Abstract
Bone marrow (BM) failure syndrome encompasses a group of disorders characterized by BM stem cell dysfunction, resulting in varying degrees of hypoplasia and blood pancytopenia, and in many patients is autoimmune and inflammatory in nature. The important role of T helper 1 (Th1) polarized CD4+ T cells in driving BM failure has been clearly established in several models. However, animal model data demonstrating a functional role for CD8+ T cells in BM dysfunction is largely lacking and our objective was to test the hypothesis that CD8+ T cells play a non-redundant role in driving BM failure. Clinical evidence implicates a detrimental role for CD8+ T cells in BM failure and a beneficial role for Foxp3+ regulatory T cells (Tregs) in maintaining immune tolerance in the BM. We demonstrate that IL-2-deficient mice, which have a deficit in functional Tregs, develop spontaneous BM failure. Furthermore, we demonstrate a critical role for CD8+ T cells in the development of BM failure, which is dependent on the cytokine, IFNγ. CD8+ T cells promote hematopoietic stem cell dysfunction and depletion of myeloid lineage progenitor cells, resulting in anemia. Adoptive transfer experiments demonstrate that CD8+ T cells dramatically expedite disease progression and promote CD4+ T cell accumulation in the BM. Thus, BM dysregulation in IL-2-deficient mice is mediated by a Th1 and IFNγ-producing CD8+ T cell (Tc1) response.
Keywords: Interleukin-2, Bone marrow failure, Autoimmune, T cells, Interferon gamma, Hematopoietic stem cell
1. Introduction
Development and differentiation of hematopoietic stem cells (HSC) into hematopoietic progenitors follows a tightly regulated pattern of lineage-specific transcription factor expression resulting in commitment to defined lineages. Regulation of this developmental program, controlled largely by growth factors and cytokines produced by the bone marrow (BM) local stromal cells, results in HSC differentiation into multiple, progressively restricted lineages from multipotent, self-renewing HSCs to committed progenitors [1]. This dynamic developmental pathway is thought to be influenced by systemic inflammation and peripheral cells entering the bone [2]. Although there has been extensive study of hematopoiesis under normal homeostatic conditions, much less is known about the contribution of inflammation and effector T cells that infiltrate the BM during abnormal immune activation.
Foxp3+ regulatory T cells (Tregs) have been implicated in maintaining tolerance to the BM niche both in allogeneic lymphocyte transfer models and in spontaneously arising BM failure in Foxp3 mutant Scurfy mice [3-5]. Both clinical and model data implicate a role for CD4+Foxp3+ Tregs in maintaining immune tolerance in the BM, considered to be an “immune privileged site” [3, 6]. Upon loss of tolerance in the BM, a T helper 1 (Th1) immune mediated CD4+ response has been demonstrated to be critical in the development of BM alterations [7-9]. However, CD8+ T cells are also speculated to be important in disease pathology based largely on clinical correlative studies showing CD8+ T cell expansion that mirrors disease severity [10-12].
IL-2−/− mice have a documented deficit in Treg survival and function [13, 14]. IL-2−/− mice also develop severe anemia [15-18]. On the BALB/c background, disease is rapid with all mice dying between 19 and 35 days of age due to complications associated with anemia [18]. Anemia has been largely attributed to the generation of red blood cell (RBC) directed antibodies, resulting in destruction of mature RBCs [17, 18]. We propose that the rapid death observed in BALB/c IL-2−/− mice is a result of both autoimmune hemolytic anemia and a lack of new RBC production in the BM due to abnormal HSC function and altered lineage commitment. We further propose that the changes to the BM are induced by infiltrating effector T cells.
The IL-2−/− model has allowed us to study the specific T cells infiltrating the BM and initiating alterations to hematopoiesis. Here we show that in IL-2−/− mice BM dysregulation is largely mediated by CD4+ T cells, without which anemia and BM alterations do not develop. However, IFNγ-production by CD8+ Tc1 cells critically influences disease severity. Through both depletion and adoptive transfer strategies, we demonstrate that despite being unable to cause disease on their own, CD8+ T cells amplify the changes in hematopoiesis. Our data provide evidence that dysregulated hematopoiesis resulting in BM failure is largely mediated by CD8+ Tc1 cells that enter and expand within the BM during systemic autoimmune disease in IL-2−/− mice. Thus, this IL-2−/− model demonstrates a functional role for CD8+ T cells in HSC dysregulation, and provides a rapid autoimmune model to study the mechanisms of HSC-mediated disease ontogeny and evaluate therapeutic interventions in multiple BM diseases.
2. Materials and Methods
2.1. Mice
All mice used in this study were on the BALB/c background. BALB/cJ mice (stock #000651) and CD45.1+ mice (stock #006584), were purchased from Jackson Laboratory. These were interbred to generate CD45.1+CD45.2+ F1 mice. Use of IL-2−/−, IL-2−/−IFNγ−/−, Rag−/−, and TCRα−/− were previously described [16, 17]. Littermates were used as controls in all experiments and both male and female mice were used, unless otherwise indicated. IL-2WT and IFNγWT groups were a mixture of +/+ and +/− genotypes as no hemizygosity effect is observed. TCRα−/− and Rag−/− mice used as recipients in adoptive transfer experiments were 5-9 weeks old at the time of transfer. Mice were housed in specific pathogen-free sterile microisolator cages. Mice were euthanized by CO2 asphyxiation followed by cervical dislocation. All mouse experiments were approved by the UC Merced Institutional Animal Care and Use Committee.
2.2. Cell staining and flow cytometry
For BM samples, both femurs were extracted and flushed with PBS + 2% FBS using a syringe and 27g needle. For analysis of stem cells and lymphocytes, RBCs were lysed prior to staining. RBCs were not lysed for experiments examining TER119+ RBC progenitor populations. Cellular viability was assessed by DAPI (Sigma-Aldrich) exclusion or with the Fixable Viability Dye eFluor780 (eBioscience). Dead cells were gated out from analysis. Lineage negative (Lin−) cells in the BM were gated as CD3−CD4−CD8−TER119−CD11b−Gr1−B220−. For all surface staining, except BM progenitor stains, Fc receptors were blocked using cell supernatants containing the CD16/32 monoclonal antibody (clone 2.4G2, ATCC). All staining and wash steps were performed in PBS + 2% FBS.
For T cell analysis in the BM, femurs and tibiae of IL-2WT or IL-2−/− mice at 12, 16 and 19 days of age were harvested and crushed using mortar and pestle. BM was collected in PBS + 2% FBS and RBCs were lysed prior to staining. All T cell analysis was performed on TCRβ+-gated events. For proliferation staining, 2×l06 cells were Fc blocked and surface stained for 30 minutes before washing in PBS+ 2% FBS. Cells were fixed and permeabilized with FoxP3 Staining Buffer Set (Cat # 005523, eBiosciences) following the manufacturer's instructions. Briefly, cells were incubated in l× Fix/Perm solution for 30 minutes, washed with l× Perm buffer, and then resuspended with anti-Ki-67 in l× Perm buffer for 45 minutes. Cells were washed and resuspended in 1.0 μg/ml DAPI. Cells were collected with flow rate of <500 events/sec. Flow cytometric analysis was performed on an LSR2 flow cytometer (BD Biosciences).
The following antibodies and staining reagents were purchased from eBioscience: B220-biotin (RA3-6B2), c-Kit-PE (2B8), CD3-biotin (145-2C11), CD3-PerCPCy5.5 (145-2C11), CD4-biotin (GK1.5), CD4-FITC (GK1.5), CD4-FITC (RM4-4), CD4-PerCPCy5.5 (RM4-5), CD8α-biotin (53-6.7), CD8α-APC (53-6.7), CD8β-APC (H35-17.2), CD11b-biotin (Ml/70), CD11b-APC (Ml/70), CD16/32-PerCPeFluor710 (93), CD19-PEeFluor610 (1D3), CD34-FITC (RAM34), CD45.1-APCeFluor780 (A20), CD45.2-PE (104), CD45.2-PerCPCy5.5 (104), CD48-APCeFluor780 (HM48-1), CD71-PE (R17217), CD150-FITC (mShad150), Gr1-biotin (RB6-8C5), Gr1-AlexaFluor488 (RB6-8C5), IFNγ-APC (XMG1.2), Ki-67-PECy7 (SolA15), Scal-APC (D7), Streptavidin-PECy7, Streptavidin-FITC, TCRβ-PE (H57-597), TER119-biotin (TER-119), TER119-APC (TER-119). The antibody B220-BrilliantUV395 (RA3-6B2) was purchased from BD Biosciences and CD44-FITC (IM7), CD62L-PE (MEL-14), and TCRγδ-biotin (GL3) were purchased from Biolegend.
2.3. Competitive reconstitution assays
IL-2−/− or IL-2WT littermate control BM was harvested from femurs and tibiae at 18-21 days of age by crushing bones using a mortar and pestle. Aged matched CD45.1+CD45.2+ heterozygous competitor BM was prepared at the same time. BM was stained with biotin-conjugated lineage antibodies. Lineage depletion via negative selection was performed using the EasySep Mouse Biotin Selection Kit according to the manufacturer's instructions (Stem Cell Technologies). The Lin− fraction was then stained with c-kit, Sca1, CD150, and CD48 antibodies. The Lin−Sca1+c-Kit+ (LSK) population of cells was further evaluated for CD150 and CD48 staining and the LSK CD150+CD48− HSCs were FACS sorted on an Aria II cell sorter (BD Biosciences). A 1:1 ratio of IL-2−/− or IL-2WT to competitor cells was generated and 400 total HSCs were transferred into lethally irradiated Rag−/−CD45.2+ recipient mice. Recipient mice were lethally irradiated with 1000 Rads delivered in 2 × 500 Rad split dose separated by 4 hours and cells were transferred 4 hours after the final dose of radiation. CD45.2+ (IL-2−/− or IL-2WT) and CD45.1+CD45.2+ (competitor) cells were identified by flow cytometry to indicate their origin. Five weeks after transfer mice were euthanized and peripheral blood, spleen, and BM was collected. RBCs were lysed prior to staining with CD45.1, CD45.2, and lineage-specific antibodies.
2.4. Adoptive T cell transfer
Donor mice were euthanized and lymph nodes isolated. A single cell suspension was generated over a wire mesh. Cells were Fc blocked and stained with CD4-PE, CD8α-APC, B220-PE-Cy7, and CD11c-PE-Cy7. PE-Cy7 labeled cells were excluded and CD4+ and CD8+ cells were each sorted to greater than 98.5% purity on a FACS Aria II cell sorter (BD Biosciences). 5×106 cells of each of the indicated cell type were injected into TCRα−/− or Rag−/− recipients via tail vein injection. The sex of recipient was matched to that of the donor cells when possible. Experimental groups were randomized across cages, such that each cage of recipient mice possessed at least one mouse from each experimental group. Every 2 weeks 100μl of blood was obtained via submandibular bleeding and complete blood counts were determined using a Hemavet 950 veterinary hematology system (Drew Scientific). Mice were euthanized at 7 weeks post-transfer, and spleen and BM analyzed.
2.5. T cell depletion
IL-2−/− or IL-2WT littermate control mice were treated with intraperitoneal injection of anti-CD4 (GK1.5), anti-CD8α (2.43), or PBS. Depleting antibodies were given at a dose of 20 μg/g body weight and were acquired from the UCSF Monoclonal Antibody Core. Treatment was given 3 times per week from day 8-9 until day 16. Spleen and both femurs were harvested and prepared at day 19 as above. To assess depletion efficiency, staining was performed with CD8β-APC or CD4-FITC (clone RM4-4).
3. Statistical analysis
Statistical difference between experimental groups was determined using an unpaired, two-tailed student's t test (GraphPad Prism Software). Bar graphs represent means with error bars indicating standard deviation.
4. Results
4.1. IL-2−/− mice develop HSC dysregulation and anemia
Autoimmune hemolytic anemia has been previously described in IL-2−/− mice on the BALB/c background [16-18]. Mice develop autoantibodies against RBCs, followed by reduced hematocrit and rapid death around three weeks of age. Previously, expansion of HSCs, but a reduction in their functional reconstituting ability was reported in IL-2−/− mice on the C57BL/6 background [19]. These mice develop a less severe and delayed anemia compared to IL-2−/− mice on the BALB/c background [18].
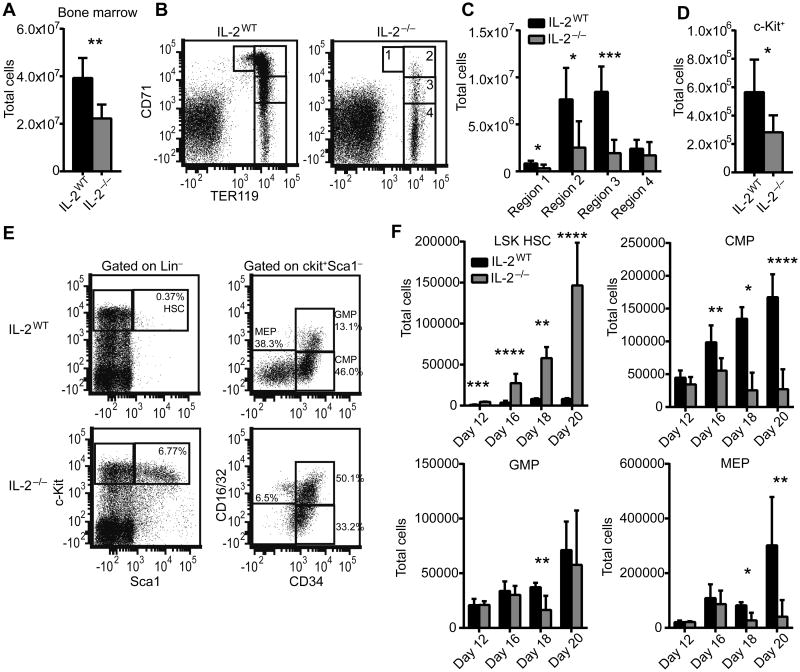
We aimed to evaluate the BM of IL-2−/− mice on the BALB/c background to determine if they suffer from the same hematopoietic failure that is evident on the C57BL/6 background. Furthermore, we aimed to characterize the cellular and molecular underpinnings of this disease. Total BM cellularity is significantly reduced in IL-2−/− mice beginning at 18 days of age and increases in severity until death at about 20 days of age (Figure 1A and not shown). In order to determine if RBC progenitors in the BM were reduced, we stained for TER119 and CD71, two markers that allow discrimination of developmentally distinct RBC progenitor populations [20]. The most immature progenitors express intermediate levels of TER119 and high levels of CD71 and progressive progenitor populations downregulate CD71 as they mature. We observed that in several mice there was a near complete absence of early RBC progenitors in the BM expressing high CD71 levels (regions 1 and 2) and overall there was a significant reduction in RBC progenitors in regions 1-3 (Figure 1B-C). However, the most mature RBC population, contained in region 4, was not numerically affected, indicating a depletion of progenitor cells rather than mature RBCs in the BM. Indeed, total c-kit+ cells in the BM, which contain HSCs and other multipotent progenitors, were depleted in IL-2−/− mice (Figure 1D). However, analysis of the HSC enriched Lin−Sca1+c-Kit+ (LSK) population showed a dramatic increase in IL-2−/− mice that amplified over time, while the common myeloid progenitor (CMP) and megakaryocyte/erythrocyte progenitor (MEP), populations upstream of RBCs, showed kinetically similar reductions (Figure 1E-F). The granulocyte/monocyte progenitor (GMP) population was less affected than progenitors of the RBC lineage (Figure 1E-F). CMP and MEP populations dramatically decreased by day 20, consistent with the lack of more mature RBC progenitors observed at that time. These results suggest a defect in differentiation toward RBCs starting with deficiency in the CMP population that can be seen as early as day 16.
Figure 1. IL-2−/− mice develop bone marrow failure and HSC dysregulation.
Total BM was isolated from 20 day old mice femurs and counted to determine total cellularity (A) and stained for TER119 and CD71 to identify red blood cell developmental stages (B-C). Regions 1-4 correlate with progressive stages of RBC differentiation with region 1 and 4 comprising the least and most mature RBCs, respectively. RBC-lysed BM was analyzed by flow cytometry for the total number of Lin−c-kit+ cells (D). BM was analyzed for the frequency and total number of Lin−Sca1+c-kit+ (LSK) HSCs and Lin−c-Kit+Sca1−CD34+CD16/32− CMPs, Lin−c-Kit+Sca1−CD34+CD16/32+ GMPs, and Lin−c-Kit+Sca1−CD34−CD16/32− MEPs from 12, 16, 18, and 20 day old mice (E-F). Flow plot shows representative data from 20 day old mice (E). (A-E) Data are from at least 2 independent experiments with n=6-10 mice per group. (F) Data are from 1-2 experiments with n=2-8 mice per group. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 based on students t test.
4.2. IL-2−/− HSCs have reduced quiescence and have a competitive disadvantage
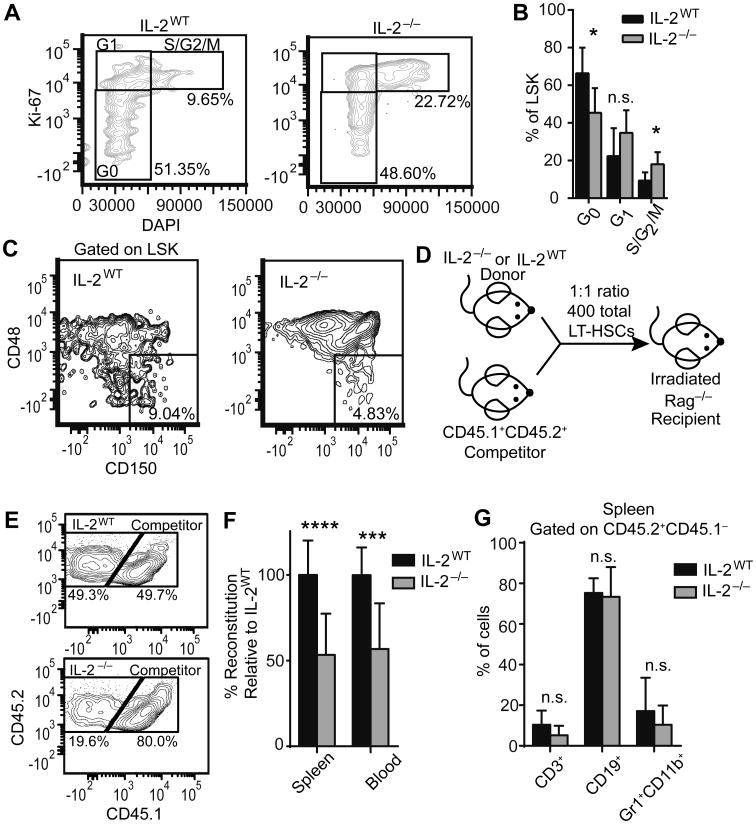
Despite the expansion of phenotypically defined HSCs, we suspected that these cells may be functionally deficient, as has been described for HSCs in other inflammatory settings [2]. To determine the proliferative and functional properties of the HSCs in IL-2−/− mice, we first stained with Ki-67 and the DNA dye DAPI (Figure 2A). Significantly fewer IL-2−/− HSCs were within the G0 stage of cell cycle, while a larger portion was in the S/G2/M stage, indicating IL-2−/− HSCs were less quiescent (Figure 2B). To further evaluate the HSCs we stained with CD150 and CD48, markers used to define long-term, quiescent HSCs (LT-HSCs) [21], and determined that there was indeed a reduction in the frequency of LSK CD150+CD48− LT-HSCs in IL-2−/− mice (Figure 2C). As quiescence is critical to the maintenance of the stem cell compartment and the avoidance of stem cell exhaustion [22], we performed a competitive reconstitution assay to assess in vivo reconstitution potential (Figure 2D). Using a 1:1 ratio of FACS sorted IL-2−/− or IL-2WT LT-HSCs to congenically marked competitor LT-HSCs, we observed an impairment of IL-2−/− BM engraftment. In the peripheral blood at 5 weeks post-transfer there was a decrease in the proportion of CD45+ cells derived from IL-2−/− donors relative to IL-2WT donors (Figure 2E-F). Despite the fact that IL-2 signaling is known to be involved in lymphoid versus myeloid cell fate decisions in vitro [23], both myeloid and lymphoid populations were represented normally among IL-2−/− cell progeny relative to IL-2WT (Figure 2G). Overall these data indicate that IL-2−/− HSCs have a functional defect, which could explain the severe anemia that develops in these mice.
Figure 2. IL-2−/− HSCs have increased proliferation and a competitive reconstitution disadvantage.
HSCs from 19-21 day old IL-2WT or IL-2−/− mice were analyzed (A-C). Representative flow plot of RBC-lysed BM stained for DAPI and Ki-67, gated on LSK HSCs, to determine proliferation stages (A). Frequencies of LSK HSCs in G0, G1, and S/G2/M stages of cell cycle (B). Representative plot of BM stained for long-term HSCs (LT-HSCs) defined as LSK CD150+CD48− (C). Schematic of LT-HSC competitive reconstitution strategy (D). LT-HSCs were FACS sorted from day 18-21 IL-2WT or IL-2−/− BM (CD45.2+CD45.1−) and age-matched CD45.2+CD45.1+ competitors. Cells were mixed at a 1:1 ratio with competitors and 400 total cells were injected via tail vein into lethally irradiated Rag−/− recipient mice. Mice were harvested at 5 weeks post-transfer to assess competitive reconstitution in peripheral blood and spleen. Representative flow plots of competitive hematopoietic reconstitution from IL-2WT LT-HSCs (E; upper) and IL-2−/− LT-HSCs (E; lower) in peripheral blood. Percentage reconstitution relative to IL-2WT LT-HSCs (F). Percentage of CD3+, CD19+ and GR1+CD11b+ cells within the CD45.2+CD45.1− fraction (G). (A-C) Data are from 2 independent experiments with n=7-10 mice per group. (D-G) Data are from 3 independent experiments with IL-2WT n=10; IL-2−/− n=12. n.s. - not significant; * p < 0.05; *** p < 0.001; **** p < 0.0001 based on students t test.
4.3. CD8+ T cells increase proliferation in IL-2−/− BM
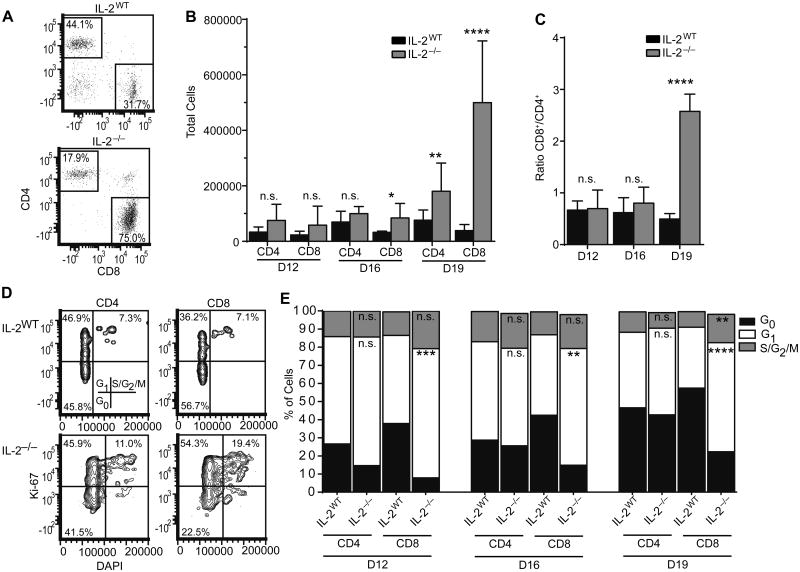
In one form of BM failure, aplastic anemia, CD8+ T cells are known to expand in the BM of patients, and the ratio of CD8+to CD4+ cells is altered [10]. Likewise, in the BM of IL-2−/− mice we observed an increase in CD8+ T cell numbers beginning at day 16, which became a profound increase by day 19, when mice begin to succumb to disease (Figure 3A-B) [18]. CD8+ cells expanded more than CD4+ cells, as indicated by a skewed CD8/CD4 cell ratio (Figure 3B-C). IL-2−/− CD8+ cells were more proliferative in the BM environment relative to IL-2WT mice beginning by day 12, as assessed by Ki-67 and DAPI co-staining (Figure 3D-E). A higher frequency of CD8+ cells were in the G1 and S/G2/M stages of the cell cycle, while CD4+ were present in increased numbers, but the frequency of cycling cells was not elevated relative to IL-2WT mice.
Figure 3. CD8+ T cells proliferate in IL-2−/− bone marrow.
Representative flow plots gated on live TCRβ+ events of day 19 RBC-lysed BM (A) and total T cell numbers from the indicated time points (B). (C) CD8+/CD4+ ratio of cells gated as in (A). (D) Representative flow plots of Ki-67 versus DAPI staining from 19 day old bone marrow. (E) Frequencies of cells in the indicated stages of cell cycle based on the staining in (D). Significance in all plots is relative to the corresponding IL-2WT group at the same time point. IL-2WT n=6-10; IL-2−/− n=5-10. Data are from 2-4 independent experiments. n.s. - not significant; * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 based on students t test.
4.4. Depletion of CD4+ and CD8+ T cells delays BM alterations in IL-2−/− mice
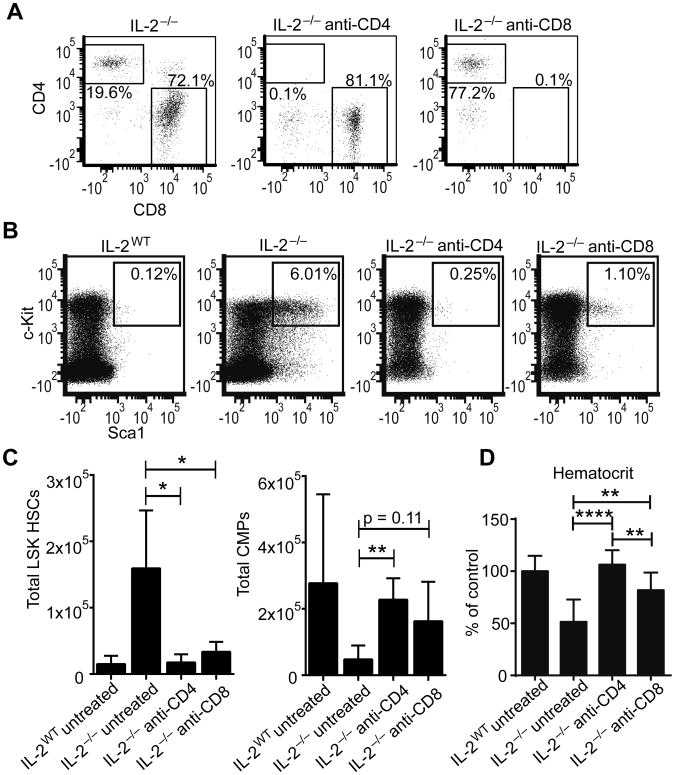
We next determined the contribution of both CD4+ and CD8+ T cells to BM dysregulation in IL-2−/− mice. First, we performed depletion studies to determine the necessity of both CD4+ and CD8+ T cells for disease development. As an increase in CD4+ and CD8+ T cell activation can be measured by 9 days of age in IL-2−/− mice based on CD44 and CD62L expression (data not shown), we performed antibody mediated depletion beginning at that point. Both CD4 and CD8 depletion was highly efficient and depletion was well maintained in the bone marrow until harvest at 19 days of age (Figure 4A). Depletion of either CD4+ or CD8+ cells largely rescued IL-2−/− mice from the HSC hyperplasia seen in untreated mice (Figure 4B-C). However, the restoration of the BM homeostasis was more complete with CD4 depletion, consistent with clinical and animal model data showing the critical role of CD4+ T cells. Likewise, CMP numbers were partially restored in both CD4 and CD8 depleted mice (Figure 4C). To further confirm a rescue of the BM failure phenotype, hematocrit was completely restored in the anti-CD4 treated IL-2−/− mice, and also significantly improved in the anti-CD8 treated IL-2−/− mice (Figure 4D). These results suggested that while CD4 T cells are pivotal for BM failure in IL-2−/− mice, CD8 T cells also play a non-redundant role in the process.
Figure 4. CD4+ and CD8+ cells contribute to bone marrow failure in IL-2−/− mice.
Mice were treated with depleting antibodies to CD4 and CD8α from 8 to 16 days of age and euthanized at day 19. Representative flow plots of bone marrow stained for CD4 and CD8 to determine peripheral depletion efficacy, gated on live CD3+TCRβ+ events (A). Representative flow plots of BM LSK HSCs gated on live Lin− cells (B). Total LSK HSCs and CMPs from BM (C). Hematocrit relative to untreated IL-2WT mice at 19 days (D). Data are from 3 independent experiments with 3-5 mice per group. * p < 0.05; ** p < 0.01 based on students t test.
4.5. CD8+ T cells are required for severe BM alterations
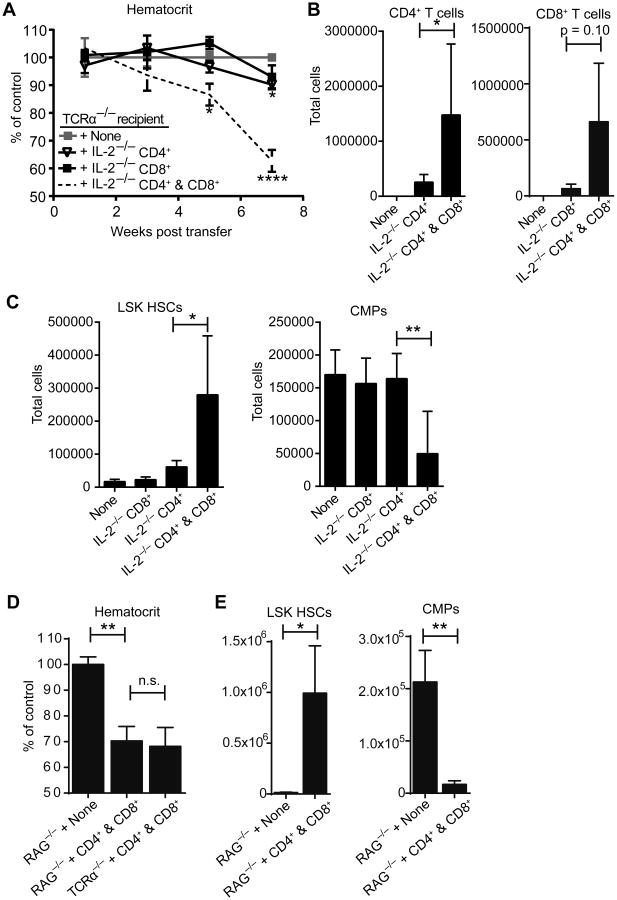
We employed an adoptive transfer model to determine if FACS-sorted IL-2−/− CD4+ or CD8+ T cells alone are capable of transferring HSC defects and anemia to T cell deficient TCRα−/− recipient mice. We performed complete blood counts and quantified hematocrit relative to control mice. IL-2−/− CD4+ T cells alone were capable of causing only mild anemia by 7 weeks post transfer (Figure 5A), which persisted out to at least 21 weeks post transfer (data not shown). In contrast, IL-2−/− CD8+ T cells alone were incapable of causing anemia during the same timeframe. However, the combination of IL-2−/− CD4+ and IL-2−/− CD8+ cells resulted in rapid and pronounced anemia (Figure 5A). CD4+ cells accumulated in great numbers in the BM only when CD8+ cells were co-transferred, indicating CD8+ help is essential for their entry or expansion at that site (Figure 5B). Likewise, CD8+ T cell expansion was dependent on the co-transfer of CD4+ T cells. Also, the characteristic HSC hyperplasia and reduction in CMPs was virtually absent when CD4+ cells were transferred alone, but evident in the double transfer of CD4+ and CD8+ cells (Figure 5C). These findings complement the antibody-mediated depletion experiments and together they demonstrate that both CD4+ and CD8+ cells are critical for the rapid onset of BM changes in IL-2−/− mice. CD4+ cells alone are sufficient to drive a mild form of the disease, but CD8+ cells greatly promote CD4+ cell accumulation in the BM and rapid disease onset.
Figure 5. Transferred IL-2−/− CD8+ cells dramatically exacerbate bone marrow failure independent of antibody response.
TCRα−/− recipient mice were injected with 5×106 FACS sorted IL-2−/− CD4+ cells, CD8+ cells, or a 1:1 mixture of both. Hematocrit from peripheral blood samples was calculated as percentage relative to hematocrit from uninjected littermate controls (A). Significance is indicated relative to uninjected littermate control mice at indicated time points. At 7 weeks post-transfer mice were euthanized and total cell numbers from RBC-lysed BM determined by flow cytometry (B-C). (D) TCRα−/− and RAG−/− recipient mice were injected with 5×106 FACS sorted IL-2−/− CD4+ cells and an equal number of IL-2−/− CD8+ cells and analyzed 7 weeks post-transfer. Relative hematocrit was calculated as in (A). (E) Total LSK HSCs and CMPs were determined from RBC-lysed BM. Data are from 4 independent experiments with 6-16 mice per group (A) and 2 independent experiments with 3-6 mice per group (B-E). n.s. - not significant; * p < 0.05; ** p < 0.01 based on students t test.
As TCRα−/− recipients have functional B cells, we next tested whether the BM disease in the T cell transferred mice is dependent on antibodies by transferring IL-2−/− CD4+ and IL-2−/− CD8+ cells into Rag−/− recipients. Comparably to TCRα−/− recipients, Rag−/− recipients had a reduction in hematocrit at 7 weeks (Figure 5D). They also exhibited an expansion of LSK HSCs and a reduction in CMPs comparable to TCRα−/− recipients (Figure 5E), indicating that BM alterations and anemia due to T cell transfer are antibody independent.
4.6. BM alteration in IL-2−/− mice is dependent on IFNγ
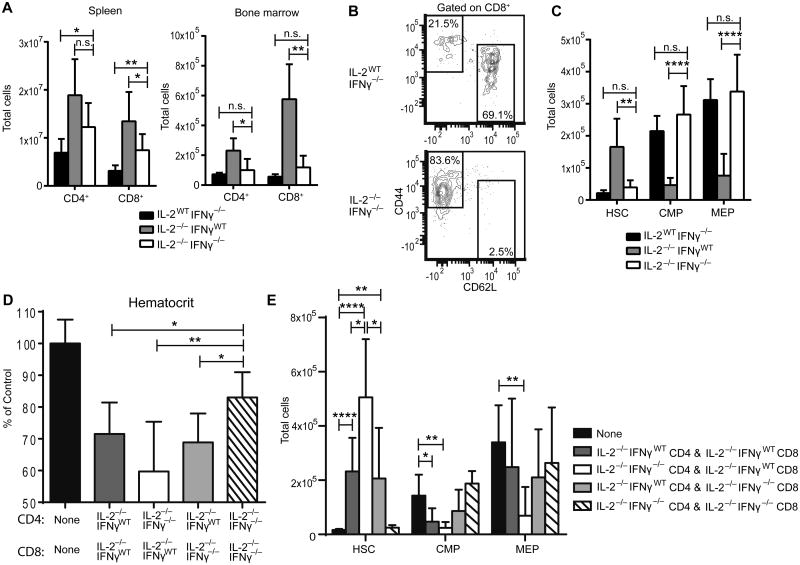
To address whether IFNγ directly plays a role in the BM changes observed in IL-2−/− mice we utilized IL-2−/−IFNγ−/− mice and evaluated their BM progenitor populations. As with IL-2−/− mice, IL-2−/−IFNγ−/− mice have a significant expansion of CD4+ and CD8+ T cells in the spleen at 3 weeks of age (Figure 6A). However, they do not have a similar expansion in the BM (Figure 6A). This lack of expansion is in spite of elevated CD8+ T cell activation in the BM, as measured by CD62L and CD44 expression (Figure 6B). Strikingly, IL-2−/−IFNγ−/− mice did not display HSC expansion, or contraction of downstream progenitor populations (Figure 6C). These data strongly suggest that BM defects in IL-2−/− mice are dependent upon the Th1/Tc1 cytokine, IFNγ.
Figure 6. IFNγ from either CD4 or CD8 T cells is required for bone marrow failure in IL-2−/− mice.
Total CD4+ and CD8+ cell numbers from 19-22 day old mice gated on live cells (A). Representative flow plot of CD44 and CD62L expression gated on BM CD8+ cells (B). Total BM LSK HSC, CMP, and MEP cell numbers from indicated mice (C). (D-E) CD4+ and CD8+ T cells were sorted from 18-21 day old IL-2−/−IFNγWT mice and IL-2−/−IFNγ−/− mice. 5×106 FACS sorted CD4+ and CD8+ cells from the indicated groups were mixed at 1:1 ratio and injected into TCRα−/− recipient mice. Hematocrit was monitored every other week by obtaining peripheral blood. At 7 weeks, mice were harvested. CBC was determined relative to uninjected TCRα−/− mice (D). BM was collected and stained for LSK HSCs, CMPs, and MEPs (E). (A-C) Data are from 4 independent experiments; IL-2WTIFNγ−/− n=7; IL-2−/−IFNγWT n=8; IL-2−/−IFNγ−/− n=8. (DE) Data are from 3 independent experiments with n=6-10 mice per group. n.s. - not significant; * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 based on students t test.
To determine which T cell population is producing the disease-mediating IFNγ, we next performed an adoptive transfer crisscross experiment of CD4 and CD8 T cells from IL-2−/− and IL-2−/−IFNγ−/− mice. Anemia occurred in recipient mice that received IL-2−/−IFNγ−/− CD4+ T cells with IL-2−/−IFNγWT CD8+ T cells (Figure 6D). Anemia also occurred in recipient mice that received IL-2−/−IFNγWT CD4+ T cells with IL-2−/−IFNγ−/− CD8+ T cells. These results indicate that anemia occurs when either CD4+ or CD8+ T cells can produce IFNγ, but only one population is the required IFNγ source for disease onset. Analysis of the BM progenitor populations from this crisscross transfer yielded similar results. The most abnormal BM progenitor profile was seen when only the transferred CD8+ T cells were capable of producing IFNγ (Figure 6E, white bar). However, when at least one population of CD4+ or CD8+ T cells could produce IFNγ, the severe abnormalities in BM progenitors were evident (Figure 6E, white and grey bars). These results suggest that T cell-produced IFNγ is critical to severe disease development and both CD4+ and CD8+ T cells are a sufficient source, but it appears CD8+ T cells may be a more potent or relevant source.
5. Discussion
We have focused on the T cell populations and pathways that are critical in HSC dysfunction in Treg defective mice. While CD4+ T cells are clearly established in their central role of inducing autoimmune BM failure, our results here demonstrate that CD8+ T cells promote the ability of CD4+ T cells to enter or expand within the BM, thus severely exacerbating BM disease. CD8+ T cells, which have clinically been known to expand in the BM during BM failure, including aplastic anemia, BM transplant and myelodysplastic syndrome [10, 11, 24-26], are similarly highly expanded in IL-2−/− BM. While IL-2−/− CD4+ T cells alone are capable of transferring mild disease, onset of BM dysregulation and development of anemia is much more severe and rapid with the co-transfer of IL-2−/− CD8+ T cells. Both autoimmune hemolytic anemia and a reduction in RBC progenitors have now been described in IL-2−/− mice [16, 17, 19]. Indeed, the rapid onset and progression of anemia in BALB/c IL-2−/− mice may be reflective of both disease processes occurring simultaneously, which would affect both the peripheral supply of RBCs and their development in the BM.
Scurfy mice lack functional Tregs due to a defect in the Foxp3 gene and exhibit similar defects in HSC function that can be attributed to metabolic alterations in the mTOR signaling pathway in HSCs [4]. In agreement with our findings, both Scurfy and IL-2−/− mice on the C57BL/6 background display an early expansion of phenotypic HSCs [4, 19], but fail to competitively reconstitute the hematopoietic system. Despite their phenotypic presence, these cells are obviously not fully functional as HSCs. An increase in Sca1 expression on BM cells has been described under inflammatory conditions [27], and we cannot rule out that c-kit+ non-HSCs may be upregulating Sca1 to phenotypically resemble HSCs. Nevertheless, regardless of Sca1 expression, we see a decrease in the Lin−c-kit+ progenitor population in the BM and a paucity of downstream myeloid progenitor populations, confirming that BM-mediated failure and anemia induction is indeed occurring.
Given the plethora of clinical evidence showing that BM failure is a T cell-mediated disease and that BM infiltration is a hallmark observation of these diseases, our findings suggest that IFNγ production by the BM-infiltrating T cells drives BM failure disease. In IL-2−/− mice and in our adoptive transfer model, the IL-2−/− BM infiltrating T cells produce high levels of IFNγ (data not shown). Chronic IFNγ results in functional impairment of HSCs through increased apoptosis and altered proliferation, leading to anemia and BM failure [28, 29]. During infection or with addition of recombinant protein, IFNγ induces myeloid differentiation, expanded BM cellularity and HSC sensitivity to CD8 T cell-mediated apoptosis [30]. On the other hand, in the ARE-del mouse model that constitutively expresses IFNγ at low levels [31], aplastic anemia is similar to the IFNγ-dependent BM failure we describe in the IL2-/- mice. However, in the ARE-del mouse, T cell activation, expansion, and migration to the BM are not required for BM dysregulation. In contrast, our adoptive transfer data indicate that the presence of T cells is necessary for severe BM defects to develop. Even without T cell production of IFNγ we observe a minor, but consistent reduction in hematocrit, indicating that T cell factors other than IFNγ may contribute to anemia. Recent reports have concluded that expression of the chemokine receptor CXCR4 on T cells is critical for BM infiltration by T cells that ultimately exacerbate aplastic anemia in an allogeneic transfer model [32] and that activated CD8+ T cells can target LSK HSCs for destruction [29], which provide further support of our data showing the requirement for T cells in BM disease.
Our results support the idea that CD4+ Th1 and CD8+ Tc1 cells are required for the damage that occurs during BM dysfunction. Our transfer data further suggests that IFNγ from CD8+ T cells may play a more substantial role in induction of HSC dysregulation and subsequent anemia. Whether this is because Tc1 differentiated CTLs are needed for BM-targeted cytolytic activity in disease induction, or CD8+ T cells produce higher levels of IFNγ and amplify the disease process, or some other reason is currently unclear. Fas-mediated killing of HSCs by CTLs has been implicated in BM failure [33, 34]. However, IL-2 is also thought to be important for Fas-mediated activation induced cell death and CTL targeted apoptosis. IL-2−/− CD8+ T cells have reduced Fas-mediated CTL activity in response to viral infection [35, 36], suggesting that other CD8+ T cell lytic mechanisms, such as secretion of perforin and granzymes, may be contributing to BM dysfunction and failure in IL-2−/− mice. In support of this idea, we have observed elevated perforin and granzyme levels in IL-2−/− CD8+ T cells (data not shown). Furthermore, perhaps the larger impact of IL-2−/− CD8+ T cells on BM failure is due to their increased proliferation and presence in higher frequency and absolute numbers in the BM.
In conclusion, we have demonstrated an essential role for CD8+ T cells in driving severe BM dysfunction and have employed a model that physiologically recapitulates the spontaneous onset of many features of clinical BM failure. The IL-2−/− mouse is a highly rapid disease induction model, enhanced by CD8+ T cells. IL-2−/− mice survive on average less than 3 weeks of age, have 100% penetrance of BM defects, and the ability to transfer disease, and thus can be used to further dissect the T cell-mediated mechanisms underlying BM failure. Future studies using this mouse model will further elucidate the mechanisms by which CD8+ T cells exacerbate disease and provide a model with which to test new therapeutics, and define the underlying mechanisms of HSC differentiation defects in BM failure.
Highlights.
IL-2-deficient BALB/c mice develop rapid bone marrow failure.
T cells and IFNγ are critical for bone marrow failure induction in this model.
CD8 T cells enhance the severity and kinetics of bone marrow failure disease.
IFNγ production restricted to CD4 or CD8 T cells alone is sufficient to cause disease.
Acknowledgments
We would like to acknowledge the staff of the UC Merced Department of Animal Research Services and the staff of the UC Merced Stem Cell Instrumentation Foundry for their support and technical assistance. We thank Dr. Kirk Jensen and members of the Hoyer lab for helpful discussions and critical evaluation of the manuscript.
Nonstandard abbreviations
- BM
bone marrow
- CMP
common myeloid progenitor
- GMP
granulocyte/monocyte progenitor
- HSC
hematopoietic stem cell
- LSK
Lin−Sca1+c-Kit+
- MEP
megakaryocyte/erythrocyte progenitor
- Tc1
T cytotoxic 1
- Th1
T helper 1
- Tregs
regulatory T cells
Footnotes
This work was supported by the National Institutes of Health grant R00HL090706, and by a UC Merced Health Sciences Research Institute Seed Grant.
Authorship: D.M.G. designed and performed experiments, analyzed data, generated figures, and wrote the manuscript; M.A.K designed, performed and analyzed experiments; D.D. and P.D.S. assisted with experiments; J.O.M. provided reagents, technical expertise, and edited the manuscript; K.K.H. designed experiments, supervised the research, and wrote the manuscript.
Conflict of Interest Disclosures: The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prohaska SS, Scherer DC, Weissman IL, Kondo M. Developmental plasticity of lymphoid progenitors. Seminars in immunology. 2002;14:377–84. doi: 10.1016/s1044532302000726. [DOI] [PubMed] [Google Scholar]
- 2.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11:685–92. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–9. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C, Liu Y, Zheng P. Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice. J Clin Invest. 2010;120:4091–101. doi: 10.1172/JCI43873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Ellison FM, Eckhaus MA, Smith AL, Keyvanfar K, Calado RT, et al. Minor antigen h60-mediated aplastic anemia is ameliorated by immunosuppression and the infusion of regulatory T cells. J Immunol. 2007;178:4159–68. doi: 10.4049/jimmunol.178.7.4159. [DOI] [PubMed] [Google Scholar]
- 6.Shi J, Ge M, Lu S, Li X, Shao Y, Huang J, et al. Intrinsic impairment of CD4(+)CD25(+) regulatory T cells in acquired aplastic anemia. Blood. 2012;120:1624–32. doi: 10.1182/blood-2011-11-390708. [DOI] [PubMed] [Google Scholar]
- 7.Tong Q, He S, Xie F, Mochizuki K, Liu Y, Mochizuki I, et al. Ezh2 Regulates Transcriptional and Posttranslational Expression of T-bet and Promotes Th1 Cell Responses Mediating Aplastic Anemia in Mice. J Immunol. 2014 doi: 10.4049/jimmunol.1302943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannakoulas NC, Karakantza M, Theodorou GL, Pagoni M, Galanopoulos A, Kakagianni T, et al. Clinical relevance of balance between type 1 and type 2 immune responses of lymphocyte subpopulations in aplastic anaemia patients. Br J Haematol. 2004;124:97–105. doi: 10.1046/j.1365-2141.2003.04729.x. [DOI] [PubMed] [Google Scholar]
- 9.Zeng W, Kajigaya S, Chen G, Risitano AM, Nunez O, Young NS. Transcript profile of CD4+ and CD8+ T cells from the bone marrow of acquired aplastic anemia patients. Exp Hematol. 2004;32:806–14. doi: 10.1016/j.exphem.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Kook H, Zeng W, Guibin C, Kirby M, Young NS, Maciejewski JP. Increased cytotoxic T cells with effector phenotype in aplastic anemia and myelodysplasia. Exp Hematol. 2001;29:1270–7. doi: 10.1016/s0301-472x(01)00736-6. [DOI] [PubMed] [Google Scholar]
- 11.Wu Q, Zhang J, Shi J, Ge M, Li X, Shao Y, et al. Increased bone marrow (BM) plasma level of soluble CD30 and correlations with BM plasma level of interferon (IFN)-γ, CD4/CD8 T-cell ratio and disease severity in aplastic anemia. PLoS One. 2014;9:e110787. doi: 10.1371/journal.pone.0110787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosokawa K, Muranski P, Feng X, Townsley DM, Liu B, Knickelbein J, et al. Memory Stem T Cells in Autoimmune Disease: High Frequency of Circulating CD8+ Memory Stem Cells in Acquired Aplastic Anemia. J Immunol. 2016;196:1568–78. doi: 10.4049/jimmunol.1501739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–7. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Pénit C. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int Immunol. 1998;10:371–8. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]
- 15.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O'Gorman WE, et al. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol. 2010;185:6426–30. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoyer KK, Kuswanto WF, Gallo E, Abbas AK. Distinct roles of helper T-cell subsets in a systemic autoimmune disease. Blood. 2009;113:389–95. doi: 10.1182/blood-2008-04-153346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoyer KK, Wolslegel K, Dooms H, Abbas AK. Targeting T cell-specific costimulators and growth factors in a model of autoimmune hemolytic anemia. J Immunol. 2007;179:2844–50. doi: 10.4049/jimmunol.179.5.2844. [DOI] [PubMed] [Google Scholar]
- 18.Sadlack B, Lohler J, Schorle H, Klebb G, Haber H, Sickel E, et al. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25:3053–9. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Astle CM, Harrison DE. Hematopoietic stem cell functional failure in interleukin-2-deficient mice. J Hematother Stem Cell Res. 2002;11:905–12. doi: 10.1089/152581602321080565. [DOI] [PubMed] [Google Scholar]
- 20.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(-/-)5b(-/-) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–73. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 21.Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13:102–16. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–8. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 23.Kondo M, Scherer DC, Miyamoto T, King AG, Akashi K, Sugamura K, et al. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407:383–6. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- 24.Sugimori C, List AF, Epling-Burnette PK. Immune dysregulation in myelodysplastic syndrome. Hematol Rep. 2010;2:e1. doi: 10.4081/hr.2010.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashida JN, Nakamura S, Toyoshima T, Moriyama M, Sasaki M, Kawamura E, et al. Possible involvement of cytokines, chemokines and chemokine receptors in the initiation and progression of chronic GVHD. Bone Marrow Transplant. 2013;48:115–23. doi: 10.1038/bmt.2012.100. [DOI] [PubMed] [Google Scholar]
- 26.van der Voort R, Volman TJ, Verweij V, Linssen PC, Maas F, Hebeda KM, et al. Homing characteristics of donor T cells after experimental allogeneic bone marrow transplantation and posttransplantation therapy for multiple myeloma. Biol Blood Marrow Transplant. 2013;19:378–86. doi: 10.1016/j.bbmt.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–8. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 28.Selleri C, Maciejewski JP, Sato T, Young NS. Interferon-gamma constitutively expressed in the stromal microenvironment of human marrow cultures mediates potent hematopoietic inhibition. Blood. 1996;87:4149–57. [PubMed] [Google Scholar]
- 29.Chen J, Feng X, Desierto MJ, Keyvanfar K, Young NS. IFN-γ-mediated hematopoietic cell destruction in murine models of immune-mediated bone marrow failure. Blood. 2015;126:2621–31. doi: 10.1182/blood-2015-06-652453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schurch CM, Riether C, Ochsenbein AF. Cytotoxic CD8+ T cells stimulate hematopoietic progenitors by promoting cytokine release from bone marrow mesenchymal stromal cells. Cell Stem Cell. 2014;14:460–72. doi: 10.1016/j.stem.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Lin FC, Karwan M, Saleh B, Hodge DL, Chan T, Boelte KC, et al. IFN-γ causes aplastic anemia by altering hematopoietic stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699–708. doi: 10.1182/blood-2014-01-549527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arieta Kuksin C, Gonzalez-Perez G, Minter LM. CXCR4 expression on pathogenic T cells facilitates their bone marrow infiltration in a mouse model of aplastic anemia. Blood. 2015;125:2087–94. doi: 10.1182/blood-2014-08-594796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gersuk GM, Beckham C, Loken MR, Kiener P, Anderson JE, Farrand A, et al. A role for tumour necrosis factor-alpha, Fas and Fas-Ligand in marrow failure associated with myelodysplastic syndrome. Br J Haematol. 1998;103:176–88. doi: 10.1046/j.1365-2141.1998.00933.x. [DOI] [PubMed] [Google Scholar]
- 34.Mori T, Nishimura T, Ikeda Y, Hotta T, Yagita H, Ando K. Involvement of Fas-mediated apoptosis in the hematopoietic progenitor cells of graft-versus-host reaction-associated myelosuppression. Blood. 1998;92:101–7. [PubMed] [Google Scholar]
- 35.Cousens LP, Orange JS, Biron CA. Endogenous IL-2 contributes to T cell expansion and IFN-gamma production during lymphocytic choriomeningitis virus infection. J Immunol. 1995;155:5690–9. [PubMed] [Google Scholar]
- 36.Bachmann MF, Schorle H, Kuhn R, Muller W, Hengartner H, Zinkernagel RM, et al. Antiviral immune responses in mice deficient for both interleukin-2 and interleukin-4. J Virol. 1995;69:4842–6. doi: 10.1128/jvi.69.8.4842-4846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]