Abstract
Research has recently identified a promising neurophysiological marker of approach motivation involving posterior versus frontal (Pz-Fz) electroencephalographic (EEG) theta activity (PFTA; Wacker, Chavanon, & Stemmler, 2006). Preliminary evidence indicates that PFTA is modulated by dopaminergic activity thought to underlie appetitive tendencies, and that it indexes self-reported Behavioral Approach System (BAS) sensitivity. To date, research has largely relied on resting indices of PFTA and has yet to examine the relationship between PFTA and specific approach-related affective states generated by emotionally salient laboratory tasks. Accordingly, the present study evaluated PFTA both at rest and during an ecologically valid autobiographical memory task in which participants recalled personal life experiences involving a goal-striving, an anxious apprehension, a low-point (i.e., difficult) and a neutral memory while EEG data were recorded. In line with prediction, elevated PFTA was observed during both goal-striving and anxious apprehension autobiographical memories. PFTA was particularly elevated during anxious apprehension memories coded as being high on approach-related tendencies. Elevated PFTA during anxious apprehension is consistent with a growing literature indicating that anxious apprehension is associated with elevated approach and reward-related brain function. Lastly, elevated resting PFTA was positively correlated with self-reported trait anger, a negatively valenced emotion characterized by approach-related tendencies. Results have implications for a) enhancing our understanding of the neurophysiology of approach-related emotions, b) establishing PFTA as an index of appetitive motivational states, and c) clarifying our understanding of the neurophysiology and approach-related tendencies associated with both anxious apprehension and anger.
Keywords: Posterior versus frontal EEG theta activity, approach motivation, autobiographical memories
Identifying neurophysiological markers of approach-related affect has important implications for informing our understanding of the mechanisms underlying affective or motivational tendencies, generating biosignatures of risk for psychiatric disorders characterized by abnormal approach-related affect, and developing targeted treatments for these psychiatric disorders (Gottesman & Gould, 2003). Recent data indicate that midline posterior versus frontal electroencephalographic (EEG) theta activity (PFTA) may reflect a newly identified neurophysiological index of approach motivation (Wacker, Chavanon, & Stemmler, 2006; 2010). PFTA constitutes a difference score between posterior (Pz) and frontal (Fz) midline theta activity (Pz-Fz). Importantly, PFTA has been shown to be sensitive to changes in dopamine signaling (Wacker et al., 2006; 2010), the neurotransmitter most centrally involved in reward processing and in facilitating approach-related behavior (Haber & Knutson, 2010; Hikosaka, Bromberg-Martin, Hong, & Matsumoto, 2008). To date, however, research has largely focused on resting indices of PFTA and thus it is unclear whether PFTA indexes approach-related motivation during emotionally salient laboratory tasks. Accordingly, the present study examined PFTA during an ecologically valid autobiographical memory task designed to elicit approach-related motivational states, including anxious apprehension.
Posterior versus Frontal Theta Activity and Approach Motivation
Animal and human based research highlights the role of dopamine in facilitating appetitive motivation and approach-related behavior (Haber & Knutson, 2010; Hikosaka et al., 2008). A fronto-striatal neural circuit has been implicated in approach and reward-related dopaminergic activity. This circuit includes, but is not limited to, the ventral tegmental area, ventral striatum, and the orbitofrontal cortex (Ernst et al., 2005; Haber & Knutson, 2010; Schultz, 2000). Research also implicates the anterior cingulate cortex (ACC) in reward processing (Baker & Holroyd, 2011; Hayden, Pearson, & Platt, 2009). The ACC receives concentrated dopaminergic inputs (Huang et al., 2013; Schweimer & Hauber, 2006), shows increased activity in response to dopamine agonists (Udo de Haes, Maguire, Jager, Paans, & den Boer, 2007), is modulated by genes controlling dopamine transmission (Blasi et al., 2005), projects to the ventral striatum (Haber, Kim, Mailly, & Calzavara, 2006; Öngür & Price, 2000) and is implicated in depressive anhedonia characterized by reduced approach-related motivation (Wacker, Dillon, & Pizzagalli, 2009).
There are growing lines evidence that PFTA indexes approach-related motivation and is modulated by dopamine related activity in the ACC. First, Wacker and colleagues (2010) reported a positive relationship between PFTA and self-reported behavioral approach system (BAS) sensitivity as measured by Carver and White’s (1994) BAS scale, a commonly used index of approach-related motivation. Second, theta current density in the rostral ACC has been shown to account for a significant portion of the variance in PFTA (Chavanon, Wacker, & Stemmler, 2011). Third, both anhedonia and a blunted ventral striatal BOLD response to monetary rewards, as indexed by fMRI, are associated with profiles of resting delta/theta current in the rostral ACC (Wacker et al., 2009). Lastly, PFTA is modulated by pharmacological agents that affect dopaminergic activity, such as a dopamine antagonist designed to attenuate dopamine related activity. Whereas unmedicated participants exhibited a positive relationship between self-reported BAS sensitivity and PFTA, individuals who took a dopamine antagonist prior to EEG recording displayed a negative relationship between BAS sensitivity and PFTA (Wacker et al., 2010). Furthermore, extraverts exhibited elevated PFTA and introverts exhibited reduced PFTA during a challenging working memory task, but this association was reversed among extraverts and introverts who had taken a dopamine antagonist prior to the task (Wacker et al., 2006).
The majority of research to date on PFTA and approach-related affect has relied on resting indices of PFTA designed to assess more trait like variability. Coan and colleagues Capability Model (2006), however, suggests that approach tendencies are more pronounced and more resistant to measurement error when elicited using emotionally salient tasks. We agree with this proposition of the Capability Model. However, we argue that many of the studies that have utilized task-based paradigms to assess positive affect or approach-related neurophysiology have employed relatively mild emotional stimuli (e.g., positive pictures/film clips, small monetary reward) that may be of limited salience or relevance to participants. Accordingly, the present study employed an ecologically valid autobiographical memory task to examine the effect of approach-oriented states on neurophysiological activity.
Approach Motivation and Anxious Apprehension
Whereas unipolar depression (without a history of hypo/mania) is characterized by decreased approach/reward-related neural activity (Forbes et al., 2009; Pizzagalli, Sherwood, Henriques, & Davidson, 2005; Thibodeau, Jorgensen, & Kim, 2006; Wacker et al., 2009), anxiety has been associated with contrasting profiles of such activity. Two neuroimaging studies reported that behaviorally inhibited adolescents displayed elevated ventral striatal activation to reward-relevant cues (Bar-Haim et al., 2009; Guyer et al., 2006). These findings are in line with a number of neurophysiological studies indicating that anxiety is associated with elevated approach-related neurophysiological activity (Heller, Nitschke, Etienne, & Miller, 1997; Mathersul, Williams, Hopkinson, & Kemp, 2008). Other research, however, has reported that anxiety is associated with either decreased (Crost, Pauls, & Wacker, 2008; Kemp et al., 2010; Tomarken & Davidson, 1994; Wiedemann et al., 1999), or maintained approach-related neurophysiological activity (Kentgen et al., 2000; Nitschke, Heller, Palmieri, & Miller, 1999). Thus, there is uncertainty as to where precisely anxiety resides on the continuum of approach-related neurophysiology.
To begin to reconcile these inconsistencies, Heller and colleagues (1998; Engels et al., 2007) proposed distinguishing between anxious arousal and anxious apprehension when examining approach-related neurophysiology in anxiety. Highlighting the importance of this distinction, there is growing evidence that anxious arousal and anxious apprehension are associated with distinct profiles of relative left frontal EEG activity, a commonly used neurophysiological index of approach-related motivation (Coan & Allen, 2004; Harmon-Jones, Gable, & Peterson, 2010; Sutton & Davidson, 1997). Anxious arousal, characterized by physiological hyperarousal and somatic tension, has been associated with decreased relative left frontal EEG activity (Wiedemann et al., 1999), reflecting decreased approach-related motivation.
By contrast, Barlow (1991) described anxious apprehension as a future-oriented mood state in which an individual manages anticipated threatening events via rumination and worry. Research indicates that anxious apprehension is associated with maintained (Nitschke et al., 1999) or elevated (Heller et al., 1997; Mathersul et al., 2008) relative left frontal EEG activity, reflecting increased approach-related motivation. These results suggest that, in contrast to depression and anxious arousal, anxious apprehension may involve sustained, or even elevated, approach-related neurophysiological activity. These findings are surprising given the lack of conceptual overlap between anxious apprehension on one hand, and approach motivation on the other. However, Treynor, Gonzalez, and Nolen-Hoeksema (2003) have shown that anxious apprehension, operationalized as rumination, contains an approach-oriented reflective factor aimed at introspection and problem solving. The reflective component of anxious apprehension is a protective factor against depression (Treynor et al., 2003), and can be considered approach-oriented in that it facilitates the use of adaptive problem solving inherent to goal attainment. Additional factor analytic research by Cox et al. (2001) and by Roberts et al. (1998) has revealed similar reflective components within anxious apprehension.
Extant research on the neurophysiology of anxious apprehension has focused exclusively on the relationship between self-reported trait levels of anxious apprehension and resting or baseline indices of relative left frontal EEG activity. Furthermore, no studies have examined the relationship between PFTA and anxious apprehension. Accordingly, the present study will use the autobiographical memory task to examine the effect of a laboratory-induced state of anxious apprehension on PFTA.
Present Study
The present study evaluated PFTA both at rest and during an ecologically valid autobiographical memory task designed to elicit approach-related motivational states. This study has implications for enhancing our understanding of the neurophysiology of approach-related emotions, establishing PFTA as a possible neurophysiological index of appetitive motivational states, and examining the approach-related tendencies associated with anxious apprehension.
EEG data were collected from a community sample (mean age = 23.0) both at rest and during an autobiographical memory task based on McAdams (1985) Life Story Interview (LSI), which has been used extensively in previous personality and life narrative research (McAdams et al., 2004; McAdams, Hoffman, Day, & Mansfield, 1996; Reese, Yan, Jack, & Hayne, 2010). This task was well suited to our goals because it has been shown to elicit a range of emotional response tendencies (McAdams et al., 2004), and because it confers a high degree of ecological validity. We used this task to elicit four autobiographical memories (goal-striving, anxious apprehension, low-point, and neutral) hypothesized to be associated with distinct motivational states. Following discussion of each autobiographical memory, participants completed a one-minute post-memory contemplation period in which they were asked to sit still and focus on the images, sounds, and feelings associated with the memory they just described. EEG data collected during this one-minute post-memory contemplation period served as the primary dependent variable for the present study.
The goal-striving memory was intended to elicit a state of high approach-related affect and was predicted to be associated with elevated PFTA, compared to the neutral and low-point memories. The anxious apprehension memory was intended to evoke a state of anxiety in which participants worried about the future outcome of an unresolved situation. Drawing on previous research that anxious apprehension is associated with either maintained or elevated approach-related neurophysiology (Nitschke et al., 1999; Heller et al., 1997; Mathersul et al., 2008), as well as elevated ventral striatal activity (Bar-Haim et al., 2009; Guyer et al., 2006), we predicted that the anxious apprehension memory would also be associated with elevated PFTA, compared to the neutral and low-point memories. We further predicted that PFTA would be particularly elevated during anxious apprehension memories characterized by elevated approach, as opposed to withdrawal, tendencies. To test this hypothesis, we employed a narrative coding scheme yielding separate scores for approach and withdrawal tendencies. The low-point memory was intended to induce a negative emotional state, which we predicted would be associated with reduced PFTA overall. However, given the possibility for approach tendencies to emerge during negative experiences, and that anger is an approach-oriented negative emotion (Carver & Harmon-Jones, 2009), we predicted that approach-oriented low-point memories would be associated with elevated PFTA versus withdrawal-oriented low-point memories. To evaluate this hypothesis, we employed both the approach and withdrawal coding schemes, as well as a separate coding scheme for anger. The neutral memory was designed to assess baseline task-related neurophysiological activity.
Analyses also examined the relationship between resting and task related PFTA and self-report measures of affective style. In line with existing research, we predicted that elevated PFTA would be associated with increased BAS sensitivity. We further predicted that elevated PFTA would be associated with self-reported anger, a negatively valenced emotion characterized by approach-related tendencies (Harmon-Jones et al., 2010). Results in line prediction would be the first to demonstrate a relationship between PFTA and self-reported anger.
Method
Participants
Forty-seven right-handed participants were recruited from the Evanston, IL area via flyer. Participants were excluded if they were either left-handed or ambidextrous (i.e., Chapman Handedness Inventory scores < 32), had suffered a head-related injury (e.g., concussion) in the last six months, or were not proficient in English. Five participants were excluded due to EEG artifact or other recoding-related issues, resulting in a final sample of 42 participants (24 female) with a mean age of 23.0 years (S.D. = 5.4, range = 18–44). Participants were told they were taking part in a study of brain activation related to sharing emotionally salient life events, and were asked to refrain from using caffeine for two hours prior to the study, and from using alcohol for 12 hours prior to the study. No participants reported current psychotropic medication use. Participants were paid $20 for their participation (approximately 2 hours). All participants provided informed consent.
Procedure
Participants first completed self-report questionnaires assessing state mood, and trait anxious apprehension, trait anger, and behavioral approach system sensitivity. Following electrode and EEG cap application, participants completed resting EEG recordings and then the autobiographical memory task, during which EEG data were collected.
Autobiographical Memory Task
Participants were administered an autobiographical memory task based on McAdams’ (1985) Life Story Interview (LSI), a protocol used extensively in previous personality research (McAdams et al., 1996; 2004; Reese et al., 2010). The LSI is typically used to collect detailed memories of important and emotionally salient life experiences, as well as the participant’s interpretation of these experiences. In the present study, the lead author (KW) sat face-to-face with each participant while they were wearing the EEG cap and corresponding electrodes. Participants sat in an electrically shielded and sound attenuated booth opposite the first author, who sat just outside the open doorway of the booth, and asked participants to describe four autobiographical memories: a) an emotionally neutral memory in which nothing important occurred, b) a goal-striving memory in which the participant pursued an important goal or reward, c) a low-point memory about the most difficult experience in the participant’s life, and d) an unresolved current experience about which the participant felt a sense of anxious apprehension (i.e., worried or nervous). Although the anxious apprehension experiences involved worries about a current or future situation, we refer to the four autobiographical accounts (including anxious apprehension) as memories for the sake of convention. Consistent with the LSI, participants were asked to describe in detail what happened in each memory, when and where it happened, who was involved, what thoughts and feelings arose during the experience, and what this experience said about who he or she was as a person. The interviewer solicited memories by reading from a script that included the above prompts and was not permitted to ask follow-up questions unless a response was vague or unclear. Memories were solicited in a counterbalanced order. Following participants’ descriptions of each autobiographical memory, the door to booth was closed and PFTA data were recorded during a one-minute eyes open contemplation period in which participants were asked to sit still, stare at a fixation cross, and focus on the images, sounds, and feelings associated with the memory they just described. A one-minute contemplation period was used given previous research suggesting that a one-minute recording interval is sufficient to capture neurophysiological shifts in approach-related affect (Harmon-Jones & Sigelman, 2001). PFTA data collected during this one-minute post-memory contemplation period served as the primary dependent variable for the current study. Following each post-memory contemplation period, participants completed a mood state measure.
Coding memories for approach/withdrawal tendencies
Two undergraduate research assistants who were blind to the hypotheses of the current study coded written transcriptions of neutral, goal-striving, anxious apprehension, and low-point memories for approach and withdrawal tendencies. We did this for descriptive purposes, and to portray motivational tendencies associated with each memory. In addition, for the two memory types characterized by a mix of approach and withdrawal tendencies (i.e., anxious apprehension and low-point memories), we examined how the motivational nature of these memories modulated PFTA.
Drawing on previous research (McAdams et al., 2004), all memories were coded for approach and withdrawal tendencies on a 3-point scale from 0 (no approach/withdrawal tendencies) to 2 (high approach/withdrawal tendencies). Our interview protocol asked for neutral memories in which nothing important took place. Thus, neutral memories were free of both approach and withdrawal tendencies. However, a detailed description of approach and withdrawal coding schemes for goal-striving, anxious apprehension, and low-point memories follows below.
With regard to the goal-striving memories, a code of 2 on approach was assigned to participants who described active attempts to pursue a clearly articulated goal. For example, several participants described the strenuous effort that went into gaining admission to a prestigious college or university. A code of 2 on withdrawal was assigned to participants who described a specific goal, but either procrastinated or did not pursue the goal for fear of failure.
With regard to anxious apprehension memories, a code of 2 on approach was assigned to participants who described significant evidence of attempts to proactively resolve an anxiety-producing situation. For example, one participant described a sense of high anxiety and worry about a depressed friend, along with repeated attempts to check on the friend’s welfare and to provide various forms of tangible support. In another example, a participant described worrying about future job prospects while actively networking, resume writing, and engaging in other tasks that might lead to employment. A code of 2 on withdrawal was assigned to participants who described significant evidence of anxiety avoidance (e.g., procrastination, trying not to think about anxiety provoking stimuli, etc). Anxious apprehension memories were coded for approach and withdrawal tendencies independent of the level of anxiety described by the participant.
With regard to low-point memories, a code of 2 for approach was assigned to participants who described being driven to action by a distressing experience. For example, one participant started a neighborhood petition to prevent a local bank from foreclosing on his parents’ home. A code of 2 for withdrawal was assigned to participants who narrated a distressing experience followed by depression, helplessness, denial, and/or avoidance. For instance, one participant described feelings of shame and helplessness while being publicly humiliated by an older family member for failing to observe a minor family custom.
In addition to coding low-point memories for approach and withdrawal tendencies, we coded low-point memories for anger. Low-point memories were intended to capture a distressing experience characterized by any number of possible negative emotions. While the majority of these emotions could be considered withdrawal oriented (e.g., sadness, fear, guilt, shame), anger has been shown to be approach-oriented (Carver & Harmon-Jones, 2009). Because anger has been linked with approach-oriented neurophysiological profiles (Harmon-Jones & Sigelman, 2001), low-point memories were coded for anger to determine whether anger in such memories might influence PFTA during low-point memory contemplation.
In low-point memories coded as high in anger, participants exhibited a strong degree of hostility, rage, or fury that was expressed outwardly either physically or verbally. For instance, one participant described a initiating a physical altercation after being subjected to racist epithets. Low-point memories coded as low in anger either exhibited no discernable anger, or mild irritation that was secondary to other negative emotions.
Interrater reliability for approach, withdrawal, and anger was computed using average measures intraclass correlations (ICC Case 1; Shrout & Fleiss, 1979). In the event of a disagreement between coders, the average of the two codes was used for analysis. Coding schemes for approach (neutral: 1.00, goal-striving: .75, anxious apprehension: .89, low-point: .78) and withdrawal (neutral: 1.00, goal-striving: .85, anxious apprehension: .83, low-point: .83) demonstrated good reliability. In addition, the coding scheme for anger demonstrated excellent reliability (low-point: .97).
For the purposes of EEG analyses, both anxious apprehension and low-point memories were divided into two groups: an approach-oriented group and a withdrawal-oriented group. Goal-striving and neutral memories were not divided in this way due to insufficient variance in approach and/or withdrawal tendencies. With regard to the anxious apprehension memory, the approach-oriented group included participants whose anxious apprehension memory was coded as being higher in approach than withdrawal orientation (n = 26). The withdrawal-oriented group included participants whose anxious apprehension memory was coded as either being higher in withdrawal than approach orientation, or equal in approach and withdrawal orientation (n = 16). As expected, the anxious approach-oriented group was coded as significantly greater in approach tendencies (M = 1.19, S.D. = .49) than the anxious withdrawal-oriented group (M = .34, S.D. = .35), t(40) = 6.01, p < .001. The anxious withdrawal-oriented group was coded as significantly greater in withdrawal tendencies (M = .63, S.D. = .62) than the anxious approach-oriented group (M = .02, S.D. = .10), t(40) = 4.93, p < .001.
An identical method was used to sort low-point memories into approach and withdrawal-oriented groups. The low-point approach-oriented group (n = 16) was coded as significantly greater in approach tendencies (M = 1.09, S.D. = .55) than the low-point withdrawal-oriented group (n = 26, M = .25, S.D. = .45), t(40) = 5.38, p < .001. The low-point withdrawal-oriented group was coded as significantly greater in withdrawal tendencies (M = .90, S.D. = .72) than the low-point approach-oriented group (M = .19, S.D. = .31), t(40) = 3.75, p < .001.
Self-report measures
The Affect Grid (AG; Russell et al., 1989) is a single-item measure of mood that was administered at baseline and after each post-memory contemplation period. The AG consists of a 9 x 9 square grid. The horizontal axis measures mood on a −4 to 4 scale from unpleasant to pleasant. The vertical axis measures arousal on a −4 to 4 scale from sleepiness to high arousal. The center square in the grid corresponds to a “neutral, average, everyday feeling” that is neither positive nor negative. Participants checked the square that best approximated their affective state in terms of mood and arousal. The AG shows good convergent validity with other scales of pleasure/displeasure and arousal (Yik, Russell, & Steiger, 2011).
Participants were administered the Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990) in order to assess the trait-like tendency toward frequent and intense verbal worry. The PSWQ is a 16-item measure that exhibits good psychometric properties (Molina & Borkovec, 1994). It includes items such as “My worries overwhelm me,” and “As soon as I finish one task, I start to worry about everything else I have to do.” These items are rated on a Likert scale from 1 (not at all typical of me) to 5 (very typical of me). Cronbach’s alpha for this sample was .95.
Behavioral activation system (BAS) and behavioral inhibition system (BIS) sensitivity were assessed by the Behavioral Inhibition System/Behavioral Activation System Scales (Carver & White, 1994). The 13-item BAS scale was used to assess self-reported appetitive motivation and sensitivity to potential reward and involves three subscales: Drive, or the diligent pursuit of goals; Fun-Seeking, or the desire to try new activities that may be exciting; and Reward Responsiveness, or the enjoyment of goal pursuit. The 11-item BIS scale assesses self-reported sensitivity to punishment. Cronbach’s alphas for this sample were .67 (BAS Drive), .64 (BAS Fun-Seeking), .88 (BAS Reward Responsiveness), .81 (BAS Total) and .84 (BIS).
To assess trait-related anger, participants completed the 8-item Defensive Fight subscale of the Reinforcement Sensitivity Theory Personality Questionnaire (Corr & Cooper, 2013) (no other subscales were administered). The Defensive Fight subscale measures the tendency to respond aggressively to threat and is theoretically and conceptually similar to anger (Corr, 2013). Cronbach’s alpha for the Defensive Fight subscale among this sample was .76.
Handedness was assessed using the 13-item Chapman Handedness Inventory (Chapman & Chapman, 1987). Cronbach’s alpha for this sample was .76.
EEG Recording and Reduction
Resting EEG recordings involved four 60-sec eyes-open trials. EEG data for the autobiographical memory task were collected during a 60-sec eyes-open contemplation period following each memory. Data were collected from 19 electrodes (FP1/FP2, F3/F4, F7/F8, C3/C4, T3/T4, P3/P4, T5/T6, FZ, CZA, CZ, PZA, PZ) grounded near Fz, and attached to a stretch-lycra electrode cap (Electro-Cap, Eaton, Ohio). The on-line reference was the left mastoid (M1) and data were recorded from the right mastoid (M2), enabling computation of an off-line average mastoid reference (impedances<5kΩ; homologs±1kΩ). Data were filtered (0.05–100 Hz), amplified, and digitized (500Hz). The EEG signals were visually scored and portions of data containing aberrant eye or muscle movements, or other sources of artifact were removed. PCA-based algorithms were then used to remove eye blinks, and another round of visual scoring was then conducted. Artifact-free epochs (1.024-sec) were Hamming windowed (75%-overlap) and power spectral density (μV2/Hz) computed. The mean number of artifact-free epochs across the resting and task intervals are as follows: resting: M = 448.50, S.D. = 39.25; neutral: M = 109.69, S.D. = 16.68; goal-striving: M = 106.40, S.D. = 17.25; anxious apprehension: M = 105.71, S.D. = 22.99; low-point: M = 107.71, S.D. = 16.68.1
In line with previous PFTA research (Wacker et al., 2006; 2010), total power in the theta band (4–8 Hz) was extracted from eyes-open intervals recorded both at rest and during each post-memory contemplation period. Theta power was log-transformed and, as in previous research (Wacker et al., 2006; 2010), a midline posterior minus frontal theta difference score was computed [ln(Pz)-ln(Fz)], with higher scores reflecting greater relative posterior theta activity, and putatively greater approach orientation (Wacker et al., 2006; 2010). PFTA difference scores exhibited a high degree of reliability. Across resting and task eyes-open intervals (8 total), Cronbach’s alpha was .93.
Data Analysis
Effect sizes were reported as the proportion of variance accounted for (ηp2). Fisher’s protected t-tests (Cohen, Cohen, West, & Aiken, 2003) were employed to minimize familywise error rate which requires a significant omnibus ANOVA F test to proceed to pairwise comparisons.
As a manipulation check, we first examined whether contemplation of the four autobiographical memories affected self-reported mood and arousal. This involved two separate repeated measures analyses of variance (ANOVA) on self-reported mood and arousal following the post-memory contemplation periods (goal-striving, anxious apprehension, low-point, and neutral). In the event of a significant omnibus F test, a follow-up repeated measures analysis of covariance (ANCOVA) was conducted to examine the effect of approach-relevant autobiographical memories on self-reported mood and arousal, controlling for baseline mood and arousal.
The primary analysis for the present study involved a one-way repeated measures ANOVA on PFTA collected during the post-memory contemplation periods (goal-striving, anxious apprehension, low-point, and neutral). In the event of a significant omnibus F test, a follow-up repeated measures ANCOVA was conducted to examine the influence of approach-relevant autobiographical memories on PFTA, controlling for resting PFTA. Given that our sample included a fairly large age range and both genders, additional follow-up repeated measures ANCOVAs were conducted to control for age and gender.
Our next analysis compared individuals who narrated approach versus withdrawal-oriented anxious apprehension scenes via t-test. In the event of significant findings, we examined whether the observed findings persisted when 1) resting PFTA and self-reported trait anxious apprehension were covaried, and 2) when age and gender were covaried.
We then compared participants who described approach versus withdrawal-oriented low-point scenes, and high anger versus low anger low-point scenes in two separate t-tests. Resting PFTA, age, and gender were covaried in the event of significant findings.
Correlational analyses examined the relationship between PFTA at rest and during the post-memory contemplation period, and both BIS/BAS sensitivity and trait-related anger, as indexed by the Defensive Fight subscale of the Reinforcement Sensitivity Theory Personality Questionnaire.
Results
Effect of Autobiographical Memory on Self-Reported Mood and Arousal
A repeated measures ANOVA indicated a main effect of autobiographical memory on self-reported mood, F(3,138) = 98.08, p < .001, ηp2 = .68. In line with prediction, pairwise comparisons indicated that the goal-striving autobiographical memory was associated with greater positive mood (M = 1.32, SE = .29) than the neutral (M = .60, SE = .19), anxious apprehension (M = −2.34, SE = .15), and low-point (M = −2.60, SE = .15) memories (p’s < .03). Furthermore, both the anxious apprehension and low-point autobiographical memories were associated with greater negative mood than the neutral memory (p’s < .001). There was no difference in self-reported mood between the anxious apprehension and low-point memories (p = .15). The observed differences were maintained when controlling for baseline mood F(3, 135) = 76.20, p < .001, ηp2 = .63.
A repeated measures ANOVA also revealed a main effect of autobiographical memory on arousal, F(3, 138) = 11.62, p < .001, ηp2 = .20. In line with prediction, pairwise comparisons indicated that the goal-striving (M = 1.43, SE = .24) and anxious apprehension (M = 1.21, SE = .26) memories were associated with greater self-reported arousal than the neutral (M = −.36, SE = .21) and low-point (M = .09, SE = .28) memories (p’s < .01). There were no differences in self-reported arousal between the goal-striving and the anxious apprehension memories (p = .54) or between the neutral and low-point (p = .18) memories. The observed differences were maintained when controlling for baseline arousal F(3, 135) = 11.66, p < .001, ηp2 = .21.
Effect of Autobiographical Memory on Posterior versus Frontal Theta Activity
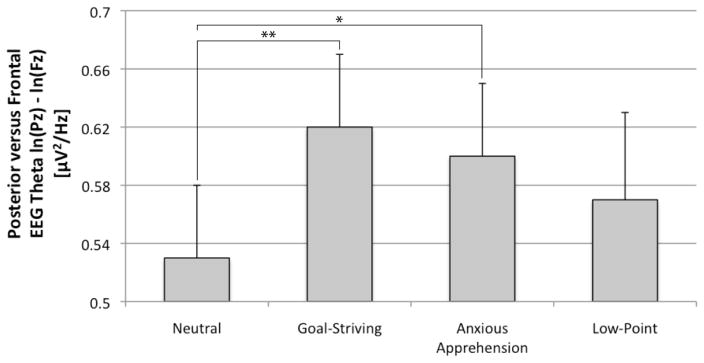
A repeated measures ANOVA revealed a main effect of autobiographical memory on PFTA, F(3, 123) = 2.78, p < .05, ηp2 = .06 (Figure 1). In line with prediction, pairwise comparisons indicated that both the goal-striving (M = .62, SE = .05; t(41) = 3.50, p < .001) and anxious apprehension (M = .60, SE = .05; t(41) = 2.37, <.05) memory contemplation periods were associated with greater PFTA compared to the neutral memory contemplation period (M = .53, SE = .05). These results were maintained after controlling for resting PFTA, F(3, 120) = 2.89, p < .05, ηp2 = .07, and gender F(3, 120) = 2.71, p < .05, ηp2 = .06. However, this effect was not maintained after controlling for age, F(3, 120) = .27, n.s. In the analysis controlling for age, there was a main effect of age F(1, 40) = 4.49, p < .05, ηp2 = .10, such that older participants exhibited elevated PFTA across autobiographical memories. Critically, however, there was no autobiographical memory x age interaction F(3, 120) = .57, n.s. This indicates that the effect of the autobiographical memory task did not vary as a function of age, and that the observed increase in PFTA during the goal-striving and anxious apprehension memory contemplation periods was not accounted for by age.2
Figure 1.
Posterior versus frontal theta activity (PFTA) during post-memory contemplation period. Note: * = p < .05, ** = p < .01. Error bars denote 1 S.E.
Contrary to prediction, pairwise comparisons indicated that the low-point contemplation period (M = .57, SE = .06) was not associated with reduced PFTA compared to the goal-striving (t(41) = 1.26, p = .21), anxious apprehension (t(41) = .84, p = .41), or neutral (t(41) = −1.27, p = .21), memory contemplation periods. Relevant to this finding, however, is the fact that, controlling for both baseline arousal and resting PFTA, there was a positive relationship between self-reported arousal following the low-point memory and PFTA during the low-point memory contemplation period, partial r(38) = .32, p < .05. Thus, participants who experienced highly arousing low-point memories tended to display elevated PFTA during these memories.
Posterior versus Frontal Theta Activity during Approach versus Withdrawal Anxious Apprehension and Low-point Memories
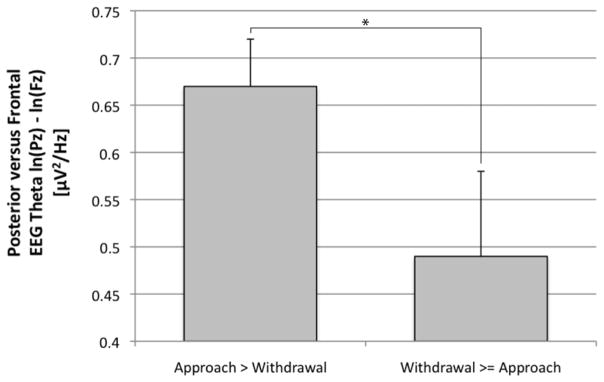
Consistent with prediction, individuals who narrated anxious apprehension memories characterized by elevated approach tendencies displayed greater PFTA during the anxious apprehension contemplation period (M = .67, SE = .05) compared to individuals who narrated anxious apprehension memories characterized by elevated withdrawal tendencies (M = .49, SE = .09), t(40) = 1.98, p = .06 (Figure 2). This effect was maintained after controlling for both resting PFTA and self-reported trait anxious apprehension using the PSWQ F(1, 38) = 6.03, p < .05, ηp2 = .14. In addition, this effect was maintained when age F(1, 39) = 3.86, p = .06, ηp2 = .09 and gender F(1, 39) = 3.80, p = .06, ηp2 = .09 were covaried.
Figure 2.
Posterior versus frontal theta activity (PFTA) during contemplation of anxious apprehension memories coded as greater in approach than withdrawal (n = 26) versus those coded as either greater in withdrawal than approach, or equal in approach and withdrawal (n = 16). Note: * = p < .05. Error bars denote 1 S.E.
In contrast to prediction, approach-oriented (M = .54, S.D. = .37) and withdrawal-oriented (M = .59, S.D. = .37) low-point memories did not differ on PFTA t(40) = .39, n.s. Lastly, low-point memories coded as being high in anger (M = .59, S.D. = .32) did not differ from low-point memories coded as being low in anger (M = .52, S.D. = .50) t(40) = .56, n.s.3
Correlational Analyses of Posterior versus Frontal Theta Activity, BAS Sensitivity, and Self-Reported Trait Anger
In line with prediction, resting PFTA was positively associated with trait-related anger, as indexed by the Defensive Fight subscale of the Reinforcement Sensitivity Theory Personality Questionnaire, r(40) = .38, p < .05) (Table 2). This relationship was particularly strong among male participants r(40)= .67, p < .001. Resting PFTA was also associated with BAS Fun-Seeking r(40) = .45, p < .01, BAS Reward Responsiveness r(40) = .26, p < .05, and BAS-Total r(40) = .26, p < .05.
Table 2.
Correlations between resting and post-memory contemplation measures of PFTA, and self-reported Defensive Fight, BIS sensitivity, and BAS sensitivity
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PFTA:Rest | - | |||||||||||
| 2. PFTA:Neut | .69** | - | ||||||||||
| 3. PFTA: Goal | .60** | .86** | - | |||||||||
| 4. PFTA: Anx | .77** | .77** | .72** | - | ||||||||
| 5. PFTA: Low | .80** | .79** | .72** | .75** | - | |||||||
| 6: PFTA: Average | .95** | .83** | .76** | .87** | .88** | - | ||||||
| 7. Def Fight | .38* | .16 | .15 | .29 | .25 | .33* | - | |||||
| 8. BAS Fun | .45** | .31* | .22 | .31* | .38* | .43** | .13 | - | ||||
| 9. BAS Drive | −.16 | .04 | .07 | −.11 | −.05 | −.11 | .14 | .16 | - | |||
| 10. BAS Reward | .26* | .20 | .04 | .31* | .14 | .27* | .18 | .37* | .40** | - | ||
| 11. BAS Total | .26* | .25 | .13 | .25 | .21 | .28* | .21 | .65** | .68** | .86** | - | |
| 12. BIS | .03 | .11 | −.08 | .23 | .02 | .06 | .22 | −.16 | .08 | .50** | .25 | - |
Note:
p < .05,
p < .01.
One-tailed tests used for relationships between resting PFTA and BAS constructs given past research suggesting a positive link (Wacker et al., 2010).Two-tailed tests were used otherwise. PFTA: Average is a composite of the 4 60-sec resting intervals and the 4 60-sec task intervals.
With respect to task-related EEG data, elevated self-reported BAS Fun-Seeking was associated with elevated PFTA during the anxious apprehension, r(40) = .31, p < .05, low-point r(40) = .38, p < .05, and neutral r(40) = .31, p < .05 contemplation periods, and elevated BAS Reward Responsiveness was associated with elevated PFTA during the anxious apprehension contemplation period, r(40) = .31, p < .05.
Discussion
The present study examined posterior versus frontal EEG theta activity, a recently proposed signature of approach-related affect, during an autobiographical memory task designed to induce approach-relevant states with a high degree of ecological validity. In line with prediction, participants displayed elevated PFTA during contemplation of goal-striving memories compared to neutral memories. This result supports the claim that PFTA may reflect a neurophysiological index of approach-related affect.
Previous research has tended to examine resting PFTA as an index of approach tendencies (Wacker et al., 2006; 2010). In these previous studies, elevated PFTA at rest was presumed to indicate a dispositional signature of approach tendencies without regard for situational demands. Dispositional models of personality contend that general tendencies hold across a variety of situations (Costa & McCrae, 2009). However, situational models propose that an individual’s behavior will vary in response to the demands of the situation (Mischel & Shoda, 2010), and that situational demands may yield a more pure measure of neurophysiological individual differences (Coan et al., 2006). Hence, our findings extend past work by suggesting that PFTA indexes appetitive tendencies not just during unconstrained resting periods, but also during emotionally demanding tasks. The proposal of elevated PFTA as an index of approach tendencies is therefore strengthened by the convergence of resting and task-based measures.
Beyond providing further validation of PFTA, the current study breaks new ground by demonstrating that contemplation of anxious apprehension memories is associated with elevated PFTA compared with contemplation of neutral memories. While no prior study has linked PFTA and anxious apprehension, previous studies have found a link between elevated relative left frontal cortical activity at rest and self-reported levels of anxious apprehension (Heller et al., 1997; Mathersul et al., 2008). Taken together, these studies highlight the potential for approach tendencies within anxious apprehension.
However, to add further clarity to this issue we compared PFTA during the contemplation of two categories of anxious apprehension memories: those demonstrating evidence of greater approach than withdrawal, and those demonstrating evidence of greater withdrawal than approach, or equal parts approach and withdrawal. The finding of elevated PFTA among approach-oriented anxious apprehension memories suggests that anxious apprehension is not a uniformly approach-oriented affect. Rather, our findings highlight the variability in approach and withdrawal tendencies within anxious apprehension, and suggest that PFTA partially indexes this variability. In addition, our findings suggest that the extent to which anxious apprehension may be considered approach-oriented depends on the participant’s subjective experience of anxiety. Participants who narrated an anxious apprehension memory in which they described taking active steps to resolve the source of the anxiety tended to show elevated PFTA, which past research has linked to high approach motivation (Wacker et al., 2010). Hence, elevated PFTA exhibited by participants who described proactively confronting their anxiety may reflect sustained approach motivation in the face of an emotionally negative experience. By contrast, participants who tended to avoid dealing with his or her anxiety in a constructive manner, and instead reported engaging in more withdrawal related behavior or cognitions, exhibited reduced PFTA. These findings are in line with research by Wacker and colleagues (2010) suggesting that reduced PFTA is characteristic of individuals low in approach motivation. This result persisted when controlling for trait worry (PSWQ) and resting PFTA, suggesting that this result was specific to state anxious apprehension elicited with our autobiographical memory paradigm.
The findings elicited by the retelling and contemplation of emotionally salient autobiographical memories underscore the importance of utilizing ecologically valid paradigms to study approach motivation. Whereas previous studies of approach-related neurophysiology have typically employed computer paradigms involving relatively mild emotional stimuli, the present study employed a more personological approach in which the participants determined which life experiences to share and why they were important. This ecologically valid approach is noteworthy for several reasons. First, traditional methods of eliciting approach motivation (e.g. monetary reward via computer task) may not be sufficiently salient or evocative to activate meaningful amounts of approach motivation. By contrast, involving the participant in the generation of approach relevant stimuli guarantees a more personological and salient emotional experience. Second, by evoking approach motivation within the context of a realistic dyadic exchange, an ecologically valid methodology may reduce measurement error stemming from the high degree of abstraction inherent to programmed computer tasks.
A secondary aim of the current study was to investigate the relationships between both resting and task-related PFTA and self-reported BAS sensitivity. Consistent with prediction, resting PFTA was significantly related to BAS Fun-Seeking. This result replicates previous studies linking elevated PFTA with various self-report measures of approach motivation (Wacker et al., 2010). There was also a relationship between post-memory contemplation PFTA and facets of BAS sensitivity. Elevated PFTA during contemplation of neutral, anxious apprehension, and low-point memories was associated with elevated BAS Fun-Seeking. In addition, participants who showed elevated PFTA during contemplation of anxious apprehension memories also tended to report elevated BAS Reward Responsiveness. These relationships, however, became non-significant when controlling for resting PFTA. This pattern suggests that dispositional (i.e., resting) and situational (i.e., task) measures of PFTA index similar variance in BAS sensitivity.
Previous research establishing PFTA at rest as a marker of approach motivation have employed measures of appetitive tendencies that confound motivational direction with affective valence. In other words, previous studies have shown that individuals exhibiting elevated resting PFTA also tend to endorse items that tap both positive affect and goal pursuit, creating ambiguity about which of these constructs is indexed by PFTA. To resolve this ambiguity, it was necessary to examine the relationship between PFTA and self-reported trait anger, a negatively valenced emotion that EEG, PET, and fMRI studies have linked to approach-related behavior (Damasio et al., 2000; Dougherty et al., 1999; Murphy, Nimmo-Smith, & Lawrence, 2003). The present study reports, for the first time, that elevated resting PFTA is associated with elevated self-reported trait anger. By demonstrating that elevated PFTA is associated with a) elevated anger, b) with a negatively valenced anxious apprehension state, and c) with a positively valenced goal-striving state, the present study suggests that PFTA indexes approach motivation regardless of valence. These results provide strong support for PFTA as an index of approach motivation, and extend previous neurophysiological research highlighting the approach orientation within anger (Harmon-Jones & Allen, 1998; Harmon-Jones & Sigelman, 2001). Future research is needed to test the hypothesis that PFTA more robustly indexes approach-oriented (e.g., expressed behaviorally) anger versus withdrawal-oriented (e.g., hidden or suppressed) anger.
Contrary to prediction, goal-striving and anxious apprehension memories did not differ from low-point memories in terms of PFTA. Important to understanding this finding, however, is the fact that elevated self-reported arousal during the low-point memory was associated with elevated PFTA during the low-point memory contemplation period. Thus, participants who experienced highly arousing low-point memories tended to display elevated PFTA during these memories. Another possibility is that elevated PFTA among low-point memories reflects approach-related tendencies embedded in these memories (e.g., rushing to the hospital after learning that a loved one has been in an accident). However, low-point memories coded as approach-oriented were not associated with greater PFTA than low-point memories coded as withdrawal-oriented. In addition, low-point memories coded as being high in anger were not associated with greater PFTA than low-point memories coded as low in anger. Therefore, neither approach, withdrawal, nor anger tendencies explain the finding of elevated PFTA during contemplation of low-point memories. This issue highlights the need for future research to examine factors that influence PFTA during different kinds of mixed negative affective states (e.g., arousal).
A limitation of the present study is that the use of a non-clinical sample prevents us from generalizing our findings to extreme populations, particularly those with clinically significant levels of anxious apprehension (i.e., generalized anxiety disorder). A second limitation is that while our main findings appear to be driven by the differing emotional states elicited by the autobiographical memory types, we are unable to precisely pinpoint which aspects of the approach-relevant emotions underlie our results. For example, while goal-striving memories were associated with greater PFTA than neutral scenes, it is unclear whether elevated goal-striving PFTA resulted from contemplation of anticipatory components (e.g., approach-oriented tasks leading to goal attainment), or from contemplation of consummatory components (e.g., re-living a sense of satisfaction stemming from the goal attainment itself) of the contemplation period. The importance of this distinction is highlighted by evidence suggesting that different neural processes underlie the emotions and motivations associated with the anticipation versus consumption of a goal or reward (Berridge, Robinson, & Aldrige, 2009). Thus, future research should examine PFTA using task paradigms that differentiate anticipatory and consummatory reward-related emotions.
In summary, elevated PFTA characterized contemplation of autobiographical memories about goal-striving experiences (e.g., applying to college), whereas reduced PFTA was exhibited during neutral memory contemplation, suggesting that PFTA indexes state approach-related motivation. In addition, contemplation of anxious apprehension memories was associated with elevated PFTA, with PFTA particularly elevated during approach-oriented anxious apprehension memories. This result suggests that PFTA taps approach motivation within the context of a negative affective state. Finally, PFTA showed a significant positive correlation with anger, further strengthening PFTA as an index of appetitive tendencies. Results from the present study enhance our understanding of the neurophysiology of approach-related emotions, helps establishes PFTA as an index of appetitive motivational states, and highlights the approach-related tendencies of both anxious apprehension and anger.
Table 1.
Means and standard deviations of approach and withdrawal codes (0–2 scale) for neutral, goal-striving, anxious apprehension, and low-point memories
| Memory | Approach Tendencies | Withdrawal Tendencies |
|---|---|---|
| Neutral | 0 (0) | 0 (0) |
| Goal-striving | 1.65 (.47) | .09 (.26) |
| Anxious apprehension | .87 (.61) | .25 (.48) |
| Low-point | .61 (.63) | .62 (.71) |
Footnotes
To test whether our primary findings were driven by outliers with a small amount of usable data, we re-ran our primary analyses excluding participants who were 3 S.D. below the mean in artifact free epochs for both resting and task conditions. This cutoff criterion corresponded to less than 62.22 epochs of useable data per minute and resulted in seven individuals being excluded. Using these exclusion criteria, the main effect of autobiographical memory on PFTA was maintained F(3, 102) = 2.95, p < .05, ηp2 = .08. In addition, the finding of elevated PFTA in approach versus withdrawal oriented anxious apprehension scenes was also maintained t(33) = 2.55, p < .05.
This finding is consistent with research showing that theta activity at frontal midline sites tends to decrease with age (Cummins & Finnigan, 2007). Because PFTA is calculated by subtracting the frontal midline electrode Fz from the posterior midline electrode Pz, lower frontal midline theta activity may have contributed to elevated PFTA among older participants.
Frontal alpha asymmetry is another commonly used neurophysiological index of approach-related affect (Sutton & Davidson, 1997; Thibodeau, Jorgensen, & Kim, 2006). As with PFTA, higher levels of relative left frontal EEG activity (as indexed by alpha) has been linked with elevated approach tendencies (Carver & Harmon-Jones, 2009). However, in the present study, there was no main effect of autobiographical memory on frontal alpha asymmetry, F(3,120) = .68, n.s. In addition, there was no difference in frontal alpha asymmetry between approach and withdrawal-oriented anxious apprehension memories t(40) = .97, n.s.
References
- Baker TE, Holroyd CB. Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biological Psychology. 2011;87(1):25–34. doi: 10.1016/j.biopsycho.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, … Ernst M. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science. 2009;20(8):1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH. Disorders of Emotion. Psychological Inquiry. 1991;2(1):58–71. doi: 10.1207/s15327965pli0201_15. [DOI] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Current Opinion in Pharmacology. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino A, Elvevag B, Callicott JH, Das S, … Weinberger DR. Effect of catechol-O-methyltransferase val158met genotype on attentional control. Journal of Neuroscience. 2005;25(20):5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Harmon-Jones E. Anger is an approach-related affect: evidence and implications. Psychological Bulletin. 2009;135(2):183–204. doi: 10.1037/a0013965. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67(2):319–333. doi: 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain and Cognition. 1987;6(2):175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Chavanon ML, Wacker J, Stemmler G. Rostral anterior cingulate activity generates posterior versus anterior theta activity linked to agentic extraversion. Cognitive Affective & Behavioral Neuroscience. 2011;11(2):172–185. doi: 10.3758/s13415-010-0019-5. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67(1–2):7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJ, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biological Psychology. 2006;72(2):198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple regression/correlation analysis for the behavioral sciences. 3. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2003. [Google Scholar]
- Corr PJ. Approach and Avoidance Behaviour: Multiple Systems and their Interactions. Emotion Review. 2013;5(3):285–290. doi: 10.1177/1754073913477507. [DOI] [Google Scholar]
- Corr PJ, Cooper A. The Corr-Cooper Reinforcement Sensitivity Theory Personality Questionnaire (RST-PQ): Development and validation. 2013. [DOI] [PubMed] [Google Scholar]
- Cox BJ, Enns MW, Taylor S. The effect of rumination as a mediator of elevated anxiety sensitivity in major depression. Cognitive Therapy and Research. 2001;25(5):525–534. [Google Scholar]
- Crost NW, Pauls CA, Wacker J. Defensiveness and anxiety predict frontal EEG asymmetry only in specific situational contexts. Biological Psychology. 2008;78(1):43–52. doi: 10.1016/j.biopsycho.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Cummins TD, Finnigan S. Theta power is reduced in healthy cognitive aging. International Journal of Psychophysiology. 2007;66(1):10–17. doi: 10.1016/j.ijpsycho.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Shin LM, Alpert NM, Pitman RK, Orr SP, Lasko M, … Rauch SL. Anger in healthy men: a PET study using script-driven imagery. Biological Psychiatry. 1999;46(4):466–472. doi: 10.1016/s0006-3223(99)00063-3. [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Mohanty A, Herrington JD, Banich MT, Webb AG, Miller GA. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology. 2007;44(3):352–363. doi: 10.1111/j.1469-8986.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, … Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, … Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, … Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26(24):6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. Journal of Neuroscience. 2006;26(32):8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology. 1998;74(5):1310–1316. doi: 10.1037//0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biological Psychology. 2010;84(3):451–462. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman J. State anger and prefrontal brain activity: evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. Journal of Personality and Social Psychology. 2001;80(5):797–803. [PubMed] [Google Scholar]
- Hayden BY, Pearson JM, Platt ML. Fictive reward signals in the anterior cingulate cortex. Science. 2009;324(5929):948–950. doi: 10.1126/science.1168488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W, Nitschke JB. The puzzle of regional brain activity in depression and anxiety: The importance of subtypes and comorbidity. Cognition & Emotion. 1998;12(3):421–447. doi: 10.1080/026999398379664. [DOI] [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106(3):376–385. doi: 10.1037//0021-843x.106.3.376. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Bromberg-Martin E, Hong S, Matsumoto M. New insights on the subcortical representation of reward. Current Opinion in Neurobiology. 2008;18(2):203–208. doi: 10.1016/j.conb.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JJ, Yen CT, Liu TL, Tsao HW, Hsu JW, Tsai ML. Effects of dopamine D2 agonist quinpirole on neuronal activity of anterior cingulate cortex and striatum in rats. Psychopharmacology. 2013;227(3):459–466. doi: 10.1007/s00213-013-2965-4. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Griffiths K, Felmingham KL, Shankman SA, Drinkenburg W, Arns M, … Bryant RA. Disorder specificity despite comorbidity: resting EEG alpha asymmetry in major depressive disorder and post-traumatic stress disorder. Biological Psychology. 2010;85(2):350–354. doi: 10.1016/j.biopsycho.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, Bruder GE. Electroencephalographic asymmetries in adolescents with major depression: influence of comorbidity with anxiety disorders. Journal of Abnormal Psychology. 2000;109(4):797–802. doi: 10.1037//0021-843x.109.4.797. [DOI] [PubMed] [Google Scholar]
- Mathersul D, Williams LM, Hopkinson PJ, Kemp AH. Investigating models of affect: relationships among EEG alpha asymmetry, depression, and anxiety. Emotion. 2008;8(4):560–572. doi: 10.1037/a0012811. [DOI] [PubMed] [Google Scholar]
- McAdams DP. Power, intimacy, and the life story: Personological inquiries into identity. New York: Guilford Press; 1985. [Google Scholar]
- McAdams DP, Anyidoho NA, Brown C, Huang YT, Kaplan B, Machado MA. Traits and stories: links between dispositional and narrative features of personality. Journal of Personality. 2004;72(4):761–784. doi: 10.1111/j.0022-3506.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- McAdams DP, Hoffman BJ, Day R, Mansfield ED. Themes of Agency and Communion In Significant Autobiographical Scenes. Journal of Personality. 1996;64(2):339–377. doi: 10.1111/j.1467-6494.1996.tb00514.x. [DOI] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behavior Research and Therapy. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y. The situated person. In: Mesquita B, Barrett LF, Smith ER, editors. The mind in context. New York: Guilford Press; 2010. pp. 149–168. [Google Scholar]
- Molina S, Borkovec TD. The Penn State Worry Questionnaire: Psychometric properties and associated characteristics. In: Davey GCL, Tallis F, editors. Worrying: Perspectives on theory, assessment and treatment. Oxford, England: John Wiley & Sons; 1994. pp. 265–283. [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognitive Affective & Behavioral Neuroscience. 2003;3(3):207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36(5):628–637. [PubMed] [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychological Science. 2005;16(10):805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Reese E, Yan C, Jack F, Hayne H. Emerging identities: Narrative and self from early childhood to early adolescence. In: McLean KC, Pasupathi M, editors. Narrative development in adolescence: Creating the storied self. Springer; 2010. pp. 23–43. [Google Scholar]
- Roberts JE, Gilboa E, Gotlib IH. Ruminative response style and vulnerability to episodes of dysphoria: Gender, neuroticism, and episode duration. Cognitive Therapy and Research. 1998;22(4):401–423. [Google Scholar]
- Russell JA, Weiss A, Mendelsohn GA. Affect Grid: A single-item scale of pleasure and arousal. Journal of Personality and Social Psychology. 1989;57(3):493–502. doi: 10.1037/0022-3514.57.3.493. [DOI] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Reviews Neuroscience. 2000;1(3):199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schweimer J, Hauber W. Dopamine D1 receptors in the anterior cingulate cortex regulate effort-based decision making. Learning & Memory. 2006;13(6):777–782. doi: 10.1101/lm.409306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal Brain Asymmetry: A Biological Substrate of the Behavioral Approach and Inhibition Systems. Psychological Science. 1997;8(3):204–210. doi: 10.1111/j.1467-9280.1997.tb00413.x. [DOI] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. Journal of Abnormal Psychology. 2006;115(4):715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ. Frontal brain activation in repressors and nonrepressors. Journal of Abnormal Psychology. 1994;103(2):339–349. doi: 10.1037//0021-843x.103.2.339. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27(3):247–259. [Google Scholar]
- Udo de Haes JI, Maguire RP, Jager PL, Paans AM, den Boer JA. Methylphenidate-induced activation of the anterior cingulate but not the striatum: a [15O]H2O PET study in healthy volunteers. Human Brain Mapping. 2007;28(7):625–635. doi: 10.1002/hbm.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Chavanon ML, Stemmler G. Investigating the dopaminergic basis of extraversion in humans: A multilevel approach. Journal of Personality and Social Psychology. 2006;91(1):171–187. doi: 10.1037/0022-3514.91.1.171. [DOI] [PubMed] [Google Scholar]
- Wacker J, Chavanon ML, Stemmler G. Resting EEG signatures of agentic extraversion: New results and meta-analytic integration. Journal of Research in Personality. 2010;44(2):167–179. http://dx.doi.org/10.1016/j.jrp.2009.12.004. [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46(1):327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann G, Pauli P, Dengler W, Lutzenberger W, Birbaumer N, Buchkremer G. Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Archives of General Psychiatry. 1999;56(1):78–84. doi: 10.1001/archpsyc.56.1.78. [DOI] [PubMed] [Google Scholar]
- Yik M, Russell JA, Steiger JH. A 12-Point Circumplex Structure of Core Affect. Emotion. 2011;11(4):705–731. doi: 10.1037/a0023980. [DOI] [PubMed] [Google Scholar]