Abstract
Modulation of the immune system through the use of micro and nano carriers offers opportunities in transplant tolerance, autoimmunity, infectious disease and cancer. In particular, polymeric, lipid and inorganic materials have been used as carriers of proteins, nucleic acids, and small drug molecules to direct the immune system toward either suppressive or stimulatory states. Current technologies have focused on the use of particulates or scaffolds, the modulation of materials properties, and the delivery of biologics or small drug molecules to achieve a desired response. Discussed are relevant immunology concepts, the types of biomaterial-carriers used for immunomodulation highlighting their benefits and drawbacks, the material properties influencing immune responses, and recent examples in the field of transplant tolerance.
INTRODUCTION
The immune system is intricately organized, composed of multiple layers that work in unison to protect the host against foreign invaders and provides homeostatic regulation of self and non-self. Appropriate immune recognition initiates isolation and elimination of pathogens, and tolerance to self or benign antigens, such as food proteins(1). Due to its crucial role in health and disease, manipulation of the immune system by therapeutic interventions is of great interest for the amelioration of malignancies. Immunostimulation may be sought, as in the case of vaccines and adjuvants for infectious diseases and cancer. Other times, immunosuppression, or diminished immune potency, is desired. While systemic immunosuppressants lowers the body’s ability to fight foreign invaders systemic treatments continue to be required for allergies, autoimmune diseases and transplant rejection.
Cell and whole-organ transplantation has become a standard procedure for the treatment of numerous conditions including cardiac, hepatic and renal failure(2). Donor tissue is normally derived from an allogenic source, which upon introduction to the recipient activates a cascade of immune responses. Much progress has been made due to immunosuppressant therapies, however chronic rejection and dysfunction persist, with only 47-61% of grafts surviving to the 10 year mark(3). Therefore, the induction of antigen specific tolerance to transplanted tissues remains a primary objective. Antigen specific therapies aim at preventing the host from rejecting cell or whole-organs while maintaining complete and functional activity to fight foreign invaders. To achieve tolerance, key cellular players must be engaged and re-programmed, including antigen presenting cells (APCs,) such as dendritic cells and macrophages, T and B lymphocytes, a strategy currently been explored through the use of nano- and micro-technologies.
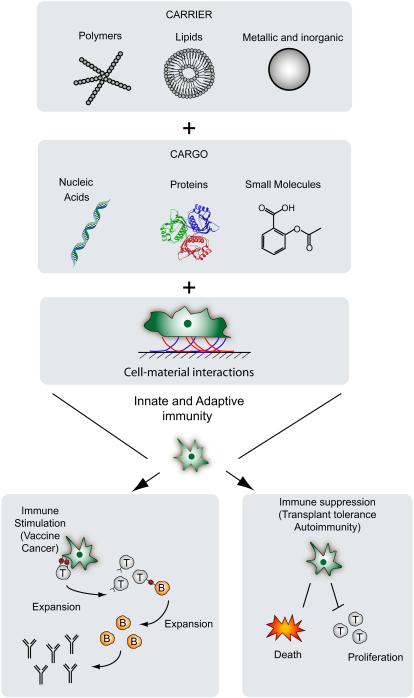
Biomaterials offer unique opportunities to modulate the immune system either toward a suppressive or stimulatory state by engaging components of the innate and adaptive immune system (Figure 1). Biomaterials are synthetic or naturally-derived materials suitable for incorporation into the human body, and are meant to perform, enhance or replace physiological functions(4). Given the variety and complexity of signals that must work together to achieve an immunological outcome, the type of biomaterial, its structure and properties should be considered when designing nano- and micro-technologies to ameliorate health concerns. In this review we focus on recent advancements of biomaterials-based nano- and micro-technologies for immunomodulation.
Figure 1.
Interaction of nano- and micro-scale biomaterial carriers with key members of the innate and adaptive immune system. Polymeric, lipid or metallic/inorganic materials have been useful as carriers of bioactive molecules or direct immunomodulators to induce either stimulation or suppression, with application toward immunogenic vaccines or tolerance.
BIOMATERIAL-CARRIERS
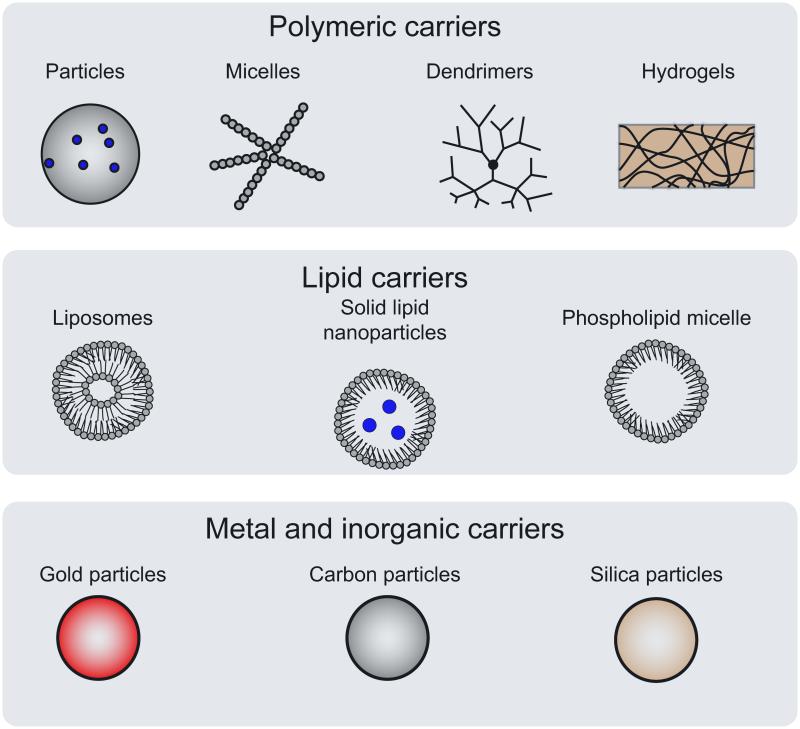
Many nano and micro-scale biomaterial systems have been described as platforms for targeting and delivery of therapeutic agents and effective immunomodulation. These agents can be encapsulated, conjugated, or adsorbed onto the material system and co-delivered with excipients or stabilizers to achieve ideal release profiles and immunological responses(5). Many carriers have been proposed and can be sorted into polymeric, lipids, metals and inorganics (Figure 2). Recent advances and limitations of each carrier type are highlighted below and summarized (Table 1).
Figure 2.
Categories of mico- and nano-carriers for immunomodulation.
Table 1.
Advantages and disadvantages of biomaterials carriers for immunomodulation
| ADVANTAGE | DISADVANTAGE | |
|---|---|---|
| POLYMERIC CARRIERS | ||
|
| ||
| MICRO AND NANO PARTICLES |
|
|
|
| ||
| MICELLES |
|
|
|
| ||
| DENDRIMERS |
|
|
|
| ||
| HYDROGELS |
|
|
|
| ||
| LIPID CARRIERS | ||
|
| ||
| LIPOSOMES |
|
|
|
| ||
| METAL AND INORGANIC | ||
|
| ||
| GOLD |
|
|
|
| ||
| CARBON |
|
|
|
| ||
| SILICA |
|
|
Polymeric
Polymeric biomaterials have been extensively investigated for the delivery of drugs, biomolecules and genes. Biocompatibility, low toxicity and biodegradability have promoted their use as a promising strategy. Additionally, chemical structures and compositions can be easily tuned to achieve desirable properties such as controlled release profiles. Examples of polymers widely used for delivery applications include polyesters (e.g., poly(lactic acid), poly(glycolic acid) and their copolymers), polyorthoesters, polyanhydrides and polycarbonates. These materials can be fabricated in the form of particles, micelles, dendrimers and hydrogels, and have each been extensively studied.
Micro- and Nano-Particles
The most common form of polymeric carriers are micro and nanoparticles, which are highly stable can effectively entrap and adsorb hydrophobic as well as hydrophilic molecules, and are easily administered through various routes. Particle sizes ranging from nanometers to micrometers can be transported through cellular and subcellular barriers making them amenable for site specific targeting. In the context of immunomodulation, for example, polymeric particles can be designed to display proteins commonly expressed by DCs, thus mimicking APCs and dictating T cell activation and differentiation. These have been referred to as artificial APCs (aAPCs), the most widely used form consists of polystyrene beads surface coated with anti-CD3/CD28 antibodies allowing the delivery of antigen-independent signal to polyclonal T cells. For antigen-specificity, polystyrene beads have also been coated with MHC-peptide single chain construct dimers or tetramers and have been used for the ex vivo expansion of tumor specific T cells.
In addition to stimulation of the T cell receptor signaling pathway through CD3/CD28, co-stimulation and inhibitory signals can be provided by covalently binding agonistic or antagonistic ligands to the beads. In a recent study, Hippen et al., demonstrated anti-CD3 antibody-loaded, aAPCs that displayed CD64 and CD86 on their surface were able to expand human natural regulatory T cells (nTregs) (6). Upon original contact nTregs numbers increased 80 fold and a single re-stimulation increased expansion to 3,000 fold while maintaining Foxp3 expression and suppressor function. These cells were then infused into an immune-deficient mice and significantly reduced graft-vs-host lethality, a disease most commonly seen following a bone marrow transplantation. In another study, Clemente-Casares et al., showed that systemic delivery of nanoparticles coated with autoimmune-disease relevant peptides bound to MHC II molecules triggers the generation and expansion of antigen-specific regulatory CD4+ T cells type 1 (Tr1)-like cells in vivo preventing and reversing type 1 diabetes(7).
Tolerance can be introduced not only by transplanting regulatory T cells but by manipulating existing ones. Low dose IL-2 treatment has been shown to increase the counts of regulatory T cells ameliorating graft vs host disease and a number of other autoimmune and inflammatory conditions (8). Biomaterials engineering has incorporated these findings and increased the functionality of aAPCs by also providing controlled release of encapsulated cytokines. For example, Steenblock et al., fabricated poly (lactic co-glycolic) acid (PLGA) microparticles surface-modified with anti-mouse CD3 and CD28 antibodies, and encapsulating IL-2. The authors demonstrated aAPCs stimulated T cells more strongly than particles with surface ligands alone, and 10-fold higher than soluble supplementation of the cytokine(9). Furthermore, they showed the response of T cells was dependent on the sustained release of IL-2.
In a different approach, polymeric particles can be designed for interactions with phagocytes and lymphocytes in mind either for the detection or prevention of transplant rejection (10-12). Early studies aimed to limit immune detection by avoiding protein adsorption and opsonization, and the use of polyethylene glycol coatings on particles rapidly emerged, as the polyethylene glycol layer sterically resists protein interactions (13). However, recent efforts have instead focused on actively directing phenotype and function of immune cells (14, 15). For example, Shirali et al., fabricated mycophenolic acid loaded PLGA nanoparticles to prolong murine skin allograft survival by upregulating PD-L1 on dendritic cells (16); Hlavaty et al., developed PLG antigen-loaded nanoparticles to promote bone marrow transplant tolerance in sex-mismatched C57BL/6 mice by interactions with CD4+ and CD8+ T cells (17) and Pan Q et al., administered corticosteroid-loaded PLGA nanoparticles weekly for the prevention of corneal allograft rejection in rats (18). These studies represent an advancement in transplant therapies without the use of a systemic immunosuppressant. Additionally, Lewis et al. developed a dual microparticle delivery system using PLGA to encapsulate combinations of immuno-suppressive factors to condition DCs toward a tolerance-inducing phenotype(19). Following up on this approach, Lewis et al., subcutaneously administered phagocytosable (encapsulating Vitamin D3 and insulin peptide as antigen) and non-phagocytosable microparticles (encapsulating TGF-β1 and GM-CSF) to non-obese diabetic mice and demonstrated 40% protection from Type 1 Diabetes development (20), representing one of the few microparticle vaccine system to successfully prevent autoimmune diabetes. Furthermore, combinatorial approaches for loading adjuvants into polymeric carriers are also being investigated, and tested using cellular based microarrays (21-23)
Micelles
Micelles are colloidal particles consisting of self-assembled aggregates of amphiphilic molecules or surfactants. In aqueous solutions and at low concentrations, amphiphiles exist as monomers. However, as their concentration increases, thermodynamic processes drive the formation of aggregates sequestering hydrophobic regions into a core like structure surrounded by hydrophilic shell(24). Micelles have been used to contain hydrophobic or poorly soluble drugs within its core. Following administration, dilution occurs rapidly and if the concentration drops below the critical micelle concentration, the stability can be compromised. However, with the addition of stabilizers, micelle carriers have successfully been employed by various groups in the context of immunomodulation. For example, Dane et al., delivered drug-loaded micelles to lymph nodes and prolonged allograft survival. Specifically, the authors used poly(ethylene glycol)-bl-poly(propylene sulfide) block copolymers 50 nm micelles to encapsulate rapamycin and tacrolimus and showed a 2-fold improvement in survival of MHC-mismatched tail skin allograft in a BALB/c mouse model (25). In another study, Miki et al., supplemented a dendritic cell vaccine with polymeric nano-micelles comprised of PEG-polyGlutamate block co-polymer carrying IL-2, and demonstrated enhanced intra-tumoral accumulation of antigen-specific cytotoxic T lymphocytes in EG7 tumor bearing mice. Furthermore, this micelle system was able to prolong IL-2 retention in blood circulation, significantly increasing DC vaccine efficacy against tumors (26).
Dendrimers
Dendrimers can be linear, cross-linked, or branched macromolecules forming a star-like structure. They have been referred to as artificial proteins based on their dimensional length scaling and narrow size distribution. Dendrimers offer a robust, covalently fixed, three dimensional structure that can be divided into three domains: (i) the multivalent surface, containing a large number of potentially reactive sites, (ii) the interior shell consisting of branches referred to as dendrons, and (iii) the core (27). The first and most extensively studied dendrimer, poly(amido amine) (PAMAM), is synthesized by using a step-wise fashion. This results in precisely defined structures with large functional groups at the surface providing opportunities for controlled conjugations of drugs and targeting moieties, separating dendrimers from other carriers by virtue of their well-controlled chemistry. However, dendrimers have shown low biocompatibility and the material selection may lead to increased toxicity.
Much of the work with dendrimers has focused on the encapsulation in its core and the covalent attachment of drugs to its surface. For example, carbohydrates constitute an important class of biological recognition molecules, displaying a wide variety of spatial structures due to their branching and various occurring isomers. In order to achieve high binding with cell surfaces, carbohydrates must be presented in a multivalent or cluster fashion(28), and functionalization of dendrimers provides an excellent platform for such multivalent presentation. For example, Heimburg et al., created a PAMAM dendrimer bearing the Thomsen-Friedenreich carbohydrate antigen (a well-documented antigen for the detection and therapy of carcinomas, particularly relevant to breast cancer) that was able to raise IgG antibodies against the antigen. This successfully impeded binding of malignant cells to vascular endothelium, blocking a metastatic step and providing a survival advantage (29, 30). Although progress has been made to understand the capability of dendrimers as therapeutics in immune applications, their clinical translation has been limited by some concerns over biocompatibility and toxicity. Dendrimers can have affinity for metal ions, lipids, proteins and nucleic acids sometimes resulting in the disruption of biological processes(31). Additionally, the expense associated with the multi-step synthesis of dendrimers has also been a concern for translation.
Hydrogels
Hydrogels are three-dimensional, cross-linked networks of highly water-soluble polymers with high porosity encompassing a wide range of chemical compositions and bulk properties. They can be formulated in a variety of different forms including micro- and nano-particles, scaffolds, coatings and films(32). Hydrogels can be tuned by controlling the density of cross-links in the gel matrix and drugs can be loaded with factors for short-term release at a rate dependent on the diffusion coefficient of the molecule through the network. Hydrogels can also be highly biocompatible, with a high water content and similar mechanical properties as the extracellular environment of soft tissues.
The utilization of hydrogels in transplant applications dates back to the 1980s when encapsulation of pancreatic islets restricted contact between the donor islets and the recipients immune cells in diabetic rats(33). The pore size of the hydrogel was large enough to allow small molecules and signaling proteins, including insulin, through but small enough to block cells and the complement system which resulted in a delayed rejection. In a more recent study conducted by Neufeld et al., encapsulation of rat islets within polymer film membranes restored normoglycemia in chemically-induced diabetic pigs for three months with no additional immunosuppression required (34). In principle, encapsulation decreases the need for systemic immunosuppression, however eventual loss of glycemic control continues to be observed (35)
Hydrogels also have limitations. For instance, low tensile strengths limits their use in load bearing applications, and can cause premature dissolution. Additionally, due to their high water content the quantity and homogeneity of agents loaded into hydrogels may be limited, particularly in the case of hydrophobic drugs. Their large water content and high porosity often results in a quick release profile of only a few hours to a few days, maximum. Clinical administration may also be a concern. Although many hydrogels can be injected, some must be implanted surgically, giving rise to a new set of complications.
Lipids
Liposomes are typically assemblies composed of one or more bilayers of amphipathic lipid molecules enclosing aqueous compartments. Their structure allows hydrophilic molecules to be incorporated within the inner compartment, while the hydrophobic compounds will be entrapped in the hydrophobic bilayer. Considering their biocompatibility, biodegradability and ability to cross lipid bilayer and cell membranes, liposomes have been widely demonstrated for various delivery platforms for vaccines(36), cancer treatments(37), gene therapy(38) and transplant(39) in various forms including single layer liposomes, solid lipid nanoparticles and phospholipid micelles. Although lipids tend to be non-immunogenic, this feature may be intentionally altered by the incorporation of antigens, and surface ligands.
Liposomes have shown much promise in advanced clinical trials for many years, with at least two adjuvant systems currently approved for human use: Inflexal®V and Epaxal® both marketed by Crucell. More specifically, both vaccines uses virosomes (unilamellar phospholipid membrane vesicle incorporating virus derived proteins) to deliver either influenza (Inflexal®V) or hepatitis A (Epaxal®) antigens to APCs in order to stimulate strong immune responses(40, 41). Through these studies it has been hypothesized that the inherent ability of APCs to sequester nanoscale liposomes more efficiently than larger-sized liposome counterparts may be a key to enhanced immune responses observed with nano or micro liposome formulations. More recently, liposomes have been investigated as a novel approach to induce long-term tolerance in organ transplantation without continuous administration of immunosuppressants. Hirai et al., at REGiMMUNE Corporation, demonstrated donor-specific tolerance can be achieved by induction of mixed chimerism in various animal models of bone transplantation (42). The authors describe a novel approach using a ligand (alpha-GalCer) for invariant Natural Killer T cells and antibody for CD40-CD40L blockage. Treatment resulted in complete acceptance of transplanted bone marrow as well as cardiac allograft from the same donor.
As with other carriers discussed here, liposomes also have limitations. They are highly susceptible to chemical and physical degradation resulting in high manufacturing cost, as conventional cost-effective sterilization techniques may not be employed. Currently, filtration and aseptic technique are recommended for the preparation of liposomes to be used in clinical settings. Liposomes also display low stability, decreasing their shelf-life and limiting their widespread application.
Metallic and Inorganic
Many inorganic materials have been studied for their use in vaccine and various immunology related applications. A well-established example is particulates of calcium phosphate, aluminum hydroxide and aluminum phosphate, collectively referred to as alum. This inorganic material causes antigen aggregation providing a depot and serving as an adjuvant for many vaccines. Interestingly, alum remains the only material approved by the U.S. Food and Drug Administration as an adjuvant in human vaccines. Although inorganic materials may be non-biodegradable, their advantage lies in their rigid structure and controllable synthesis(43). Gold, carbon, and silica particles have all been studied for their use in vaccine development.
Gold was one of the first metals to be discovered and its use in medical applications can be traced back to the seventeenth century. Gold particles can easily be fabricated into different shapes (spherical, rod, cubic, shell), with a size range of 2-150 nm, and can be surface-conjugated to achieve desired outcomes (44). Gold nanorods in particular, have been used as carriers for antigens derived from various viruses such as influenza (45), or as DNA adjuvants for human immunodeficiency virus (HIV)(46). Furthermore, gold nanoparticles have been conjugated with dye-oligonucleotide (5′-Cy5-GAG CTG CAC GCT GCC GTC AAA AAA AAA A-SH-3′) to investigate a non-viral transfection delivery system for pancreatic islet cell transplantation (47). The authors demonstrated transfected islets maintained normal mitochondrial function, calcium influx and insulin release when stimulated by glucose both in vitro and in vivo. This technology has the potential to facilitate a wide variety of applications, such as the direct manipulation of factors following transplantation using gold nanoparticles complexed with siRNA, functional proteins, and pharmacological agents that have previously been shown to improve outcomes of pancreatic islet transplants. Although widely used in experimental models, the biodistribution, circulation time and toxicity of gold particles continue to raise concerns and limit their application in clinical settings.
Carbon nanoparticles are another inorganic composition for drug and vaccine delivery. Carbon nanoparticles are easily synthesized into a variety of shapes (nanotubes, mesoporous spheres, etc), and can be made to be biocompatible. In particular, carbon nanotubes offer the possibility of multivalent surface conjugation of peptide antigens. For example, Villa et al. investigated the delivery of single-wall carbon nanotubes as antigen carriers to APCs to promote responses to human tumor antigens. The authors used covalently attached a large number of peptide ligands on to carbon nanotubes. Immunization of mice with the construct along with adjuvant induced specific IgG responses against the peptide, while in comparison, the peptide with adjuvant without the vehicle did not induce such a result (48).
Although carbon based carriers have attracted much attention because of their unique physical, chemical and mechanical properties, toxicity data at the molecular, cellular and whole animal level is often conflicting. During large-scale preparation and purification procedures impurities, mainly metal catalysts residues, are introduced and difficult to remove without destroying the structural integrity of the carrier. These impurities are often released from carbon particles leading to increased oxidative stress, inflammatory responses, malignant transformation and DNA damage or mutation (49).
Lastly, of the inorganic family, a promising material for immunomodulation is silica. Silica nanoparticles have properties amenable for various applications including tumor targeting, real time imaging, and vaccine delivery. They can be prepared with tunable properties including size, shape and porosity which can alter interaction with immune cells (50). Furthermore, its abundant surface silanol makes silica unique as it allows for further conjugation and introduction of modulation of cell recognition, absorption or uptake. For instance, Xia et al. demonstrated that polyethyleneimine coating of mesoporous silica nanoparticles enhanced pancreatic cancer cellular uptake and safely delivered siRNA and DNA constructs (51).
CARRIER PROPERTIES INFLUENCING IMMUNE RESPONSES
Pre-clinical and clinical evaluations of many types of carriers have demonstrated that material properties are related to their biological outcome. Much can be learned from nature as immune cells have evolved to respond to pathogens displaying many sizes, shapes and surface charges. These same properties are important considerations when designing carriers for immunomodulation. Carrier size appears to be a major influence in the cellular uptake and further endocytic pathway directing their intracellular fate and thus overall biological effect. Carriers may be assimilated by receptor-mediated endocytosis, which relies on the specific recognition of surface receptors and their ligands; by receptor-independent endocytosis (pinocytosis) referring to the invagination of the cell membrane encapsulating liquids from the extracellular environment; or phagocytosis in which solid factors are engulfed by the cell membrane. Carriers with diameters larger than 0.5 µm tend to be assimilated through phagocytosis(52), which is carried out by members of the innate immune system (e.g., DCs, macrophages, neutrophils and mast cells), and leads to cargo degradation in lysosomes and presentation on the cell surface for recognition by the adaptive immune system. Smaller carriers, less than 150 nm, are generally taken by cells via receptor-mediated endocytosis or pinocytosis which are involved in the uptake of essential nutrients, downregulation of cell signaling by internalization and degradation of receptors, and maintaining cellular homeostasis (53).
It has also been reported that geometrical shape of particulate carriers influences cellular uptake and trafficking. While spherical polymeric carriers are quickly internalized, anisotropic systems are poorly phagocytosed thus increasing their circulation time and systemic delivery of their cargo(54). To demonstrate this concept, Champion et al., used polystyrene particles of various sizes and shapes to study the phagocytosis of alveolar macrophages. The authors report that all shapes were able to initiate phagocytosis in at least one direction. However, it was reported that the point of contact dictated whether macrophages phagocytosed or simply spread on the particles, concluding this effect is based on the actin structure that must form around the particle to be internalized(55). Further studies elucidating the role of biomaterials shape will not only allow researchers to understand immune cells interactions with pathogens further but could inform the design of micro and nano carrier-based therapeutics.
Also important for the design on new technologies to modulate the immune system, is consideration of carriers’ surface properties, which plays a role in interactions with innate immune cells. For example, charged gold particles are reported to be more toxic than their neutral counter parts(56), cytotoxicity of PAMAM dendrimers is correlated with the number of primary amino groups (57), and DCs and macrophages preferentially interact with cationic molecules(58). The surface of biomaterials can also be modified by protein adsorption, which can direct subsequent cell-protein-material interactions (59-63). For example, Acharya et al., demonstrated that DC morphology and production of cytokines is differentially dependent upon adhesive substrates (59). Specifically, DCs cultured on albumin and serum coated surfaces maintained low levels of stimulatory and co-stimulatory molecules and produce increased levels of IL-10. Conversely, DCs cultured on collagen and vitronectin substrates expressed higher stimulatory and co-stimulatory molecules and generated higher levels of IL-12p40 indicating a suppressive and inflammatory DC phenotype respectively.
CONCLUSION
Technologies that target the immune system through the use of materials as nano- and micro carriers have gained traction in recent years. Such biomaterials are contributing to translation of basic immunology discoveries into therapies for transplant rejection, autoimmune and infectious diseases, and cancer. They offer many advantages over current clinical approaches including targeted delivery, controlled release, and stability. Expanding the implementation of materials-based technologies in clinical settings is expected to have broad impact.
ACKNOWLEDGMENTS
Support is gratefully acknowledged by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, R01 DK091658, and R01 DK098589 (to BGK).
Abbreviations
- APC
antigen-presenting cell
- nTreg
natural regulatory T cell
- PLGA
poly (lactic co-glycolic) acid
Footnotes
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Khan TA, Reddy ST. Immunological principles regulating immunomodulation with biomaterials. Acta Biomaterialia. 2014;10(4):1720–7. doi: 10.1016/j.actbio.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Hlavaty KA, Luo X, Shea LD, Miller SD. Cellular and molecular targeting for nanotherapeutics in transplantation tolerance. Clinical Immunology. 2015;160(1):14–23. doi: 10.1016/j.clim.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Page EK, Dar WA, Knechtle SJ. Tolerogenic therapies in transplantation. Front Immunol. 2012;3:198. doi: 10.3389/fimmu.2012.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bracho-Sanchez E, Lewis JS, Keselowsky BG. Biomaterials-Based Immunomodulation of Dendritic Cells. In: Santambrogio L, editor. Biomaterials in Regenerative Medicine and the Immune System. Springer International Publishing; 2015. pp. 139–56. [Google Scholar]
- 5.Lewis JS, Roy K, Keselowksy BG. MRS Bulletin. Cambridge University Press; 2014. Materials that harness and modulate the immune system; pp. 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3(83):83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530(7591):434–40. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- 8.Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. 2015;15(5):283–94. doi: 10.1038/nri3823. [DOI] [PubMed] [Google Scholar]
- 9.Steenblock ER, Fadel T, Labowsky M, Pober JS, Fahmy TM. An artificial antigen-presenting cell with paracrine delivery of IL-2 impacts the magnitude and direction of the T cell response. J Biol Chem. 2011;286(40):34883–92. doi: 10.1074/jbc.M111.276329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keselowsky BG, Xia CQ, Clare-Salzler M. Multifunctional dendritic cell-targeting polymeric microparticles: engineering new vaccines for type 1 diabetes. Hum Vaccin. 2011;7(1):37–44. doi: 10.4161/hv.7.1.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler KS, Lovato DM, Adolphi NL, Belfon R, Fegan DL, Monson TC, et al. Development of antibody-tagged nanoparticles for detection of transplant rejection using biomagnetic sensors. Cell Transplant. 2013;22(10):1943–54. doi: 10.3727/096368912X657963. [DOI] [PubMed] [Google Scholar]
- 12.Penno E, Johnsson C, Johansson L, Ahlström H. Macrophage uptake of ultra-small iron oxide particles for magnetic resonance imaging in experimental acute cardiac transplant rejection. Acta Radiol. 2006;47(3):264–71. doi: 10.1080/02841850500539041. [DOI] [PubMed] [Google Scholar]
- 13.Alcantar NA, Aydil ES, Israelachvili JN. Polyethylene glycol-coated biocompatible surfaces. J Biomed Mater Res. 2000;51(3):343–51. doi: 10.1002/1097-4636(20000905)51:3<343::aid-jbm7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.Azzi J, Tang L, Moore R, Tong R, El Haddad N, Akiyoshi T, et al. Polylactide-cyclosporin A nanoparticles for targeted immunosuppression. FASEB J. 2010;24(10):3927–38. doi: 10.1096/fj.10-154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azzi J, Yin Q, Uehara M, Ohori S, Tang L, Cai K, et al. Targeted Delivery of Immunomodulators to Lymph Nodes. Cell Rep. 2016 doi: 10.1016/j.celrep.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirali AC, Look M, Du W, Kassis E, Stout-Delgado HW, Fahmy TM, et al. Nanoparticle delivery of mycophenolic acid upregulates PD-L1 on dendritic cells to prolong murine allograft survival. Am J Transplant. 2011;11(12):2582–92. doi: 10.1111/j.1600-6143.2011.03725.x. [DOI] [PubMed] [Google Scholar]
- 17.Hlavaty KA, McCarthy DP, Saito E, Yap WT, Miller SD, Shea LD. Tolerance induction using nanoparticles bearing HY peptides in bone marrow transplantation. Biomaterials. 2016;76:1–10. doi: 10.1016/j.biomaterials.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan Q, Xu Q, Boylan NJ, Lamb NW, Emmert DG, Yang JC, et al. Corticosteroid-loaded biodegradable nanoparticles for prevention of corneal allograft rejection in rats. J Control Release. 2015;201:32–40. doi: 10.1016/j.jconrel.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis JS, Roche C, Zhang Y, Brusko TM, Wasserfall CH, Atkinson M, et al. Combinatorial delivery of immunosuppressive factors to dendritic cells using dual-sized microspheres. J Mater Chem B Mater Biol Med. 2014;2(17):2562–74. doi: 10.1039/C3TB21460E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis JS, Dolgova NV, Zhang Y, Xia CQ, Wasserfall CH, Atkinson MA, et al. A combination dual-sized microparticle system modulates dendritic cells and prevents type 1 diabetes in prediabetic NOD mice. Clin Immunol. 2015;160(1):90–102. doi: 10.1016/j.clim.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acharya AP, Clare-Salzler MJ, Keselowsky BG. A high-throughput microparticle microarray platform for dendritic cell-targeting vaccines. Biomaterials. 2009;30(25):4168–77. doi: 10.1016/j.biomaterials.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 22.Acharya AP, Carstens MR, Lewis JS, Dolgova N, Xia CQ, Clare-Salzler MJ, et al. A cell-based microarray to investigate combinatorial effects of microparticle-encapsulated adjuvants on dendritic cell activation. Journal of Materials Chemistry B. 2016 doi: 10.1039/C5TB01754H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acharya AP, Lewis JS, Keselowsky BG. Combinatorial co-encapsulation of hydrophobic molecules in poly(lactide-co-glycolide) microparticles. Biomaterials. 2013;34(13):3422–30. doi: 10.1016/j.biomaterials.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson N, Ghandehari H. Polymeric conjugates for drug delivery. Chemistry of materials : a publication of the American Chemical Society. 2012;24(5):840–53. doi: 10.1021/cm2031569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dane KY, Nembrini C, Tomei AA, Eby JK, O'Neil CP, Velluto D, et al. Nano-sized drug-loaded micelles deliver payload to lymph node immune cells and prolong allograft survival. Journal of Controlled Release. 2011;156(2):154–60. doi: 10.1016/j.jconrel.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Miki K, Nagaoka K, Harada M, Hayashi T, Jinguji H, Kato Y, et al. Combination therapy with dendritic cell vaccine and IL-2 encapsulating polymeric micelles enhances intra-tumoral accumulation of antigen-specific CTLs. Int Immunopharmacol. 2014;23(2):499–504. doi: 10.1016/j.intimp.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 27.Svenson S, Tomalia DA. Dendrimers in biomedical applications--reflections on the field. Adv Drug Deliv Rev. 2005;57(15):2106–29. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem Rev. 2002;102(2):555–78. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- 29.Heimburg J, Yan J, Morey S, Glinskii OV, Huxley VH, Wild L, et al. Inhibition of spontaneous breast cancer metastasis by anti-Thomsen-Friedenreich antigen monoclonal antibody JAA-F11. Neoplasia. 2006;8(11):939–48. doi: 10.1593/neo.06493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baek M-G, Roy R. Synthesis and protein binding properties of T-antigen containing GlycoPAMAM dendrimers. Bioorganic & Medicinal Chemistry. 2002;10(1):11–7. doi: 10.1016/s0968-0896(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Y, Zhao L, Li Y, Xu T. Design of biocompatible dendrimers for cancer diagnosis and therapy: current status and future perspectives. Chem Soc Rev. 2011;40(5):2673–703. doi: 10.1039/c0cs00097c. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. Journal of Advanced Research. 2015;6(2):105–21. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210(4472):908–10. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 34.Neufeld T, Ludwig B, Barkai U, Weir GC, Colton CK, Evron Y, et al. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS One. 2013;8(8):e70150. doi: 10.1371/journal.pone.0070150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zakrzewski JL, van den Brink MR, Hubbell JA. Overcoming immunological barriers in regenerative medicine. Nat Biotechnol. 2014;32(8):786–94. doi: 10.1038/nbt.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther Adv Vaccines. 2014;2(6):159–82. doi: 10.1177/2051013614541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown S, Khan DR. The treatment of breast cancer using liposome technology. J Drug Deliv. 2012;2012:212965. doi: 10.1155/2012/212965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu H, Li Z, Si J. Nanocarriers in gene therapy: a review. J Biomed Nanotechnol. 2014;10(12):3483–507. doi: 10.1166/jbn.2014.2044. [DOI] [PubMed] [Google Scholar]
- 39.de Kleer I, Vastert B, Klein M, Teklenburg G, Arkesteijn G, Yung GP, et al. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood. 2006;107(4):1696–702. doi: 10.1182/blood-2005-07-2800. [DOI] [PubMed] [Google Scholar]
- 40.Mischler R, Metcalfe IC. Inflexal®V a trivalent virosome subunit influenza vaccine: production. Vaccine. 2002;20(Supplement 5):B17–B23. doi: 10.1016/s0264-410x(02)00512-1. [DOI] [PubMed] [Google Scholar]
- 41.Bovier PA. Epaxal: a virosomal vaccine to prevent hepatitis A infection. Expert Rev Vaccines. 2008;7(8):1141–50. doi: 10.1586/14760584.7.8.1141. [DOI] [PubMed] [Google Scholar]
- 42.Hirai T, Ishii Y, Ikemiyagi M, Fukuda E, Omoto K, Namiki M, et al. A novel approach inducing transplant tolerance by activated invariant natural killer T cells with costimulatory blockade. Am J Transplant. 2014;14(3):554–67. doi: 10.1111/ajt.12606. [DOI] [PubMed] [Google Scholar]
- 43.Jiao Q, Li L, Mu Q, Zhang Q. Immunomodulation of Nanoparticles in Nanomedicine Applications. BioMed Research International. 2014;2014:426028. doi: 10.1155/2014/426028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao L, Seth A, Wibowo N, Zhao C-X, Mitter N, Yu C, et al. Nanoparticle vaccines. Vaccine. 2014;32(3):327–37. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 45.Tao W, Ziemer KS, Gill HS. Gold nanoparticle-M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine (Lond) 2014;9(2):237–51. doi: 10.2217/nnm.13.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu L, Liu Y, Chen Z, Li W, Wang L, Wu X, et al. Surface-engineered gold nanorods: promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012;12(4):2003–12. doi: 10.1021/nl300027p. [DOI] [PubMed] [Google Scholar]
- 47.Vega RA, Wang Y, Harvat T, Wang S, Qi M, Adewola AF, et al. Modified gold nanoparticle vectors: a biocompatible intracellular delivery system for pancreatic islet cell transplantation. Surgery. 2010;148(4):858–65. doi: 10.1016/j.surg.2010.07.036. discussion 65-6. [DOI] [PubMed] [Google Scholar]
- 48.Villa CH, Dao T, Ahearn I, Fehrenbacher N, Casey E, Rey DA, et al. Single-walled carbon nanotubes deliver peptide antigen into dendritic cells and enhance IgG responses to tumor-associated antigens. ACS Nano. 2011;5(7):5300–11. doi: 10.1021/nn200182x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Zhao Y, Sun B, Chen C. Understanding the Toxicity of Carbon Nanotubes. Accounts of Chemical Research. 2013;46(3):702–13. doi: 10.1021/ar300028m. [DOI] [PubMed] [Google Scholar]
- 50.Niut Y, Popatt A, Yu M, Karmakar S, Gu W, Yu C. Recent advances in the rational design of silica-based nanoparticles for gene therapy. Ther Deliv. 2012;3(10):1217–37. [PubMed] [Google Scholar]
- 51.Xia T, Kovochich M, Liong M, Meng H, Kabehie S, George S, et al. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano. 2009;3(10):3273–86. doi: 10.1021/nn900918w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298(2):315–22. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 53.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of Nanomedicines. Journal of controlled release : official journal of the Controlled Release Society. 2010;145(3):182–95. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Control Release. 2007;121(1-2):3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103(13):4930–4. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaeublin NM, Braydich-Stolle LK, Schrand AM, Miller JM, Hutchison J, Schlager JJ, et al. Surface charge of gold nanoparticles mediates mechanism of toxicity. Nanoscale. 2011;3(2):410–20. doi: 10.1039/c0nr00478b. [DOI] [PubMed] [Google Scholar]
- 57.Naha PC, Davoren M, Lyng FM, Byrne HJ. Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cells. Toxicol Appl Pharmacol. 2010;246(1-2):91–9. doi: 10.1016/j.taap.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 58.Kwon YJ, Standley SM, Goh SL, Fréchet JMJ. Enhanced antigen presentation and immunostimulation of dendritic cells using acid-degradable cationic nanoparticles. Journal of Controlled Release. 2005;105(3):199–212. doi: 10.1016/j.jconrel.2005.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Acharya AP, Dolgova NV, Clare-Salzler MJ, Keselowsky BG. Adhesive substrate-modulation of adaptive immune responses. Biomaterials. 2008;29(36):4736–50. doi: 10.1016/j.biomaterials.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 60.Acharya AP, Dolgova NV, Xia CQ, Clare-Salzler MJ, Keselowsky BG. Adhesive substrates modulate the activation and stimulatory capacity of non-obese diabetic mouse-derived dendritic cells. Acta Biomater. 2011;7(1):180–92. doi: 10.1016/j.actbio.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 61.Lewis JS, Zaveri TD, Crooks CP, 2nd, Keselowsky BG. Microparticle surface modifications targeting dendritic cells for non-activating applications. Biomaterials. 2012;33(29):7221–32. doi: 10.1016/j.biomaterials.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acharya AP, Dolgova NV, Moore NM, Xia CQ, Clare-Salzler MJ, Becker ML, et al. The modulation of dendritic cell integrin binding and activation by RGD-peptide density gradient substrates. Biomaterials. 2010;31(29):7444–54. doi: 10.1016/j.biomaterials.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 63.Zaveri TD, Lewis JS, Dolgova NV, Clare-Salzler MJ, Keselowsky BG. Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials. 2014;35(11):3504–15. doi: 10.1016/j.biomaterials.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]