Abstract
Extracellular matrix (ECM) supplies both physical and chemical signals to keratocytes which can impact their differentiation to fibroblasts and/or myofibroblasts. It also provides a substrate through which they migrate during wound repair. We have previously shown that following transcorneal freeze injury (FI), migrating corneal fibroblasts align parallel to the stromal lamellae during wound repopulation. In this study, we compare cell and ECM patterning both within and on top of the stroma at different time points following lamellar keratectomy (LK) in the rabbit. Twelve rabbits received LK in one eye. Rabbits were monitored using in vivo confocal microscopy at 3, 7, 21 and 60 days after injury. A subset of animals was sacrificed at each time point to further investigate cell and matrix patterning. Tissue was fixed and labeled in situ with Alexa Fluor 488 phalloidin (for F-actin), and imaged using multiphoton fluorescence and second harmonic generation (SHG) imaging (for collagen). Immediately following LK, cell death occurred in the corneal stroma directly beneath the injury. At 7 and 21 days after the procedures, analysis of fluorescence (F-actin) and SHG results (collagen) indicated that fibroblasts were co-aligned with the collagen lamellae within this region. In contrast, stromal cells accumulating on top of the stromal wound bed were randomly arranged, contained more prominent stress fibers, and expressed alpha smooth muscle actin (α-SMA) and fibronectin. At 60 days, cells and matrix in this region had become co-aligned into lamellar-like structures; cells were elongated but did not express stress fibers. Corneal haze measured using in vivo confocal microscopy peaked at 21 days after LK, and was significantly reduced by 60 days. Cell morphology and patterning observed in vivo was similar to that observed in situ. Our results suggest that the topography and alignment of the collagen lamellae direct fibroblast patterning during repopulation of the native stroma after LK injury in the rabbit. In contrast, stromal cells accumulating on top of the stromal wound bed initially align randomly and produce a fibrotic ECM. Remarkably, over time, these cells appear to remodel the ECM to produce a lamellar structure that is similar to the native corneal stroma.
Keywords: Confocal microscopy, Corneal Wound Healing, Extracellular Matrix, SHG Imaging
1. Introduction
Stromal keratocytes play a central role in mediating the corneal response to injury or refractive surgery (Netto et al., 2005). During wound healing, quiescent corneal keratocytes surrounding the area of injury generally become activated, proliferate, and transform into a fibroblastic phenotype (Jester et al., 1999c; Stramer et al., 2003). In certain wound types, fibroblasts further differentiate into myofibroblasts, which generate stronger forces and synthesize a disorganized fibrotic extracellular matrix (ECM) (Blalock et al., 2003; Jester et al., 1999a). Following vision correction procedures such as photorefractive keratectomy (PRK) or laser assisted in situ keratomileusis (LASIK), cellular force generation and fibrosis can alter corneal shape and reduce corneal transparency. In addition, a decrease in the concentration of keratocyte-specific “corneal crystallin” proteins has been associated with an increase in cellular light scattering during wound healing, which also contributes to clinical haze (Jester et al., 2012; Jester et al., 1999b). Both PRK and LASIK result in a region of keratocyte death beneath the laser-treated area (Mohan et al., 2000; Møller-Pedersen et al., 1998; Wilson, 2002). Stromal cell death can also be induced by toxic injury (Jester et al., 1998; Maurer et al., 1997) as well as UV cross-linking of the cornea in keratoconus patients (Knappe et al., 2011; Mencucci et al., 2010; Wollensak et al., 2004). Ideally, repopulation of damaged stromal tissue following these insults should occur via intra-stromal migration of keratocytes from the surrounding stromal tissue, without generation of contractile forces that could disrupt the collagen architecture or the production of fibrotic ECM which can reduce transparency. Previous work has shown that myofibroblast transformation of corneal keratocytes during wound healing is mediated by transforming growth factor beta (TGF-β) in combination with other growth factors; (Chen et al., 2009; Etheredge et al., 2009; Funderburgh et al., 2001; Jester et al., 1999a; Jester et al., 2002; Jester et al., 1995; Jester et al., 1999c) however, less is known about the biochemical and biophysical signals that regulate cell and matrix patterning during wound healing
We recently used in vivo confocal microscopy to assess keratocyte backscattering, alignment, morphology and connectivity during intra-stromal wound healing, following a full-thickness corneal freeze injury (FI) in the rabbit (Petroll et al., 2015). We also correlated these findings with en bloc 3-D confocal fluorescence imaging of cellular patterning, and second harmonic generation (SHG) imaging of the corneal collagen lamellae. Interestingly, we found that keratocyte alignment during wound repopulation was highly correlated with the structural organization of the lamellae, suggesting contact guidance of intra-stromal cell migration.
Following FI, the epithelial basement membrane remains intact, and stromal healing involves fibroblast migration into the injured tissue, without myofibroblast transformation, fibrosis or matrix remodeling. In contrast, healing following keratectomy wounds in the rabbit has three phases: stromal repopulation (migration), fibrosis, and regeneration and/or remodeling. Specifically, using in vivo confocal microscopy, Jester and coworkers demonstrated that following PRK, corneal fibroblasts migrated into the wounded stromal tissue by 7 days after injury, without transforming into myofibroblasts (Moller-Pedersen et al., 1998). By 21 days, significant sub-epithelial haze, myofibroblast transformation and associated fibrosis were detected. Interestingly, by 6 months, both stromal thickness and haze values returned to near pre-operative levels, suggesting regeneration and/or remodeling of corneal tissue. In addition to changes in cell phenotype and backscatter during stromal wound healing, others have studied ECM organization following keratectomy wounds (Farid et al., 2008; Fitzsimmons et al., 1992; Latvala et al., 1995; Moller-Pedersen et al., 1998; Rawe et al., 1992); however, the temporal and spatial correlation between cell alignment and ECM patterning during the fibrosis, remodeling and/or regeneration phases of wound healing has not been established.
In this study, we investigate the relationships between cell and ECM organization during healing following lamellar keratectomy (LK). Using a custom modified Heidelberg Retinal Tomograph with Rostock Corneal Module (HRT-RCM; Heidelberg Engineering, GmbH, Dossenheim, Germany) in vivo confocal microscope (Petroll et al., 2013), we assess keratocyte patterning, tissue growth, and corneal haze development throughout the full thickness of the cornea at various time points post-injury. Additionally, we use in situ multiphoton fluorescence and SHG imaging to determine the correlation between cell and ECM alignment in the migratory, fibrotic and regenerative/remodeling phases of wound healing (Han et al., 2005; Morishige et al., 2006).
2. Materials and Methods
2.1 Animal Model
All animal procedures were in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research as well as approved by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee. 12 New Zealand white rabbits were anesthetized using 50 mg/kg intramuscular ketamine and 5.0 mg/kg xylazine and locally anesthetized in the left eye with 1 drop of proparacaine. LK was performed following anesthesia. A speculum was placed into the left eye and a 150 μm deep incision was made in the peripheral cornea using a diamond knife. A spatula was used at the base of the incision to separate the layers of collagen within the stroma. Once a resection plane was created, a 5 mm trephine punch was used to remove the anterior corneal tissue in the central cornea. Immediately following surgery, 0.3 mg/kg of buprenorphine SR (slow release) was injected. Gentamicin eye drops were administered as an antibiotic in the left eye twice a day for 7 days following injury.
2.2 In Vivo Confocal Microscopy
Rabbits were monitored using an HRT-RCM in vivo confocal microscopy with Confocal Microscopy Through Focusing (CMTF) software for analysis as previously described (Petroll et al., 2015; Petroll et al., 2013). Rabbits were scanned 1 week before LK (Pre-Op), and at 3, 7, 21, and 60 days after LK. Rabbits were anesthetized prior to scanning with 50 mg/kg intramuscular ketamine and 5.0 mg/kg xylazine and locally anesthetized in each eye with 1 drop of proparacaine. Since the reflection from the Tomocap can obscure images of the superficial epithelial cells, a thin PMMA (poly(methyl methacrylate)) washer was placed on the Tomocap to eliminate these reflections, as previously described (Zhivov et al., 2009). The objective was positioned on the cornea to create a flat field-of-view image in the central cornea. CMTF scans were collected by starting the scan in the anterior chamber and finishing above the epithelium with a constant speed of 60 μm per second. Images were acquired with the rate set to 30 frames per second. To allow quantitative assessment of haze, scans were collected using a constant gain setting, by unchecking the “auto brightness” box in the HRT software interface. Each scan was conducted using a gain of 6, which was set by moving the horizontal slider under the “auto brightness” box six mouse clicks to the right. At least 3 scans were collected within the central area of the cornea where LK was performed. Each scan contained a 3-D stack of 384 × 384-pixel images (400 × 400 μm), with a step size of approximately 2 μm between images. Additional scans were collected closer to the wound edge in some animals. In some cases, a portion of the scan was saturated when using manual gain settings due to strong cell/matrix reflectivity. In these cases, additional scans were taken using the “auto brightness” enabled so that changes in cell patterning and morphology could be documented. Only the scans taken with a gain of 6 were used for quantitative analysis.
After image acquisition, scans were saved as “.vol” files, which could be opened into our in-house CMTF software to analyze the 3-D changes in cell morphology and cell/ECM reflectivity (Petroll et al., 2013). The program generates an intensity vs. depth curve, corresponding to the average pixel intensity of each image and the z-depth of that image within the scan, respectively. The relative amount of backscatter, or haze, associated with the stromal keratocytes and ECM was measured by taking the area under the curve between the location of the basal lamina peak (top of the stroma) and the endothelial peak. A baseline of 13 was chosen for haze calculations, since this value was below the baseline intensity for the normal stroma and above the intensity of the anterior chamber. The thicknesses for the epithelial and stroma layer were also calculated by the CMTF program using the interfaces (peaks on CMTF curve) between each layer in the cornea.
2.3 In Situ Multiphoton Imaging
A subset of rabbits was sacrificed at 7, 21, and 60 days to further investigate cell and matrix patterning. The corneal tissue was fixed via anterior chamber perfusion with a PBS solution containing: 1% paraformaldehyde, 1% dimethyl sulfoxide, 1% Triton-X-100, and 5% Dextran for 5 minutes, followed by additional submersion ex vivo for 15 minutes, as previously described (Jester et al., 1994). Tissues were washed with 1X PBS twice for 10 minutes each, then labeled in situ with Alexa Fluor 488 phalloidin (Molecular Probes, Thermo Fisher Scientific, Waltham, MA, USA) at a concentration of 1:20 in PBS for 3 hours at 37°C. Next, the tissues were washed with PBS three times for 30 minutes each.
Labeled tissues were imaged using multiphoton fluorescence and SHG imaging on a laser scanning confocal microscope (Leica SP8, Heidelberg, Germany). Corneas were blocked to localize the wound area, and placed with the epithelium facing the bottom of the Mattek dish for imaging. A glycerol:PBS solution (2:1) was used to help maintain normal corneal hydration. Multiphoton fluorescence and SHG images were generated using a wavelength of 880 nm (Coherent Chameleon Vision II, ultrafast Ti: Sapphire laser, Santa Clara, CA). SHG forward scatter, phalloidin (F-actin), and SHG backscatter images were captured simultaneously, as described previously (Petroll et al., 2015). Stacks of optical sections were collected using a 25x water immersion objective lens (0.95 NA, 2.4 mm free working distance).
2.4 Image Analysis for Alignment Measurements
Cell and/or matrix alignment from both in vivo HRT-RCM scans and in situ laser confocal scans were quantified using the “Directionality” plugin in ImageJ, which uses a Fourier Transform algorithm to determine the percent of image content aligned at each radial angle within the image (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA). Plots showing both cell and matrix directionality were generated from in situ F-actin and SHG images to allow direct comparison of the angle distributions. Regions for analysis were selected in the anterior and posterior stroma using the first stromal image below the epithelium and the last stromal image above the endothelium, respectively. Analysis was conducted in grid-like sub-regions in each image (256 × 256 pixel sub-regions from 1024 × 1024 pixel images) as previously described (Sivaguru et al., 2010). Sub-regions that did not contain cells or collagen were not included in the analysis.
2.5 Statistical Methods
SigmaPlot (version 12.5; Systat Software, Inc., San Jose, CA, USA) was used for statistical analysis. Linear regression analysis was used to determine the correlation coefficients between the angular distributions of F-actin and the forward scattered SHG signals, as previously described (Petroll et al., 2015). One way analysis of variance (ANOVA) was used for comparing haze values, epithelial and stromal thicknesses, and correlation coefficients from directional analysis. Post-hoc analysis using the Holm-Sidak Method was conducted to compare between groups.
3. Results
3.1 Time-dependent tracking of cellular changes using in vivo confocal microscopy
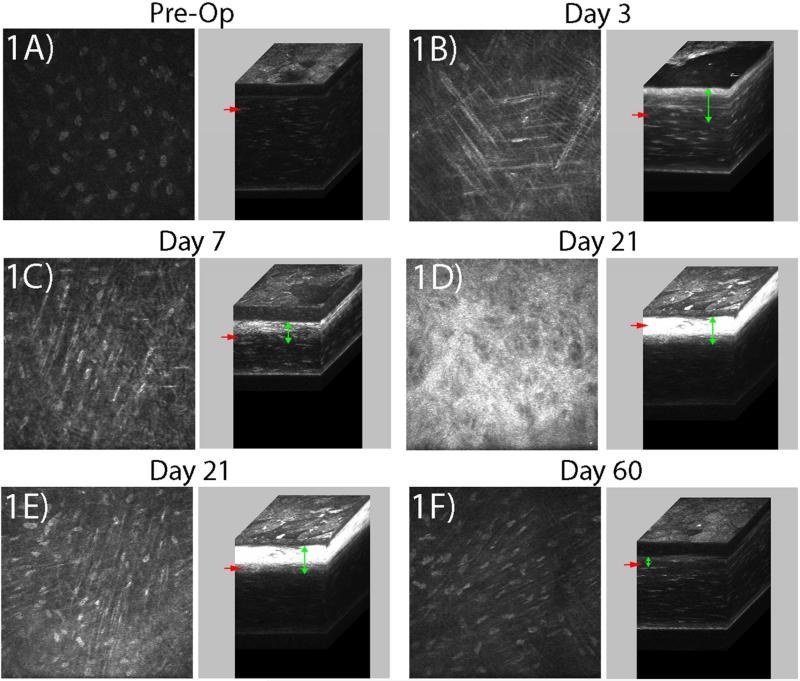
The CMTF scans were obtained without difficulty using the HRT-RCM at all of the time points evaluated. Corneal keratocytes were quiescent and did not appear elongated in the pre-operative (Pre-Op), normal cornea; backscatter was primarily from the nuclei with only a faint signal from cell bodies (Figure 1A). After keratectomy, an area of keratocyte death is created directly beneath the tissue removal site within the native ECM (Moller-Pedersen et al., 1998). By 3 days post-injury, elongated, reflective cells (presumably corneal fibroblasts) had migrated into this acellular wound region (Figure 1B), which was approximately 100 microns thick (vertical green arrows). The epithelium was only partially resurfaced at 3 days after injury, and significant stromal edema was present. At 7 days, elongated corneal fibroblasts were again observed throughout the anterior stromal region (Figure 1C). Backscattering from these cells was brightest near the surface of the injured stroma (Supplemental Movie 1 and 2). The epithelium had completely resurfaced at 7 days, and there was less stroma edema. At 21 days, a highly reflective, disorganized layer of cells was present directly beneath the epithelium (Figure 1D, note that automatic brightness image is shown). Below this layer, elongated cells were still present in the anterior stroma (Figure 1E), however they were generally less reflective than at day 7. At 60 days, cells in both the sub-epithelial region and anterior stroma had a more quiescent phenotype, as indicated by reduced backscatter primarily originating from the cell nuclei (Figure 1F). Cells in the posterior stroma remained quiescent and the endothelium appeared normal at all of the time points studied (not shown).
Figure 1.
Representative images from in vivo CMTF scans. The depth of each 2-D image within the cornea is indicated by the red arrows in the corresponding 3-D reconstruction (immediately to the right of each 2-D image). Green arrows indicate the region of fibroblastic transformation and/or remodeling in the anterior stroma. (A) Pre-operative, uninjured eye, (B) 3 days post-injury, (C) 7 days post-injury, (D, E) 21 days post-injury, and (F) 60 days post-injury. A, B, C, E, and F were acquired using a manual gain of 6. The 2-D image shown in (D) was acquired using auto brightness enabled, since the manual gain image was saturated. Images are 330 μm × 330 μm.
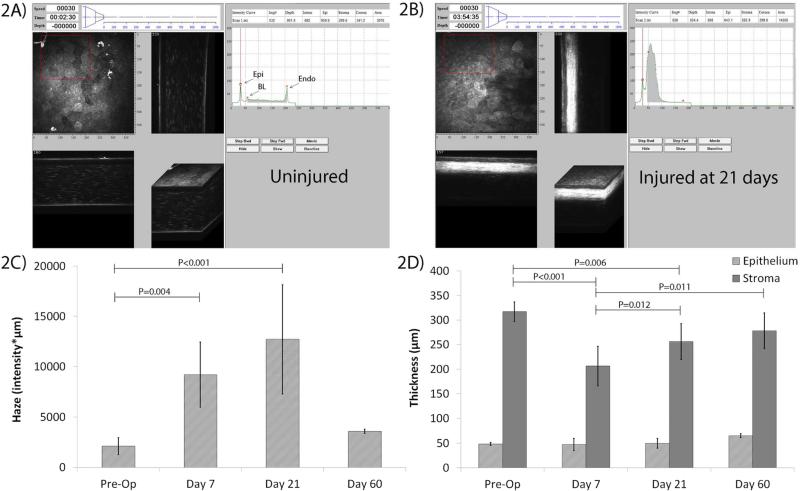
For quantitative analysis, intensity vs. depth curves were calculated and plotted in the CMTF software (Figure 2) (Petroll et al., 2013). In the normal cornea, 3 distinct peaks can be found in the curve (Figure 2A), which correspond to the boundaries of the 3 layers of the cornea (epithelial surface “Epi”, basal lamina “BL”, endothelium “Endo”). At 21 days, a large peak is observed in the anterior stroma due to cell activation and fibrosis (Figure 2B), and the haze measured (shaded region under the CMTF curve) is greater than that of uninjured corneas (compared with Figure 2A). We used these CMTF scans to measure the average haze for each time point (Figure 2C). Quantitative analysis reveals a substantial increase in backscatter post-injury at 7 days compared to the Pre-Op cornea, and maximum haze at 21 days (Figure 1D and 2C). A reduction in haze to near Pre-Op levels occurs by 60 days. There was a decrease in stromal thickness at day 7 as compared to Pre-Op due to LK tissue resection; however, stromal thickness progressively increased at 21 days and 60 days (Figure 2D). The epithelial thickness had returned to Pre-Op values by day 7, and there were no significant changes at 21 or 60 days after injury (Figure 2D).
Figure 2.
CMTF results from in vivo HRT-RCM scans. A) Example of output from a HRT-RCM scan in an uninjured cornea uploaded into CMTF. In the normal cornea, 3 distinct peaks can be found in the curve, which correspond to the boundaries of the 3 layers of the cornea (epithelium “epi”, stroma “BL”, endothelium “Endo”). B) Example of output from a scan in an injured cornea at 21 days post-injury. A large peak is observed in the anterior stroma due to cell activation and fibrosis (arrow). The haze measured is greater post-injury than pre-injury (shown in A). C) Haze at each time point. (* P < 0.01 compared to Pre-Op, ANOVA). D) Epithelial and stromal thickness at each time point (P values are shown as indicated between groups, Repeated Measures ANOVA).
3.2 Assessment of in vivo changes in cell patterning
Images obtained with in vivo confocal microscopy also revealed changes in cell patterning. At 3 days post-injury, cells in the anterior stroma had an elongated morphology, and groups of cells aligned in parallel were often observed (Figure 1B). A similar pattern was observed at 7 days, although cells were more reflective (Figure 1C). At 21 days, cells that were in the reflective layer directly beneath the epithelium were not elongated or aligned in any particular pattern (Figure 1D). Below this layer, cells were elongated and generally aligned in interconnected, parallel lines (Figure 1E). By 60 days, cells directly beneath the epithelium were elongated and highly aligned with narrow processes (Figure 1F).
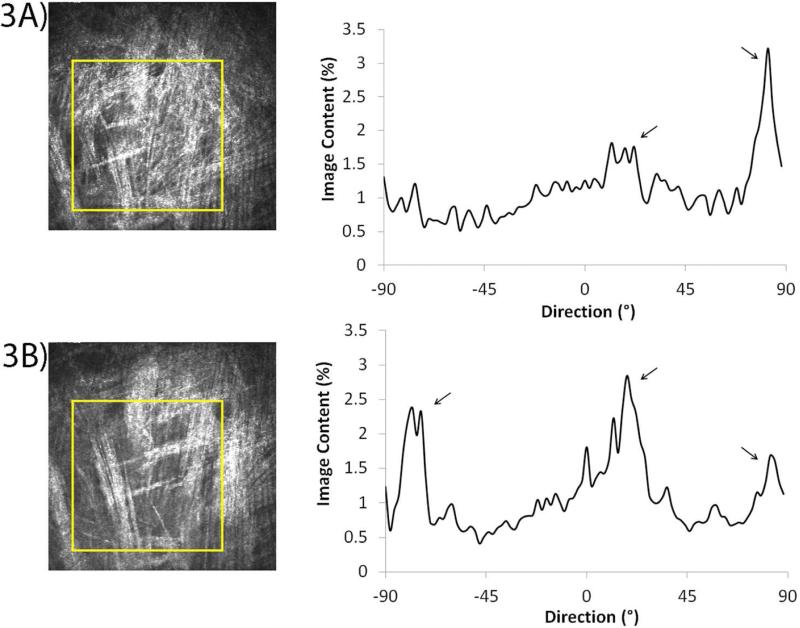
To investigate whether cell patterning could be quantified from the in vivo confocal images, the “Directionality” plugin in ImageJ (Fiji version) was used. Sequential images within in vivo confocal scans reveal that cell alignment shifts from one layer to the next (arrows, Figure 3A and 3B), consistent with the orthogonal layering of the collagen lamellae within the cornea (also see Supplemental Movies 1 and 2). Corresponding graphs for each image show the percentage of image content at each angle. As the scan progresses towards the epithelium (scan endpoint), a new layer of cells oriented at a different direction gradually comes into focus (Supplemental Movie 1 and 2), which correlates with a decrease in one peak and an increase in another (depending on the prominence of cell direction at a specific angle).
Figure 3.
Directionality analysis using in vivo HRT-RCM scans. (A, B) Sequential images taken from an in vivo scan 7 days post-LK, and the corresponding graphs revealing the highest percentage of image content at specified angles (arrows).
3.3 Temporal and spatial analysis of cell/ECM alignment
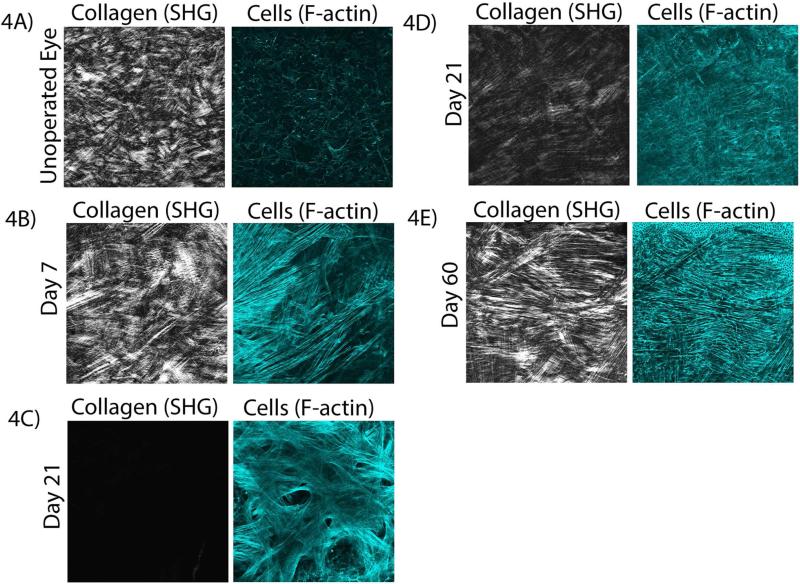
In order to assess changes in keratocyte cytoskeletal organization, F-actin labeling was used. To determine if there was any co-alignment between cells and collagen lamallae, SHG images were collected simultaneously. In the control (un-operated eye), keratocytes are quiescent, and collagen fibers are interwoven in the anterior region of the uninjured stroma with no apparent cell/ECM correlation (Figure 4A). Consistent with a study by Young, et al. (Young et al., 2014), dendritic processes appeared to be aligned in correlation with the collagen lamellae of the posterior stroma, although no measurable correlation was found since the cell bodies were not aligned. By 7 days, keratocytes transformed into a fibroblastic phenotype, as indicated by an elongated morphology and more intense F-actin labeling. These cells appeared to be arranged into parallel groups that were co-aligned with the collagen lamellae in the anterior stroma (Figure 4B).
Figure 4.
Multiphoton fluorescent and SHG images at each time point. A) Collagen and cells in an uninjured cornea (anterior stroma), at 7 days (B), at 21 days on top of the wound (C) and in the anterior stroma (D), and at 60 days (E). Images in (A,B,D,E) are 465 μm × 465 μm. Image (C) is 230 μm × 230 μm.
At 21 days, a region containing a thin additional layer of cells is present directly beneath the epithelium (on top of the stromal wound bed), where little or no SHG signal is detected (Figure 4C). These cells are randomly arranged with a broad morphology and prominent intracellular stress fibers. Alpha smooth muscle actin (α-SMA) and fibronectin labeling are also observed in this region (Supplemental Figure 1), indicating myofibroblast transformation. In contrast, cells directly underneath this myofibroblastic layer, within the native stroma, appear to be co-aligned with the collagen at 21 days; these cells are thinner and do not have prominent stress fibers (Figure 4D and Supplemental Movie 3).
By 60 days, all cells in the anterior stroma are highly aligned with the collagen. These cells are thinner, and have less intense F-actin labeling (Figure 4E and Supplemental Movie 4). Overall, the cell morphology and patterning observed in situ is highly comparable to that observed in vivo with the HRT-RCM.
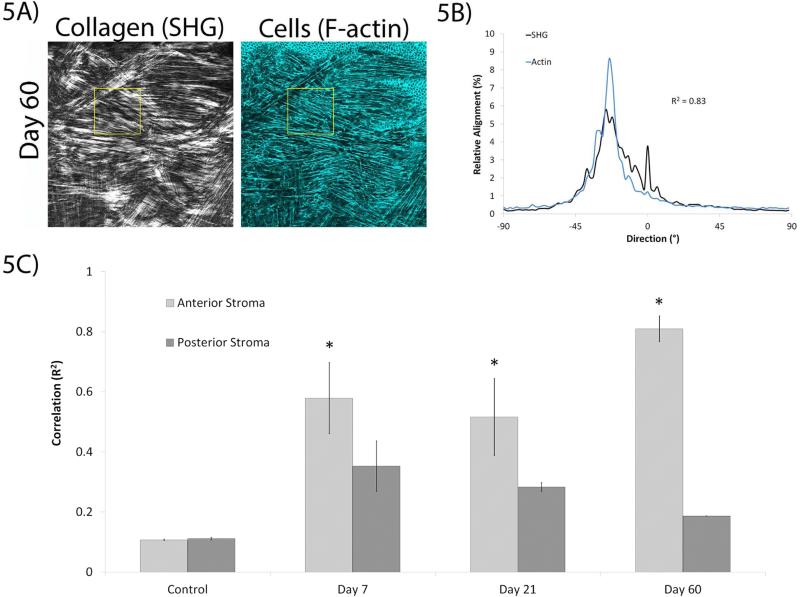
To quantify the extent of alignment between the collagen and the keratocytes, we used directionality analysis at various sites in the cornea. The images were divided into sub-regions for this analysis (Figure 5A and 5B), since larger images often contained multiple lamellae oriented at different angles, which can introduce uncertainty in the interpretation of cell/matrix correlations. The average correlation values from the designated regions (as specified in the Methods) indicated that cells and collagen were generally co-aligned in the anterior stroma of the injured corneas, whereas minimal cell/ECM co-alignment was observed in the posterior cornea or in uninjured control corneas (Figure 5C). At 7 days, there was greater cell/ECM alignment in the anterior stroma compared to the posterior stroma. Likewise, the anterior stroma had greater co-alignment of cells/ECM compared to the posterior stroma at 21 days. The greatest difference in correlation between the anterior and posterior stroma at any given time point was at 60 days, with the cell/ECM alignment also being the greatest in the anterior stroma compared to all other time points studied.
Figure 5.
Directionality analysis was used to quantify correlation at each time point. Directionality analysis was conducted using smaller regions within an image (the yellow boxed region in (A)), which generated corresponding output shown in (B, from the yellow boxed region). C) Alignment of cells with collagen in different regions of the cornea at all time points. Images in (A-E) are 465 μm × 465 μm. (* P < 0.05 compared to posterior stroma and control, ANOVA).
4. Discussion
We recently studied cell and matrix patterning following FI, which involves intrastromal cell migration to repopulate the injured region (Petroll et al., 2015). At 3 days after FI, we observed elongated, reflective cells in the posterior stroma near the wound edge; while at 7 days, cells had migrated throughout the thickness of the cornea. Using SHG imaging, we demonstrated that migrating corneal fibroblasts were aligned parallel to the stromal lamellae. In the current study, we follow up on this important observation by assessing cell and matrix patterning following LK. Using LK, two distinct patterns of wound healing were observed.
First, beginning at 3 days after injury, elongated fibroblasts within the anterior of the native stroma were organized into groups of parallel cells, whose alignment was highly correlated with that of the collagen lamellae, consistent with our observations after FI. The backscattering from these cells peaked at day 7 and gradually decreased at days 21 and 60. These results demonstrate how collagen lamellae patterning may provide topographical cues that modulate corneal fibroblast migration following injury. Supporting our findings, in vitro studies have shown that cell morphology, migration, and phenotype are directly affected by localized substrate topography (Ghibaudo et al., 2009; Teixeira et al., 2003; Teixeira et al., 2004). More specifically, corneal fibroblasts have been previously found to co-align to collagen-coated substrates containing ridges and grooves aligned parallel with a pitch greater than 1 μm (Pot et al., 2010). In contrast, on planar surfaces or on surfaces with smaller pitch sizes, fibroblasts were randomly oriented and migrated slower.
In contrast to the aligned cells observed within the stroma, a second population of cells was observed on top of the native stroma, beginning at 21 days following LK. This thin layer of cells formed an interconnected network that was randomly arranged, and produced significant backscatter and haze. Cells also had prominent stress fibers and expressed α-SMA and fibronectin at 21 days, suggesting myofibroblast transformation. Importantly, we found a substantial reduction in sub-epithelial haze between 21 and 60 days, as well as stromal rethickening and a significant increase in cell/matrix co-alignment. These results suggest that there was remodeling of the fibrotic tissue and regeneration of corneal stroma at the wound site.
The epithelial basement membrane (EBM) plays an important role in differentiation of migrating keratocytes. After LK, the EBM is removed and growth factors, such as TGF-β and platelet derived growth factor (PDGF; from the tears and epithelial cells), are allowed to infiltrate into the stroma and modulate keratocyte phenotype to initiate wound healing (Jester et al., 2002; Stramer et al., 2003; Wilson et al., 1999; Wilson et al., 2001). Most notably, TGF-β induces myofibroblast transformation of corneal keratocytes, particularly in the anterior stroma (Fini, 1999; Funderburgh et al., 2001; Jester et al., 1997; Jester et al., 1999a; Masur et al., 1996; Mohan et al., 2003; Petridou et al., 2000). Our results show myofibroblast localization directly beneath the epithelial layer at the wound site by 21 days. However, by 60 days, these myofibroblasts disappear and are replaced by narrow-bodied cells that do not express prominent stress fibers. The mechanism for the transition in the cell phenotype between 21 days to 60 days is unknown. One possible mechanism is that the myofibroblasts become apoptotic and are replaced by more quiescent, elongated keratocytes (Torricelli et al., 2016). Some reports have shown apoptosis of myofibroblasts in the anterior stroma 4 weeks to 3 months post-PRK, likely due to reestablishment of epithelial basement membrane and decreased levels of TGF-β and PDGF (Mohan et al., 2003; Torricelli et al., 2013). Maintenance of myofibroblast phenotype is dependent upon TGF-β and PDGF, (Mohan et al., 2003) therefore; a phenotypic switch (transdifferentiation) to more passive fibroblasts and keratocytes is also a possibility at decreased levels of these growth factors (Jester et al., 1999b; Jester et al., 1999c; Masur et al., 1996; Mohan et al., 2003).
As mentioned above, in addition to changes in cell morphology and phenotype, dramatic changes in cell/matrix alignment were observed between 21 and 60 days after injury. Previous studies by Jester and coworkers (Moller-Pedersen et al., 1998) have shown that following PRK, significant sub-epithelial haze, myofibroblast transformation and associated fibrosis initially develops, peaking at 21 days. However, by 6 months, both stromal thickness and haze values return to near Pre-Op levels, suggesting possible regeneration and/or remodeling of corneal tissue. Our results following LK are consistent with these findings, and provide new data demonstrating that this remodeled stromal tissue is highly aligned, similar to the native stroma. A study conducted by Wang, et al. demonstrated that cell-secreted collagen was patterned in correlation to the direction of micro-grooved silicone membranes (Wang et al., 2003). Thus, corneal fibroblasts accumulating on top of the stroma may use the topography of the base (most anterior) collagen layer after LK as a template, and align and secrete collagen based on the native collagen patterning of this layer (Gouveia et al., 2013; Gouveia et al., 2015). Interestingly, ascorbic acid promotes human corneal fibroblasts plated on aligned substratum to produce collagen that closely mimics corneal ECM, unlike cells plated on unaligned substrates. Thus, it is possible that at later time points, as new cells begin to fill in the tissue void, they spread and/or migrate using cell-secreted collagen from the layer beneath them as a guide, and secrete collagen that is patterned similarly to the native stroma (Guillemette et al., 2009; Guo et al., 2007; Karamichos et al., 2014; Karamichos et al., 2010; Ren et al., 2008; Saeidi et al., 2012). This mechanism of collagen deposition and cell alignment is a potential explanation for why the patterning at 60 days resembles the less interwoven collagen patterning observed at the mid-stroma (template layer location) of the normal cornea, instead of the highly interwoven pattern normally observed in the anterior stroma (Morishige et al., 2006).
Another possible mechanism that could contribute to collagen alignment at 60 days is the remodeling of corneal collagen due to cell mechanical activity. Cell contractility forces can change the orientation of collagen fibers within non-cell-derived collagen gels to match the cell alignment during spreading and migration (Eastwood et al., 1998; Guido and Tranquillo, 1993; Kim et al., 2006). In this study, fibroblasts are highly elongated at 60 days, which would result in contractile forces being exerted on the ECM parallel to the direction of the long cell axis (Karamichos et al., 2007; Wang et al., 2002). This could contribute to the similar alignment of extracellular collagen.
In this study, we used in vivo confocal microscopy to measure the progress of corneal wound healing and cell activity, at various time points after injury in the cornea. In vivo confocal microscopy has been used in a variety of corneal research and clinical applications since its development over 25 years ago, (Dhaliwal et al., 2007; Efron, 2007; Erie et al., 2009; Labbe et al., 2009; Patel and McGhee, 2013; Petroll et al., 2011; Petroll et al., 1998; Petroll and Robertson, 2015; Tervo and Moilanen, 2003; Villani et al., 2014; Zhivov et al., 2010; Zhivov et al., 2006) and is ideally suited to monitoring the cellular events of wound healing (Bouheraoua et al., 2014; Jester et al., 1999c; Kaufman and Kaufman, 2006; Petroll et al., 2011; Petroll et al., 1998; Tervo and Moilanen, 2003). Previous studies have used HRT-RCM to investigate quantitative changes in cell morphology, density, and reflectivity (Petroll et al., 2013). Here, we show for the first time that cell alignment can be quantified from CMTF scans obtained using the HRT-RCM in the cornea of a live rabbit using the “Directionality” plugin in ImageJ.
One limitation of this study is the use of the LK model, which creates a rough wound bed due to tearing of lamellae and is less reproducible than laser tissue ablation. The use of a clinical PRK laser to create an injury would reduce variability in the surgical procedure and would also be more clinically relevant. It would also be interesting to evaluate more extended time points after injury, since complete re-thickening (regeneration) of the corneal stroma has been shown at 6 months after PRK surgery (Moller-Pedersen et al., 1998). Additionally, labeling native collagen with DTAF (5([4,6-dichlorotriazin-2yl]amino)fluorescein) immediately after surgery would allow native versus newly secreted collagen to be distinguished at different time points after injury (Jester et al., 1992).
Overall, based on our findings, we hypothesize that the topography and alignment of the collagen lamellae direct fibroblast patterning during repopulation of the native stroma after LK injury in the rabbit. Within the stroma, fibroblasts in the wound region had an elongated morphology and were co-aligned with the collagen lamellae. In contrast, cells accumulating on top of the native stroma were randomly organized, expressed stress fibers, and produced a fibrotic ECM. Remarkably, over time, cells appeared to remodel this fibrotic ECM to produce a lamellar structure that is similar in organization (or patterning) and reflectivity to the native corneal stroma. Corneal scarring is a major cause of blindness worldwide. Thus, identifying the changes in gene and protein expression that occur during the remodeling/regenerative phase of healing in the rabbit could potentially lead to novel therapies for preventing or reversing corneal scarring in human patients.
Supplementary Material
Highlights.
Cell and matrix patterning were assessed after lamellar keratectomy
Fibroblasts migrating within the stroma after injury co-aligned with the lamellae
Myofibroblasts in the fibrotic layer on top of the stroma are randomly aligned
Over time, fibrosis and haze are reduced and the lamellar pattern returns
Cells and collagen are co-aligned, suggesting tissue regeneration and/or remodeling
Acknowledgements
This study was supported by NIH Grants R01 EY013322, P30 EY020799 and Research to Prevent Blindness, Inc., NY, NY, HHMI Med into Grad Grant (Grant# 56006776) to the Mechanisms of Disease and Translational Science PhD Track which is also supported by a NIH T32 (Grant# 1T32GM10977601). Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number TL1TR001104. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blalock TD, Duncan MR, Varela JC, Goldstein MH, Tuli SS, Grotendorst GR, Schultz GS. Connective tissue growth factor expression and action in human corneal fibroblast cultures and rat corneas after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2003;44:1879–1887. doi: 10.1167/iovs.02-0860. [DOI] [PubMed] [Google Scholar]
- Bouheraoua N, Jouve L, El Sanharawi M, Sandali O, Temstet C, Loriaut P, Basli E, Borderie V, Laroche L. Optical coherence tomography and confocal microscopy following three different protocols of corneal collagen-crosslinking in keratoconus. Invest Ophthalmol Vis Sci. 2014;55:7601–7609. doi: 10.1167/iovs.14-15662. [DOI] [PubMed] [Google Scholar]
- Chen J, Guerriero E, Sado Y, SundarRaj N. Rho-mediated regulation of TGF-beta1-and FGF-2-induced activation of corneal stromal keratocytes. Invest Ophthalmol Vis Sci. 2009;50:3662–3670. doi: 10.1167/iovs.08-3276. [DOI] [PubMed] [Google Scholar]
- Dhaliwal JS, Kaufman SC, Chiou AG. Current applications of clinical confocal microscopy. Curr Opin Ophthalmol. 2007;18:300–307. doi: 10.1097/ICU.0b013e3281b11665. [DOI] [PubMed] [Google Scholar]
- Eastwood M, Mudera VC, McGrouther DA, Brown RA. Effect of precise mechanical loading on fibroblast populated collagen lattices: morphological changes. Cell Motil Cytoskeleton. 1998;40:13–21. doi: 10.1002/(SICI)1097-0169(1998)40:1<13::AID-CM2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Efron N. Contact lens-induced changes in the anterior eye as observed in vivo with the confocal microscope. Prog Retin Eye Res. 2007;26:398–436. doi: 10.1016/j.preteyeres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Erie JC, McLaren JW, Patel SV. Confocal microscopy in ophthalmology. Am J Ophthalmol. 2009;148:639–646. doi: 10.1016/j.ajo.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Etheredge L, Kane BP, Hassell JR. The effect of growth factor signaling on keratocytes in vitro and its relationship to the phases of stromal wound repair. Invest Ophthalmol Vis Sci. 2009;50:3128–3136. doi: 10.1167/iovs.08-3077. [DOI] [PubMed] [Google Scholar]
- Farid M, Morishige N, Lam L, Wahlert A, Steinert RF, Jester JV. Detection of corneal fibrosis by imaging second harmonic-generated signals in rabbit corneas treated with mitomycin C after excimer laser surface ablation. Invest Ophthalmol Vis Sci. 2008;49:4377–4383. doi: 10.1167/iovs.08-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons TD, Fagerholm P, Harfstrand A, Schenholm M. Hyaluronic acid in the rabbit cornea after excimer laser superficial keratectomy. Invest Ophthalmol Vis Sci. 1992;33:3011–3016. [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta -induced keratocytemyofibroblast transdifferentiation. J Biol Chem. 2001;276:44173–44178. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghibaudo M, Trichet L, Le Digabel J, Richert A, Hersen P, Ladoux B. Substrate topography induces a crossover from 2D to 3D behavior in fibroblast migration. Biophys J. 2009;97:357–368. doi: 10.1016/j.bpj.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia RM, Castelletto V, Alcock SG, Hamley IW, Connon CJ. Bioactive films produced from self-assembling peptide amphiphiles as versatile substrates for tuning cell adhesion and tissue architecture in serum-free conditions. Journal of Materials Chemistry B. 2013;1:6157–6169. doi: 10.1039/c3tb21031f. [DOI] [PubMed] [Google Scholar]
- Gouveia RM, Castelletto V, Hamley IW, Connon CJ. New Self-Assembling Multifunctional Templates for the Biofabrication and Controlled Self-Release of Cultured Tissue. Tissue Eng Part A. 2015;21:1772–1784. doi: 10.1089/ten.tea.2014.0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido S, Tranquillo RT. A methodology for the systematic and quantitative study of cell contact guidance in oriented collagen gels. Correlation of fibroblast orientation and gel birefringence. J Cell Sci. 1993;105(Pt 2):317–331. doi: 10.1242/jcs.105.2.317. [DOI] [PubMed] [Google Scholar]
- Guillemette MD, Cui B, Roy E, Gauvin R, Giasson CJ, Esch MB, Carrier P, Deschambeault A, Dumoulin M, Toner M, Germain L, Veres T, Auger FA. Surface topography induces 3D self-orientation of cells and extracellular matrix resulting in improved tissue function. Integr Biol (Camb) 2009;1:196–204. doi: 10.1039/b820208g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Hutcheon AE, Melotti SA, Zieske JD, Trinkaus-Randall V, Ruberti JW. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2007;48:4050–4060. doi: 10.1167/iovs.06-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Giese G, Bille J. Second harmonic generation imaging of collagen fibrils in cornea and sclera. Opt Express. 2005;13:5791–5797. doi: 10.1364/opex.13.005791. [DOI] [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Petroll WM, Olsen DR, Cavanagh HD. Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF beta in the rabbit. Cornea. 1997;16:177–187. [PubMed] [Google Scholar]
- Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994;35:730–743. [PubMed] [Google Scholar]
- Jester JV, Brown D, Pappa A, Vasiliou V. Myofibroblast differentiation modulates keratocyte crystallin protein expression, concentration, and cellular light scattering. Invest Ophthalmol Vis Sci. 2012;53:770–778. doi: 10.1167/iovs.11-9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999a;40:1959–1967. [PubMed] [Google Scholar]
- Jester JV, Huang J, Petroll WM, Cavanagh HD. TGFbeta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFbeta, PDGF and integrin signaling. Exp Eye Res. 2002;75:645–657. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- Jester JV, Li HF, Petroll WM, Parker RD, Cavanagh HD, Carr GJ, Smith B, Maurer JK. Area and depth of surfactant-induced corneal injury correlates with cell death. Invest Ophthalmol Vis Sci. 1998;39:922–936. [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal transparency: evidence for 'corneal crystallins'. J Cell Sci. 1999b;112(Pt 5):613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Barry PA, Cavanagh HD. Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Invest Ophthalmol Vis Sci. 1995;36:809–819. [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999c;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Feng W, Essepian J, Cavanagh HD. Radial keratotomy. 1. The wound healing process and measurement of incisional gape in two animal models using in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 1992;33:3255–3270. [PubMed] [Google Scholar]
- Karamichos D, Funderburgh ML, Hutcheon AE, Zieske JD, Du Y, Wu J, Funderburgh JL. A role for topographic cues in the organization of collagenous matrix by corneal fibroblasts and stem cells. PLoS One. 2014;9:e86260. doi: 10.1371/journal.pone.0086260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Guo XQ, Hutcheon AEK, Zieske JD. Human Corneal Fibrosis: An In Vitro Model. Invest Ophthalmol Vis Sci. 2010;51:1382–1388. doi: 10.1167/iovs.09-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Lakshman N, Petroll WM. Regulation of corneal fibroblast morphology and collagen reorganization by extracellular matrix mechanical properties. Invest Ophthalmol Vis Sci. 2007;48:5030–5037. doi: 10.1167/iovs.07-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman SC, Kaufman HE. How has confocal microscopy helped us in refractive surgery? Curr Opin Ophthalmol. 2006;17:380–388. doi: 10.1097/01.icu.0000233959.73262.99. [DOI] [PubMed] [Google Scholar]
- Kim A, Lakshman N, Petroll WM. Quantitative Assessment of Local Collagen Matrix Remodeling in 3-D Culture: The Role of Rho Kinase. Exp Cell Res. 2006;312:3683–3692. doi: 10.1016/j.yexcr.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappe S, Stachs O, Zhivov A, Hovakimyan M, Guthoff R. Results of confocal microscopy examinations after collagen cross-linking with riboflavin and UVA light in patients with progressive keratoconus. Ophthalmologica. 2011;225:95–104. doi: 10.1159/000319465. [DOI] [PubMed] [Google Scholar]
- Labbe A, Khammari C, Dupas B, Gabison E, Brasnu E, Labetoulle M, Baudouin C. Contribution of in vivo confocal microscopy to the diagnosis and management of infectious keratitis. Ocul Surf. 2009;7:41–52. doi: 10.1016/s1542-0124(12)70291-4. [DOI] [PubMed] [Google Scholar]
- Latvala T, Tervo K, Mustonen R, Tervo T. Expression of cellular fibronectin and tenascin in the rabbit cornea after excimer laser photorefractive keratectomy: a 12 month study. The British Journal of Ophthalmology. 1995;79:65–69. doi: 10.1136/bjo.79.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci U S A. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer JK, Li HF, Petroll WM, Parker RD, Cavanagh HD, Jester JV. Confocal microscopic characterization of initial corneal changes of surfactant-induced eye irritation in the rabbit. Toxicol Appl Pharmacol. 1997;143:291–300. doi: 10.1006/taap.1996.8097. [DOI] [PubMed] [Google Scholar]
- Mencucci R, Marini M, Paladini I, Sarchielli E, Sgambati E, Menchini U, Vannelli GB. Effects of riboflavin/UVA corneal cross-linking on keratocytes and collagen fibres in human cornea. Clin Experiment Ophthalmol. 2010;38:49–56. doi: 10.1111/j.1442-9071.2010.02207.x. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrosio R, Jr., Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Mohan RR, Kim WJ, Wilson SE. Modulation of TNF-alpha-induced apoptosis in corneal fibroblasts by transcription factor NF-kappaB. Invest Ophthalmol Vis Sci. 2000;41:1327–1336. [PubMed] [Google Scholar]
- Møller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Corneal Haze Development After PRK Is Regulated by Volume of Stromal Tissue Removal. Cornea. 1998;17:627. doi: 10.1097/00003226-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Moller-Pedersen T, Li HF, Petroll WM, Cavanagh HD, Jester JV. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 1998;39:487–501. [PubMed] [Google Scholar]
- Morishige N, Petroll WM, Nishida T, Kenney MC, Jester JV. Noninvasive corneal stromal collagen imaging using two-photon-generated second-harmonic signals. J Cataract Refract Surg. 2006;32:1784–1791. doi: 10.1016/j.jcrs.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Ambrosio R, Jr., Hutcheon AE, Zieske JD, Wilson SE. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea. 2005;24:509–522. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
- Patel DV, McGhee CN. Quantitative analysis of in vivo confocal microscopy images: a review. Surv Ophthalmol. 2013;58:466–475. doi: 10.1016/j.survophthal.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Petridou S, Maltseva O, Spanakis S, Masur SK. TGF-beta receptor expression and smad2 localization are cell density dependent in fibroblasts. Invest Ophthalmol Vis Sci. 2000;41:89–95. [PubMed] [Google Scholar]
- Petroll W, Cavanagh H, Jester J. Confocal microscopy. Cornea. Elsevier, Inc; St. Louis: 2011. pp. 205–220. [Google Scholar]
- Petroll WM, Cavanagh HD, Jester JV. Clinical confocal microscopy. Curr Opin Ophthalmol. 1998;9:59–65. doi: 10.1097/00055735-199808000-00011. [DOI] [PubMed] [Google Scholar]
- Petroll WM, Kivanany PB, Hagenasr D, Graham EK. Corneal Fibroblast Migration Patterns During Intrastromal Wound Healing Correlate With ECM Structure and Alignment. Invest Ophthalmol Vis Sci. 2015;56:7352–7361. doi: 10.1167/iovs.15-17978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroll WM, Robertson DM. In Vivo Confocal Microscopy of the Cornea: New Developments in Image Acquisition, Reconstruction, and Analysis Using the HRT-Rostock Corneal Module. Ocul Surf. 2015;13:187–203. doi: 10.1016/j.jtos.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroll WM, Weaver M, Vaidya S, McCulley JP, Cavanagh HD. Quantitative 3-dimensional corneal imaging in vivo using a modified HRT-RCM confocal microscope. Cornea. 2013;32:e36–43. doi: 10.1097/ICO.0b013e31825ec44e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot SA, Liliensiek SJ, Myrna KE, Bentley E, Jester JV, Nealey PF, Murphy CJ. Nanoscale Topography–Induced Modulation of Fundamental Cell Behaviors of Rabbit Corneal Keratocytes, Fibroblasts, and Myofibroblasts. Invest Ophthalmol Vis Sci. 2010;51:1373–1381. doi: 10.1167/iovs.09-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawe IM, Zabel RW, Tuft SJ, Chen V, Meek KM. A morphological study of rabbit corneas after laser keratectomy. Eye. 1992;6:637–642. doi: 10.1038/eye.1992.137. [DOI] [PubMed] [Google Scholar]
- Ren R, Hutcheon AE, Guo XQ, Saeidi N, Melotti SA, Ruberti JW, Zieske JD, Trinkaus-Randall V. Human primary corneal fibroblasts synthesize and deposit proteoglycans in long-term 3-D cultures. Dev Dyn. 2008;237:2705–2715. doi: 10.1002/dvdy.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeidi N, Guo X, Hutcheon AE, Sander EA, Bale SS, Melotti SA, Zieske JD, Trinkaus-Randall V, Ruberti JW. Disorganized collagen scaffold interferes with fibroblast mediated deposition of organized extracellular matrix in vitro. Biotechnol Bioeng. 2012;109:2683–2698. doi: 10.1002/bit.24533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaguru M, Durgam S, Ambekar R, Luedtke D, Fried G, Stewart A, Toussaint KC. Quantitative analysis of collagen fiber organization in injured tendons using Fourier transform-second harmonic generation imaging. Optics Express. 2010;18:24983–24993. doi: 10.1364/OE.18.024983. [DOI] [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J Cell Sci. 2003;116:1881–1892. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira AI, Nealey PF, Murphy CJ. Responses of human keratocytes to micro- and nanostructured substrates. J Biomed Mater Res A. 2004;71:369–376. doi: 10.1002/jbm.a.30089. [DOI] [PubMed] [Google Scholar]
- Tervo T, Moilanen J. In vivo confocal microscopy for evaluation of wound healing following corneal refractive surgery. Prog Retin Eye Res. 2003;22:339–358. doi: 10.1016/s1350-9462(02)00064-2. [DOI] [PubMed] [Google Scholar]
- Torricelli AA, Santhanam A, Wu J, Singh V, Wilson SE. The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res. 2016;142:110–118. doi: 10.1016/j.exer.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Agrawal V, Santhiago MR, Wilson SE. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest Ophthalmol Vis Sci. 2013;54:4026–4033. doi: 10.1167/iovs.13-12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani E, Baudouin C, Efron N, Hamrah P, Kojima T, Patel SV, Pflugfelder SC, Zhivov A, Dogru M. In Vivo Confocal Microscopy of the Ocular Surface: From Bench to Bedside. Current eye research. 2014;39:213–231. doi: 10.3109/02713683.2013.842592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Jia F, Gilbert TW, Woo SL. Cell orientation determines the alignment of cell-produced collagenous matrix. J Biomech. 2003;36:97–102. doi: 10.1016/s0021-9290(02)00233-6. [DOI] [PubMed] [Google Scholar]
- Wang N, Tolic-Norrelykke IM, Chen J, Mijailovich SM, Butler JP, Fredberg JJ, Stamenovic D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol. 2002;282:C606–616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- Wilson SE. Analysis of the keratocyte apoptosis, keratocyte proliferation, and myofibroblast transformation responses after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2002;100:411–433. [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 1999;18:293–309. doi: 10.1016/s1350-9462(98)00017-2. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Mohan RR, Ambrosio R, Jr., Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20:625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E, Wilsch M, Seiler T. Keratocyte apoptosis after corneal collagen cross-linking using riboflavin/UVA treatment. Cornea. 2004;23:43–49. doi: 10.1097/00003226-200401000-00008. [DOI] [PubMed] [Google Scholar]
- Young RD, Knupp C, Pinali C, Png KMY, Ralphs JR, Bushby AJ, Starborg T, Kadler KE, Quantock AJ. Three-dimensional aspects of matrix assembly by cells in the developing cornea. Proc Natl Acad Sci U S A. 2014;111:687–692. doi: 10.1073/pnas.1313561110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivov A, Guthoff RF, Stachs O. In vivo confocal microscopy of the ocular surface: from bench to bedside and back again. Br J Ophthalmol. 2010;94:1557–1558. doi: 10.1136/bjo.2010.187906. [DOI] [PubMed] [Google Scholar]
- Zhivov A, Stachs O, Kraak R, Stave J, Guthoff RF. In vivo confocal microscopy of the ocular surface. Ocul Surf. 2006;4:81–93. doi: 10.1016/s1542-0124(12)70030-7. [DOI] [PubMed] [Google Scholar]
- Zhivov A, Stachs O, Stave J, Guthoff RF. In vivo three-dimensional confocal laser scanning microscopy of corneal surface and epithelium. Br J Ophthalmol. 2009;93:667–672. doi: 10.1136/bjo.2008.137430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.