Abstract
Background
Side effects prompt some patients to forego otherwise-beneficial therapies. This study explored which characteristics make side effects particularly aversive.
Methods
We used a psychometric approach, originating from research on risk perception, to identify the factors (or components) underlying side effect perceptions. Women (N=149) aged 40–74 were recruited from a patient registry to complete an online experiment. Participants were presented with hypothetical scenarios in which an effective and necessary medication conferred a small risk of a single side effect (e.g., nausea, dizziness). They rated a broad range of side effects on several characteristics (e.g., embarrassing, treatable). In addition, we collected four measures of aversiveness for each side effect: choosing to take the medication, willingness to pay to avoid the side effect (WTP), negative affective attitude associated with the side effect, and how each side effect ranks among others in terms of undesirability. A principle-components analysis (PCA) was used to identify the components underlying side effect perceptions. Then, for each aversiveness measure separately, regression analyses were used to determine which components predicted differences in aversiveness among the side effects.
Results
The PCA revealed four components underlying side effect perceptions: affective challenge (e.g., frightening), social challenge (e.g., disfiguring), physical challenge (e.g., painful), and familiarity (e.g., common). Side effects perceived as affectively and physically challenging elicited the highest levels of aversiveness across all four measures.
Conclusions
Understanding what side effect characteristics are most aversive may inform interventions to improve medical decisions and facilitate the translation of novel biomedical therapies into clinical practice.
Keywords: medical decision making, affect, risk perception, tradeoff, psychometric paradigm
Introduction
Informed decision making is a cornerstone of modern medical practice. It emphasizes that medical decisions should be based on a combination of patient preferences and values and an objective evaluation of the probability and severity of the benefits and side effects of treatment [1]. However, the mere presence of a side effect may interfere with the objective evaluation of the information [2]. Interference from side effects can result in the rejection of an otherwise beneficial treatment [i.e., side effect aversion, 3]. The goal of this study is to improve understanding of what makes some side effects particularly aversive. The results will inform the development of patient decision support tools, provide healthcare providers insight they can apply to patient consultations, and facilitate the translation of existing and novel biomedical therapies into clinical practice.
Side Effects Influence Medical Decisions
Concerns about side effects can discourage people from accepting preventive medical therapies for the primary prevention of disease. For example, young men who had sex with men cited side effects as a reason for declining pre-exposure prophylaxis for HIV transmission [4]. Side effects were also mentioned as a reason for declining influenza immunization [5] and for declining to take tamoxifen for primary prevention of breast cancer [6].
It is understandable that patients who have neither symptoms nor a diagnosis might be reluctant to take a medication that could cause harm in order to reduce the likelihood of experiencing an illness that may never become manifest. However, individuals who have been diagnosed with a specific health condition face an urgent need to carefully consider the tradeoffs of accepting or forgoing treatment. Whereas some patients agree to treatment regardless of the number or toxicity of side effects [7, 8], others are reluctant to agree to potentially life-saving therapies. This has been demonstrated in many contexts, including taking adjuvant chemotherapy for breast cancer [9], undergoing biologic therapy for rheumatoid arthritis [10], and adhering to antidepressant medication [11].
A critical factor contributing to aversion to side effects may be the negative affect associated with them [12]. Negative affective responses can discourage the effective use of probabilistic information [13]. For instance, when choosing between risky options, people tended to pay less attention to probability information when the risky option was a medication that included a side effect than when the risky option was a gamble that included an equivalent monetary loss. Instead, they simply chose the scenario with the outcome that elicited the least negative affect [2]. Little is known, however, about the characteristics that make side effects aversive and which characteristics may make them more acceptable. Understanding the key factors that structure beliefs about side effects and how those side effect representations affect decisions may improve the ability to identify medications that may elicit aversion.
Side Effect Representations
Based on their knowledge and experiences, people develop cognitive representations of illnesses (e.g., cardiovascular disease) and symptoms (e.g., rash) [14, 15]. These representations can guide care-seeking and treatment decisions. For example, sneezing, itchy nose, and nasal congestion are more likely to be attributed to hay fever than strep throat because the symptoms more closely resemble the representation of hay fever [16]. The resulting hay fever self-diagnosis is more likely to be treated using home remedies than by visiting the emergency department.
Affect is one potential determinant of the underlying structures of symptom representations. Some research has examined the potential for side effects to be embarrassing or shameful [17], but the role of other emotions such as fear, worry, or dread have been under-explored. However, dread is an important factor that structures health risk perceptions. In their psychometric paradigm, Slovic and colleagues used factor-analytic techniques to examine the interrelationships among the characteristics of 81 health hazards. The more a hazard could be described as having “dreadful” characteristics, the more it elicited higher perceptions of risk and higher desires for risk reduction measures [18]. This is consistent with later work illustrating that negative affect can increase perceptions of risk, reduce perceptions of benefit, and lead people to neglect critical probability information [19].
Given the importance of illness and symptom representations for treatment decisions and the role of negative affect in influencing risk perceptions and the use of probability information, it seems plausible that increasing understanding of side effect representations and the role of affect in these representations may improve understanding of medication tradeoff decisions. These insights will help identify medical treatments that may be at risk for producing side effect aversion (i.e., ignoring probability information when making tradeoffs) and inform the development of tools to reduce side effect aversion and support informed decision making.
Objective and Research Questions
This study examined how laypeople conceptualize side effects and how those conceptualizations influence the aversiveness of a medication that confers a small risk of a side effect. To accomplish this, we connect previous approaches to examining the underlying structure of symptom representations [17] and risk perceptions [18]. Specifically, participants rated a broad range of side effects on several characteristics (e.g., visibility, dreadful). The key factors (or components) underlying side effect perceptions were then extracted. The research questions were: (1) What is the structure underlying side effect perceptions? and (2) How are the components structuring side effect perceptions related to aversiveness as defined by (a) choosing to accept the medication; (b) willingness to pay to avoid the side effect; (c) negative affective attitude associated with a side effect; and (d) undesirability of experiencing the side effect?
The current research included only women because several studies suggest that concerns about side effects may be more common and/or influential among women than men [3, 5, 12, 20]. Furthermore, men—particularly white men in the United States—often view the same hazards as less risky than women [21]. Excluding men reduces the variability in responses due to influences other than side effects, such as this so-called “white male effect”. Reducing this variability will facilitate the identification of distinct categories of side effect characteristics.
Methods
Participants
All study materials and procedures were approved by the Human Research Protection Office at Washington University in St. Louis. Eligibility criteria were: being a woman aged 40–74, being white or African American, having no prior cancer history, having a working computer at home, and using the Internet at least three times per week for work or leisure. The latter two criteria were added because some participants from pilot testing were unable to complete the study independently due to very limited computer literacy.
Participants were recruited from a participant registry and biorepository. Individuals were recruited into the registry by clinical staff immediately after undergoing annual mammographic screening. Of the 12,227 individuals in the registry, 10,239 consented to be recontacted for future research. The registry data manager identified potentially eligible individuals for this study based on age, race, and health history. She randomly selected 1,400 women and sent the names and contact information to the research team in batches of 200. A research assistant contacted potentially eligible individuals by telephone, verified eligibility, and enrolled participants. Seven individuals were excluded due to age, one for race, six for computer literacy, and 49 for receiving a cancer diagnosis after joining the registry. Several participants were ineligible for multiple reasons. Of the 402 individuals reached by phone, 270 were screened, 213 enrolled, 151 consented, and 149 completed the survey.
The sample was relatively uniform in terms of race and ethnicity. Of the 149 women, 114 (76.5%) were white, 31 (20.8%) were African American, and 3 (2.0%) were Hispanic; one person (0.7%) indicated African American and Hispanic ancestry. The sample was also highly educated: 79 (53.0%) women reported having a Bachelor’s or postgraduate degree, and 47 (31.5%) had some college experience or an Associate’s degree. Only 23 (15.5%) women had no college experience. The mean age was 56.3 years (SD = 7.5). Although the age of the sample participants was nearly identical to the age of all registry participants (i.e., 56.4 years), the registry included slightly more African Americans (26.6%). Only 1.5% of the registry sample reported being a race other than African American or white. The registry does not include data about educational attainment. According to the U.S. Census Bureau, the city of St. Louis is predominantly African American (47.7%) and white (46.6%), and 30.4% of residents have a Bachelor’s or postgraduate degree.
Materials and Measures
An initial pool of 52 side effects was created by reviewing the side effects associated with the 25 most commonly-mentioned medications in physician office visits [22]. Side effects associated with disease-modifying anti-rheumatic drugs, raloxifene, and antidepressants were also included because the diseases for which these drugs are prescribed are more prevalent among women than men. The final 20 side effects were chosen to ensure variability in characteristic ratings, which were obtained in a small pilot study (so that not all side effects had high ratings for any single characteristic). The final 20 side effects were: nausea, diarrhea, constipation, rash, dizziness, muscle pain, headache, exhaustion, ulcer, increased appetite, loss of appetite, hair loss, depression, decreased sex drive, hot flashes, excessive sweating, mouth sores, dry mouth, weight gain, and blurry vision.
In a side effect perception task, participants rated each of these side effects on several characteristics (“How much do you think [SIDE EFFECT] can be described by the words below? 1 = Not at all; 10 = Perfectly; I don’t want to answer). An initial list of 18 potential side effect characteristics was generated by reviewing the literatures about risk perception [18], illness and symptom representations [17, 23], and the role of affect and emotions in risk perception [19, 24]. The list was reduced and modified during pilot testing. Ambiguous terms were clarified when possible or removed when necessary. The final 15 characteristics were: painful, disabling, embarrassing, disfiguring, gross, common, symptomatic, visible, dreadful, frightening, treatable, angry, sad, chronic, and delayed.
In addition to the side effect perception task, participants were presented for each side effect with three items and a ranking task to measure side effect aversiveness: a choice item (“If this were a real choice, do you think you would take this drug? (1) Definitely would not – (5) Definitely would; Don’t know; Don’t want to answer”); a willingness to pay to avoid the side effect (WTP) item (“Imagine a new drug to treat the illness is discovered. It works just as well as the one your doctor mentioned, but does not cause [SIDE EFFECT]. How many extra dollars would you pay for a 1-month supply of this drug? $_____”), and a negative affective attitude item (“How bad would it be to have [SIDE EFFECT]? (1) Not bad at all – (100) The worst experience you can imagine”). In the ranking task, participants rated the undesirability of each of the same ten side effects (“Rank the side effects so that the side effect that you think would be the WORST to have is AT THE TOP of the list”).
Procedure
Participants completed the study on the Internet at home. The research assistant emailed participants a link to the survey-hosting website Unipark. Up to two reminder emails were sent, each two weeks apart. Participants who completed the study were entered into a raffle to win one of six $75 discount store gift cards.
Participants provided informed consent by reading an information sheet and clicking a button labeled “I agree.” Participants noted whether they had ever experienced a side effect and, if so, the severity of the effect. Next, they indicated the first three things that came to mind when hearing the words “side effect.” Then, respondents completed the first of ten blocks. Each block included three tasks and was specific to one side effect. Over the course of the study, all 20 side effects were presented to participants. To minimize participant burden and maintain data quality, each participant responded to only ten side effects (drawn randomly from the pool of 20). Each side effect was rated by at least 45 participants.
Each block began with a hypothetical scenario, which read, “Imagine a doctor tells you that you have a serious illness. The illness can be treated, but you’ll have to take a small pill every day for the next 5 years. Unfortunately, this is the only treatment available. The doctor says that 1 out of 100 people who take the pill have [SIDE EFFECT].” Participants were then presented with the choice item, the WTP item, the negative affective attitude item, and the side effect perception task. Then the next block of four tasks followed, based on a new side effect. At the end of ten blocks, participants completed the ranking task for each of the ten side effects that they evaluated previously. The study took approximately 30 minutes to complete, with a range of approximately 15–70 minutes.
Results
Aversiveness Measures
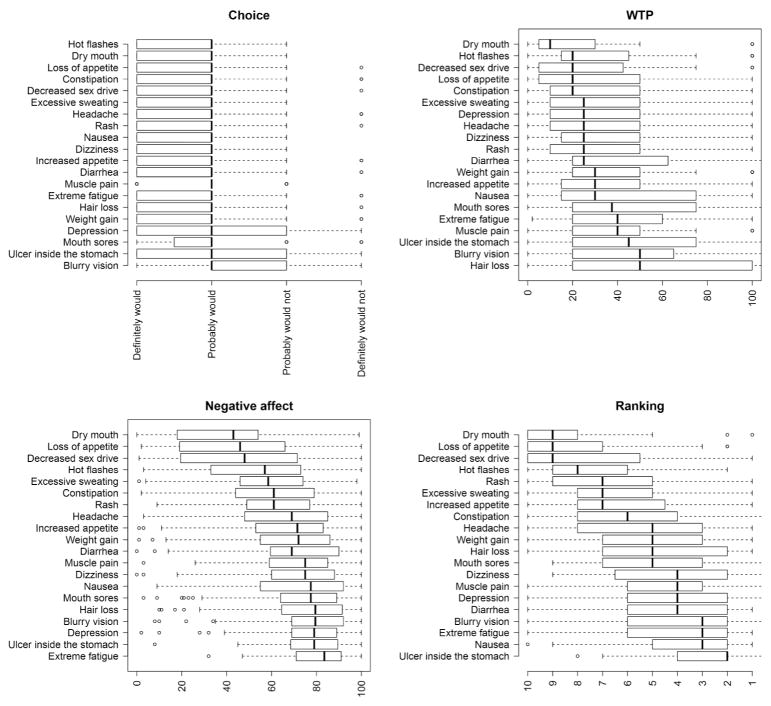
Figure 1 shows the distribution of responses for each measure of side effect aversiveness (i.e., choice, WTP, negative affective attitude, and undesirability ranking). For the choice item, a large majority of respondents (range 65%–95%) indicated that they would “probably” or “definitely” take the drug. Nevertheless, there were differences between the side effects, with the lowest medication choice ratings expressed for blurry vision, followed by stomach ulcer, mouth sores, depression and weight gain. Hot flashes, dry mouth, and loss of appetite were associated with the highest choice ratings. For the WTP item, the highest amounts were elicited from hair loss, blurry vision, stomach ulcer, muscle pain, and extreme fatigue. The side effects with the lowest WTPs were dry mouth, hot flashes, and decreased sex drive. Negative affective attitude was most pronounced for extreme fatigue, followed by stomach ulcer, depression, blurry vision, and hair loss. Dry mouth, loss of appetite, and decreased sex drive elicited the least negative affective attitude. Finally, when sorting the side effects in terms of overall undesirability in the ranking task, stomach ulcer, nausea, extreme fatigue, and blurry vision were ranked as worst. Dry mouth, loss of appetite, and decreased sex drive were ranked as least undesirable. Table 1 shows that these four facets of respondents’ reactions to the side effects were strongly correlated with each other (across side effects, in terms of Spearman rank correlation).
Figure 1.
Boxplots showing the distribution of the responses on the four aversiveness measures for each side effect, ordered by their respective means from least to most unattractive. Aversiveness ratings include the choice item (upper-left panel), the willingness-to-pay item (WTP; upper-right panel), the negative affective attitude item (bottom-left panel), and the ranking task (bottom-right panel).
Table 1.
Spearman rank correlations among the four measures of side effect aversiveness
| Aversiveness measure | Negative affective attitude | WTP | Undesirability rank |
|---|---|---|---|
| Choice | −0.88 | −0.86 | −0.77 |
| Negative affective attitude | 0.83 | 0.74 | |
| WTP | 0.91 |
Note. All ps < .001; WTP = willingness to pay
Cognitive Representations of Side Effects
To examine the structure of respondents’ cognitive representations of the side effects, we first determined for each side effect its average rating (across participants) on each characteristic in the side effect perception task.1 Table 2 shows the intercorrelations between the individual items. Using the principal() function of the psych package [25] in R, a principal component analysis (PCA) was conducted on the average ratings (with an oblique rotation).2 Bartlett’s test of sphericity indicated that the correlations between the items were sufficiently large for conducting a PCA, χ2(171) = 2,050.4, p < .001. The scree plot suggested a four-component solution, which explained 82% of the total variance. The individual components accounted for 27%, 24%, 19%, and 12% of the variance. The Kaiser-Meyer-Olkin (KMO) measure indicated some sampling inadequacy for the analysis; specifically, the KMO values were below the desirable value of .5 for 6 of the 15 items. Although this might indicate that the number of side effects of 20 was too small to reliably extract components, it is important to note that the KMO value does not take into account that the ratings of each side effect represent averages across participants, and are thus based on a considerably higher number of data points than what is being considered in the KMO. The individual components were only slightly correlated, with the highest intercorrelation between the first and the second component (r = .25); the intercorrelations between the other components ranged between r = −.04 (Components 2 and 3) to .18 (Components 1 and 3).
Table 2.
Intercorrelations among the 15 Side Effect Characteristics
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| 1 Painful | 0.57 | −0.19 | −0.10 | 0.10 | 0.33 | 0.35 | −0.31 | 0.29 | 0.26 | 0.78 | −0.06 | 0.03 | 0.49 | 0.37 |
| 2 Disabling | 1 | −0.19 | −0.29 | −0.17 | 0.09 | 0.45 | −0.17 | 0.49 | 0.69 | 0.41 | 0.28 | 0.41 | 0.52 | 0.49 |
| 3 Embarrassing | 1 | 0.68 | 0.80 | −0.09 | 0.36 | 0.77 | 0.63 | 0.09 | −0.06 | 0.45 | 0.50 | 0.48 | 0.45 | |
| 4 Disfiguring | 1 | 0.49 | −0.12 | 0.21 | 0.83 | 0.55 | 0.19 | −0.12 | 0.51 | 0.52 | 0.43 | 0.50 | ||
| 5 Gross | 1 | −0.14 | 0.35 | 0.47 | 0.48 | −0.08 | 0.23 | 0.07 | 0.24 | 0.39 | 0.36 | |||
| 6 Common | 1 | −0.21 | −0.16 | −0.02 | −0.21 | 0.44 | 0.11 | 0 | 0.23 | 0.06 | ||||
| 7 Symptomatic | 1 | 0.34 | 0.62 | 0.27 | 0.20 | 0 | 0.20 | 0.45 | 0.32 | |||||
| 8 Visible | 1 | 0.56 | 0.14 | −0.27 | 0.46 | 0.48 | 0.41 | 0.44 | ||||||
| 9 Dread | 1 | 0.65 | 0.16 | 0.69 | 0.84 | 0.88 | 0.86 | |||||||
| 10 Frightening | 1 | 0.08 | 0.64 | 0.76 | 0.54 | 0.71 | ||||||||
| 11 Treatable | 1 | −0.14 | −0.10 | 0.35 | 0.24 | |||||||||
| 12 Sad | 1 | 0.93 | 0.64 | 0.74 | ||||||||||
| 13 Angry | 1 | 0.73 | 0.83 | |||||||||||
| 14 Chronic | 1 | 0.85 | ||||||||||||
| 15 Delayed | 1 | |||||||||||||
Table 3 reports how the different characteristics loaded on the four components after rotation. The first component showed high loadings of emotional characteristics (e.g., sad, frightening), so it could be called an affective challenge component. The second component had high loadings of items that relate to characteristics associated with social interactions and embarrassment (e.g., gross, disfiguring), yielding a social challenge component. The third component had high loadings of items referring to being constrained (e.g., painful, symptomatic); we refer to it as a physical challenge component. The fourth component had high loadings on items capturing the degree to which the side effect was familiar (e.g., common, treatable), so we refer to it as a familiarity component.
Table 3.
Component loadings from the principle component analysis (oblique rotation) of the average (across participants) side effect characteristics as obtained in the side effect perception task.
| Characteristic | Componenta
|
Communality | |||
|---|---|---|---|---|---|
| 1 (affective challenge) | 2 (social challenge) | 3 (physical challenge) | 4 (familiarity) | ||
| Sad | 0.96 | 0.94 | |||
| Angry | 0.91 | 0.90 | |||
| Frightening | 0.75 | 0.38 | −0.31 | 0.90 | |
| Delayed | 0.69 | 0.41 | 0.69 | ||
| Dread | 0.55 | 0.49 | 0.45 | 0.95 | |
| Chronic | 0.51 | 0.41 | 0.39 | 0.77 | |
| Embarrassing | 0.88 | 0.88 | |||
| Gross | 0.88 | 0.74 | |||
| Visible | 0.76 | 0.81 | |||
| Disfiguring | 0.69 | 0.72 | |||
| Painful | 0.79 | 0.35 | 0.81 | ||
| Disabling | 0.37 | −0.36 | 0.76 | 0.89 | |
| Symptomatic | 0.55 | 0.70 | 0.73 | ||
| Common | 0.90 | 0.79 | |||
| Treatable | 0.54 | 0.58 | 0.78 | ||
|
| |||||
| Variance accounted for | 27% | 24% | 19% | 12% | |
Loadings < .30 not shown. Bolded text denotes characteristics that were included in the component.
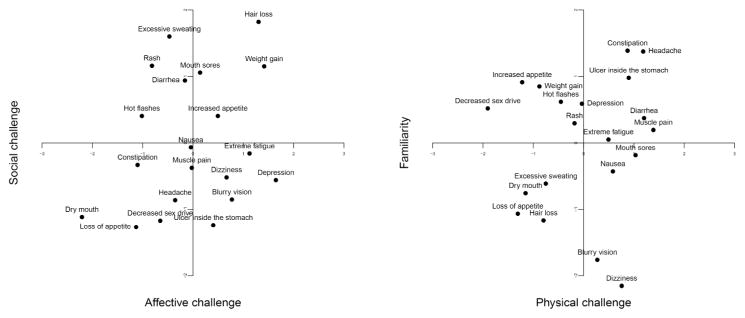
Figure 2 plots how the side effects are represented on these components. For simplicity, the representations are shown for the combination of the affective challenge and the social challenge components, as well as for the combination of the physical challenge and familiarity components. As can be seen, side effects with particularly high values on the affective challenge component are depression, weight gain, hair loss, and extreme fatigue, whereas dry mouth and constipation have rather low values on this component. Hair loss and extreme sweating have the highest values on the social challenge component, and loss of appetite the lowest. Muscle pain and diarrhea are viewed as most physically challenging, decreased sex drive and loss of appetite as least. Finally, constipation and headache have highest values on the familiarity component, whereas dizziness and blurry vision score lowest.
Figure 2.
Location of the side effects on the four components (affective challenge, social challenge, physical challenge, familiarity) derived from the relationship among 15 characteristics of the side effects. For better readability, only two components are shown at a time; the panels represent the pairwise combinations of the first and the second component, and the third and the fourth component, respectively.
Which Components Underlying Side-Effect Perceptions Predict Aversiveness?
Regression analyses were used to examine the extent to which, across all 20 side effects, the different components structuring respondents’ perceptions of the side effects were predictive of their variability in aversiveness (i.e., choice, WTP to avoid the side effect, negative affective attitude, undesirability rank). Analyses were conducted for each of the four measures of aversiveness separately. Specifically, for a given measure of aversiveness (e.g., choice), the average value for each side effect was regressed onto the side effect’s scores for all of the four components structuring side effect perception (i.e., affective challenge, social challenge, physical challenge, and familiarity). For WTP we used the median value to reduce the influence of extreme responses. Table 4 shows the results of the four regression analyses. Across all four measures of aversiveness, affective and physical challenge were the strongest predictors. Side effects that were perceived as being the most affectively and physically challenging elicited the lowest choice ratings and the highest WTP, negative affective attitude, and undesirability ratings. Interestingly, the social challenge and familiarity components predicted none of the aversiveness measures. In other words, although the affective and social components were the most prominent in shaping people’s perceptions of side effects, only the affective and physical challenge components were relevant for predicting aversion when a medication had a particular side effect.
Table 4.
Regression analyses predicting side effect aversiveness from the component scores underlying side effect representations.
| Predictor | Aversiveness Measurea
|
|||
|---|---|---|---|---|
| Choice | Negative Affective Attitude | WTP | Undesirability Rank | |
| Intercept | 5.45 [5.17, 5.72] | 64.96 [63.39, 66.53] | 50.32 [46.50, 54.13] | 5.45 [5.18, 5.72] |
| Affective challenge | −1.04 [−1.34, −0.74] | 8.55 [6.84, 10.26] | 8.73 [4.58, 12.89] | −1.04 [−1.34, −0.74] |
| Social challenge | 0.08 [−0.21, 0.37] | 0.79 [−0.88, 2.47] | 2.38 [−1.69, 6.45] | 0.08 [−0.21, 0.37] |
| Physical challenge | −1.00 [−1.29, −0.71] | 5.96 [4.30, 7.62] | 6.87 [2.83, 10.92] | −1.00 [−1.29, −0.71] |
| Familiarity | 0.07 [−0.21, 0.36] | −0.24 [−1.87, 1.39] | −2.98 [−6.95, 0.99] | 0.07 [−0.21, 0.36] |
| R2 = .34 | R2 = .94 | R2 = .77 | R2 = .90 | |
Shown are the regressions for the four measures of aversiveness separately. The 95% confidence intervals for the beta coefficients are in brackets. Predictors that are statistically significant at p < .05 are bolded.
Discussion
This study examined how laypeople conceptualize side effects and how those conceptualizations may influence the aversiveness of a medication that confers a small risk of a side effect. In doing so, it extends prior research on medical treatment tradeoff decisions to incorporate research related to illness and symptom representations, as well as research examining the affective influences on risk perceptions and decisions.
The results indicate four key findings. First, there is considerable variability among the side effects in terms of how strongly people find them aversive. Second, the underlying structure of side effect perceptions can be described as having four components: as being related to one’s feelings, social interactions, and physical well-being, and the extent to which they are familiar. With the major exception of the affective challenge component, these results are mostly consistent with prior research on symptom perceptions [C.D. Jenkins as cited by 16, 17, 26]. Unlike other studies, we did not identify location of the body, viral etiology, and psychological etiology as key factors [17]. However, it is not possible to draw a direct comparison between those results and our own. Because our interest was in identifying the characteristics of side effects that are related to common medications [22] instead of illness symptoms more generally, we excluded from our list of side effects any symptoms that were not common side effects of common medications, but that Bishop identified in his research as being associated with those aspects (e.g., kidney pain, nasal congestion, and nervousness, respectively [17]). A study that examines conceptualizations of rarer side effects may uncover aspects related to bodily location.
The third key finding was that emotional components may be key drivers of aversive responses to side effects. Specifically, medications with side effects that people find affectively challenging were rated as the most aversive; the degree to which the side effect was socially challenging or familiar played little to no role. This aversion manifested as lower willingness to take the medication, higher willingness to pay for an equally effective medication that did not have the side effect, higher negative affective attitude, and higher ratings of undesirability. That affect might have a key role in medical treatment decisions that require trading off risks and benefits has been examined only relatively recently [2, 27–29]. Even less research has focused on particular side effects (e.g., [30]). Ours is the first study to identify affect as an integral component of the underlying structure of people’s conceptualizations of medication side effects and to link this component directly to multiple measures of aversiveness.
The consistent and profound association of affect with multiple measures of aversiveness highlights its importance for perceptions of side effects. It also provides support for the hypothesis that the disproportionately high negative impact of a side effect on treatment decisions (i.e., side effect aversion) may be due in part to the non-normative influence of affect that people associate with side effects [2, 3, 12, 13]. These results are also consistent with the role of affect in the formation of risk perceptions [31] and on judgment and decision making processes [19]. Indeed, decision makers need affect and emotions to be able to draw meaning from a given situation or piece of information [32] and to evaluate options that have very different characteristics [27]. Nevertheless, the direct causal link between affect and side effect aversion has yet to be established.
The fourth key finding was that side effects that were perceived as being physically challenging were nearly as aversive as those that elicited negative affect. This result was not anticipated, but it is consistent with public health research demonstrating that structural barriers, such as lack of employer-paid leave in the U.S., can thwart patients’ desire and intentions to obtain needed healthcare [33]. Further research needs to identify the interpersonal, institutional, and policy conditions under which medications that have physically challenging side effects are most likely to be perceived as most aversive.
Additional research is also needed to understand the process by which people integrate various cognitive, affective, practical, and social considerations when making treatment decisions. Furthermore, it is unclear whether decisions that incorporate both key normative criteria (e.g., probability) and non-normative considerations (e.g., affect, practical) are more consistent with key patient-centered outcomes than are decisions that rely solely on one or the other. Addressing these empirical questions will produce both theoretical and practical advances in medical decision making.
Practical Implications
These results could be translated to clinical and community settings in several ways. Assuming additional research identifies effective strategies to do so, physicians who prescribe medications that have a high potential for being perceived as affectively or physically challenging, such as depression, extreme fatigue, dizziness, and muscle pain could initiate conversations with patients about feelings they might have about taking these medications. Health psychologists, social workers, occupational therapists, and nurses might work with patients to develop coping strategies to overcome psychosocial concerns or practical daily living difficulties that arise due to experiencing side effects. More broadly, insurance companies and hospitals might explore whether in-home support services provided by visiting nurses might alleviate the psychological and practical burden conferred by these side effects.
The results of this study could also inform the development of patient and clinical decision support tools. Research examining whether and how decision tools can acknowledge the negative affect associated with side effects without causing undue concern in patients is needed. Research should also investigate the feasibility of prompting people to attend to probabilistic information [34]. However, these efforts should also ensure that any strategies that are developed are not misleading. For example, a visual display can draw attention to probabilistic information and reduce aversion [20], but using numerical formats that reduce patients’ comprehension of important risk information (e.g., 1 in X [35] and number-needed-to-treat/number-needed-to-harm [36]) is not acceptable.
Limitations
These results should be considered in the context of several limitations. First, this study utilized hypothetical scenarios that described a treatment as having only one side effect. Although this might raise concerns about the applicability of the findings to real medical decisions, our decision allowed us to examine a wider variety of potential side effects that potentially affect a larger number of patients than had we restricted our study to a single patient population (e.g., rheumatoid arthritis). Nevertheless, this research could be used to guide research examining specific diseases in more depth. Second, the sample was fairly homogenous: all participants were women and the vast majority were white and highly educated. It is critical that future research examines the content and behavioral consequences of side effect representations among socio-demographically diverse patients who are facing real medical decisions that have multiple side effects. It would also be interesting to see if the results of this study – particularly the results related to physical challenge – are as important in countries that have more extensive worker, parental, and health support policies (e.g., visiting nurses) than are available in the U.S.
It should also be noted that, due to concerns about over-burdening participants with a highly cognitively demanding study, we did not include the Beliefs about Medication scale. Examining whether representations vary among people with different medication beliefs is an important next step. Another important question is whether the underlying structures of potentially life-threatening side effects (e.g., cancer resulting from disease-modifying antirheumatic drugs) differ from those presented here.
Conclusions
Patient decisions are often acutely sensitive to the possibility of experiencing side effects of medical treatments. Our study suggests that affective responses to side effects and the potential for side effects to become physically challenging may have important roles in shaping acceptance of medications with side effects. Our study also offers insights regarding the basic components that structure perceptions of side effects. Together, these results help explain and predict which side effects might prompt people to reject otherwise helpful and necessary treatments.
Supplementary Material
Acknowledgments
Financial support for this study was provided by the Barnes-Jewish Hospital Foundation and the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Footnotes
We also conducted all analyses when z-standardizing the responses for each participant within each scale (but across side effects), because participants might vary systematically in their use of the rating scales. Although the resulting component structure was very similar to the one obtained with unstandardized ratings (see Supplemental Material), the Components 3 and 4 were considerably less interpretable. In addition the regression results accounted for less variance and were less consistent with public health research regarding the role of structural barriers in health decisions [33]. Therefore, we base our main conclusions on the analyses with the nonstandardized responses.
An exploratory factor analysis (oblique rotation) with weighted least-squares parameter estimation and using the fa() function of the psych package [25] in R yielded highly similar results as the PCA. Parameter estimation of the exploratory factor analysis with maximum likelihood, iterated principal axis, and minimum residual, however, resulted in factor loadings larger than 1 (“Heywood cases”) for some items, which is likely due to the rather small set of side effects relative to the number of items [37].
The authors have no conflicts of interest to report.
Contributor Information
Erika A. Waters, Division of Public Health Sciences, Washington University in St. Louis.
Thorsten Pachur, Center for Adaptive Rationality, Max Planck Institute for Human Development.
Graham A. Colditz, Division of Public Health Sciences, Washington University in St. Louis.
References
- 1.U.K. General Medical Council. Consent: patients and doctors making decisions together. London, UK: 2008. Available from: http://www.gmc-uk.org/guidance/ethical_guidance/consent_guidance_index.asp. [Google Scholar]
- 2.Pachur T, Galesic M. Strategy Selection in Risky Choice: The Impact of Numeracy, Affect, and Cross-Cultural Differences. J Behav Decis Mak. 2013;26(3):260–71. [Google Scholar]
- 3.Waters EA, Weinstein ND, Colditz GA, Emmons K. Aversion to side effects in preventive medical treatment decisions. Br J Health Psychol. 2007;12:383–401. doi: 10.1348/135910706X115209. [DOI] [PubMed] [Google Scholar]
- 4.Bauermeister JA, Meanley S, Pingel E, Soler JH, Harper GW. PrEP awareness and perceived barriers among single young men who have sex with men. Current HIV research. 2013;11(7):520–7. doi: 10.2174/1570162x12666140129100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraut A, Graff L, McLean D. Behavioral change with influenza vaccination: factors influencing increased uptake of the pandemic H1N1 versus seasonal influenza vaccine in health care personnel. Vaccine. 2011;29(46):8357–63. doi: 10.1016/j.vaccine.2011.08.084. [DOI] [PubMed] [Google Scholar]
- 6.Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol. 2001;8(7):580–5. doi: 10.1007/s10434-001-0580-9. [DOI] [PubMed] [Google Scholar]
- 7.Vickers AJ, Elkin EB, Peele PB, Dickler M, Siminoff LA. Long-term health outcomes of a decision aid: Data from a randomized trial of Adjuvant! in women with localized breast cancer. Med Decis Making. 2009;29:461–7. doi: 10.1177/0272989X08329344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen LA, Appelbaum PS, Klein WM, Weinstein ND, Cook W, Fogel JS, et al. Unrealistic optimism in early-phase oncology trials. IRB. 2011;33(1):1–8. Epub 2011/02/15. [PMC free article] [PubMed] [Google Scholar]
- 9.Harder H, Ballinger R, Langridge C, Ring A, Fallowfield LJ. Adjuvant chemotherapy in elderly women with breast cancer: patients’ perspectives on information giving and decision making. Psychooncology. 2013;22(12):2729–35. doi: 10.1002/pon.3338. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe F, Michaud K. Resistance of rheumatoid arthritis patients to changing therapy: discordance between disease activity and patients’ treatment choices. Arthritis Rheum. 2007;56(7):2135–42. doi: 10.1002/art.22719. [DOI] [PubMed] [Google Scholar]
- 11.Aikens JE, Nease DE, Jr, Nau DP, Klinkman MS, Schwenk TL. Adherence to maintenance-phase antidepressant medication as a function of patient beliefs about medication. Ann Fam Med. 2005;3(1):23–30. doi: 10.1370/afm.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waters EA, Weinstein ND, Colditz GA, Emmons K. Explanations for side effect aversion in preventive medical treatment decisions. Health Psychol. 2009;28(2):201–9. doi: 10.1037/a0013608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pachur T, Hertwig R, Wolkewitz R. The affect gap in risky choice: Affect-rich outcomes attenuate attention to probability information. Decision. 2014;1(1):64. [Google Scholar]
- 14.Bishop GD, Converse SA. Illness representations: a prototype approach. Health Psychol. 1986;5(2):95–114. doi: 10.1037//0278-6133.5.2.95. Epub 1986/01/01. [DOI] [PubMed] [Google Scholar]
- 15.Leventhal H, Nerenz DR, Steel DJ. Illness representations and coping with health threats. In: Baum A, Taylor SE, Singer DJ, editors. Handbook of Psychology and Health. Vol. 4. Hillsdale, NJ: Lawrence Erlbaum Associates; 1984. pp. 219–52. [Google Scholar]
- 16.Bishop GD. Understanding the understanding of illness: lay disease representations. In: Skelton JA, Croyle RT, editors. Mental Representation in Health and Illness. New York: Springer-Verlag; 1992. [Google Scholar]
- 17.Bishop GD. Lay conceptions of physical symptoms. J Appl Soc Psychol. 1987;17(2):127–46. [Google Scholar]
- 18.Slovic P. Perception of risk. Science. 1987;236:280–5. doi: 10.1126/science.3563507. [DOI] [PubMed] [Google Scholar]
- 19.Slovic P, Peters E, Finucane ML, Macgregor DG. Affect, risk, and decision making. Health Psychol. 2005;24(4 Suppl):S35–40. doi: 10.1037/0278-6133.24.4.S35. Epub 2005/07/28. [DOI] [PubMed] [Google Scholar]
- 20.Waters EA, Weinstein ND, Colditz GA, Emmons K. Reducing aversion to side effects in preventive medical treatment decisions. J Exp Psychol Appl. 2007;13(1):11–21. doi: 10.1037/1076-898X.13.1.11. [DOI] [PubMed] [Google Scholar]
- 21.Finucane ML, Slovic P, Mertz CK, Flynn J, Satterfield TA. Gender, race, and perceived risk: The ‘white male’ effect. Health, risk & society. 2000;2(2):159–72. [Google Scholar]
- 22.National Center for Health Statistics. National Ambulatory Medical Care Survey: 2008 Summary Tables: Ambulatory and Hospital Care Statistics Branch. Centers for Disease Control and Prevention, National Center for Health Statistics; 2008. [cited 2011 September 29]. Available from: http://www.cdc.gov/nchs/ahcd.htm. [Google Scholar]
- 23.Leventhal H, Brissette I, Leventhal EA. The common-sense model of self-regulation of health and illness. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behaviour. New York, NY: Routledge; 2003. pp. 42–65. [Google Scholar]
- 24.Lerner JS, Keltner D. Beyond valence: Toward a model of emotion-specific influences on judgement and choice. Cognition and Emotion. 2000;14(4):473–93. [Google Scholar]
- 25.Revelle W. psych: Procedures for Personality and Psychological Research, Version 1.5.8. Evanston, Illinois, USA: Northwestern University; 2015. [Google Scholar]
- 26.Jones RA, Wiese HJ, Moore RW, Haley JV. On the perceived meaning of symptoms. Med Care. 1981;19(7):710–7. doi: 10.1097/00005650-198107000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Peters E, Lipkus I, Diefenbach MA. The functions of affect in health communications and in the construction of health preferences. J Commun. 2006;56:S140–S62. [Google Scholar]
- 28.Dillard AJ, Ferrer RA, Ubel PA, Fagerlin A. Risk perception measures’ associations with behavior intentions, affect, and cognition following colon cancer screening messages. Health Psychol. 2011 doi: 10.1037/a0024787. Epub 2011/08/03. [DOI] [PubMed] [Google Scholar]
- 29.Pachur T, Hertwig R, Steinmann F. How do people judge risks: availability heuristic, affect heuristic, or both? J Exp Psychol Appl. 2012;18(3):314–30. doi: 10.1037/a0028279. Epub 2012/05/09. [DOI] [PubMed] [Google Scholar]
- 30.Phillips LA, Diefenbach MA, Abrams J, Horowitz CR. Stroke and TIA survivors’ cognitive beliefs and affective responses regarding treatment and future stroke risk differentially predict medication adherence and categorised stroke risk. Psychol Health. 2015;30(2):218–32. doi: 10.1080/08870446.2014.964237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slovic P, Fischhoff B, Lichtenstein S. Facts and fears: Understanding perceived risk. In: Schwing RC, Albers WAJ, editors. Societal risk assessment: How safe is safe enough? New York: Plenum; 1980. pp. 181–214. [Google Scholar]
- 32.Damasio A. Descartes’ error: Emotion, reason, and the human brain. New York, NY: Quill; 1994. [Google Scholar]
- 33.Hunleth JM, Steinmetz EK, McQueen A, James AS. Beyond Adherence: Health Care Disparities and the Struggle to Get Screened for Colon Cancer. Qual Health Res. 2015 doi: 10.1177/1049732315593549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rottenstreich Y, Kivetz R. On decision making without likelihood judgment. Organ Behav Hum Decis Process. 2006;101:74–88. [Google Scholar]
- 35.Zikmund-Fisher BJ. Time to retire the 1-in-X risk format. Med Decis Making. 2011;31(5):703–4. doi: 10.1177/0272989X11418238. Epub 2011/09/17. [DOI] [PubMed] [Google Scholar]
- 36.Sheridan SL, Pignone MP, Lewis CL. A randomized comparison of patients’ understanding of number needed to treat and other common risk reduction formats. J Gen Intern Med. 2003;18:884–92. doi: 10.1046/j.1525-1497.2003.21102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costello AB, Osborne JW. Exploratory Factor Analysis: Four recommendations for getting the most from your analysis. Practical Assessment, Research, and Evaluation. 2005;10(7):1–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.