Abstract
RATIONALE
Structural analogs of the bioactive lipid, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol, were synthesized with a xylitol polar headgroup and both diacyl and diether radyl groups. Mass spectral characterization of xylitol phospholipids (PX) was carried out using collisional activation and high resolution mass measurements of positive molecular ion species and compared with the phosphatidylglycerol (PG) analogs.
METHODS
Xylitol phospholipids were synthesized using a transphosphatidylation reaction catalyzed by phospholipase D and purified by HPLC. Compounds were subjected to electrospray ionization and CID was performed using a tandem quadrupole mass spectrometer to generate positive and negative molecular ions. Diether phospholipids were additionally analyzed by high resolution mass spectrometry as protonated and sodiated molecular species in positive ion mode.
RESULTS
Ester linked xylitol phospholipid analogs behaved similar to PG after collisional activation of [M-H]−. The product ions formed by CID of the diether PG and PX negative ions only revealed information about the headgroup with no information about the aliphatic chains. In contrast, CID of protonated and sodiated diether phospholipid positive ions, revealed reactions corresponding to cleavage of the ether chain, likely occurring by charge driven reaction mechanisms.
CONCLUSIONS
Novel xylitol phospholipid analogs with diacyl and diether radyl substituents of the glycerol backbone were characterized by tandem mass spectrometry. These unique diether phospholipid analogs enabled exploration of ether cleavage reactions of the positive molecular ion species induced by collision induced decomposition.
Keywords: Phospholipid, Tandem Mass Spectrometry, Ether Lipid, PG, Xylitol
INTRODUCTION
Phospholipids are a large family of lipids that are the major structural constituents of nearly all biological membranes. Phospholipids also play additional roles in living organisms involving intracellular and extracellular signalling processes mediated by specific interactions with proteins. One very specialized pool of phospholipids is located in the extracellular alveolar compartment of the lung, in a lipid and protein complex known as pulmonary surfactant. Pulmonary surfactant plays an essential role in lung homeostasis by regulating both biophysical and innate immune properties in the alveoli.[1, 2] Pulmonary surfactant consists of 90% lipid and 10% protein by weight.[3] The majority of lipids in pulmonary surfactant are phospholipids with the most abundant class being phosphatidylcholine (PC). The second most abundant phospholipid class in pulmonary surfactant is phosphatidylglycerol (PG). Lung alveoli are the only tissue location where PG is found abundantly in humans, reaching concentrations between 5 and 10 mg/mL.[4] Recent studies have demonstrated that PG is an important regulator of the innate anti-viral defense and inflammation.[5–10]
The molecule 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (PG(16:0/18:1)) has been previously found to have potent antiviral activity against respiratory syncytial virus (RSV) and influenza A.[5, 7–9] PG(16:0/18:1) also suppressed inflammation by antagonizing multiple toll-like receptors (TLR).[6, 10, 11] The biological activity of PG(16:0/18:1) has prompted studies to explore structure/function relationships of structurally related molecules that might surpass PG(16:0/18:1) in efficacy, potency and biological half-life.[10] This led to the synthesis of novel phospholipid analogs of PG(16:0/18:1) with a xylitol head group which we have termed phosphatidylxylitol (PX).[12] Xylitol is a five-carbon sugar alcohol approved for use by the FDA as a bulk sweetener and has a similar structure to glycerol. For these reasons it was a favorable option as a substrate in a transphosphatidylation reaction. The majority of these analogs, like PG(16:0/18:1), have ester-linked fatty acids, however two compounds have long-chain alkyl radyl groups attached as ether moieties at the sn-1 and sn-2 positions of the glycerol backbone. The dialkyl PG analogs are resistant to acyl hydrolase degradation and could have an extended half-life in the lung as well as prolonged biological activity. Only a few detailed mass spectrometric studies have been reported in the literature that have focused on behavior of diether phospholipids by identifying the product ions that reveal the methyl branching positions of the prenol-derived alkyl chains. [13–15] The PX compounds reported here were not methyl branched and represent a novel class of diether and diacyl xylitol phospholipids that have not been previously characterized by tandem mass spectrometry.
MATERIALS AND METHODS
Materials
Phospholipids purchased from Avanti Polar Lipids (Alabaster, AL) were: 1,2-di-O-tetradecyl-sn-glycero-3-phosphocholine, PC(O-14:0/O-14:0); 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine, PC(16:0/18:1); 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol, PG(16:0/18:1); and 1,2-di-O-(9Z-octadecenyl)-sn-glycero-3-phosphocholine, PC(O-18:1/O-18:1). Xylitol, phospholipase D from cabbage, 8-anilino-1-naphthalenesulfonic acid (ANSA) and diethyl ether were purchased from Sigma-Aldrich (St. Louis, MO). All other solvents were HPLC grade and were purchased from Thermo Fisher Scientific (Waltham, MA).
Transphosphatidylation reaction
A transphosphatidylation reaction with the enzyme phospholipase D (PLD) was utilized to synthesize analogs of PG(16:0/18:1) for testing structural features of the lipid important for immunoregulatory activity. PLD catalyzes the hydrolytic cleavage of terminal phosphate ester bonds of glycerophospholipids with a choline head group; and in the presence of a primary alcohol utilizes transphosphatidylation activity to exchange the choline head group for that of the primary alcohol.[12] This method was used to synthesize analogs that contain a xylitol polar head group and vary in their hydrophobic chains. Aliquots of 5 – 10 mg of diacyl and dialkyl choline phospholipid species in chloroform were dried under a stream of nitrogen gas. Diethyl ether was added to the dried PC species and once again dried using nitrogen gas to eliminate all of the chloroform. Dried PC species were resuspended in 3.1 mL of diethyl ether. Xylitol 40%, w/v) was dissolved in pH 5.5 sodium acetate buffer that contained 120 mM calcium chloride for a final volume of 500 µL. This aqueous solution was then added to the ether phase of the reaction followed by the addition of PLD at a final concentration of 120 units/mL which was empirically found to be optimal. The reaction mixture was vigorously mixed using a Vortex apparatus for 18–24 hr, at temperatures ranging from 22°C to 42.5°C. Reactions were stopped by the addition of 50 µL 0.5 M EDTA. Ether was evaporated under a stream of nitrogen and the lipids were extracted using the Bligh-Dyer method.[16] Reaction progress was assessed using thin layer chromatography followed by spraying the plate with 0.1% aqueous ANSA and visualization using UV light.
High performance liquid chromatography (HPLC)
Normal phase HPLC was used to purify phosphatidyl xylitol phospholipids on a 150 mm×30 mm silica column. The HPLC solvents consisted of (solvent A) and (solvent B). Solvent A contained hexane:isopropanol (3:4) and solvent B contained hexane:isopropanol:aqueous 1 mM ammonium acetate (3:4:0.7). The starting HPLC solvent consisted of 55% solvent A and 45% solvent B. From 0 min to 20 min the % solvent B was increased incrementally to 60%. At 20 min, solvent B was increased to 100% until the end of the elution. A photodiode array detector was used to monitor eluting lipid species at 200 nm. The desired xylitol phospholipid peak was recovered, dried under nitrogen gas, and subjected to Bligh-Dyer extraction.[16]
Mass spectrometry
Characterization of all phospholipids including the PX lipids were carried out on Applied Biosystems (AB) Sciex QTrap 4000 (Thornhill, Ontario, Canada). Purified lipids were infused into the mass spectrometer electrospray interface in a methanol:water (2:1) solution containing 1 mM ammonium acetate. QTrap conditions for negative ion mode: declustering potential −90 V, ion spray voltage −4000 V, entrance potential −10 V. The parameters for positive ion mode were the same except for the ion spray voltage, which was 4500 V. Collision energy for CID of PX lipids varied with each compound ranging from 10 V to 70 V for positive ions and −10 V to −40 V for negative ions.
The high resolution analysis of PG and PX lipids was performed on the Synapt G2-S mass spectrometer (Waters, Manchester, U.K.) in positive ion mode. Samples were infused directly into the electrospray source at a concentration of 100 nM via a syringe pump (1 µL/min). The settings for the mass spectrometer included setting the mass spectrometer to high resolution mode, ESI voltage 3000 V, sampling cone 40 V, source offset 80 V, source temperature 110°C, desolvation temperature 150°C, desolvation gas 500 L/h, nebulizer gas 6.0 bar and trap collision energy of 24 V for protonated ions and 38 V for sodiated ions. Leucine enkephalin was used as the lockspray compound to calibrate each spectrum obtained for high resolution analysis. Once the accurate mass was obtained for each ion in the CID spectrum, a table was generated of possible identifications of each ion within a mass range of ± 5 ppm.
RESULTS AND DISCUSSION
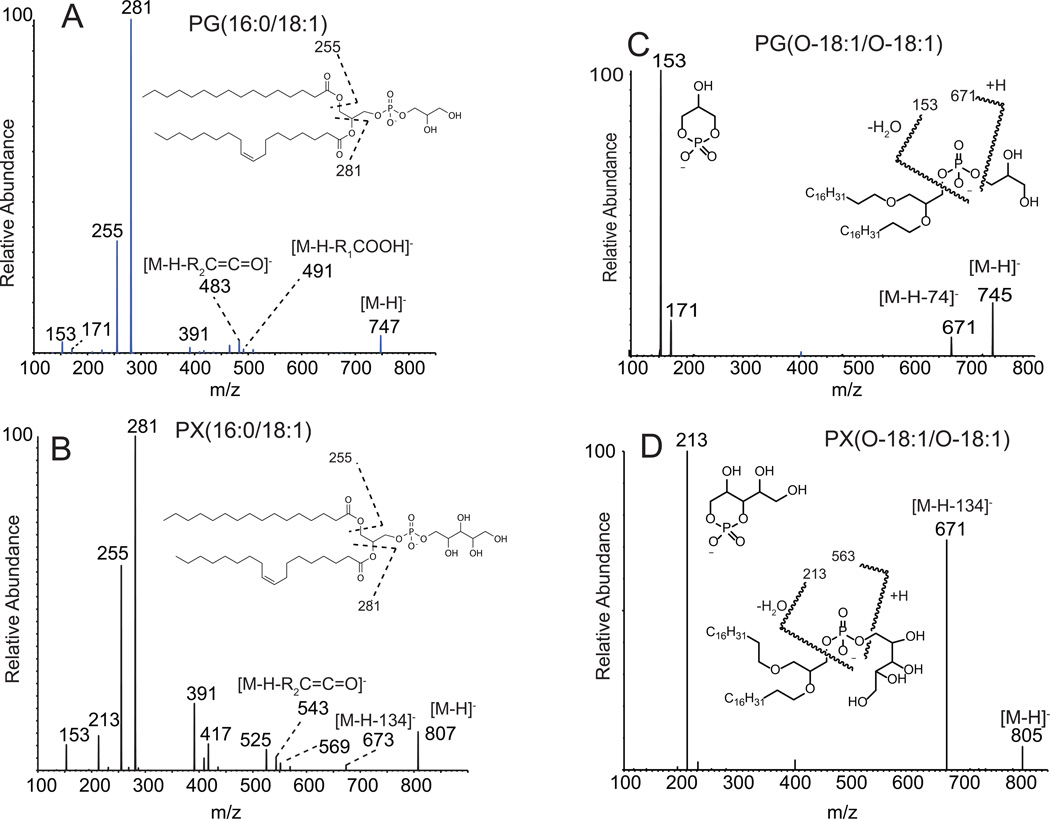
The tandem mass spectrometry of PG molecular species has been well documented for both positive ions and negative ions.[17] Collisional activation of molecular anions from diacyl PG yield abundant carboxylate anions at m/z 255 and 281 (Figure 1A) as previously reported, as well as loss of the fatty acyl group at the sn-2 position as a neutral ketene and free carboxylic acid from the sn-1 position (m/z 483 and 491, respectively).[18] The corresponding PX analog of the same phospholipid molecular species behaved in an almost identical fashion with abundant carboxylate anions from each of sn-1 and sn-2 positions of the glycerol backbone (Figure 1B). In contrast to the CID behavior of diacyl PG and diacyl PX, the negative ion collisional activation of the diether analogs of both PG (Figure 1C) and PX (Figure 1D) display very different collision induced decomposition reactions. The most abundant high mass product ion corresponded to the neutral loss of the polar head group, which was 74 Da for PG and 134 Da for PX. The ion corresponding to the cleavage of the phosphodiester bond immediately adjacent to the alkyl chain followed by loss of water to form a cyclic phosphodiester ion were also abundant product ions for both the diether PG and PX at m/z 153 (Figure 1C) and m/z 213 (Figure 1D). These latter phosphate ester cleavage reactions were present but not abundant in the product ion spectra of diacyl PG and PX (Figures 1A and 1B) and represent a reaction pathway of product ion formation that was less favorable because it competed poorly with carboxylate anion formation. No information was obtained from the negative product ion spectra concerning the nature of the ether-linked groups at sn-1 and sn-2 positions and one could only assume, from the measured mass of the [M-H]−, the total number of alkyl carbon atoms and double bonds. It was clear from the negative ion spectra of the diether analogs that no ester moieties were present in these phospholipid species, due to the absence of carboxylate anions in the product ion spectra.
Figure 1.
Electrospray ionization (negative ions) and tandem mass spectrometry of (A) PG(16:0/18:1), (B) PX(16:0/18:1), (C) PG(O-18:1/O-18:1), (D) PX(O-18:1/O-18:1). These MS/MS spectra were obtained using a tandem quadrupole mass spectrometer.
Positive ion [M+H]+ and [M+Na]+ collisional activation
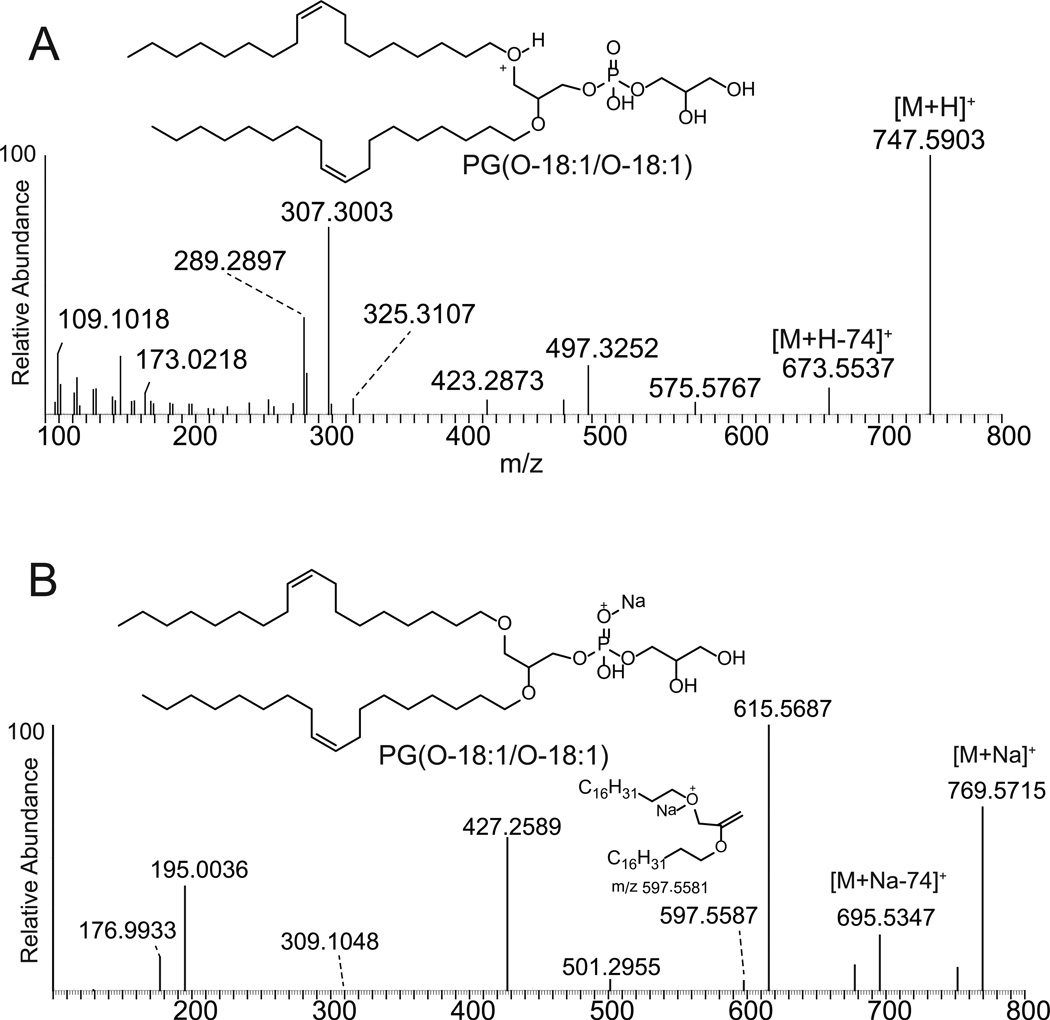
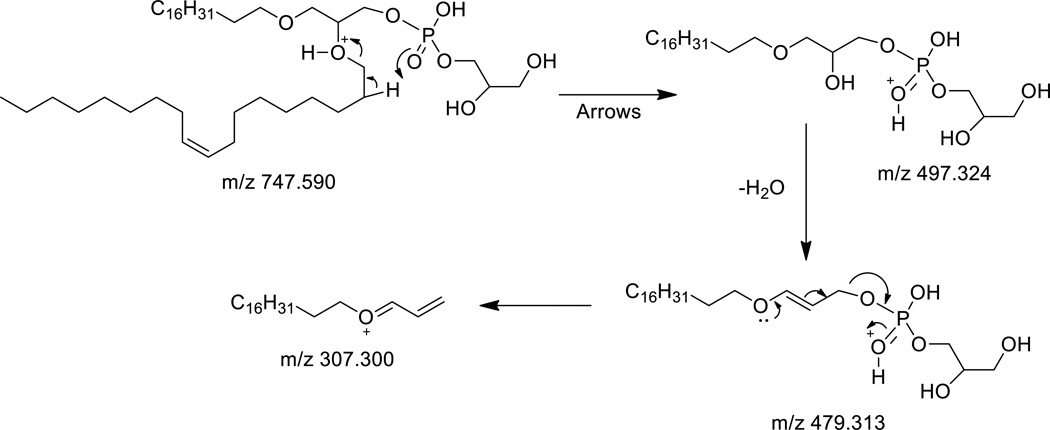
Considerably more fragmentation was observed when the positive ions corresponding to either the protonated or sodiated adducts of the diether PG and PX analogs were collisionally activated. High resolution analysis of the major product ions following collisional activation of the diether PG(O-18:1/O-18:1) [M+H]+ at m/z 747.590 did yield interesting information concerning the nature of the alkyl chains (Figure 2A). Likely, this was due to a population of ions with a protonated charge site on either of the two ether moieties. With charging at these sites, charge-remote fragmentation reactions could be observed corresponding to loss of the phosphate and polar head group from both the protonated (m/z 575.577) (C39H75O2)+ and sodiated species (m/z 597.559, C39H74NaO2+) (Figure 2A and 2B) which provided evidence that the charge site was on the alkyl portion of these diether phospholipids. There were other differences in CID behavior of the protonated and sodiated diether species that were observed in the mass spectra. An interesting ion at m/z 497.325 (C24H50O8P)+ corresponded to loss of one of the fatty alkyl groups likely as a charge-driven product, as suggested in Scheme 1. This would be a valuable fragmentation to ascertaining the exact nature of each of the fatty alkyl groups and in this case with symmetrical phospholipid, where both of the fatty alkyl radyl groups were identical, led to a single product ion. The protonated species had the most abundant ion appearing at m/z 307.300 (C21H39O)+, most likely consisting of one of the alkyl ether chains which retains the 3-carbon atoms of glycerol. This involved dehydration of m/z 497.325 (C24H50O8P)+ followed by charge driven rearrangement of the molecule; the proposed structure for m/z 307.300 (Scheme 1) was consistent with the high resolution analysis.
Figure 2.
Electrospray ionization (positive ions) and tandem mass spectrometry of diether PG phospholipids. (A) Product ion spectrum following collisional activation of [M+H]+ of PG(O-18:1/O-18:1). (B) Product ions obtained following collisional activation of the sodiated adduct [M+Na]+ of PG(O-18:1/O-18:1).
Scheme 1.
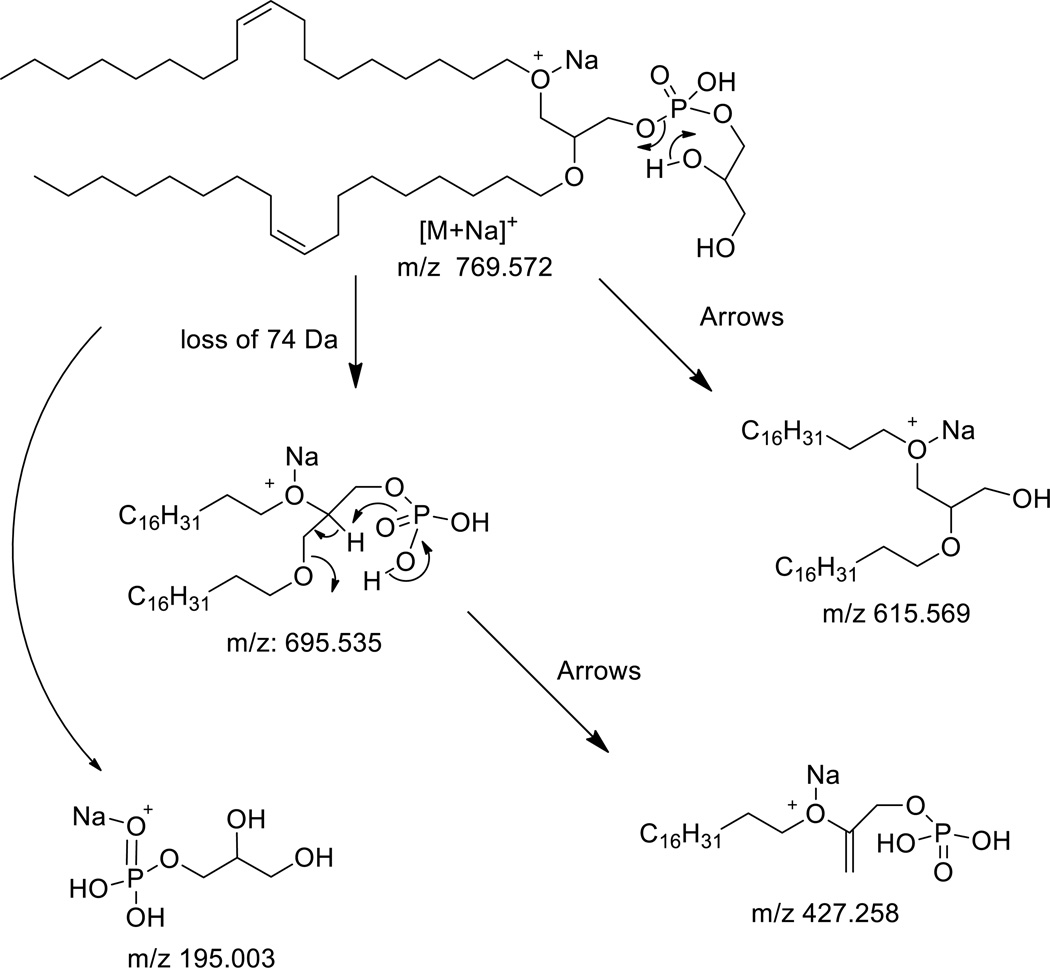
The high resolution analysis of product ions obtained after collisional activation of the diether PG analog as a sodium adduct also generated an ion corresponding to the loss of the polar head group, in this case being m/z 695.535 (Figure 2B). The most abundant product ion appeared at m/z 615.569 (C39H76NaO3) + and likely corresponded to a loss of the polar head group plus the phosphodiester as a neutral cyclic phosphate. Formation of such a cyclic phosphate neutral has been described previously as a common reaction product of most phospholipids, but in particularly PG.[18] The structure of this ion consistent with high resolution mass spectra data is shown in Scheme 2. The ion at m/z 427.259 (C21H41NaO5P)+ is an interesting product ion corresponding to the loss of one of the alkyl groups, likely the one that is not charged by the sodium attachment site, but leads to formation of monoalkylphosphate ester (Scheme 2). Most likely this originates from the ion at m/z 695.535, since it does not contain the elements of the polar head group. The sodiated glycerophosphate cation is also an abundant product ion (m/z 195.004, C3H9NaO6P+).
Scheme 2.
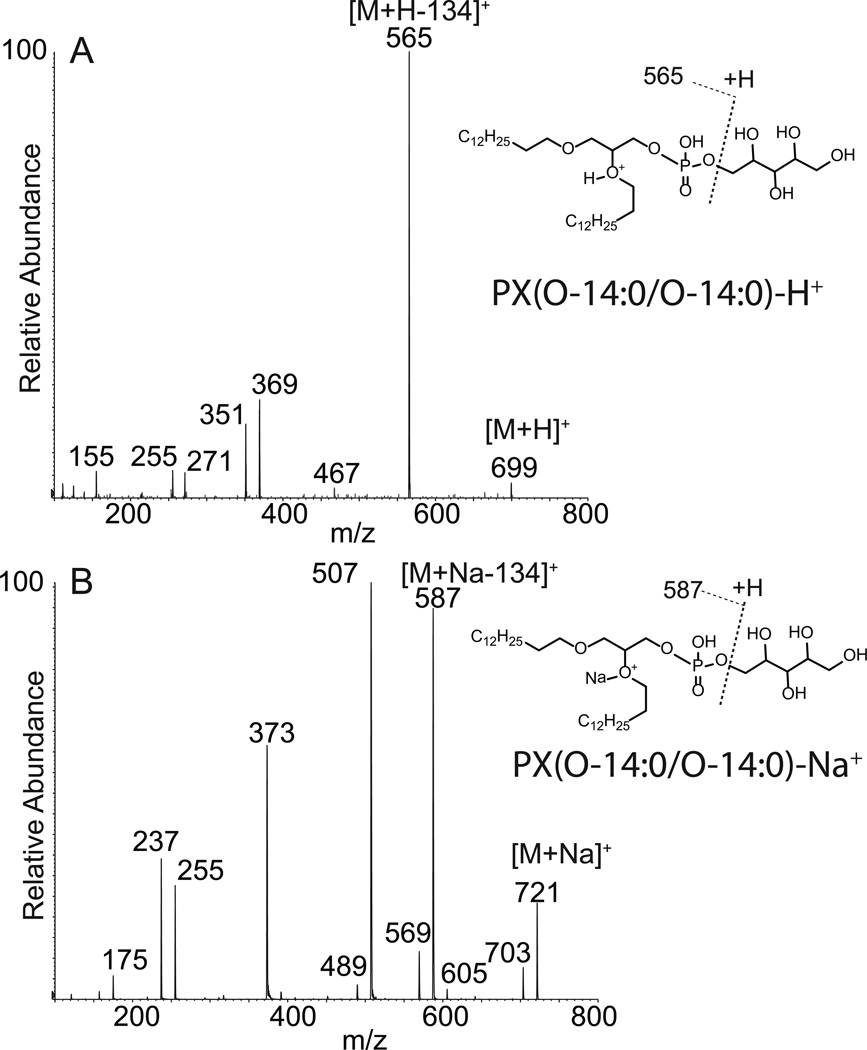
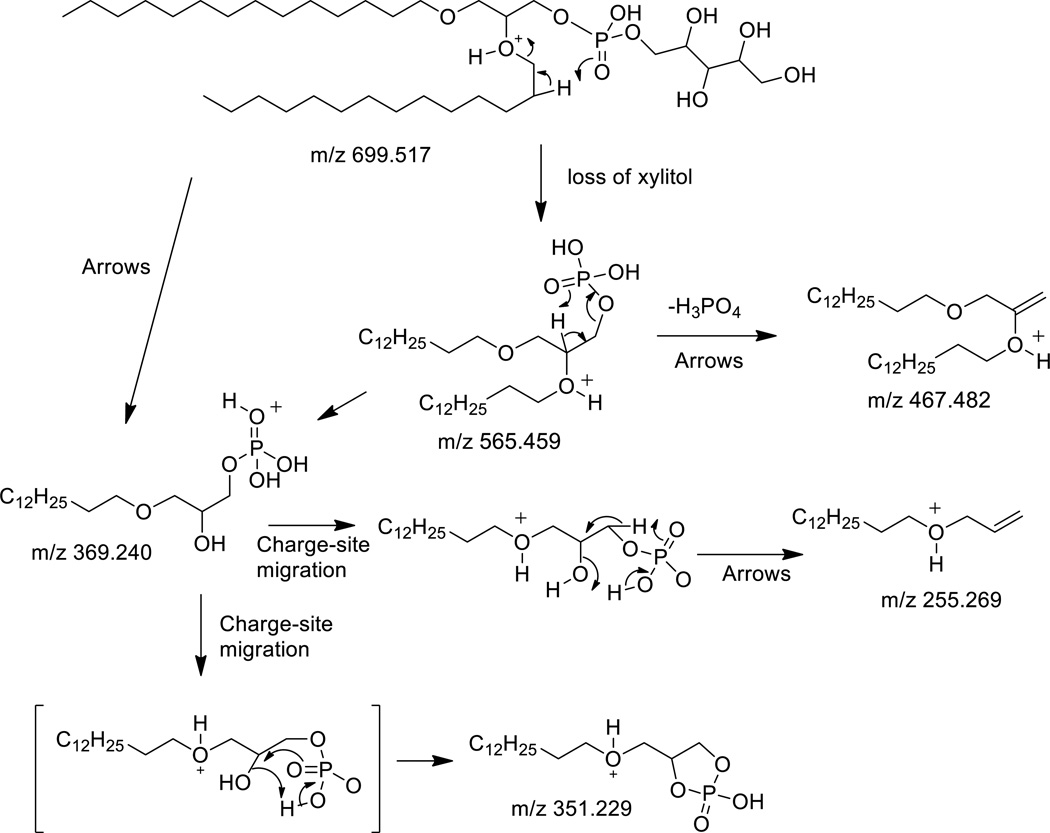
The [M+H]+ of the diether PX lipid appears to lose the polar head group in a much more facile reaction than the glycerol polar head group (Figure 3), likely a consequence of the additional hydroxyl moieties in the polar head group, which could facilitate rearrangement reactions with loss of the neutral sugar species. There were ions directly analogous to the PX and PG molecular species, including m/z 467 (Figure 3A) which would be identical to the ion annotated as m/z 575.577 in the diether PG(O-18:1/O-18:1) molecular species (Figure 2A). Just as the decomposition mechanisms appear to be charge-remote fragmentations where the protonation of the ether group tags the fragment ion for detection in the mass spectrometer, the PX(O-14:0/O-14:0) major fragment ions at m/z 351 and 369 could be suggested to be formed by a mechanism similar to that described for PG(O-18:1/O-18:1) at m/z 479 and 497, respectively. These ions were likely derived from the abundant fragment ion corresponding to the loss of the polar head group (m/z 565, [M+H-134]+) or directly from the molecular ion (Scheme 3). These were interesting ions because they would contain only one of the alkyl groups and in the case of m/z 369 retained the hydroxyl group corresponding to one of the ether positions in PX(O-14:0/O-14:0). The loss of water from this ion at m/z 369 would yield the ion at m/z 351. This ion was abundant and likely the result of phosphate ester participation, which facilitated the loss of water which normally is very difficult to achieve by dehydration mechanisms.
Figure 3.
Electrospray ionization (positive ions) and tandem mass spectrometry of diether xylitol glycerophospholipids. (A) Product ions obtained following the collisional activation of PX(O-14:0/O-14:0). (B) Product ions obtained following collisional activation of the sodiated adduct of PX(O-14:0/O-14:0).
Scheme 3.
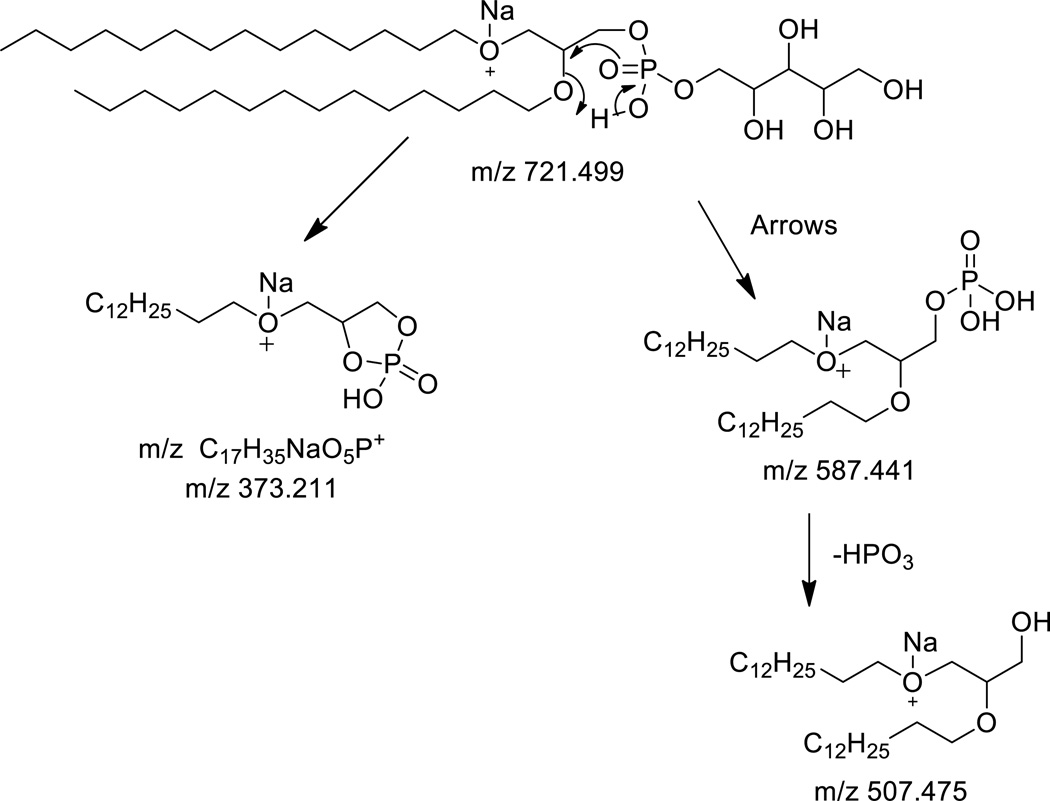
The sodiated adduct of the diether PX readily lost the polar head group as observed as the ion at m/z 587 ([M+Na-134]+). This ion appeared to further lose neutral HPO3 in a very common neutral loss mechanism observed for phosphopeptides,[19] phosphohistidines,[20] and other phosphorylated biomolecules. This finding reinforces the likelihood that the charge site is remote from the phosphate diether. The ion at m/z 373 could result from favorable cyclic phosphate ester formation (Scheme 4). The more abundant formation of m/z 373 likely represents the stability of the phospholipid fragment ion when sodium is attached to one of the alkyl ether groups. These are very informative ions concerning the nature of the diether analogs since they involve loss of one of the alkyl chains and thus can provide information as to the nature of the alkyl chain that is lost as well as the alkyl chain remaining as this ion.
Scheme 4.
Conclusion
The tandem mass spectrometry of diacyl PX lipids are quite similar to that known for diacyl PG molecular species.[19] The major product ions following collisional activation of the molecular anions [M-H]− correspond to stable carboxylate anions as expected for the negative ion tandem mass spectrum of virtually all diacyl phospholipids.[21, 22] There were additional product ions corresponding to the loss of the xylitol head group to form a phosphatidic acid-like product ion. The negative ion tandem mass spectrum of diether PG and PX phospholipids did not yield information about the alkyl chain but only information about the polar head group being either glycerol or xylitol attached to the phosphate ester moiety. Specific information about the nature of the alkyl ether groups was obtained by positive ion collisional activation of either the protonated [M+H]+ or sodiated positive ions [M+Na]+. Both cations yield abundant ions corresponding to the loss of one of the alkyl chains following collisional activation. This behavior had been reported for the alkyl, acyl phospholipids such as 1-O-alkyl-2-acyl-phosphatidylcholine which is a relatively abundant phospholipid found in many cells.[23] Considering the report that other diether phosphonolipids have been reported as useful synthetic surfactants, [24] the unique collisional activated ion transitions for dialkyl PX as well as dialkyl PG from both positive and negative molecular ions could be used in further studies to monitor metabolism and distribution of other bioactive ether lipids in vivo.
Acknowledgments
This work was supported by a grant from the National Institutes of Health HL094629 (DRV) and ES022171 (RCM) as well as support from NIH/NCATS Colorado CTSA Grant Number UL1 TR001082 (RCM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Batenburg JJ. Surfactant phospholipids: synthesis and storage. Am. J. Physiol. 1992;262:L367. doi: 10.1152/ajplung.1992.262.4.L367. [DOI] [PubMed] [Google Scholar]
- 2.Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L259. doi: 10.1152/ajplung.00112.2007. [DOI] [PubMed] [Google Scholar]
- 3.Creuwels LA, van Golde LM, Haagsman HP. The pulmonary surfactant system: biochemical and clinical aspects. Lung. 1997;175:1. doi: 10.1007/PL00007554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis JF, Jobe AH. Surfactant and the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1993;147:218. doi: 10.1164/ajrccm/147.1.218. [DOI] [PubMed] [Google Scholar]
- 5.Numata M, Chu HW, Dakhama A, Voelker DR. Pulmonary surfactant phosphatidylglycerol inhibits respiratory syncytial virus-induced inflammation and infection. Proc. Natl. Acad. Sci. U.S.A. 2010;107:320. doi: 10.1073/pnas.0909361107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandasamy P, Zarini S, Chan ED, Leslie CC, Murphy RC, Voelker DR. Pulmonary surfactant phosphatidylglycerol inhibits Mycoplasma pneumoniae-stimulated eicosanoid production from human and mouse macrophages. J. Biol. Chem. 2011;286:7841. doi: 10.1074/jbc.M110.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Numata M, Kandasamy P, Nagashima Y, Posey J, Hartshorn K, Woodland D, Voelker DR. Phosphatidylglycerol suppresses influenza A virus infection. Am. J. Respir. Cell Mol. Biol. 2012;46:479. doi: 10.1165/rcmb.2011-0194OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Numata M, Grinkova YV, Mitchell JR, Chu HW, Sligar SG, Voelker DR. Nanodiscs as a therapeutic delivery agent: Inhibition of respiratory syncytial virus infection in the lung. Int. J. Nanomedicine. 2013;8:1417. doi: 10.2147/IJN.S39888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Numata M, Nagashima Y, Moore ML, Berry KZ, Chan M, Kandasamy P, Peebles RS, Murphy RC, Voelker DR. Phosphatidylglycerol provides short-term prophylaxis against respiratory syncytial virus infection. J. Lipid Res. 2013;54:2133. doi: 10.1194/jlr.M037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandasamy P, Numata M, Berry KZ, Fickes R, Leslie CC, Murphy RC, Voelker DR. Structural analogs of pulmonary surfactant phosphatidylglycerol inhibit toll-like receptor 2 and 4 signaling. J. Lipid Res. 2016;57:993. doi: 10.1194/jlr.M065201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuronuma K, Mitsuzawa H, Takeda K, Nishitani C, Chan ED, Kuroki Y, Nakamura M, Voelker DR. Anionic pulmonary surfactant phospholipids inhibit inflammatory responses from alveolar macrophages and U937 cells by binding the lipopolysaccharide-interacting proteins CD14 and MD-2. J. Biol. Chem. 2009;284:25488. doi: 10.1074/jbc.M109.040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang SF, Freer S, Benson AA. Transphosphatidylation by phospholipase D. J. Biol. Chem. 1967;242:477. [PubMed] [Google Scholar]
- 13.Knappy CS, Chong JP, Keely BJ. Rapid discrimination of archaeal tetraether lipid cores by liquid chromatography-tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2009;20:51. doi: 10.1016/j.jasms.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Yoshinaga MY, Kellermann MY, Rossel PE, Schubotz F, Lipp JS, Hinrichs KU. Systematic fragmentation patterns of archaeal intact polar lipids by high-performance liquid chromatography/electrospray ionization ion-trap mass spectrometry. Rapid Commun. Mass Spectrom. 2011;25:3563. doi: 10.1002/rcm.5251. [DOI] [PubMed] [Google Scholar]
- 15.Hsu FF, Lobasso S, Turk J, Gaskell S. Structural studies on archaeal phytanyl-ether lipids isolated from membranes of extreme halophiles by linear ion-trap multiple-stage tandem mass spectrometry with electrospray ionization. Anal. Chim. Acta. 2013;771:73. doi: 10.1016/j.aca.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 17.Hsu FF, Turk J. Studies of phosphatidylglycerol with triple quadrupole tandem mass spectrometry with electrospray ionization: Fragmentation processes and structural characterization. J. Am. Soc. Mass Spectrom. 2001;12:1036. [Google Scholar]
- 18.Hsu FF, Turk J. Charge-driven fragmentation processes in diacyl glycerophosphatidic acids upon low-energy collisional activation. A mechanistic proposal. J. Am. Soc. Mass Spectrom. 2000;11:797. doi: 10.1016/S1044-0305(00)00151-3. [DOI] [PubMed] [Google Scholar]
- 19.Palumbo AM, Smith SA, Kalcic CL, Dantus M, Stemmer PM, Reid GE. Tandem mass spectrometry strategies for phosphoproteome analysis. Mass. Spectrom. Rev. 2011;30:600. doi: 10.1002/mas.20310. [DOI] [PubMed] [Google Scholar]
- 20.Oslund RC, Kee JM, Couvillon AD, Bhatia VN, Perlman DH, Muir TW. A phosphohistidine proteomics strategy based on elucidation of a unique gas-phase phosphopeptide fragmentation mechanism. J. Am. Chem. Soc. 2014;136:12899. doi: 10.1021/ja507614f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy RC. Tandem Mass Spectrometry of Lipids: Molecular analysis of Complex Lipids. London: Royal Society of Chemistry; 2015. [Google Scholar]
- 22.Pulfer M, Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrom. Rev. 2003;22:332. doi: 10.1002/mas.10061. [DOI] [PubMed] [Google Scholar]
- 23.Hsu FF, Turk J. Electrospray ionization/tandem quadrupole mass spectrometric studies on phosphatidylcholines: The fragmentation processes. J. Am. Soc. Mass Spectrom. 2003;14:352. doi: 10.1016/S1044-0305(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Chang Y, Schwan AL, Notter RH. Activity and Inhibition Resistance of a Phospholipase-Resistant Synthetic Surfactant in Rat Lungs. Am J of Respir Cell Mol Biol. 2007;37:387. doi: 10.1165/rcmb.2006-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]