Abstract
Objective
To investigate the risk of malignancy in patients with sarcoidosis in a population-based cohort.
Methods
A cohort of Olmsted County, Minnesota residents diagnosed with sarcoidosis between January 1, 1976 and December 31, 2013 was identified based on individual medical record review. For each sarcoidosis subject, two sex and aged-matched comparator subjects without sarcoidosis were randomly selected. Cases and comparators were then cross-indexed with the Mayo Clinic Cancer Registry, which collected data on every type of malignancy except for non-melanoma skin cancer, for malignancy ascertainment.
Results
345 incident cases of sarcoidosis and 690 comparators were identified. There was no difference in the prevalence of malignancy at index date between the two groups (4.3% among cases and 4.3% among comparators, P = 1.0). During follow-up, 36 patients with sarcoidosis and 91 subjects without sarcoidosis developed malignancy with a cumulative incidence at 10 years of 3.8% and 7.1%, respectively. The difference corresponded to a hazard ratio (HR) of 0.72 (95% confidence interval (CI), 0.49 -1.06). The cumulative incidences at 10 years for individual types of malignancy were also similar between the two groups with non-significant HRs. However, subgroup analysis found that cases with extra-thoracic involvement were at higher risk of incident hematologic malignancy compared with cases without extra-thoracic involvement (HR 1.87; 95% CI 1.09-3.22)
Conclusion
Risk of malignancy was similar among patients with sarcoidosis compared to non-sarcoidosis subjects. However, the risk of incident hematologic malignancy was significantly higher among patients with sarcoidosis with extra-thoracic involvement compared to patients without extra-thoracic disease.
Keywords: Sarcoidosis, Epidemiology, Malignancy, Comorbidity
Introduction
Sarcoidosis is a chronic inflammatory disorder that typically affects the lungs and intrathoracic lymph nodes although it can affect virtually any organ. Non-caseating granuloma is the histo-pathological hallmark of the disease [1]. The reported annual incidence varies among different ethnic groups, ranging from less than 1 per 100,000 to 71 per 100,000 [2]. The etiology of sarcoidosis is not known but interaction between genetic predisposition and environmental factors is believed to play a pivotal role in its pathogenesis. Several studies have demonstrated an association between risk of sarcoidosis and some major histocompatibility complex alleles and tumor necrosis factor (TNF) polymorphism [3].
The possible relationship between malignancy and sarcoidosis has been a subject of debate for decades. Several case reports and case series had observed patients who were diagnosed with cancer shortly before or after the diagnosis of sarcoidosis [4]. Nonetheless, subsequent epidemiologic studies have yielded inconsistent results, ranging from no association to a significantly elevated risk with relative risk (RR) of 1.7 [5, 6]. A meta-analysis published in 2014 summarizing the effect estimates from all available studies found a small but significantly increased risk for malignancy (RR 1.21; 95% CI 1.04 -1.40). A sensitivity analysis from that study that included only cases of malignancy diagnosed after the first year of diagnosis of sarcoidosis did not show a significantly elevated risk [7].
To shed more light on this controversial issue, this population-based cohort study was pursued to compare the risk of malignancy among patients with sarcoidosis and age and sex-matched comparators without a diagnosis of sarcoidosis.
Methods
Data source and study population
Through the resources of the Rochester Epidemiology Project (REP), the population of Olmsted County, Minnesota, is well suited for investigation of the association between sarcoidosis and malignancy as complete medical records for all residents seeking medical care for over 60 years are available. The record linkage system allows ready access to the inpatient and outpatient medical records from all health care providers for the local population, including the Mayo Clinic, the Olmsted Medical Center, local nursing homes, and the few private practitioners. The application of this data system for use in epidemiologic studies has previously been described and assures virtually complete clinical information for all sarcoidosis and comparator subjects among Olmsted County, Minnesota residents [8]. This study was approved by the institutional review boards of the Mayo Clinic and the Olmsted Medical Center.
Study design
A cohort of Olmsted County, Minnesota residents diagnosed with sarcoidosis between January 1, 1976 and December 31, 2013 was identified. Potential cases were initially screened using diagnostic codes related to sarcoid, sarcoidosis, and non-caseating granuloma. Diagnosis of sarcoidosis was then verified based on individual medical record review. Inclusion required physician diagnosis supported by histopathologic evidence of non-caseating granuloma, radiographic evidence of intrathoracic sarcoidosis, compatible clinical presentation, and exclusion of other granulomatous diseases. The only exception to the requirement of histopathology was stage I pulmonary sarcoidosis that required only the radiographic feature of symmetric bilateral hilar adenopathy. Biopsy-proven isolated granulomatous disease of a specific organ except for the skin was also included if there were no better alternative diagnosis.
The case definition for sarcoidosis was based on the Statement on Sarcoidosis by the American Thoracic Society [9]. Cases with a diagnosis of sarcoidosis prior to becoming an Olmsted County resident (prevalent cases) were excluded. All confirmed cases of sarcoidosis were then further categorized into two subgroups including sarcoidosis with extra-thoracic involvement and sarcoidosis without extra-thoracic involvement (in other words, sarcoidosis with only intra-thoracic involvement). Extra-thoracic involvement was defined as the presence of organ other than lungs and intra-thoracic lymph node involvement by sarcoidosis including uveitis, conjunctivitis, central nervous system involvement, sarcoidosis-specific skin lesions, erythema nodosum, extra-thoracic lymph node involvement, gastrointestinal sarcoidosis, hepatic sarcoidosis, cardiac sarcoidosis, arthritis, bone and muscle involvement, splenic involvement, renal involvement, exocrine gland involvement and endocrine gland involvement.
For each sarcoidosis subject, two comparator subjects without sarcoidosis at the time of the patient's sarcoidosis diagnosis were randomly selected and assigned an index date that corresponded to the sarcoidosis incidence date. Matching criteria were similar age (±3 years) and same sex. For malignancy ascertainment, cases and comparators were then cross-indexed with the Mayo Clinic Cancer Registry, which collected data on every type of malignancy except for non-melanoma skin cancer.
Statistical analysis
Descriptive statistics (means, proportions etc.) were used to summarize the data for the sarcoidosis and non-sarcoidosis cohorts. The proportion of patients with malignancies prior to sarcoidosis incidence/index date was compared between the cohorts using chi-square tests. The cumulative incidence of malignancy in patients with and without sarcoidosis adjusted for the competing risk of death was estimated. These methods are similar to the Kaplan-Meier method with censoring of patients who are still alive at last follow up. However, patients who died prior to experiencing malignancy were appropriately accounted for to avoid overestimation of the rate of occurrence of malignancy, which may have occurred if such subjects were simply censored at death. Patients/comparators with a history of malignancy prior to sarcoidosis incidence/index date were removed from these analyses because they were not at risk of developing malignancy. Cox proportional hazards models adjusted for age, sex and calendar year of sarcoidosis incidence/index date were used to compare the rate of development of malignancy, both overall and by site, between patients with sarcoidosis and the non-sarcoidosis comparison cohort. These same models were also used to assess the risk of malignancy associated with extra-thoracic involvement among the patients with sarcoidosis.
Results
For the years 1976-2013, 345 incident cases of sarcoidosis were identified. 205 of them had only intra-thoracic sarcoidosis while 140 cases had with extra-thoracic disease (130 in association with intra-thoracic disease and 10 without intra-thoracic involvement). The most common type of extra-thoracic involvement in this cohort was skin rash (18%) followed by arthritis (12%), ophthalmologic involvement (6%) and splenomegaly (4%). The details of the clinical characteristics of this cohort of sarcoidosis were reported previously [10]. There were 690 sex and age-matched subjects without sarcoidosis identified as comparators. The mean age and percentage of female were similar in the two cohorts (cases, 45.6 years and 50.4%; comparators, 45.4 years and 50.4%). The median time of follow up for cases and comparators was 12.9 years and 12.5 years, respectively.
Overall, there was no difference in the prevalence of malignancy at sarcoidosis incidence/index date among patients with sarcoidosis (15 cases, 4.3%) compared to non-sarcoidosis subjects (30 cases, 4.3%), P = 1.0. However, there were more prevalence cases of cancer diagnosed within three months prior to sarcoidosis incidence/index date among patients with sarcoidosis (6 cases, 1.7%) compared to non-sarcoidosis subjects (1 case, 0.1%), P = 0.007. During follow-up, 36 patients with sarcoidosis and 91 subjects without sarcoidosis developed malignancy with cumulative incidences at 10 years of 3.8% and 7.1%, respectively. The difference corresponded to hazard ratio (HR) of 0.72 (95% confidence interval (CI), 0.49 -1.06) (Figure 1A). The cumulative incidences at 10 years for individual type of malignancy were also similar between patients with sarcoidosis and non–sarcoidosis comparators; hazard ratios were non-significant (Table 1). However, subgroup analysis found that cases with extra-thoracic involvement were at higher risk of incident hematologic malignancy compared with cases without extra-thoracic involvement. There were 5 incident cases of hematologic malignancy (2 chronic myelogenous leukemias, 2 diffuse large B-cell lymphomas and 1 Hodgkin's lymphoma) in sarcoidosis with extra-thoracic involvement group while there was only 1 incident case of hematologic malignancy (acute myeloid leukemia) in sarcoidosis without extra-thoracic involvement group which corresponded to HR of 1.87, 95% CI 1.09-3.22) (Figure 1B). Risk of overall and solid malignancy was also higher among those with extra-thoracic disease but was not statistically significant (HR 1.26; 95% CI 0.64-2.49 and HR 1.17; 95% CI 0.73-1.88, respectively) (Table 2).
Figure 1.
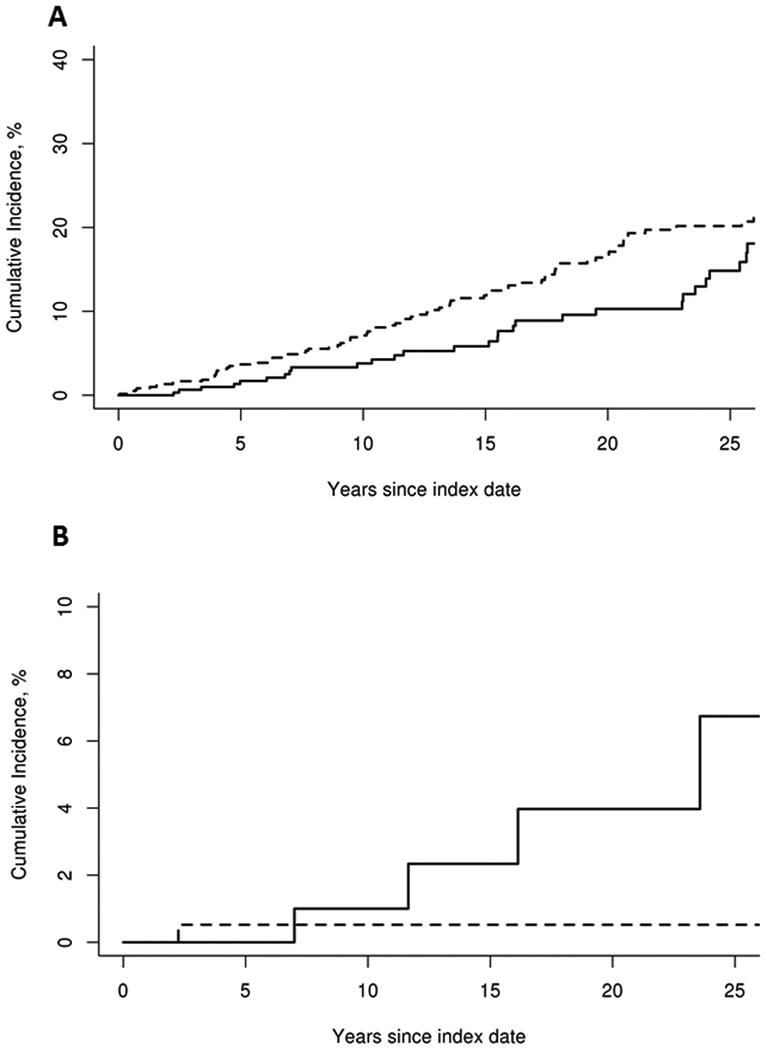
A: Cumulative incidence of any cancer excluding non-melanoma skin cancers (sarcoidosis-solid line; non-sarcoidosis dashed line).
B: Cumulative incidence of hematologic cancer (sarcoidosis with extra-thoracic involvement: solid line; sarcoidosis without extra-thoracic involvement: dashed line)
Table 1. Cumulative incidence rate of malignancy in 345 patients with sarcoidosis in 1976-2013 compared to 690 subjects without sarcoidosis.
| Malignancy Site | Number of events after incidence/index in sarcoidosis / non-sarcoidosis subjects | Cumulative incidence at 10 years in patients with sarcoidosis (± SE)* | Cumulative incidence at 10 years in comparator subjects without sarcoidosis (± SE)* | Hazard ratio (95% confidence interval)** |
|---|---|---|---|---|
| Any malignancy (excluding non-melanoma skin cancers) | 36 / 91 | 3.8 ± 1.2 | 7.1 ± 1.1 | 0.72 (0.49, 1.06) |
| Solid | 30 / 86 | 3.0 ± 1.1 | 6.7 ± 1.1 | 0.90 (0.68, 1.19) |
| Hematologic | 6 / 6 | 0.7 ± 0.5 | 0.6 ± 0.3 | 0.98 (0.70, 1.35) |
| Specific type of cancer*** | ||||
| Head/neck | 3 / 5 | 0.0 ± 0.0 | 0.3 ± 0.2 | 0.94 (0.68, 1.30) |
| Colon/rectal | 3 / 12 | 0.3 ± 0.3 | 0.7 ± 0.3 | 0.93 (0.67, 1.29) |
| Other digestive | 3 /24 | 0 | 0.2 ± 0.2 | 0.94 (0.68, 1.31) |
| Lung | 3 / 14 | 0.3 ± 0.3 | 0.8 ± 0.4 | 0.94 (0.68, 1.30) |
| Skin (excluding non-melanoma) | 6 / 15 | 0.4 ± 0.4 | 1.4 ± 0.5 | 0.90 (0.66, 1.23) |
| Breast (female only; no male events) | 5 / 15 | 0.8 ± 0.8 | 2.0 ± 0.8 | 0.79 (0.52, 1.19) |
| Prostate (male only) | 7 / 12 | 1.5 ± 1.0 | 2.5 ± 1.0 | 1.09 (0.69, 1.73) |
Cumulative incidence is adjusted for the competing risk of death
Adjusted for age, sex and calendar year of sarcoidosis incidence/index date
Data on gastric, pancreatic, liver, bone, ovary, kidney, bladder and ophthalmologic malignancies were not shown as the number of events was small (<5 per group) and cumulative incidence was not be estimated
Table 2. Cumulative incidence rate of malignancy in 345 patients with sarcoidosis in 1976-2013 (comparing 140 patients with systemic involvement to 205 patients without systemic involvement.
| Malignancy Site | Prior to Sarcoidosis incidence: Systemic / non-systemic, no. | p-value comparing prior events* | Number of events after incidence: systemic / non-systemic | Cumulative incidence at 10 years for systemic Sarcoidosis patients (± SE)** | Cumulative incidence at 10 years for non-systemic Sarcoidosis subjects (± SE)** | Hazard ratio (95% confidence interval)*** |
|---|---|---|---|---|---|---|
| Any malignancy (excluding non-melanoma skin cancers) | 5 / 10 | 0.60 | 14 / 22 | 3.7 ± 1.8 | 3.9 ± 1.8 | 1.26 (0.64, 2.49) |
| Solid malignancy | 5 / 8 | 1.0 | 9 / 21 | 2.7 ± 1.5 | 3.3 ± 1.5 | 1.17 (0.73, 1.88) |
| Hematologic malignancy | 0 / 2 | 0.52 | 5 / 1 | 1.0 ± 1.0 | 0.5 ± 0.5 | 1.87 (1.09, 3.22) |
Fisher's exact test
Cumulative incidence is adjusted for the competing risk of death.
adjusted for age, sex and calendar year of sarcoidosis incidence/index date
Discussion
A possible causal relationship between sarcoidosis and malignancy has been a subject of debate for decades, since the first epidemiologic study by Brincker and Wilbek reporting a higher than expected incidence of malignancy in the nationwide Danish sarcoidosis registry was published in 1974 [11]. However, the validity of the study was questioned as almost one-third of patients in the registry may have been misdiagnosed [12]. Moreover, subsequent epidemiologic studies have yielded inconsistent results [7].
The current study was conducted in an attempt to shed more light on this controversial issue, and is the first study that utilized population-based cohort to investigate the relationship between sarcoidosis and malignancy. Neither the overall incidence of malignancy, not the incidence of specific types of malignancy was increased in patients with sarcoidosis. However, subgroup analysis revealed that the risk of incident hematologic malignancy was almost double among those with extra-thoracic involvement compared to those with only intra-thoracic disease. This finding suggests that the risk of malignancy is elevated only in the subgroup of patients with more extensive disease.
It has been postulated that the dysregulation of the immune system in patients with sarcoidosis could predispose then to the development of malignancy due to the impaired cancer-surveillance immunity. Moreover, the increased mitotic activity of lymphocytes in the lymph nodes and involved tissues of these patients could increase the likelihood of mutation and subsequent malignant transformation [13, 14]. This hypothesis is well supported by the results of the current study as hematologic malignancy was the only subtype of malignancy that was markedly more frequent.
Abnormal antigens found in malignant tissue can trigger immune reactions. Non-caseating epithelioid granulomas are frequently observed in the lymph nodes that receive drainage from cancerous tissue. This phenomenon is sometimes called a sarcoid-like reaction and is reported in up to 13% of patients with lymphoma and 4% of patients with carcinoma [15]. It has been postulated that the presence of these non-caseating epithelioid granulomas might account for the apparent increased incidence of malignancy shortly after the diagnosis of sarcoidosis, as those patients might present with enlarged lymph nodes and be diagnosed with sarcoidosis before the primary tumor is found [14].
This hypothesis is supported by previous studies that observed a significantly increased incidence of malignancy within the first years of sarcoidosis diagnosis, with a sharp decline of the incidence afterward [6]. However, the current study does not support this hypothesis as the cumulative incidence of malignancy among patients with sarcoidosis was consistently lower than non-sarcoidosis comparators, even during the first year after diagnosis/incidence date. Moreover, the cumulative incidence of hematologic malignancy among patients with extra-thoracic involvement was not higher compared to those without extra-thoracic disease until approximately seven years after diagnosis. Nonetheless, the prevalence of malignancy diagnosed within three months prior to index date was significantly higher among patients with sarcoidosis, possibly supporting the notion that some cancers may be associated with sarcoid-like reactions.
The major strengths of this study are that it is population-based with a long period of follow-up. The comprehensive record-linkage system allows capture of nearly all diagnosed cases of sarcoidosis in this population. Therefore, the disease expression in this cohort should represent the actual spectrum of the disease in the community, unlike hospital-based cohorts. Diagnosis of sarcoidosis was verified by medical record review, minimizing the risk of disease misclassification, a common concern in coding-based studies. The duration of follow-up was long with median of over 12 years which would allow detection of malignancy that developed long after the diagnosis/incidence date.
The major limitations of the study are those of the retrospective study design as the case ascertainment depends on diagnosis being made by the physicians and other health-care providers. Generalizability of the findings is another concern as the population of Olmsted County is predominately of Northern European ancestry with a higher proportion of health-care workers and correspondingly higher education level and socioeconomic status. The health-care system in Olmsted County is relatively more unified. As the clinical presentation of sarcoidosis varies among ethnic groups [1, 2], it is likely that the risk of malignancy could be different in a more diverse population. Finally, statistical power was limited for comparisons among sarcoidosis patients with and without extra-thoracic involvement, a potential subject for a future larger study.
Conclusion
There was no difference in the prevalence and cumulative incidence of malignancy among patients with sarcoidosis compared to non-sarcoidosis subjects. However, the risk of incident hematologic malignancy was significantly higher among patients with sarcoidosis with extra-thoracic involvement compared to patients without extra-thoracic disease.
Significance and innovation.
-
-
Cumulative incidence of malignancy at 10 year was not increased in patients with sarcoidosis compared to non-sarcoidosis subjects
-
-
Risk of incident hematologic malignancy was significantly higher among patients with sarcoidosis with extra-thoracic involvement compared to patients without extra-thoracic disease.
Acknowledgments
Funding: This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Thomas WK, Hunninghake GW. Sarcoidosis. JAMA. 2003;289:3300–3. doi: 10.1001/jama.289.24.3300. [DOI] [PubMed] [Google Scholar]
- 2.Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: A 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 3.Newman LS, Rose CS, Bresnitz EA, Rossman MD, Barnard J, Frederick M, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324–30. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 4.Suen JS, Forse MS, Hyland RH, Chan CK. The malignancy-sarcoidosis syndrome. Chest. 1990;96:1300–1. doi: 10.1378/chest.98.5.1300. [DOI] [PubMed] [Google Scholar]
- 5.Askling J, Grunewald J, Eklund A, Hillerdl G, Ekbom A. Increased risk of cancer following sarcoidosis. Am J Respir Crit Care Med. 1999;160:1668–72. doi: 10.1164/ajrccm.160.5.9904045. [DOI] [PubMed] [Google Scholar]
- 6.Ji J, Shu X, Li X, Sundquist K, Sunquist J, Hemminki K. Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann Oncol. 2009;20:1121–6. doi: 10.1093/annonc/mdn767. [DOI] [PubMed] [Google Scholar]
- 7.Ungprasert P, Srivali N, Wijarnpreecha K, Thongprayoon C, Cheungpasitporn W, Knight EL. Is the incidence of malignancy increased in patients with sarcoidosis? A systematic review and meta-analysis Respirology. 2014;19:993–8. doi: 10.1111/resp.12369. [DOI] [PubMed] [Google Scholar]
- 8.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 9.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, et al. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–73. [PubMed] [Google Scholar]
- 10.Ungprasert P, Carmona EM, Utz JP, Ryu JH, Crowson CS, Matteson EL. Epidemiology of sarcoidosis 1946-2013: A population-based study. Mayo Clin Proc. 2016;91:183–8. doi: 10.1016/j.mayocp.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer. 1974;29:247–51. doi: 10.1038/bjc.1974.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romer FK. Is there any causal relationship between sarcoidosis and malignancy? Sarcoidosis. 1990;7:80–4. [PubMed] [Google Scholar]
- 13.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006;203:359–70. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma OP, Lamb C. Cancer in interstitial pulmonary fibrosis and sarcoidosis. Curr Opin Pulm Med. 2003;9:398–401. doi: 10.1097/00063198-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Brincker H. Sarcoid reactions in malignant tumours. Cancer Treat Rev. 1986;13:147–56. doi: 10.1016/0305-7372(86)90002-2. [DOI] [PubMed] [Google Scholar]


