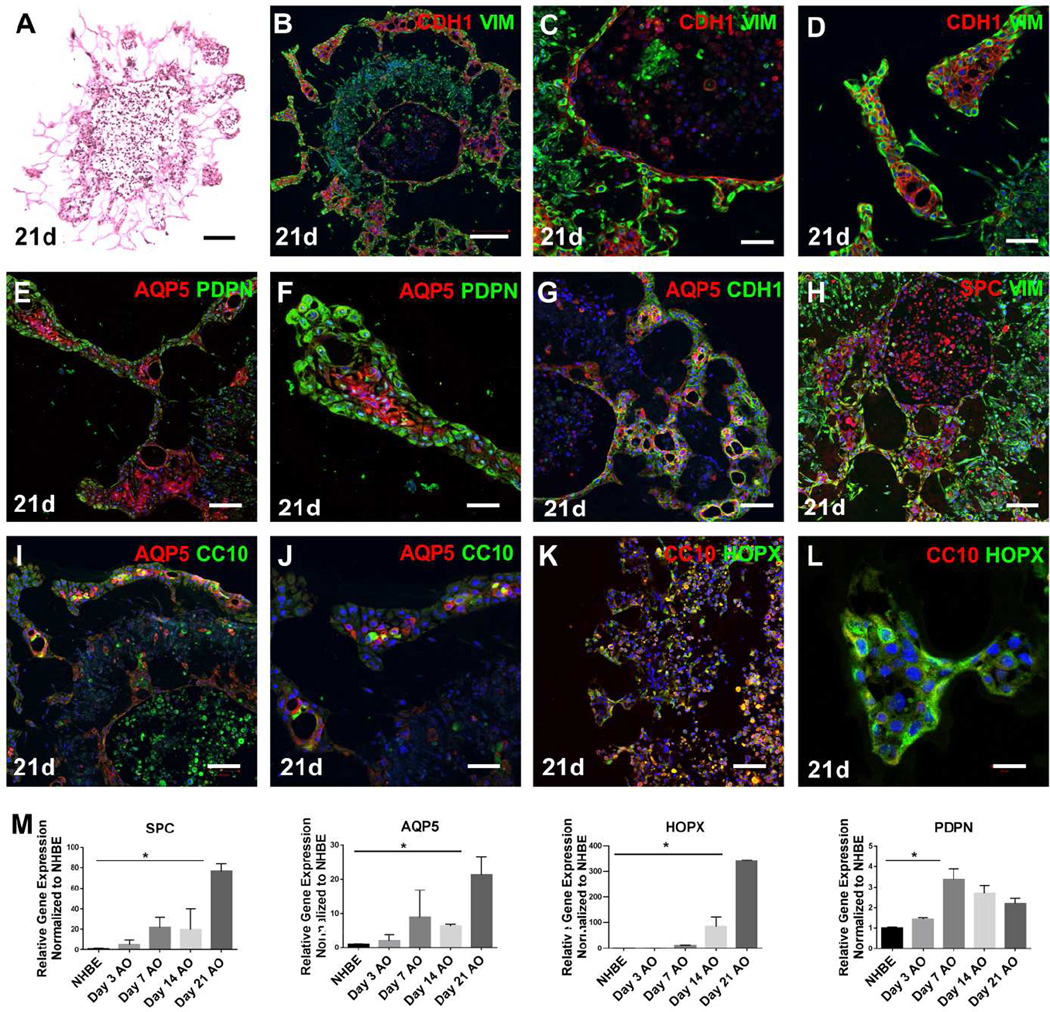
In addition to epithelial clustering within compacted organoids, we also observed invasive tubular structures emanating from airway organoids with prolonged culture, as shown in a representative H&E stained section (Fig 4A) and brightfield images of live organoid cultures (Fig S3A, S3B). In contrast, we did not observe any invasive tubular structures forming from long-term NHBE mono-cultures (day21, Figure S3C) which instead remained uniformly spherical in shape. Organoid invasive structures invariably demonstrated close apposition of E-cadherin and vimentin immunostaining, suggesting close epithelial-mesenchymal cell interactions (Fig 4B). E-cadherin positive epithelial cells were typically tightly surrounded by vimentin positive cells (Fig 4C), recapitulating epithelial-mesenchymal orientation during airway branching morphogenesis [14, 37]. Some epithelial-fibroblast invasive structures extended far from the originating organoid, with a transition to a more flattened and extended epithelial morphology (Fig 4D) suggesting the possibility that aspects of distal lung development might also be evoked within airway organoid culture.
Figure 4.
Expression of distal lung epithelial lineage markers in airway organoids. H&E staining of airway organoid section (A). Co-staining of E-cadherin (red) and Vimentin (green) reveals diverse architecture of epithelial and mesenchymal compartments within airway organoids at day 21 in vitro with low- and high-power imaging (B–D). AECI markers AQP5 (red) and PDPN (green) were seen throughout the airway organoids with low- and high- power magnification (E, F), as well as with E-cadherin (green) in the airway organoids (G). Co-staining AQP5 (red) with CC10 (green) in the airway organoids with low- and high- power magnification (I, J). AECII marker SPC (red) co-staining with Vimentin (green) the airway organoids (H). Co-staining CC10 (red) with HOPX (green) in the airway organoids with low- and high- power magnification (K, L). Relative gene expression of SPC, AQP5, HOPX, and PDPN in airway organoids at different time points compared to NHBE 2D cell culture (M). Values in graphs represent mean ± SEM; n=3, *P < 0.05; **P < 0.01; t-test. Scale bars, 200µm (A, B), 100µm (E, G, H, I, K), 50µm (C, D, F, J, L).
The distal epithelium of the lung is lined by Type I and type II alveolar epithelial cells (AECI, AECII). The AECI marker Aquaporin 5 (AQP5) [38–40] was strongly expressed within tubular structures near the periphery of the main airway organoid body, and within the thin epithelial lining of invasive projections (Fig 4E, 4F), where it co-stained with E-cadherin (Fig 4G). Podoplanin (PDPN), another marker of AECs highly expressed in lung parenchyma [41], but also in lung fibroblasts [42], was much more broadly distributed in the fibroblast-populated area of human airway organoids, but not typically in AQP5+ or E-cadherin+ cells (Fig 4E, 4F, Fig S3D). In multiple instances we observed co-staining of the more proximal epithelial marker CC10 with the distal epithelial markers AQP5 and HOPX (Fig 4I–L), implying considerable complexity and the presence of immature or mixed-lineage expression within areas of organoid-derived tubular structures, consistent with noted epithelial cell type diversity in distal lung development [43]. In other locations, relatively strong and exclusive expression of distal epithelial markers were observed within tubular structures (Fig 4F, L), consistent with cells undergoing further distal lineage-specific differentiation. Additional evidence for AECI lineage expression within airway organoids was obtained by qPCR, which demonstrated prominent upregulation of AECI markers AQP5, PDPN and HOPX [5, 23] over time (Fig 4M). Within organoids, but distinct from invasive structures, we also observed large epithelial clusters that were relatively devoid of interacting fibroblasts (Fig 4H), and stained positively for CC10, the AECII marker SPC, or both [31, 44, 45] (Fig 4H), suggesting further regional complexity in epithelial lineage marker expression within airway organoids, and the potential emergence of unique mixed lineage cells distinct from the original basal bronchial epithelial cell population. Taken together, our data demonstrates that the epithelium within airway organoids displays remarkable plasticity, and in a limited and surprising fashion recapitulates aspects of both proximal airway and distal alveolar cell self-assembly and differentiation.
3.5 Invasive tubular structures require actomyosin contraction and localized YAP activation
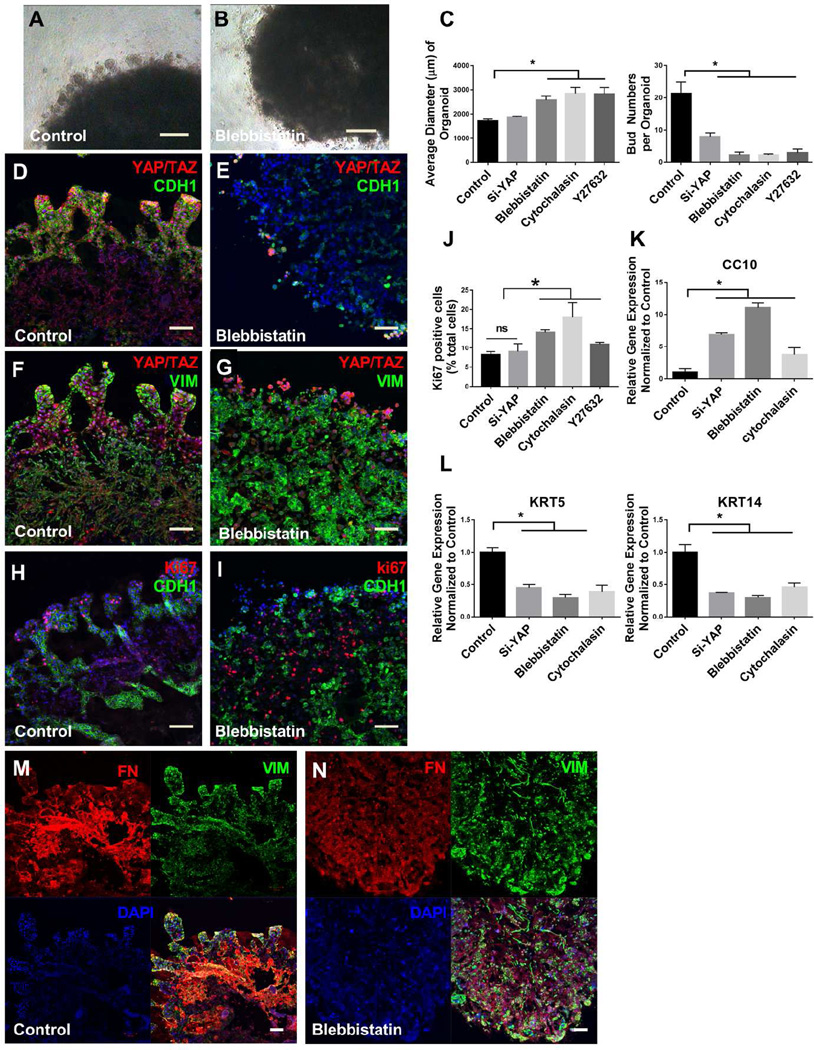
The omission of fibroblasts in our organoid culture led not only to formation of smaller aggregates, but also to a failure of invasive tubular structures to form (Fig 1D, Fig S3C). These observations suggested to us that both organoid formation and invasion requires mechanical contributions, particularly those generated by contractile fibroblasts. In agreement with this concept, inhibition of myosin II with blebbistatin both attenuated organoid compaction and reduced the number and extent of invasive bud formation at the periphery of airway organoids (Fig. 5A–C). Similar findings were observed with the Rho kinase inhibitor Y27632 and the inhibitor of actin polymerization cytochalasin D (Fig. 5C), consistent with an essential role for actomyosin-mediated contractile forces in organoid formation as previously observed [25, 28], and also for tubular invasion as noted here. Interestingly, we observed that the overall levels of cell proliferation, as quantified by Ki67 immunopositive cells, were significantly higher in organoids treated with blebbistatin, cytochalasin D and Y-27632 (Fig 5H–J, S3J, S3K) consistent with proliferative effects of actomyosin inhibition in other contexts [46, 47], and suggestive of enhanced proliferation also contributing to greater organoid size under these conditions.
Figure 5.
Invasive tubular structures require localized YAP and actomyosin. Tubular outgrowths from airway organoids invade surrounding matrix shown in the control (A), loss of such structures with blebbistatin treatment (B). Quantification the number of invasive tubular structures (number of buds) and the average diameter of airway organoids in different treatment groups comparing with control (C). Co-staining of YAP/TAZ (red) and E-cadherin (CDH1, green) reveals high expressing YAP/TAZ cells within invasive epithelial tubular structures of airway organoids in the control (D) and their relative loss and disorganization in blebbistatin treatment group (E). Co-staining of YAP/TAZ (red) and Vimentin (green) in the control (F) and blebbistatin treatment group (G). Co-staining of Ki67 (red) and Vimentin (green) in the control (H) and blebbistatin treatment group (I), along with quantification of Ki67 postitive cells (J). Co-staining of Fibronectin (red) and Vimentin (green) in the control (M) and blebbistatin treatment group (N). Relative gene expression of KRT5, KRT14 and CC10 in airway organoids with and without Si-YAP, blebbistatin and cytochalasin D treatment (K, L). Values in graphs represent mean ± SEM; n=3, *P < 0.05; **P < 0.01; t-test. Scale bars, 200µm (A–B), 100µm (D, E, F, G, H, I, M, N).
The mechanoresponsive transcriptional regulator YAP has been widely implicated in tissue morphogenesis [48, 49], and plays important roles in both lung epithelium [50–52] and mesenchyme [53–55]. Therefore, we hypothesized that YAP activation may play key roles in the formation of organoids and invasive tubular structures. Knockdown of YAP by siRNA did not significantly reduce organoid compaction (Fig. 5C). However, immunostaining for YAP demonstrated robust nuclear localization at the periphery of organoids associated with invasive tubular structures (Fig. 5D, 5F). Knockdown of YAP by siRNA (Fig S4D) significantly diminished invasive bud formation (Fig. 5C), demonstrating an essential role for YAP in formation and invasion of these structures. Notably, YAP knockdown disrupted the organization of epithelial and mesenchymal cells, as well as deposition of an organized fibronectin matrix (Fig 5M), suggesting profound reliance on this mechanoresponsive pathway for tissue organization and morphogenesis (S4A, S4E). Consistent with the mechanoresponsive nature of this process, blebbistatin (Fig 5E, 5G and 5N), cytochalasin D (Fig S4B, S4F) and Y-27632 (Fig S4C, S4G) disrupted the localized pattern of YAP expression, as well as dramatically disorganizing epithelial-mesenchymal distribution and patterns of fibronectin deposition within airway organoids. While overall cell proliferation was not significantly altered with YAP siRNA knockdown (Fig 5J, 5I, S4I), the localized pattern of proliferation typically observed in invasive tubular structures at the organoid edge (Fig 5H) was lost with YAP knockdown (Fig S4I). Similar disorganization of proliferation patterns was observed with blebbistatin (Fig 5I), cytochalasin D (Fig S4J) and Y-27632 treatment (S4K), consistent with prior observations of mechanical patterning of proliferation via YAP [56].
Based on previous observations that YAP plays a critical role in airway epithelial patterning, we also examined expression of basal and luminal epithelial markers after treatment to disrupt actomyosin or knockdown YAP by siRNA. Real-time PCR analysis demonstrated that basal cell markers KRT5 and KRT14 were significantly reduced (Fig 5L) and airway club cell differentiation marker CC10 were up-regulated with YAP siRNA or actomyosin disruption (Fig 5K). We also observed YAP nuclear localization and expression of CC10 were exclusive of one another in the organoid (Fig S4H, S4L). These observations are consistent with prior in vivo findings that YAP expression controls progenitor cell fate in the lungs and promotes a basal cell expression program [52, 57], demonstrating that the organoid system recapitulates aspects of YAP-mediated epithelial fate determination observed in mouse models of lung development.
3.6 Modeling a fibrogenic stimulus response in human airway organoids
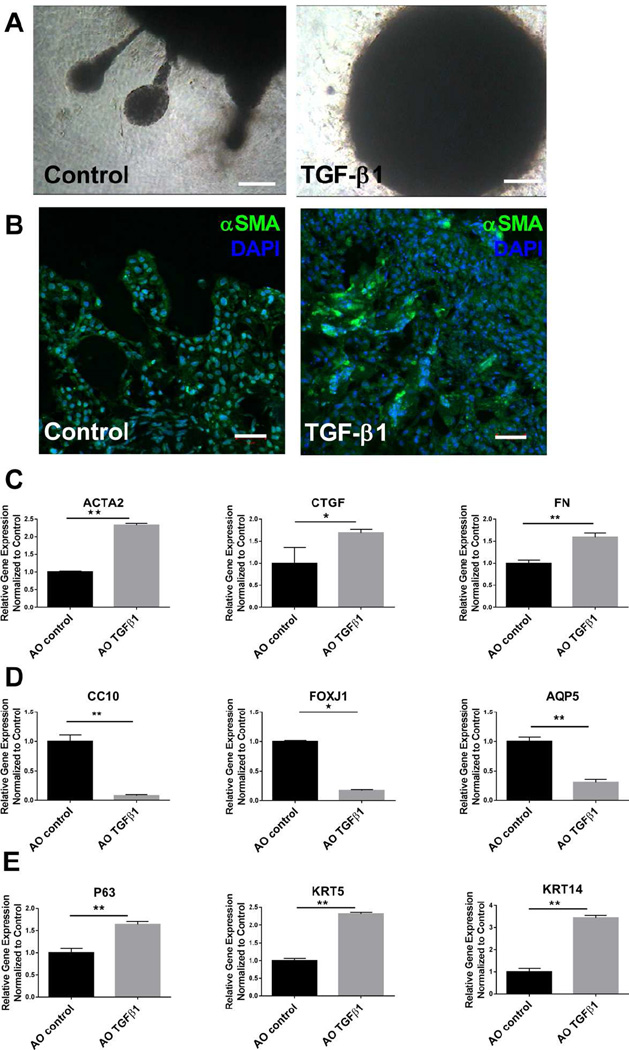
Currently, in vitro studies of lung fibrosis typically focus on a single cell types, especially fibroblasts[58] or lung epithelial cells[59]. The close proximity of primary lung epithelial cells and mesenchymal cells within airway organoids prompted us to investigate whether our model could be useful for studying the orchestrated responses of multiple cell types to a fibrogenic stimulus. Therefore we employed a pathophysiologically relevant pro-fibrotic stimulus, TGF-β1[60]. Not only is TGF-β1 highly relevant in the context of pulmonary fibrosis [61], it is implicated in lung development [62], airway and alveolar remodeling [63], and is known to evoke profound responses in primary human epithelial, endothelial and mesenchymal cell types [60]. To evaluate the response to TGF-β1, we treated airway organoids (AOs) with 5 ng/mL TGF-β1 for 96 hours and then analyzed gene expression by qPCR and organoid morphology by immunofluorescence. We found that TGF-β1 treatment inhibited/reversed tubular invasion of the matrix at the periphery of airway organoids (Fig 6A), consistent with known inhibitory effects of TGF-β1 on lung branching and epithelial differentiation in embryonic and fetal lung in vivo [64]. Immunofluorescence imaging demonstrated increasing αSMA expression (Fig 6B) with TGF-β1 treatment, and qPCR confirmed significant induction of several fibrogenic genes in airway organoids, including FN(1.5-fold), ACTA2 (2.3-fold), CTGF(1.6-fold) as shown in Figure 6C. To address which cell types contribute to increased αSMA, we labeled either fibroblasts (HLF) or epithelial cells (NHBE) with CellTracker™ Red CMTPX prior to organoid formation, then treated with TGF-β1 as above and used immunofluorescence imaging to visualize αSMA+ cells. Co-staining of αSMA was observed with both CellTracker+ fibroblasts (Fig S5A) and epithelial cells (Fig S5B), indicating the both fibroblasts and epithelial cells contribute to the enhanced αSMA expression under TGF-β1 treatment, echoing prior observations of TGF-β1 responses in primary bronchial epithelial cells[65]. Interestingly, TGF-β1 had the opposite effect on expression of proximal lung epithelial cell markers CC10 and FOXJ1 and the distal lung epithelial cell marker AQP5, all of which decreased dramatically after treatment (Fig 6D). Meanwhile, the expression of airway epithelial basal cell markers (P63, KRT5) were increased by TGF-β1 treatment (Fig 6E). These observations highlight the robust multicellular effects of TGF-β1 in airway organoids, with simultaneous increases in fibrogenic gene expression and decreases in expression of epithelial differentiation markers, and support the potential for this model to be used in elucidating cell-cell interactions that regulate tissue level responses to fibrogenic stimuli.
Figure 6.
TGF-β1 responses in human airway organoids. Tubular outgrowths from airway organoids invade surrounding matrix without TGF-β1 treatment, absence of such outgrowths with TGF-β1 treatment (A). αSMA (green) staining of airway organoids with and without TGF-β1 treatment (B). Relative gene expression of ACTA2, CTGF, FN (C), CC10, FOXJ1, AQP5 (D) and P63, KRT5, KRT14 (E) in airway organoids with and without TGF-β1 treatment. Values in graphs represent mean ± SEM; n=4, *P < 0.05; **P < 0.01; t-test. Scale bars, 100µm (A), 50µm (B).
3.7 Ectopic transplantation of human airway organoids
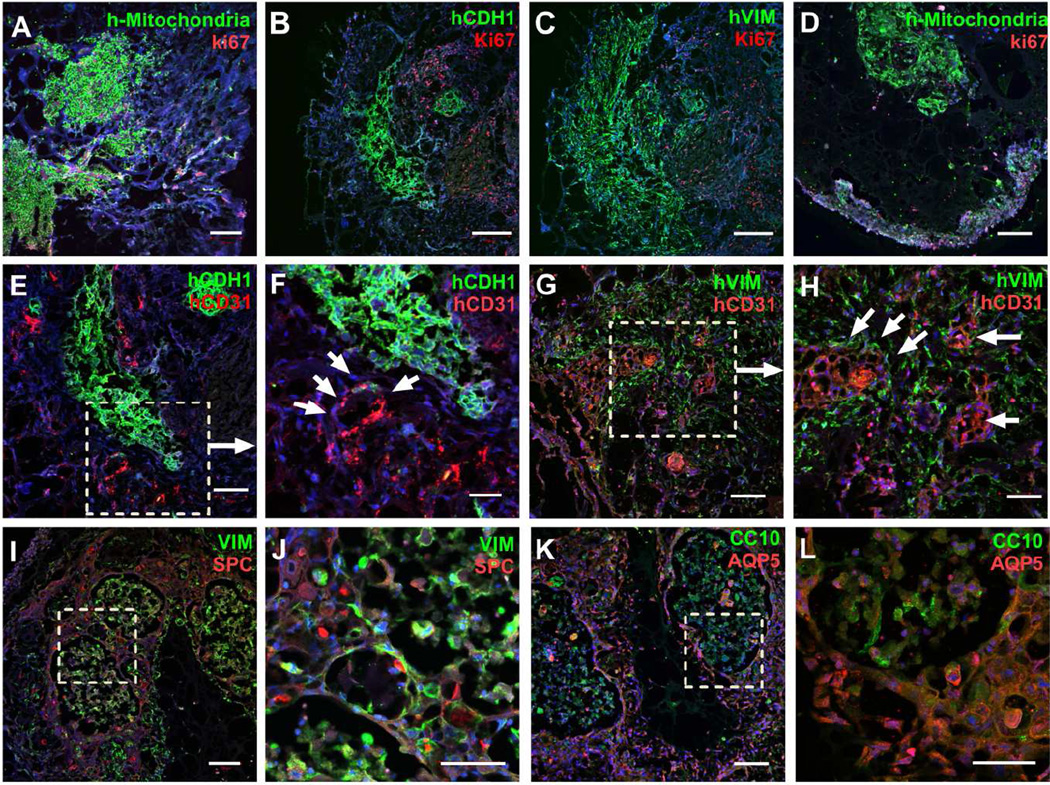
To evaluate whether airway organoids can be transplanted in vivo and are capable of surviving and vascularizing in vivo, we transplanted day 7 airway organoids, embedded within Matrigel, under the kidney capsule of NSG mice. Kidneys were harvested after 1 week or 6 weeks for visual and microscopic examination. Organoids were easily visible after 1 week in the kidney capsule (Fig S6A). At 1 week after implantation there was prominent proliferation in the vicinity of the implanted organoid, particularly at the interface between host tissue and airway organoid grafts. However, co-staining with human specific E-cadherin (Fig 7B) and vimentin (Fig 7C), along with an antibody specific to human mitochondria (Fig 7A), demonstrated that the vast majority of proliferation was not within airway organoid cells, but rather within host tissue. Human specific CD31+ endothelial cells were observed within airway organoids (Fig 7E–H), with some in close proximity to epithelial tubular structures (Fig 7E–F), while others were distributed outside epithelial structures surrounded by fibroblasts (Fig 7G–H).
Figure 7.
Ectopic transplantation of human airway organoids. Human mitochondria (green, A, D), human E-Cadherin (green, B) human Vimentin (green, C), and proliferating cells (Ki67, red, A–D) were assessed by immunostaining at the week 1(A–C) and week 6 (D). Human endothelial cells were assessed by human CD31 (red) co-staining with human E-cadherin (green, E, F) and human Vimentin (green, G, H) at week 1. SPC (red) were used for additional epithelial staining with low- and high- power magnification (I, J), as was double staining for AQP5 (red) and CC10 (green) with low- and high- power magnification (K, L). Scale bars, 200µm (B, C), 100µm (A, D, E, G, I, K), 50µm (F, H, J, L). n=4.
By week 6 after implantation organoids regressed in size. Under microscopic visualization the remaining human cells appeared to be fewer in number (Fig 7D), and although human specific CD31+ endothelial cells were observed, they were less abundant than at 1 week, and restricted to the edge of the implanted organoid (Fig S6B, S6C). In contrast, abundant host vasculature, as indicated by anti-CD31 antibody that reacts with both mouse and human (green) was found to have broadly invaded the organoid area (Fig S6B, S6C). Thus, while we did not observe any direct human to mouse vasculature connection, these observations are consistent with a robust capability of airway organoids to recruit host vasculature. Further immunostaining with lung epithelial differentiation markers demonstrated enriched and relatively distinct expression of proximal secretory airway epithelial cell marker CC10 (Fig 7K, L, S6D) or distal alveolar epithelial markers AQP5 (Fig 7K, L, S6E) and SPC (Fig 7I, J), consistent with the remarkable epithelial plasticity observed in airway organoids in vitro, but with less evidence of the mixed-lineage cells observed in vitro. In combination with the relative paucity of proliferation observed at 1 week, and the general lack of ectopic tissue growth from week 1 to 6, our results suggest that ectopic transplantation of airway organoids in vivo prompted a shift toward cell lineage commitment and differentiation toward mature, non-proliferating states. Thus our data demonstrate that the bronchial epithelial, mesenchymal and vascular cells in the airway organoid can survive and undergo considerable maturation following engraftment in vivo. However, under the present conditions adult human airway organoids did not undergo robust growth and expansion when ectopically transplanted under the kidney capsule, suggesting a somewhat limited regenerative potential of the current system.
4. Discussion
We report the development of airway organoids from a mixed population of human adult lung epithelial, mesenchymal and endothelial cells. We observed a remarkable capacity for self-assembly, morphogenesis, and differentiation within airway organoids that mimicked many aspects of lung tissue formation and maturation. Lung organoids have recently been reported from human iPS cells [19], and smaller organoids have been formed from defined lung progenitors alone or in combination with supportive niche cells [21–23]. Our study differs from these efforts in two key ways. First, we used an unsorted population of human adult-derived cells. This is advantageous from a tissue sourcing perspective as all of the cells were commercially available, but clearly limiting in delineating mechanisms of differentiation, and in the observed limited proliferative potential of human adult cell-derived airway organoids. Second, we used organ bud culture conditions [25] which allowed a mixed population of cells to rapidly aggregate and self-condense, organize and engage in reciprocal cell-cell interactions. Such a method is ideal for engineering organ buds from a large mixed cell population, and offers the potential to create relatively large and transplantable tissue-like aggregates, but is clearly less amenable to mechanistic dissection of cellular lineage and fate choices during organoid culture.
Interestingly, we observed expression of both proximal and distal epithelial markers within airway organoids. Our source for lung epithelium was commercially available normal human bronchial epithelial cells with a predominant basal cell phenotype. Lung epithelial cells in general display tremendous plasticity in regenerative contexts [66]. Recent studies have shown that adult airway epithelial cells preserve the potential, when needed, to proliferate, migrate and differentiate into several cell types during the severe lung injury [8, 67, 68]. Our study shows that an unsorted population of NHBE cells maintains surprising potential to express hallmarks of both proximal and distal epithelial lineages found in the adult lung. Recent studies have shown that the fate and multilineage potential of epithelial stem cells can change depending on whether a stem cell exists within its resident niche and responds to normal tissue homeostasis, whether it is mobilized to repair a wound, or whether it is taken from its niche and undergoes de novo tissue morphogenesis after transplantation [69]. Additional efforts will be needed to determine if the remarkable epithelial plasticity observed here reflects the unique niche(s) provided by organoid culture, or instead reflects the mixed starting epithelial population and the possible presence of alveolar (or other) lung progenitors with distal lineage potential. In addition, further investigation of additional niche cells specific to the proximal airways, such as airway smooth muscle cells [70], may offer additional insight into the cell-cell interactions that regulate airway epithelial differentiation within our organoid system. Whatever the outcome of these future studies, the results provided here demonstrate a remarkable potential for an adult human lung epithelial population to self-organize and mature toward both airway and alveolar lineages, suggesting the presence of important cell-cell interactions within airway organoids that merit further investigation.
One striking observation from our airway organoids was the formation of invasive tubular structures that recapitulated the basic architecture of epithelial and mesenchyme juxtaposition observed during lung development. Multicellular tubular structures are essential elements in formation of complex organs and tissues, and their formation here by budding from the central organoid mass mimics, though clearly in a limited fashion, a basic aspect of organ development. Despite the formation of these invasive structures, we note that distinct highly-organized and integrated proximal and distal structures mimicking mature airway and alveoli were not commonly observed in the organoids. Rather, while the organoids display remarkable self-organization capacity, the patterns of cellular differentiation and morphogenesis suggest a relatively stochastic process, as is apparent in detailed IF images (Fig 3–4). This is perhaps due to the organoid formation process we have adopted, in which compaction of the mixed cell population generates local variations in epithelial, endothelial and fibroblast cell densities, superimposed on the cellular variability inherent to these cell populations. Thus while the organoids display robust self-organizing capacity and potential for differentiation in vitro, they remain far from mature lung tissue. Our observations are similar to other organoids studies [25, 71, 72] that show that in vivo incubation is essential to further maturation and organization of tissue like functionality in organoids beyond the relatively primitive embryonic tissue-like states that form in vitro.
Excitingly, we were able to demonstrate that molecular control over organoid invasion and differentiation appears to be governed by YAP, consistent with its important role in airway branching, morphogenesis and differentiation in the developing lung [50–52, 54, 55, 57], suggesting that airway organoid formation recapitulates key signaling aspects of lung development. Better understanding the morphogenetic function of YAP could facilitate advances in generating complex tissue engineered structures such as organoids, and given YAP’s mechanoresponsive nature [53, 56], our findings suggests that exogenous control of mechanical forces to better mimic the developmental environment may be necessary to promote enhanced organoid growth and morphogenesis [73]. A major goal going forward will be to focus on cell signaling and genetic factors that guide branching morphogenesis of airway organoids, as well as regulatory cues from ECM, mesenchyme and vascular endothelial cells that influence cellular differentiation and organoid morphogenesis.
We also demonstrated the capacity for ectopic transplantation of human adult airway organoids into immuno-deficient mice, with the presence of both recruited host vascular cells and lineage-specific differentiation of graft epithelial cells. While the observed in vivo differentiation potential was supportive of possible future application of this approach in regenerating functional lung tissue, several barriers remain. Notably, engrafted airway organoids did not grow over time, suggesting limited proliferative potential of the adult-derived cells used in our approach. More proliferative responses have been previously reported when using selected resident lung epithelial progenitor subpopulations [67, 74] or immortalized airway derived cells [75]; thus, sorting and utilization of resident progenitor populations may provide improvements in organoid growth [76]. Alternatively, fetal-derived lung cells may offer growth advantages innate to their developmental state, and have been shown previously to undergo organoid-like organization and growth [77, 78]. Ultimately, human iPS-derived cells driven toward lung lineages may provide the clinically-relevant cell source needed for patient-specific applications [79, 80]. Moving from ectopic transplantation of airway organoids to lung-specific application will require further development of tissue implantation strategies. Recent work has demonstrated successful attachment and vascular recruitment into biocompatible hydrogels on the pleural surface of the lung [81], suggesting one possible path forward. Further efforts to improve vascularization may also be critical in supporting organoid engraftment and growth. A recent study demonstrated that co-seeding of endothelial and perivascular cells is essential to successful revascularization of decellularized lung tissue [82]. Sorting and seeding of mesenchymal cell subpopulations that support vascularization in organoids may be one means by which to enhance organoid growth and engraftment potential. Finally, inclusion of additional matrix and soluble cues [13] will likely be essential to further optimize cell organization, growth and differentiation, and in vivo integration of airway organoid grafts with host tissue.
A more immediate opportunity for use of airway organoids is apparent in the realm of disease-modeling. There is a clear rationale and drive to develop new 3D multi-cell culture models that overcome limitations of traditional 2D culture systems, allowing cells to grow and respond in environments that mimic native tissue and promote close interactions between cells, ECM, and soluble cues[83]. In this study, we demonstrated that the fibrogenic factor TGF-β1 not only enhances expression of mesenchymal genes implicated in fibrosis, but simultaneously and dramatically attenuates expression of both proximal and distal lung epithelial differentiation markers. The airway organoid method developed here provides a tractable method by which cellular and molecular responses to disease-relevant perturbations can be studied within a 3D tissue-like environment approximating the native lung. Because mixed human cell populations are used, extension of this method to human primary disease-derived cells is straight-forward, and will allow comparison of normal and pathological cell population interactions under baseline and disease modeling conditions. The resulting platform has the potential to become a valuable tool for assessing tissue-, cell- and molecular-level responses, and for evaluating novel therapies and patient-specific interventions.
5. Conclusion
Our results demonstrate that multicellular airway organoids derived from adult human cells are able to self-organize and mature toward lung tissue-like structures. Fibroblasts are essential for the self-condensation of aggregates into mechanically stable airway organoids, and likely play a continuing role in reciprocal interactions with endothelial and epithelial cells. YAP was necessary for organization and invasion of tubular structures, and controlled aspects of epithelial differentiation, mimicking key aspects of in vivo lung development. Despite the proximal source of primary epithelium used in the airway organoids, both proximal and distal epithelial markers were expressed over time both in vitro and in vivo, demonstrating remarkable epithelial plasticity within organoid cultures. Successful ectopic engraftment of airway organoids represented an important initial step toward future application in lung regenerative approaches, though many challenges remain. Finally, airway organoids assembled from adult human primary cells represent a new and potentially powerful tool within which to study physiologic and pathologic cell-cell interactions.
Supplementary Material
Acknowledgments
This work was supported by NIH HL092961, the Caerus Foundation, as well as a Postdoctoral Fellowship Award in Regenerative Medicine and Science from the Mayo Clinic Center for Regenerative Medicine. We thank Dr. Gary C. Sieck and Dr. Y.S. Prakash for providing access to cryosection facility and other equipment support. We thank Dr. Andrew Haak for image analysis support, and Dr. Giovanni Ligresti for helpful discussion. We thank Yunhua Fang and Kristin J. Mantz for their technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc. 2014;11:404–406. doi: 10.1513/AnnalsATS.201311-405PS. [DOI] [PubMed] [Google Scholar]
- 2.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 3.Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med. 1999;340:1081–1091. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 4.Lau AN, Goodwin M, Kim CF, Weiss DJ. Stem cells and regenerative medicine in lung biology and diseases. Mol Ther. 2012;20:1116–1130. doi: 10.1038/mt.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med. 2014;20:822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang SX, Green MD, de Carvalho AT, Mumau M, Chen YW, D'Souza SL, et al. The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat Protoc. 2015;10:413–425. doi: 10.1038/nprot.2015.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen C, Shezen E, Aronovich A, Klionsky YZ, Yaakov Y, Assayag M, et al. Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat Med. 2015;21:869–879. doi: 10.1038/nm.3889. [DOI] [PubMed] [Google Scholar]
- 10.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 11.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581–2591. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prakash YS, Tschumperlin DJ, Stenmark KR. Coming to terms with tissue engineering and regenerative medicine in the lung. Am J Physiol Lung Cell Mol Physiol. 2015;309:L625–L638. doi: 10.1152/ajplung.00204.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasai Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12:520–530. doi: 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 17.Hattori N. Cerebral organoids model human brain development and microcephaly. Mov Disord. 2014;29:185. doi: 10.1002/mds.25740. [DOI] [PubMed] [Google Scholar]
- 18.Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, Enomura M, et al. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 2014;9:396–409. doi: 10.1038/nprot.2014.020. [DOI] [PubMed] [Google Scholar]
- 19.Dye BR, Hill DR, Ferguson MA, Tsai YH, Nagy MS, Dyal R, et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife. 2015;4 doi: 10.7554/eLife.05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 25.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 26.Greggio C, De Franceschi F, Figueiredo-Larsen M, Gobaa S, Ranga A, Semb H, et al. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development. 2013;140:4452–4462. doi: 10.1242/dev.096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thundat T, Warmack RJ, Chen GY, Allison DP. Thermal and Ambient-Induced Deflections of Scanning Force Microscope Cantilevers. Appl Phys Lett. 1994;64:2894–2896. [Google Scholar]
- 28.Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, et al. Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell Stem Cell. 2015;16:556–565. doi: 10.1016/j.stem.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Wansleeben C, Barkauskas CE, Rock JR, Hogan BL. Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip Rev Dev Biol. 2013;2:131–148. doi: 10.1002/wdev.58. [DOI] [PubMed] [Google Scholar]
- 30.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 31.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fanucchi MV, Murphy ME, Buckpitt AR, Philpot RM, Plopper CG. Pulmonary cytochrome P450 monooxygenase and Clara cell differentiation in mice. Am J Respir Cell Mol Biol. 1997;17:302–314. doi: 10.1165/ajrcmb.17.3.2774. [DOI] [PubMed] [Google Scholar]
- 33.Rogers DF. Airway goblet cells: responsive and adaptable front-line defenders. Eur Respir J. 1994;7:1690–1706. [PubMed] [Google Scholar]
- 34.Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L231–L234. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishikawa H, Marshall WF. Ciliogenesis: building the cell's antenna. Nat Rev Mol Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 37.Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McElroy MC, Kasper M. The use of alveolar epithelial type I cell-selective markers to investigate lung injury and repair. Eur Respir J. 2004;24:664–673. doi: 10.1183/09031936.04.00096003. [DOI] [PubMed] [Google Scholar]
- 39.Flodby P, Borok Z, Banfalvi A, Zhou B, Gao D, Minoo P, et al. Directed expression of Cre in alveolar epithelial type 1 cells. Am J Respir Cell Mol Biol. 2010;43:173–178. doi: 10.1165/rcmb.2009-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC, Gabriel SE. Expression and localization of epithelial aquaporins in the adult human lung. Am J Respir Cell Mol Biol. 2001;24:224–234. doi: 10.1165/ajrcmb.24.3.4367. [DOI] [PubMed] [Google Scholar]
- 41.Ramirez MI, Millien G, Hinds A, Cao Y, Seldin DC, Williams MC. T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev Biol. 2003;256:61–72. doi: 10.1016/s0012-1606(02)00098-2. [DOI] [PubMed] [Google Scholar]
- 42.Hoshino A, Ishii G, Ito T, Aoyagi K, Ohtaki Y, Nagai K, et al. Podoplanin-positive fibroblasts enhance lung adenocarcinoma tumor formation: podoplanin in fibroblast functions for tumor progression. Cancer Res. 2011;71:4769–4779. doi: 10.1158/0008-5472.CAN-10-3228. [DOI] [PubMed] [Google Scholar]
- 43.Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH, et al. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature. 2014;509:371–375. doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 45.Kim CF. Paving the road for lung stem cell biology: bronchioalveolar stem cells and other putative distal lung stem cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1092–L1098. doi: 10.1152/ajplung.00015.2007. [DOI] [PubMed] [Google Scholar]
- 46.Suprynowicz FA, Upadhyay G, Krawczyk E, Kramer SC, Hebert JD, Liu X, et al. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc Natl Acad Sci U S A. 2012;109:20035–20040. doi: 10.1073/pnas.1213241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mih JD, Marinkovic A, Liu F, Sharif AS, Tschumperlin DJ. Matrix stiffness reverses the effect of actomyosin tension on cell proliferation. J Cell Sci. 2012;125:5974–5983. doi: 10.1242/jcs.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porazinski S, Wang H, Asaoka Y, Behrndt M, Miyamoto T, Morita H, et al. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature. 2015;521:217–221. doi: 10.1038/nature14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588:2663–2670. doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell. 2014;30:151–165. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell. 2014;30:137–150. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szymaniak AD, Mahoney JE, Cardoso WV, Varelas X. Crumbs3-Mediated Polarity Directs Airway Epithelial Cell Fate through the Hippo Pathway Effector Yap. Dev Cell. 2015;34:283–296. doi: 10.1016/j.devcel.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 54.Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308:L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, et al. Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell Rep. 2015;13:1016–1032. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 57.Lange AW, Sridharan A, Xu Y, Stripp BR, Perl AK, Whitsett JA. Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. J Mol Cell Biol. 2015;7:35–47. doi: 10.1093/jmcb/mju046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blackwell TS, Tager AM, Borok Z, Moore BB, Schwartz DA, Anstrom KJ, et al. Future directions in idiopathic pulmonary fibrosis research. An NHLBI workshop report. Am J Respir Crit Care Med. 2014;189:214–222. doi: 10.1164/rccm.201306-1141WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. Faseb J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 61.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest. 2004;125:754–765. doi: 10.1378/chest.125.2.754. [DOI] [PubMed] [Google Scholar]
- 63.Lee CG, Kang HR, Homer RJ, Chupp G, Elias JA. Transgenic modeling of transforming growth factor-beta(1): role of apoptosis in fibrosis and alveolar remodeling. Proc Am Thorac Soc. 2006;3:418–423. doi: 10.1513/pats.200602-017AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeng X, Gray M, Stahlman MT, Whitsett JA. TGF-beta1 perturbs vascular development and inhibits epithelial differentiation in fetal lung in vivo. Dev Dyn. 2001;221:289–301. doi: 10.1002/dvdy.1140. [DOI] [PubMed] [Google Scholar]
- 65.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, et al. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med. 2009;180:122–133. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 66.Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344:1242281. doi: 10.1126/science.1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donati G, Watt FM. Stem cell heterogeneity and plasticity in epithelia. Cell Stem Cell. 2015;16:465–476. doi: 10.1016/j.stem.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 70.Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, et al. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest. 2011;121:4409–4419. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watson CL, Mahe MM, Munera J, Howell JC, Sundaram N, Poling HM, et al. An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 2014;20:1310–1314. doi: 10.1038/nm.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xinaris C, Benedetti V, Rizzo P, Abbate M, Corna D, Azzollini N, et al. In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J Am Soc Nephrol. 2012;23:1857–1868. doi: 10.1681/ASN.2012050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nelson CM, Gleghorn JP. Sculpting organs: mechanical regulation of tissue development. Annu Rev Biomed Eng. 2012;14:129–154. doi: 10.1146/annurev-bioeng-071811-150043. [DOI] [PubMed] [Google Scholar]
- 74.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, et al. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franzdottir SR, Axelsson IT, Arason AJ, Baldursson O, Gudjonsson T, Magnusson MK. Airway branching morphogenesis in three dimensional culture. Respir Res. 2010;11:162. doi: 10.1186/1465-9921-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruiz EJ, Oeztuerk-Winder F, Ventura JJ. A paracrine network regulates the cross-talk between human lung stem cells and the stroma. Nat Commun. 2014;5:3175. doi: 10.1038/ncomms4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mondrinos MJ, Koutzaki S, Lelkes PI, Finck CM. A tissue-engineered model of fetal distal lung tissue. Am J Physiol Lung Cell Mol Physiol. 2007;293:L639–L650. doi: 10.1152/ajplung.00403.2006. [DOI] [PubMed] [Google Scholar]
- 78.Mondrinos MJ, Jones PL, Finck CM, Lelkes PI. Engineering de novo assembly of fetal pulmonary organoids. Tissue Eng Part A. 2014;20:2892–2907. doi: 10.1089/ten.tea.2014.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, et al. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest. 2013;123:4950–4962. doi: 10.1172/JCI68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang SX, Islam MN, O'Neill J, Hu Z, Yang YG, Chen YW, et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32:84–91. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mammoto T, Mammoto A. Implantation of fibrin gel on mouse lung to study lung-specific angiogenesis. J Vis Exp. 2014 doi: 10.3791/52012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ren X, Moser PT, Gilpin SE, Okamoto T, Wu T, Tapias LF, et al. Engineering pulmonary vasculature in decellularized rat and human lungs. Nat Biotechnol. 2015;33:1097–1102. doi: 10.1038/nbt.3354. [DOI] [PubMed] [Google Scholar]
- 83.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.