Abstract
Transforming growth factor β1 (TGFβ1) plays a key role in T cell homeostasis and peripheral tolerance. We evaluated the influence of a novel human mutant TGFβ1/Fc protein on memory CD4+ and CD8+ Tcell (Tmem) responses in vitro and their recovery following anti-thymocyte globulin (ATG)-mediated lymphodepletion in monkeys. TGFβ1/Fc induced Smad-2/3 protein phosphorylation in rhesus and human peripheral blood mononuclear cells and augmented the suppressive effect of rapamycin on rhesus Tmem proliferation after either alloactivation or anti-CD3/CD28 stimulation. In combination with IL-2, the incidence of CD4+CD25hiFoxp3hi regulatory T cells (Treg) and Treg/Th17 ratios were increased. In lymphodepleted monkeys, whole blood trough levels of infused TGFβ1/Fc were maintained between 2–7 μg/mL for 35 days. Following ATG administration, total T cell numbers were reduced markedly. In those given TGFβ1/Fc infusion, CD8+T cell recovery to pre-depletion levels was delayed compared to controls. Additionally, numbers of CD4+CD25hiCD127lo Treg increased at 4–6 weeks after depletion but subsequently declined to pre-depletion levels by 12 weeks. In all monkeys, CD4+CD25hiFoxp3hi Treg/CD4+IL-17+ cell ratios were reduced, particularly after stopping TGFβ1/Fc infusion. Thus, human TGFβ1/Fc infusion may delay Tmem recovery following lymphodepletion in nonhuman primates. Combined (low-dose) IL-2 infusion may be required to improve the Treg/Th17 ratio following lymphodepletion.
Introduction
Lymphocyte depletion is currently performed at the time of transplantation and is associated with both prolonged graft survival and reduced intensity maintenance immunosuppression (1–4). In both rodents and humans however, lymphodepletion is followed by compensatory lymphopenia-induced homeostatic proliferation that results in rapid expansion of memory T cells (Tmem), mainly of an effector phenotype (5, 6). These Tmem are resistant to immunosuppressive (IS) therapy (7), paradoxically favor rejection and present a barrier to transplantation tolerance (8–11). Indeed, T cell lymphopenia is associated with the breakdown of immune tolerance in rodents and humans (7, 12–15).
Transforming growth factor beta 1 (TGFβ1) is a pleiotropic cytokine that is critical for T cell homeostasis and regulatory T cell (Treg) development (16–20). In rodents, TGFβ1 signaling is required to prevent autoimmune responses during lymphopenia-induced proliferation (21). TGFβ1 signaling is also critical for Tmem suppression (22). Previously, it has been shown that in rodents, infusion of the novel immunoligand human mutant TGFβ1/Fc protein (TGFβ1/Fc), especially in combination with rapamycin, promotes Treg generation while inhibiting Th17 differentiation. In addition, short-term combined TGFβ1/Fc and rapamycin administration induces tolerance to pancreatic islet allografts (23).
Nonhuman primates (NHP) are robust pre-clinical models for testing promising new approaches to overcoming barriers to tolerance induction. As in rodents and humans (5, 8), lymphodepletion in NHP is associated with enhanced recovery of effector T cells and effector Tmem (24, 25). Here, we report for the first time on the impact of human TGFβ1/Fc infusion on the recovery of Tmem and Treg in rhesus monkeys following T cell depletion.
Materials and Methods
Animals
Healthy male juvenile rhesus macaques (Macaca mulatta) of Indonesian origin, weighing 5–7 kg, were obtained from the NIAID-sponsored rhesus macaque colony (Yamassee, SC). All monkeys were maintained in the NHP Research Facility of the Department of Laboratory Animal Resources at the University of Pittsburgh School of Medicine. All animal procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Experiments were conducted according to the guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Specific environment enrichment was provided.
Construction and characterization of human TGFβ1/Fc
Human TGFβ1/Fc was constructed as described (23). Briefly, human mutant TGFβ1 cDNA was fused with human Fcγ4 cDNA to extend its circulating t1/2. This cDNA was subcloned into an expression vector and packaged in a retroviral vector used to transduce Chinese hamster ovary cells using the GPEx™ expression technology (Catalent Pharma Solutions, Madison, WI). A pool of transduced cells was grown in serum-free medium and secreted fusion protein was purified by protein A affinity chromatography. The purified final product was diafiltered into phosphate buffer, pH 7.2. The mature, biologically active TGFβ1/Fc fusion protein has a mass of 190 kDa, exclusive of glycosylation. The capacity of the final TGFβ1/Fc product to activate the Smads pathway was assessed using Western blot.
T cell proliferation assays
Fresh peripheral blood mononuclear cells (PBMC) were isolated from healthy monkeys. To assess T cell proliferation by CD3/CD28 activation, flat-bottom 96-well plates were coated with 1μg/mL anti-human CD3 Ab (Clone SP34-2; BD Pharminogen). PBMC were then labeled with 4 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) and plated at a final density of 2×105 cells/well in the presence of 1μg/mL soluble anti-human CD28 Ab (Clone CD28.2; eBioscience) for 3–4 days. Additionally, PBMC were cultured in the absence or presence of rapamycin (1 ng/mL, LC Laboratories, Woburn, MA) and human TGFβ1/Fc (1 μg/mL or 10 μg/mL), with or without recombinant human IL-2 (300 U/mL, R&D Systems, Minneapolis, MN). In other experiments, PBMC were cocultured with allogeneic T cell-depleted PBMC for 4–5 days to evaluate the proliferation of alloreactive T cells.
Proliferation was determined by CFSE dye dilution. Percent suppression was determined as: (percent dye dilution without additional agents – percent dye dilution with indicated agents)/percent dye dilution without additional agents X 100%. Cultures of responder cells without stimulation were used as controls.
Smads pathway activation by western blot
Rhesus and human PBMC were rested in serum-free IMDM medium overnight, followed by exposure to 10 ng/mL human recombinant (r) TGFβ1 (R&D Systems, Minneapolis, MN) or TGFβ1/Fc (5 μg/mL) for 30 min. Protein extracts were obtained using RIPA buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton x-100, 1% sodium deoxycholate, 0.1%SDS, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride and 1 protease inhibitor mini tablet (Pierce, Rockford, lL)/10 mL. Protein content was determined using the Pierce™ BCA Protein Assay Kit (Rockford, IL). Samples were resolved in an SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to a nitrocellulose membrane. Western blotting was performed with phospho-Smad2/3 (Ser465/467) Abs (Cell Signaling, Boston, MA).
TGFβ1/Fc infusion and immunosuppressive (IS) drug regimen
To determine its pharmacokinetics, one dose of TGFβ1/Fc (5mg/kg) was infused intravenously in 2 healthy naïve rhesus monkeys, followed by serum sample collection at 30 min, 4, 24, 48 hours and 5 days after infusion. To achieve lymphodepletion, four monkeys received 4 consecutive intravenous (i.v.) infusions of rabbit anti-thymocyte globulin (ATG; Genzyme, Boston, MA) at 10 mg/kg on day 0, then 5 mg/kg on days 2, 9 and 16. Intramuscular rapamycin (target blood trough levels 5–10 ng/mL; LC Laboratories) was given daily, starting on day -2 up to 3 months. TGFβ1/Fc (5mg/kg) was infused intravenously to two IS monkeys starting after the first dose of ATG, twice a week for 4 weeks. Blood samples were collected twice a week to monitor TGFβ1/Fc kinetics.
Measurement of serum TGFβ1/Fc levels
Plates were coated with monoclonal anti-human IgG4 (clone HP6025, Life Technologies, Grand Island, NY) at a concentration of 1 μg/mL and blocked with Super Block (Thermo Scientific, Woodstock, GA). This capture antibody reacts with the human IgG4 Fc portion of the fusion protein but lacks reactivity with macaque immunoglobulins. Pre- and post- treatment samples were serially diluted in phosphate-buffered saline (PBS; 2%) bovine serum albumin, plated for 1 hour, and washed with PBS/0.05% Tween 20. TGFβ1/Fc was detected by incubating with polyclonal goat anti-human IgG - horseradish peroxidase (Jackson ImmunoResearch Labs, West Grove, PA). Plates were then incubated with 1-Step Ultra TMB ELISA Substrate (Thermo Scientific). TMB stop solution (KPL, Gaithersburg, MD) was added and the absorbance read on Mithras LB 940 microplate reader (Berthold Technologies, Calmbacher, Germany) at 450nm. A standard curve was generated using serum spiked with known quantities of TGFβ1/Fc and used to calculate the TGFβ1/Fc concentration present in specimens.
Phenotypic analysis of T cells
As described previously (26), PBMC were stained at 4°C with the following fluorochrome-labeled anti-human Abs: anti-CD3 (clone SP34-2), anti-CD4 (OKT4), anti-CD8 (RPA-T8), anti-CD25 (BC96), anti-CD28 (CD28.2), anti-CD45RA (5H9), anti-CD62L (SK11) and anti-CD127 (HIL-7R-M21) from BD Pharminogen (Franklin Lakes, NJ). Anti-Foxp3 (206D) was obtained from BioLegend (San Diego, CA). Anti-IL-17 (eBio64Dec17) and anti-Ki67 (20Raj1) were obtained from eBioscience (San Diego, CA). Data were acquired on a LSR II or LSR Fortessa (BD Bioscience) and analyzed with FlowJo software (Tree Star, Ashland, OR, USA). Absolute counts of Tmem and Treg were determined using CountBright™ absolute counting beads (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol.
Following ATG-mediated lymphocyte depletion, whole blood samples were collected once a week to determine T cell subsets. At one month post-infusion, inguinal lymph nodes (LN) were collected and lymphocyte phenotypic analysis performed. Data were analyzed with FlowJo software (Tree Star) and graphed with GraphPad Prism (Graph Pad Software, San Diego, CA).
Statistical analysis
Differences between means were evaluated using the Mann-Whitney test or ANOVA, as appropriate. Statistical analyses were conducted using Prism Graphpad software.
Results
TGFβ1/Fc acts together with rapamycin to markedly suppress T cell proliferation and reduces Ki67 expression in vitro
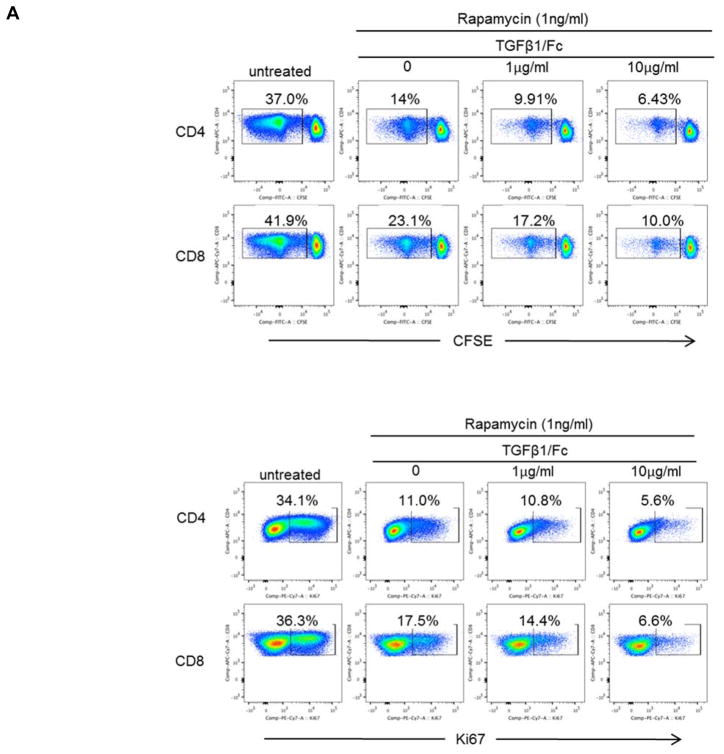
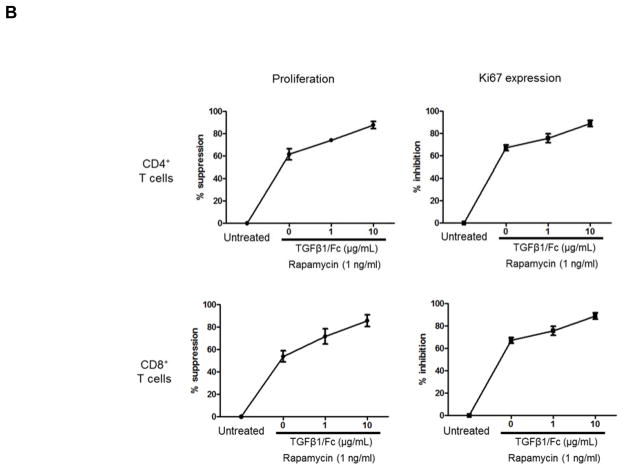
To examine the influence of TGFβ1/Fc combined with rapamycin on rhesus monkey CD4+ and CD8+ T cell proliferation, healthy rhesus PBMC were stimulated with either allogeneic T cell-depleted PBMC or anti-CD3/CD28 mAb. In the presence of rapamycin alone (1 ng/mL), proliferation of allo-stimulated CD4+ and CD8+ T cell were reduced by 40–60% (Figure 1A and 1B), whereas combination of TGFβ1/Fc and rapamycin resulted in stronger suppression in a TGFβ1/Fc concentration-related manner. Consistent with the observed suppressive effect on proliferation, TGFβ1/Fc together with rapamycin markedly inhibited expression of Ki67, a key marker associated with cell proliferation (Figure 1A and 1B).
Figure 1. TGFβ1/Fc augments the suppressive effects of rapamycin on rhesus monkey global T cell proliferation.
(A) CFSE-labeled rhesus monkey PBMC were cultured for 4 days with T cell-depleted allogeneic stimulators in the absence or presence of rapamycin (1 ng/mL) and the indicated concentrations of TGFβ1/Fc (0, 1, 10 μg/mL). CD4+ and CD8+ T cell proliferation was measured by CFSE dilution (top). Additionally, inhibition of Ki67 expression by CD4+ and CD8+ T cells following allostimulation was evaluated (bottom). (B) Combined data from 3 different experiments for 3 different monkey pairs. Data represent means ± SD of three independent experiments using different responders and stimulator-responder pairs. PBMC, peripheral blood mononuclear cell.
Similarly, stronger inhibitory effects of CD4+ and CD8+ T cell proliferation were observed in the presence of TGFβ1/Fc and rapamycin following CD3/CD28 mAb activation (Supplementary Figure 1).
TGFβ1/Fc augments the suppressive effects of rapamycin on CD4+ and CD8+ memory T cell proliferation in vitro
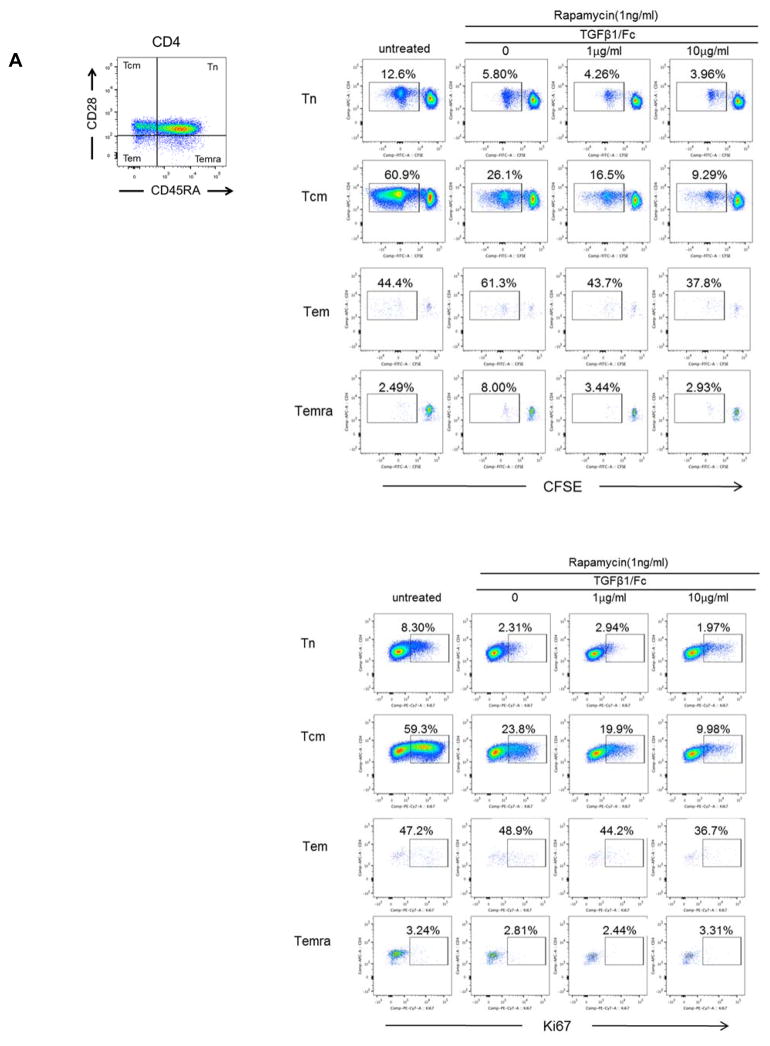
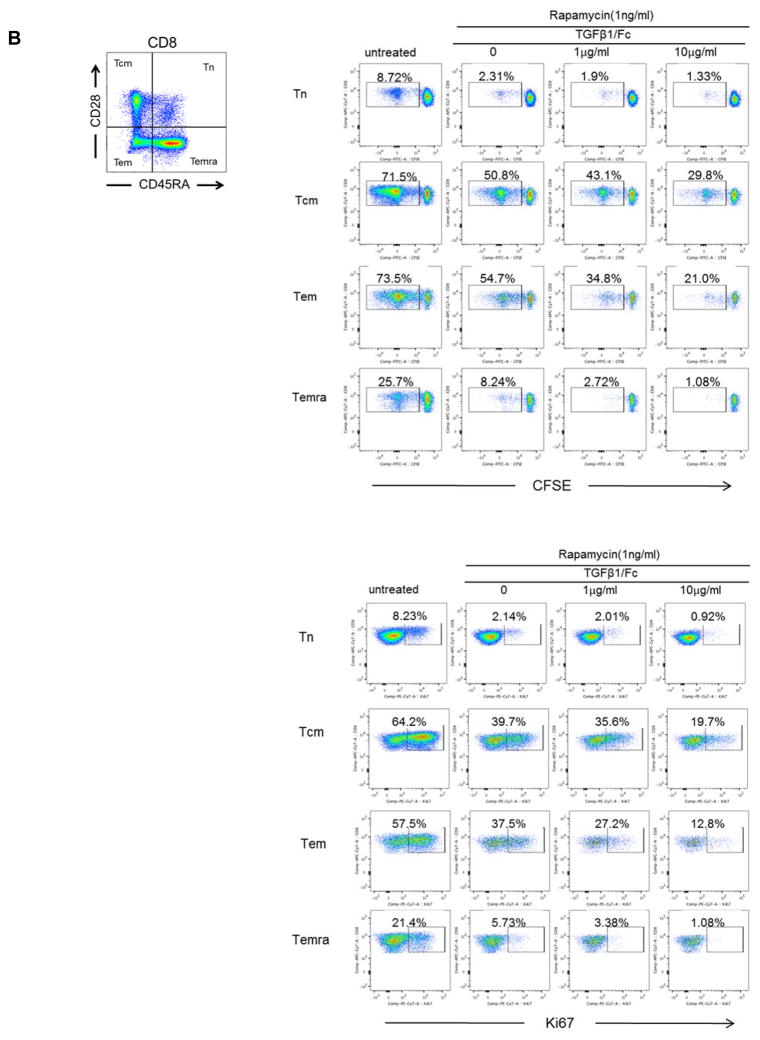
We next evaluated the influence of TGFβ1/Fc on Tmem subsets following alloAg stimulation. Rhesus CD4+ (Figure 2A) and CD8+ (Figure 2B) Tmem subsets were determined based on their differential expression of CD28 and CD45RA, as described (27). Following allo-stimulation, TGFβ1/Fc combined with rapamycin more markedly suppressed both CD4+ and CD8+ central memory (Tcm) and effector memory (Tem) proliferation and Ki67 expression compared to rapamycin alone.
Figure 2. TGFβ1/Fc augments the suppressive effects of rapamycin on memory T cell proliferation.
(A) Percent of proliferation and Ki67 expression by CD4+ naïve and Tmem subsets in response to allostimulation in the presence of rapamycin ± TGFβ1/Fc. (B) Similarly, CD8+ naïve and Tmem subsets were also evaluated for percent of proliferation and Ki67 expression. CFSE-labeled PBMC were cultured for 4 – 5 days with T cell-depleted allogeneic PBMC in the absence or presence of rapamycin ± TGFβ1/Fc. At the end of the culture, T cell subsets were assessed by flow cytometry based on CD45RA and CD28 expression: naïve (Tn)- CD45RA+CD28+, central memory (Tcm)- CD45RA−CD28+, effector memory (Tem)- CD45RA−CD28−, and terminally-differentiated effector memory (Temra)-CD45RA+CD28−. All results are representative data of three independent experiments using different responders and stimulator-responder pairs. PBMC, peripheral blood mononuclear cell.
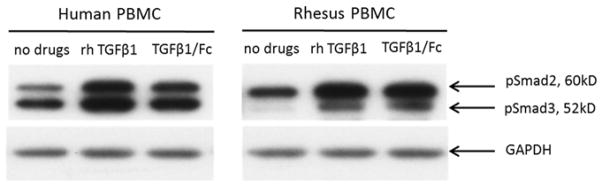
TGFβ1/Fc activates human and rhesus monkey Smad2/3 pathway
To assess the effect of TGFβ1/Fc on intracellular signal transduction, we evaluated activation of the receptor-regulated Smad2/3 pathway (28) in both human and rhesus PBMC. For comparison, we also evaluated the influence of recombinant human TGFβ1 (rhTGFβ1). TGFβ1/Fc-induced smad-2 and smad-3 phosphorylation in human PBMC as efficiently as rhTGFβ1. The TGFβ1/Fc induced smad-2 and smad-3 phosphorylation in rhesus PBMC was similar to that achieved by rhTGFβ1 (Figure 3).
Figure 3. TGFβ1/Fc activates the Smads (2/3) pathway.
Western blotting was used to determine the expression of Smad2/3 by rhesus and human PBMC treated with rhTGFβ1 (10 ng/mL) or TGFβ1/Fc (5 μg/mL) for 30 min. The same cell lysates were used to evaluate the expression of GAPDH as a loading control. One representative experiment of two performed is shown. PBMC, peripheral blood mononuclear cell.
TGFβ1/Fc blood levels in naïve and lymphodepleted monkeys
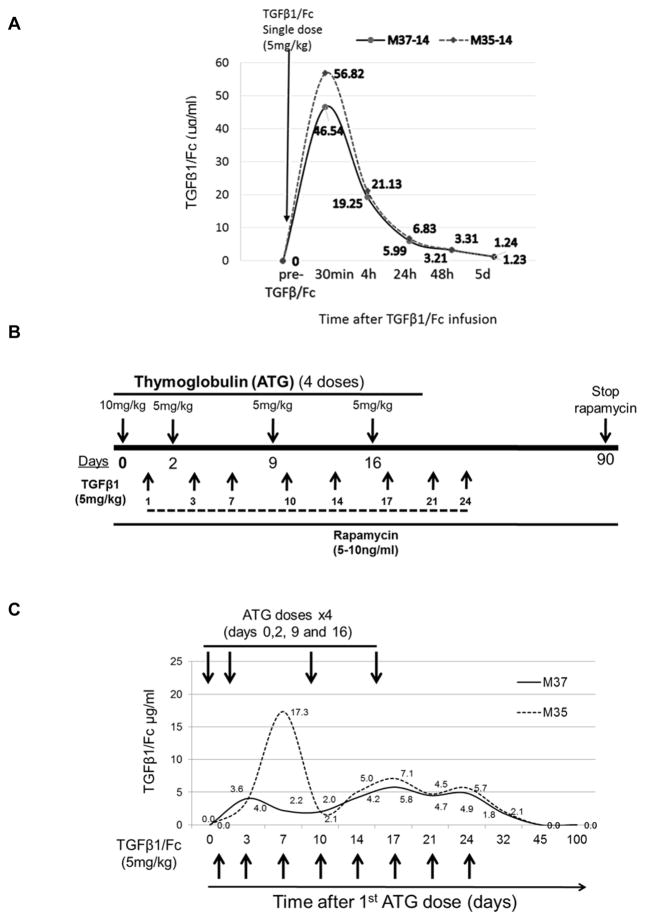
Recently, it was reported in mice (23) that the circulating t1/2 of the active form of TGFβ1 was very short (3 min), but that the circulating t1/2 of human TGFβ1/Fc was markedly extended to 32h. First, we examined TGFβ1/Fc levels in peripheral blood of two healthy rhesus monkeys. As shown in Figure 4A, following a single i.v. infusion, TGFβ1/Fc reached peak levels at 30 min, declined thereafter, but remained detectable at 24 and 48 hours, and could no longer be detected in the blood beyond 5 days after infusion.
Figure 4. Pharmacokinetics of TGFβ1/Fc following i.v. infusion in healthy and lymphodepleted monkeys.
(A) Time course of serum concentration of TGFβ1/Fc in 2 healthy naïve rhesus monkeys after a single i.v. infusion (5 mg/kg). (B) Protocol for immunosuppression and TGFβ1/Fc infusion in lymphodepleted monkeys. The monkeys received i.v. ATG on days 0, 2, 9, 16. Intramuscular rapamycin was commenced on day 0 and whole blood trough levels maintained at 5–10 ng/mL until day 90. In 2 IS monkeys, TGFβ1/Fc was infused twice a week for 4 weeks starting after the first dose of ATG. (C) Serum TGFβ1/Fc levels in TGFβ1/Fc-infused monkeys (n=2) at various times up to 3 months after the start of ATG administration.
We then established an IS protocol to achieve lymphodepletion in four healthy monkeys. Four doses of ATG were given on days 0 (10 mg/kg), 2 (5 mg/kg), 9 (5 mg/kg) and 16 (5 mg/kg). Rapamycin was injected daily from day 0 with levels maintained between 10 and 15 ng/mL (Figure 4B). TGFβ1/Fc was administered at 5 mg/kg into two of the lymphodepleted monkeys twice a week for 4 weeks following the first dose of ATG. The other two lymphodepleted monkeys were used as controls. As shown in Figure 4C, in lymphodepleted monkeys receiving TGFβ1/Fc, trough levels of TGFβ1/Fc between 2 and 8 μg/mL (except for day 7 in one monkey) were achieved and maintained in the peripheral blood between days 3 and 35). TGFβ1/Fc was detected in the blood for at least 1 week after the last dose (day 24) and was not detected in blood more than 2 weeks after the last infusion.
TGFβ1/Fc in combination with ATG and rapamycin delays the recovery of Tmem in vivo
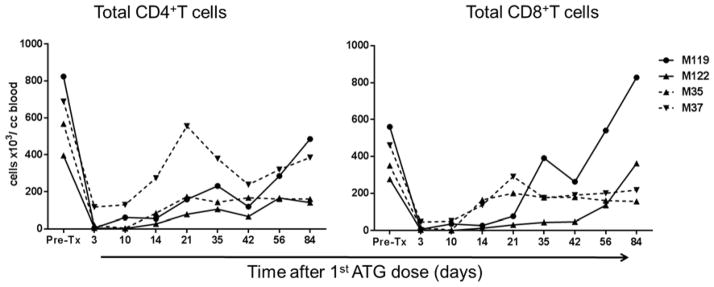
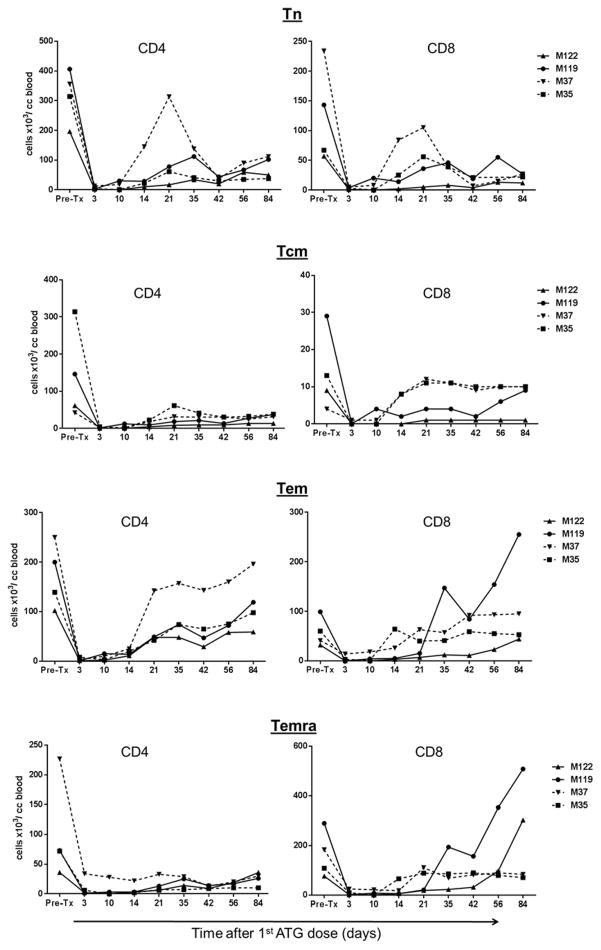
We next evaluated the influence of the TGFβ1/Fc on the recovery of T cells and Tmem populations in monkeys receiving IS. Both naive and memory CD4+ and CD8+ T cell subsets in fresh blood samples were enumerated by flow cytometry based on their differential expression of CD62L and CD45RA (Figure 5). Total CD4+ T cells were reduced in all monkeys and remained below pre-depletion levels for at least 12 weeks. In one monkey with TGFβ1/Fc infusion (M37), there was comparatively early recovery of CD4+ Tem, but numbers still remained below pre-depletion levels at day 84. While total CD8+ T cells were depleted as efficiently as CD4+ T cells in all monkeys, earlier recovery of total CD8+ T cells to or above pre-depletion levels was observed without compared to with TGFβ1/Fc infusion. With no TGFβ1/Fc infusion, there was earlier recovery of CD8+ Tem in one monkey (M119) and of CD8+ Temra in both monkeys, compared to those with protein infusion. These data suggest that combined administration of TGFβ1/Fc with rapamycin may delay recovery of effector and memory CD8+ T cells in lymphodepleted monkeys.
Figure 5. Influence of TGF-β1/Fc infusion on circulating CD4+ and CD8+ naïve and memory T cell subsets.
Absolute numbers of circulating total CD4+ and CD8+ T cells as well as naïve and memory T cell subsets, in control monkeys M119 and M122 (solid lines) and TGFβ1/Fc–infused monkeys M35 and M37 (dashed lines) at various times after the start of ATG administration are shown. CD4+ and CD8+ naïve and memory T cell subsets in peripheral blood were distinguished based on CD45RA and CD62L expression: Naïve (Tn) CD45RAhiCD62Lhi, effector memory (Tem) CD45RAloCD62Llo, terminally-differentiated effector memory (Temra) CD45RAhiCD62Llo, and central memory (Tcm) CD45RAloCD62Lhi.
In these studies, we did not observe consistent CD62L expression by stored PBMC (compared to fresh blood samples). Accordingly, we evaluated naive and memory CD4+ T cell subsets (1 month after the first dose of ATG) in inguinal lymph nodes based on their differential expression of CD28 and CD45RA. No striking differences in the incidences of naive and memory T cell subsets in LN were observed, with or without TGFβ1/Fc infusion (data not shown).
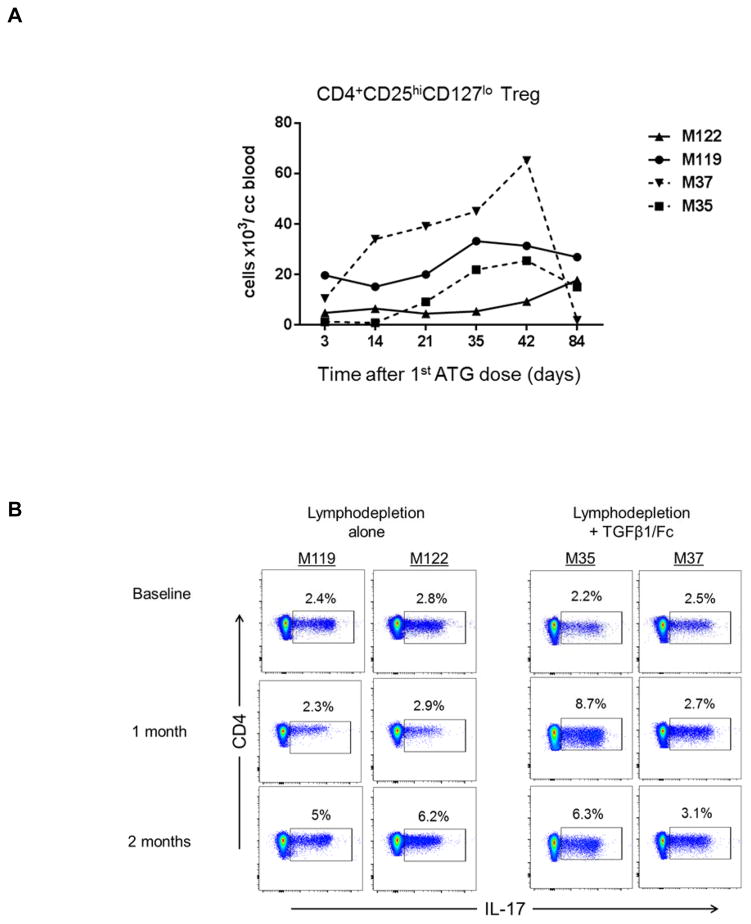
Combined TGFβ1/Fc and rapamycin administration induces a transient increase in CD4+CD25hiCD127lo Treg in peripheral blood
We then determined the influence of TGFβ1/Fc infusion on regulatory CD4+CD25hiCD127lo T cell absolute numbers in peripheral blood. As shown in Figure 6A, following T cell depletion, there was a steady recovery in Treg absolute numbers between days 14 and 42 with TGFβ1/Fc infusion that was less evident in controls. Additionally, we calculated the ratios of Treg to CD4+Tem and CD4+Temra using absolute cell numbers, but inter-individual variability made interpretation of the results in the small numbers of animals difficult.
Figure 6. Influence of TGFβ1/Fc infusion on regulatory T cells.
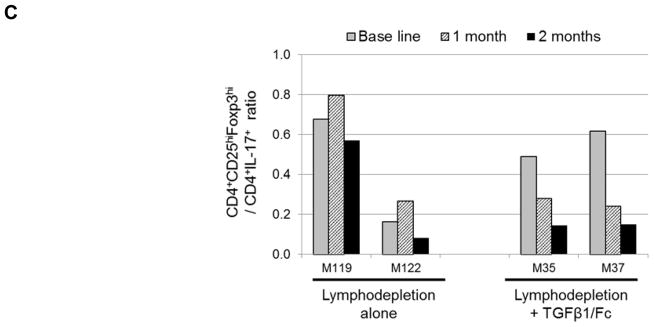
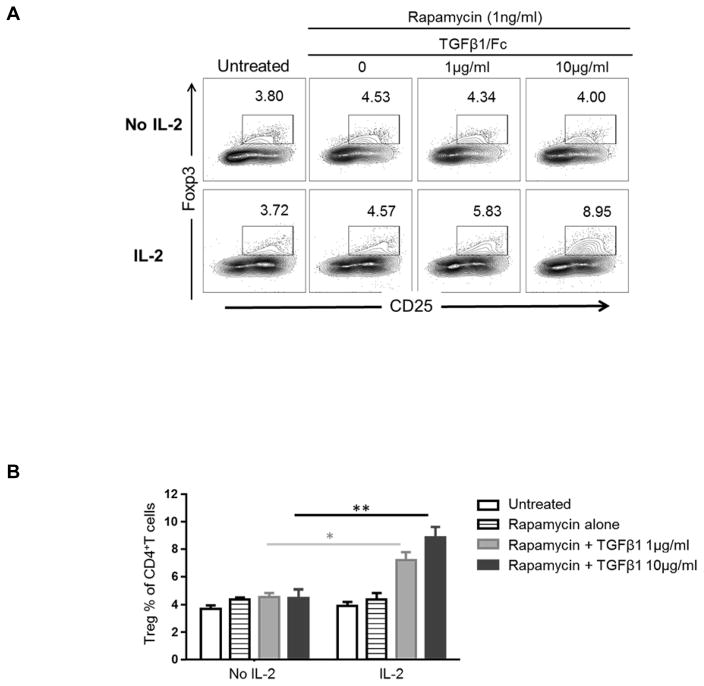
(A) Absolute numbers of Treg (CD4+CD25+CD127−) in peripheral blood of lymphodepleted monkeys without (solid lines) and with TGFβ1/Fc infusion (dashed lines) at various times after the first ATG infusion. (B) Percentages of CD4+IL-17+ T cells in each monkey, before, 1 month and 2 months after lymphodepletion. PBMC were activated for 4h with PMA and ionomycin in the presence of GolgiStop (BD Bioscience). (C) In stored PBMC, the ratio of CD4+CD25hiFoxp3hi Treg to CD4+IL-17+ cells was determined for each monkey at each time point examined. PBMC, peripheral blood mononuclear cell.
Combined TGFβ1/Fc and rapamycin administration does not promote Treg to Th17 ratios in blood
As TGFβ1 plays a critical role in the balance between Treg and Th17 cells (29), we measured the incidences of CD4+IL-17+ cells in PBMC 1 and 2 months after lymphodepletion (Figure 6B). In all monkeys, the percentages of CD4+IL-17+ T cells increased at 2 months following lymphodepletion. We then evaluated the ratio of CD4+CD25+Foxp3hi Treg to CD4+IL-17+ cells in PBMC. With no TGFβ1/Fc, the Treg/Th17 ratio one month after depletion was similar to before depletion, and then slightly reduced at 2 months. On the other hand, the Treg/Th17 ratio was markedly reduced at 1 and 2 months in TGFβ1/Fc-treated monkeys (Figure 6C).
Combination TGFB1/Fc with IL-2 promotes the Treg/Th17 ratio
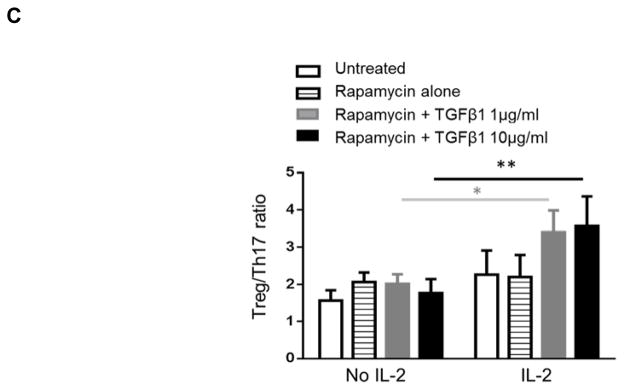
The bi-functional role of TGFβ1/Fc in regulation of immune reactivity depends on the cytokine milieu. It is known that both TGFβ1 and IL-2 are essential for Treg induction. To further define the influence of TGFβ1/Fc on the Treg/Th17 ratio during T cell activation, CD3/CD28 activation of rhesus monkey PBMC was induced in the presence or absence of TGFβ1/Fc, with or without IL-2. As shown in Figure 7A, T cell activation in the presence of rapamycin and/or TGFβ1/Fc alone did not induce Treg. However, the incidence of Treg was increased by TGFβ1/Fc in a concentration-dependent manner only in the presence of IL-2 (Figure 7B). This was associated with an increased Treg/Th17 ratio only in the presence of IL-2 (Figure 7C). These data suggest that addition of low dose IL-2 infusion (30) may be required to improve the Treg to Th17 ratio following TGFβ1/Fc administration in vivo.
Figure 7. Combination of IL-2 and TGFβ1/Fc enhances Treg and increases the Treg/Th17 ratio.
Rhesus monkey PBMC were stimulated with anti-CD3 and anti-CD28 Abs and cultured without or with rapamycin (1 ng/mL) ± the indicated concentrations of TGF-β1/Fc (0, 1, 10 μg/mL) and with or without exogenous IL-2. On day 4, the cells were harvested and further activated with PMA, ionomycin and GolgiStop for 4 h, then stained for intracellular IL-17 and Foxp3. (A) Representative percentages of CD4+CD25+Foxp3+ Treg. (B) Overall Treg percentages and (C) Treg/Th17 ratios plotted as means ± SD. n=5; **, p<0.001; *, P<0.01.
Discussion
TGFβ exists in 3 different isoforms (β1, β2 and β3), the most prevalent of which, TGFβ1, is an important regulator of immune responses and exerts powerful anti-inflammatory effects (18, 31). Inhibitory effects of TGFβ1 were first described in 1986 (32), when its suppressive effect on T and B lymphocytes was reported. The later development of TGFβ1-deficient mice established the crucial roles of TGFβ1 in immune responses (33, 34). TGFβ1 plays a complex role in the development of T cell lineages and the induction of immune tolerance. It has been suggested that TGFβ1 may be indispensable for the development and maintenance of Treg in the periphery (16, 17), as well as Treg function (19). Furthermore, recent studies indicate that naive CD4+CD25−Foxp3− T cells in the periphery can be converted into CD4+CD25+Foxp3+ Treg by TGFβ1 during T cell receptor stimulation (16).
Recently, a mutant human TGFβ1/Fc has been generated (23). In order to promote the half-life of TGFβ1, the Cys codons in the pro region of the human TGFβ1 precursor were changed into serine (Ser). The mutant TGFβ1 cDNA was then fused with human IgG4 Fc to produce an autoactive TGFβ1/Fc that does not depend on acidification for activation. In combination with rapamycin monotherapy, infusion of human mutant TGFβ1/Fc induces islet allograft tolerance in a rodent model, associated with increased Treg and reduction of pro-inflammatory cytokines, including IL-17 (23).
Lymphocyte depletion has been used increasingly in clinical organ transplantation as a basis for tolerance induction strategies. Studies in rodents, NHP and humans indicate that lymphodepletion is associated with reduced cellular rejection and prolonged allograft survival. However, there is also recent evidence that T cell depletion at the time of transplant may enhance the recovery of pro-inflammatory T cells with effector and memory phenotypes after transplantation, where this enhanced recovery is due to homeostatic proliferation of Tmem in lymphopenic hosts (11). Additionally, lymphodepletion-resistant Tmem contribute to allograft rejection, as demonstrated by studies in rodents (35) and humans (36).
In addition to its Treg-promoting effects, TGFβ1 has been shown to be critical for the prevention of dysregulated expansion of Tmem (22). Furthermore, immunomodulatory effects of TGFβ1 may prevent inflammatory responses (37) after transplantation (38). Here, we evaluated for the first time, the influence of human TGFβ1/Fc protein on rhesus monkey T cells in vitro and also in vivo following lymphodepletion. In vitro, TGFβ1/Fc induced Smad 2 and 3 phosphorylation in rhesus PBMC and augmented the suppressive effect of rapamycin on both CD4+ and CD8+ Tmem proliferation in response to either CD3/CD28 activation or allo-stimulation.
We were able to achieve trough levels of TGFβ1/Fc between 2 and 8 μg/mL in lymphodepleted monkeys where TGFβ1/Fc could be detected in the blood for at least 1 week after the final dose. We then evaluated the influence of TGFβ1/Fc on the recovery of CD4+ and CD8+ Tmem in lymphodepleted monkeys. With no TGFβ1/Fc administration, recovery of effector CD8+ Tmem was observed by 6–12 weeks after depletion, while in monkeys with TGFβ1/Fc administration, effector CD8+ Temra remained below pre-depletion levels. Of note, TGFβ1/Fc was given for only 4 weeks, and trough levels were not detected by day 45 after combined lymphodepletion and TGFβ1/Fc infusion.
In all lymphodepleted monkeys (with or without TGFβ1/Fc infusion), there were increased incidences of CD4+IL-17+ T cells at 1 and 2 months after lymphodepletion compared to baseline. However, the Treg to Th17 ratio was reduced markedly in TGFβ1/Fc-treated compared to control monkeys. It is known that TGFβ1 and IL-2 orchestrate Treg induction (39), where IL-2 is critical for Treg development (40, 41) and TGFβ1 mediates Treg induction (42). Notably, our in vitro data indicate that combined TGFβ1/Fc and rapamycin dose not enhance the Treg to Th17 ratio despite reduced Tmem proliferation. However, the Treg to Th17 ratio was enhanced significantly when TGFβ1/Fc and rapamycin were combined with IL-2.
The potential requirement of combined low dose IL-2 infusion with TGFβ1/Fc to increase the incidence of Treg compared to Th17 or effector T cells may introduce significant complexity to clinical application. Furthermore, It has been reported that TGFβ1 can induce the production of connective tissue growth factor, that promotes fibrosis in rodents (43, 44) in the presence of IL-6, and is IL-17-dependent.
In conclusion, human TGFβ1/Fc infusion may delay Tmem recovery following lymphodepletion in nonhuman primates. However, following the final dose of TGFβ1/Fc, enhanced Th17 cell: Treg ratios were observed. As TGFβ1-mediated regulation of T cell homoestasis is dependent on the cytokine milieu, therapeutic infusion of TGFβ1/Fc requires further evaluation regarding timing and dosage. Additional administration of low dose IL-2 (30), in combination of TGFβ1/Fc, may be required for sustaining enhanced Treg to Th17 T cell ratios, particularly in the setting of transplantation.
Supplementary Material
Suppression of CD4+ and CD8+ T cell proliferation and ki67 expression in response to anti-CD3/CD28 stimulation in the presence or absence of rapamycin with or without TGFβ1/Fc as described in materials and methods. Results are representative of three independent experiments using PBMC from three different monkeys.
Acknowledgments
The work was supported by National Institutes of Health (NIH) grant U01 AI102456, part of the NIH NHP Transplantation Tolerance Cooperative Study Group and sponsored by the NIAID and NIDDK. Fusion protein used in these studies was produced by the NIH Nonhuman Primate Reagent Resource funded by NIH grant R24 OD010976 and NIAID contract HHSN272200130031C. T.L. Sumpter is a recipient of 1 K01 AR067250-01A1 award.
Abbreviations
- NHP
non-human primate
- TGFβ1
transforming growth factor β1
- Tem
effector memory T cells
- Temra
terminally-differentiated memory T cells
- Tmem
memory T cells
- Tn
naïve T cells
- Treg
regulatory T cells
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Calne R, Friend P, Moffatt S, Bradley A, Hale G, Firth J, et al. Prope tolerance, perioperative campath 1H, and low-dose cyclosporin monotherapy in renal allograft recipients. Lancet. 1998;351(9117):1701–1702. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- 2.Margreiter R, Klempnauer J, Neuhaus P, Muehlbacher F, Boesmueller C, Calne RY. Alemtuzumab (Campath-1H) and tacrolimus monotherapy after renal transplantation: results of a prospective randomized trial. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(7):1480–1485. doi: 10.1111/j.1600-6143.2008.02273.x. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro R, Ellis D, Tan HP, Moritz ML, Basu A, Vats AN, et al. Alemtuzumab pre-conditioning with tacrolimus monotherapy in pediatric renal transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(12):2736–2738. doi: 10.1111/j.1600-6143.2007.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teuteberg JJ, Shullo MA, Zomak R, Toyoda Y, McNamara DM, Bermudez C, et al. Alemtuzumab induction prior to cardiac transplantation with lower intensity maintenance immunosuppression: one-year outcomes. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(2):382–388. doi: 10.1111/j.1600-6143.2009.02856.x. [DOI] [PubMed] [Google Scholar]
- 5.Valujskikh A. Targeting T-cell memory: where do we stand? Current opinion in organ transplantation. 2008;13(4):344–349. doi: 10.1097/MOT.0b013e3283061126. [DOI] [PubMed] [Google Scholar]
- 6.Moxham VF, Karegli J, Phillips RE, Brown KL, Tapmeier TT, Hangartner R, et al. Homeostatic proliferation of lymphocytes results in augmented memory-like function and accelerated allograft rejection. Journal of immunology. 2008;180(6):3910–3918. doi: 10.4049/jimmunol.180.6.3910. [DOI] [PubMed] [Google Scholar]
- 7.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. Journal of immunology. 1999;163(4):2267–2275. [PubMed] [Google Scholar]
- 8.Valujskikh A, Li XC. Frontiers in nephrology: T cell memory as a barrier to transplant tolerance. Journal of the American Society of Nephrology: JASN. 2007;18(8):2252–2261. doi: 10.1681/ASN.2007020151. [DOI] [PubMed] [Google Scholar]
- 9.Bingaman AW, Farber DL. Memory T cells in transplantation: generation, function, and potential role in rejection. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(6):846–852. doi: 10.1111/j.1600-6143.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- 10.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nature medicine. 2004;10(1):87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tchao NK, Turka LA. Lymphodepletion and homeostatic proliferation: implications for transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(5):1079–1090. doi: 10.1111/j.1600-6143.2012.04008.x. [DOI] [PubMed] [Google Scholar]
- 12.Barrett SP, Toh BH, Alderuccio F, van Driel IR, Gleeson PA. Organ-specific autoimmunity induced by adult thymectomy and cyclophosphamide-induced lymphopenia. European journal of immunology. 1995;25(1):238–244. doi: 10.1002/eji.1830250139. [DOI] [PubMed] [Google Scholar]
- 13.Gleeson PA, Toh BH, van Driel IR. Organ-specific autoimmunity induced by lymphopenia. Immunological reviews. 1996;149:97–125. doi: 10.1111/j.1600-065x.1996.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 14.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117(2):265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 15.Khoruts A, Fraser JM. A causal link between lymphopenia and autoimmunity. Immunology letters. 2005;98(1):23–31. doi: 10.1016/j.imlet.2004.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26(5):579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annual review of immunology. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nature immunology. 2008;9(6):632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 20.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. Journal of immunology. 2004;172(9):5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 21.Zhang N, Bevan MJ. TGF-beta signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation. Nature immunology. 2012;13(7):667–673. doi: 10.1038/ni.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas PJ, Kim SJ, Mackall CL, Telford WG, Chu YW, Hakim FT, et al. Dysregulation of IL-15-mediated T-cell homeostasis in TGF-beta dominant-negative receptor transgenic mice. Blood. 2006;108(8):2789–2795. doi: 10.1182/blood-2006-05-025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Zhang D, Shen M, Liu Y, Tian Y, Thomson AW, et al. Combined administration of a mutant TGF-beta1/Fc and rapamycin promotes induction of regulatory T cells and islet allograft tolerance. Journal of immunology. 2010;185(8):4750–4759. doi: 10.4049/jimmunol.1000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Windt DJ, Smetanka C, Macedo C, He J, Lakomy R, Bottino R, et al. Investigation of lymphocyte depletion and repopulation using alemtuzumab (Campath-1H) in cynomolgus monkeys. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(4):773–783. doi: 10.1111/j.1600-6143.2010.03050.x. [DOI] [PubMed] [Google Scholar]
- 25.Marco MR, Dons EM, van der Windt DJ, Bhama JK, Lu LT, Zahorchak AF, et al. Post-transplant repopulation of naive and memory T cells in blood and lymphoid tissue after alemtuzumab-mediated depletion in heart-transplanted cynomolgus monkeys. Transplant immunology. 2013;29(1–4):88–98. doi: 10.1016/j.trim.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ezzelarab MB, Zahorchak AF, Lu L, Morelli AE, Chalasani G, Demetris AJ, et al. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(8):1989–2005. doi: 10.1111/ajt.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. Journal of immunology. 2002;168(1):29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 28.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nature reviews Molecular cell biology. 2007;8(12):970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 29.O’Garra A, Stockinger B, Veldhoen M. Differentiation of human T(H)-17 cells does require TGF-beta! Nature immunology. 2008;9(6):588–590. doi: 10.1038/ni0608-588. [DOI] [PubMed] [Google Scholar]
- 30.Aoyama A, Klarin D, Yamada Y, Boskovic S, Nadazdin O, Kawai K, et al. Low-dose IL-2 for In vivo expansion of CD4+ and CD8+ regulatory T cells in nonhuman primates. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(9):2532–2537. doi: 10.1111/j.1600-6143.2012.04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nature reviews Immunology. 2002;2(1):46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 32.Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, et al. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayasoufi K, Yu H, Fan R, Wang X, Williams J, Valujskikh A. Pretransplant antithymocyte globulin has increased efficacy in controlling donor-reactive memory T cells in mice. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(3):589–599. doi: 10.1111/ajt.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenblum JM, Kirk AD. Recollective homeostasis and the immune consequences of peritransplant depletional induction therapy. Immunological reviews. 2014;258(1):167–182. doi: 10.1111/imr.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ezzelarab MB, Ekser B, Azimzadeh A, Lin CC, Zhao Y, Rodriguez R, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22(1):32–47. doi: 10.1111/xen.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong AS, Alegre ML. Transplantation tolerance and its outcome during infections and inflammation. Immunological reviews. 2014;258(1):80–101. doi: 10.1111/imr.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu L, Kitani A, Strober W. Molecular mechanisms regulating TGF-beta-induced Foxp3 expression. Mucosal Immunol. 2010;3(3):230–238. doi: 10.1038/mi.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson BH. IL-2, regulatory T cells, and tolerance. Journal of immunology. 2004;172(7):3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 41.Cheng G, Yu A, Malek TR. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunological reviews. 2011;241(1):63–76. doi: 10.1111/j.1600-065X.2011.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. Journal of immunology. 2007;178(4):2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 43.Booth AJ, Csencsits-Smith K, Wood SC, Lu G, Lipson KE, Bishop DK. Connective tissue growth factor promotes fibrosis downstream of TGFbeta and IL-6 in chronic cardiac allograft rejection. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(2):220–230. doi: 10.1111/j.1600-6143.2009.02826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Csencsits K, Wood SC, Lu G, Faust SM, Brigstock D, Eichwald EJ, et al. Transforming growth factor beta-induced connective tissue growth factor and chronic allograft rejection. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(5 Pt 1):959–966. doi: 10.1111/j.1600-6143.2006.01292.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppression of CD4+ and CD8+ T cell proliferation and ki67 expression in response to anti-CD3/CD28 stimulation in the presence or absence of rapamycin with or without TGFβ1/Fc as described in materials and methods. Results are representative of three independent experiments using PBMC from three different monkeys.