Abstract
Although climate change is predicted to affect methane (CH4) emissions in paddy soil, the dynamics of methanogens and methanotrophs in paddy fields under climate change have not yet been fully investigated. To address this issue, a multifactor climate change experiment was conducted in a Chinese paddy field using the following experimental treatments: (1) enrichment of atmospheric CO2 concentrations (500 ppm, CE), (2) canopy air warming (2°C above the ambient, WA), (3) combined CO2 enrichment and warming (CW), and (4) ambient conditions (CK). We analyzed the abundance of methanogens and methanotrophs, community structures, CH4 production and oxidation potentials, in situ CH4 emissions using real-time PCR, T-RFLP, and clone library techniques, as well as biochemical assays. Compared to the control under CE and CW treatments, CH4 production potential, methanogenic gene abundance and soil microbial biomass carbon significantly increased; the methanogenic community, however, remained stable. The canopy air warming treatment only had an effect on CH4 oxidation potential at the ripening stage. Phylogenic analysis indicated that methanogens in the rhizosphere were dominated by Methanosarcina, Methanocellales, Methanobacteriales, and Methanomicrobiales, while methanotrophic sequences were classified as Methylococcus, Methylocaldum, Methylomonas, Methylosarcina (Type I) and Methylocystis (Type II). However, the relative abundance of Methylococcus (Type I) decreased under CE and CW treatments and the relative abundance of Methylocystis (Type II) increased. The in situ CH4 fluxes indicated similar seasonal patterns between treatments; both CE and CW increased CH4 emissions. In conclusion results suggest that methanogens and methanotrophs respond differently to elevated atmospheric CO2 concentrations and warming, thus adding insights into the effects of simulated global climate change on CH4 emissions in paddy fields.
Keywords: elevated CO2, warming, methanogen, methanotroph, paddy field
Introduction
Climate model projections suggest that atmospheric carbon dioxide (CO2) concentrations are likely to double by the end of the century, with mean global temperatures potentially increasing by a further 1.4–5.8°C (IPCC, 2007). Methane (CH4) is the second most abundant greenhouse gas (GHG) after CO2, accounting for about 20% of anthropogenic radiative forcing (Nisbet et al., 2014); atmospheric CH4 concentrations have increased from about 715 ppb before the industrial revolution to 1800 ppb in 2008 (Montzka et al., 2011). The global warming potential of CH4 is 25 times that of CO2, thus small changes in atmospheric CH4 concentrations will significantly contribute to future climate warming (Bridgham et al., 2013). Global climate change, such as elevated CO2 and warming, have been reported to dramatically alter the properties and functioning of terrestrial ecosystems (Rosenzweig et al., 2007; Austin et al., 2009; Singh et al., 2010).
One of the most important sources of atmospheric CH4 are rice paddies (Yan et al., 2009; Liu et al., 2012), accounting for 5–19% of global CH4 emissions (IPCC, 2007). An increase in CH4 emissions from these sources in response to elevated atmospheric CO2 and increased temperatures have already been identified, an occurrence which results in a positive feedback in the global warming process (Allen et al., 2003; Tokida et al., 2010; van Groenigen et al., 2011). Recent evidence has shown that elevated CO2 concentrations increased CH4 emissions from paddy soils by an average of 43% (van Groenigen et al., 2011); increased soil temperatures (2°C) resulted in a 42% increase in CH4 emissions (Tokida et al., 2010). It is generally assumed that elevated CO2 enhances photosynthesis, root biomass and exudates of rice (Pritchard, 2011; Okubo et al., 2014) which may provide more substrate for CH4 production (Inubushi et al., 2003). As methanogens and methanotrophs regulate CH4 emissions in rice soil (Conrad, 2007; Høj et al., 2008; Knoblauch et al., 2008), it is therefore important to understand how climate change factors affect microbial community structures and functions involved in the CH4 cycle.
CH4 production, the final microbial decomposition process of organic matter in paddy fields, is produced by methanogens (Bridgham et al., 2013; Breidenbach and Conrad, 2015), of which there are two main types of methanogenic pathways: acetate- and H2/CO2-dependent methanogenesis (Conrad and Klose, 2006; Breidenbach et al., 2015). Most biogenically produced methane is oxidized by methanotrophs at the soil surface (Conrad, 2007; Yun et al., 2013). CH4 oxidation can proceed both aerobically and anaerobically (Lüke et al., 2014; Knief, 2015). Aerobic methanotrophs are a subset of methylotrophs which can utilize CH4 as sole C and energy source (Chen et al., 2014). Aerobic methanotrophs in rice field consist mainly of proteobacterial lineages (Hu and Lu, 2015), while verrucomicrobial methanotrophs are restricted to extreme environments (Op den Camp et al., 2009). The proteobacterial methanotrophs can be separated into Type I and Type II groups belonging to Gammaproteobacteria and Alphaproteobacteria, respectively (Chen et al., 2014; Lee et al., 2014). Anaerobic methane oxidation can be coupled to sulfate reduction, metal reduction, nitrite dismutation, disulphide disproportionation (Ettwig et al., 2010; Joye, 2012). To study the diversity of methanogens and methanotrophs, we selected the genes coding for subunit A of the methyl coenzyme-M reductase enzyme (mcrA) and particulate methane monooxygenase enzyme (pmoA), respectively.
Methanogens have been identified to be sensitive to global climate change; atmospheric CO2 enrichment and warming alter the composition of methanogenic archaea and increase their abundance and activity in paddy soils (Peng et al., 2008; Liu et al., 2012). During a short-term incubation of paddy soil, due to reduced soil redox potential, increased available C and methanogens (Das and Adhya, 2012), elevated atmospheric CO2 and temperature interaction significantly increased CH4 production under flooded conditions. Elevated CO2 and increased carbon input from plants to soil may, have a positive effect on methanogenic archaea. However, Angel et al. (2012) identified that atmospheric CO2 enrichment had no significant impact on methanogenic community and CH4 production potential in a waterlogged grassland. On the other hand, CH4 oxidation decreased under elevated CO2 concentrations from different forest soils (McLain and Ahmann, 2008; Dubbs and Whalen, 2010), which may be due to increased soil moisture, the availability of carbon and reduced soil O2 concentrations under elevated CO2 conditions. Dijkstra et al. (2010) suggested that CH4 oxidation may be enhanced under drier soil conditions with increasing temperatures. However, studies investigating the responses of methanotrophic communities under paddy fields to elevated CO2 levels and atmospheric warming are limited.
While most experimental designs have studied the effects of a single climate factor (e.g., CO2 enrichment or increased temperatures) on soil CH4 cycling, the microbial responses in multi-factorial experiments have not been thoroughly investigated (Singh et al., 2010). In previous studies, elevated CO2 levels and atmospheric warming had either an additive or an antagonistic effect on soil microbial communities and functions in temperate agricultural soils (French et al., 2009; Pritchard, 2011). In order to assess how multiple climate change variables synergistically interact to affect soil methanogen and methanotroph microorganisms, we simultaneously artificially elevated atmospheric CO2 conditions (500 ppm, ambient) and air temperatures (+2°C, ambient) in a Chinese paddy field. The objective of this study was to address how CO2 enrichment, warming and their interaction affected the abundance and community composition of methanogens and methanotrophs in a rice paddy, and to determine if these changes could be linked to CH4 production and oxidization. The microbial abundance, community structure and composition were quantified and fingerprinted with real-time PCR (qPCR), terminal-restriction fragment length polymorphism (T-RFLP) and clone library techniques; CH4 production and oxidization potentials were assessed using biochemical assays.
Materials and Methods
Site Description and Experimental Setup
The field experiment simulating climate change was established in 2010 in Kangbo village (31°30′N, 120°33′E), Changshu Municipality, Jiangsu, China. The area experiences a subtropical monsoon climate with an annual mean temperature (2004–2013) of 16°C and mean precipitation of 1100–1200 mm. The soil is a Gleyic Stagnic Anthrosol formed on a clayey lacustrine deposit which has been cultivated with a rice-wheat rotation for hundreds of years. The basic properties of the topsoil prior to the experiment in 2010 were: soil pH of 7.0, organic carbon of 1.6%, total nitrogen of 1.9 g kg-1 and bulk density 12 g cm-3.
The experimental details with elevated CO2 and warming are presented in the study of Liu et al. (2014). In brief, with a block split-plot design, one field plot was artificially treated with a continuous atmospheric CO2 concentration enrichment up to 500 ppm (CE) using a liquid CO2 supply. The crop canopy air of another field plot was warmed by 2°C (WA) above the ambient temperature with infrared heaters. A further field plot was subjected to both CO2 enrichment and warming (CW), and a final field plot was maintained at an ambient condition as a control (CK). Treatment levels for our investigation were defined according to IPCC (2007): the A2 emission scenario predicts atmospheric CO2 concentrations to increase by 500 ppm and global mean air temperatures to increase by 2°C. For the elevated CO2 treatment, pure CO2 gas from a liquid tank was injected into the plots via perforated pipes surrounding the ring. Sixteen Li-820 CO2 sensors (Li-COR Inc., Lincoln, NE, USA) were installed and evenly distributed above the canopy to automatically control CO2 concentrations. CO2 concentration consistency over the ring was controlled by automatic adjustment to wind direction and velocity; when weed speed was more than 5 m/s, or if it was raining, CO2 spraying ceased. For the warming treatment, 12 infrared heaters (2000 W, 240 V, 1.65 m long × 0.14 m wide; HS-2420, Kalglo Electronics Co., Inc., Bethlehem, PA, USA) were situated on each ring. The heaters were adjusted every week to maintain a clearance height of 1.2 m above the top of the canopy during the growth period. The air temperature in the experimental plot was monitored by 6 infrared thermometers (Model SI- 121, Apogee instruments Inc., Logan, UT, USA) which were arranged in a hexagonal array (Liu et al., 2015; Cai et al., 2016; Wang et al., 2016). During the rice season, the average CO2 concentration under elevated CO2 plots was 515 ± 40 ppm and the increase of canopy air temperature under the warming plots was 1.98 ± 0.2°C. The control plots, surrounded by the same infrastructure, did not receive CO2 enrichment or any warming treatments. Each treatment was replicated in three rings with the same infrastructure, having 8-m-diameter and covering 50 m2 per ring. All the rings were buffered by an adjacent field to avoid treatment cross-over, and the distance between treatment plots was around 28 m.
In the experimental season, rice (Oryza sativa L. cv., Changyou No.5) was transplanted at a density of three seedlings per hill on 20th June, 2013. Plots were treated with local conventional practices, including a soil water regime of flooding during seedling to tillering stages, intermittent irrigation during heading, and drainage for ripening. Urea and ammonium bicarbonate were applied as basal fertilizers at a rate of 150 kg N ha-2 (120 kg-N ha-2 as urea and 30 kg-N ha-2 as ammonium bicarbonate) on 21th June, 2013. Chlorpyrifos was applied as a pesticide at a rate of 800–1000 g ha-2 at the heading stage. The management practices were consistent across all the treatments.
Sample Collection
Rice rhizosphere soils were sampled at the tillering (19th July), heading (4th September) and ripening (24th October) stages in 2013. The rhizosphere of five individual rice plants were randomly collected at a depth of 0–15 cm from each plot, following the procedure described by Butler et al. (2003). The rhizosphere soil (being tightly adhered to the plant roots with about 1 cm thickness) was carefully removed and evenly mixed to form a composite sample. These soil samples were passed through a 2-mm sieve and immediately sealed in a plastic bag before being transferred to the laboratory (within 1 day after sampling). Fresh samples were stored at 4°C and analyzed for soil physico-chemical analyses within 1 week of sampling. A sub-sample of the soil was stored at –20°C prior to DNA extraction; this was undertaken within 1 week of sampling.
Soil Property Analysis
Soil microbial biomass carbon (SMBC) was determined by a fumigation-extraction method following Wu et al. (1990). The samples were fumigated with ethanol free chloroform for 24 h at 25°C before being extracted with 0.5 mM K2SO4 for 30 min on a shaker; unfumigated samples were also processed using the same method. The extracts were analyzed for extractable C using an automated TOC Analyzer (TOC-500, Japan). A KEC of 0.45 was used to convert the measured C to SMBC values. Inorganic N (NH4+ -N and NO3- -N) was extracted by shaking with 0.5 mol L-1 K2SO4 (1: 5 (w/w) soil: K2SO4 solution) for 1 h and then filtering through a 0.45-um-pore-size polysulfone membrane, before colorimetric determination using an automated flow injection analyzer (Skalar Analytical B.V., The Netherlands).
Measurement of Induced CH4 Production and Oxidation Potential
The CH4 production and oxidation potentials of soil were analyzed with a laboratory incubation method. CH4 production potentials in the soil samples were determined following the methods of Singh et al. (2012). In summary, 15 g of sample was transferred into a 120 ml glass jar, amended with 25 ml of sterile distilled deionized water and sealed with a butyl rubber stopper. The headspace in the jar was flushed with N2 for 10 min. Each soil sample was repeated in triplicate and incubated at 28°C in the dark. CH4 production was analyzed periodically by gas chromatography (Agilent 4890D, USA) equipped with a flame-ionization detector (FID). The CH4 concentration in the headspace was measured every 24 h for 1 week, and CH4 production potential was calculated using a linear regression of increased CH4 concentration with time.
CH4 oxidation potential was measured following the protocol of Vishwakarma et al. (2010). In summary, 15 g of sample was transferred into gas-tight 120 ml glass jars and incubated at 28°C for 7 days in the dark. All samples were analyzed in triplicate. The headspace in the jars contained 5% v/v methane in air. The CH4 concentration in the headspace was measured every 24 h for 1 week. CH4 oxidation potentials were calculated from the initial linear reduction of CH4 concentration with time and expressed as mg CH4-C per hour per gram dry weight.
Monitoring CH4 Emissions
A static closed chamber-GS method was used to monitor CH4 flux (Zou et al., 2009). Gas samples were collected once a week during the rice growing season. Samples were collected between 08:00 and 10:00 on the collection day and 4 individual gas samples were collected with a syringe at 0, 10, 20, and 30 min after chamber closure. The concentration of CH4 in a sample was analyzed using a gas chromatograph (Agilent 7890A) equipped with a flame ionization detector (FID). The carrier gas was nitrogen and a flow rate of 40 ml/min was maintained. The oven and FID were operated at 50 and 300°C, respectively.
DNA Extraction and Real-Time PCR Assay
Total DNA was extracted from 0.35 g of fresh soil with a PowerSoilTM DNA isolation kit (MoBio, Carlsbad, CA, USA) following the manufacturer’s instructions. DNA quality was assessed on an agarose gel while DNA quantity was determined using a Nanodrop spectrophotometer (Thermo Scientific, DA, USA).
Real-time PCR was performed in a 7500 real-time PCR system (Applied Biosystems, Germany) via fluorometric monitoring with SYBR Green 1 dye. The primer pair mcrAF/mcrAR (Luton et al., 2002) and A189f/mb661r (Horz et al., 2001) were used to quantify methanogenic archaeal mcrA genes and methanotrophic bacterial pmoA genes of all samples, respectively. Each reaction was performed in a 25 μl volume containing 15 ng of DNA, 1 μl of 10 μM of each primer and 12.5 μl of SYBR premix EX TaqTM (Takara Shuzo, Shinga, Japan). A melting curve analysis was conducted following each assay to confirm specific amplification was not from primer-dimers or other artifacts. A single clone containing the target region was grown in Luria-Bertani media and plasmid DNA was extracted using a plasmid-extraction kit (Takara, Japan). Standard curves were generated using a 10-fold dilution of plasmid DNA, from 103 to 109 copies of the template. PCR efficiencies were obtained between 98% and 106%, with R2 values >0.99. The final methanogenic mcrA gene and methanotrophic pmoA gene copy numbers were calibrated against total DNA amounts and soil water content.
Terminal-Restriction Fragment Length Polymorphism Analysis of Soil Microbial Communities
Terminal-restriction fragment length polymorphism was used for analyzing the community structure of methanogens and methanotrophs. Briefly, the functional genes mcrA and pmoA were amplified by PCR using the primer pairs mcrAF/mcrAR and A189f/mb661r, as previously mentioned with the 5′ end of the mcrAF and A189f primers labeled with 6-carboxyfluorescein (6-FAM). All PCRs were performed in duplicate and pooled for subsequent restriction and T-RFLP analysis. PCR products were separated by 1.5% agarose gel, and purified using the PCR solution purification kit (Takara, Dalian, China). Purified PCR products were used in a restriction digest TaqI and MspI (Takara, Dalian, China) for mcrA and pmoA genes as per the manufacturer’s instructions, respectively. Fragment analysis was achieved by capillary electrophoresis (ABI 3100 Genetic Analyzer; Applied Biosystems, Carlsbad, CA, USA) using a GeneScan ROX-labeled GS500 internal size standard. T-RFLP patterns were analyzed using GeneMapper software (Applied Biosystems) by peak height integration of different terminal restriction fragments (T-RFs). The fluorescence intensity (%) represented by a single T-RF was calculated relative to the total fluorescence intensity of all T-RFs. Peaks with heights that were less than 2% of the total peak height were excluded from further analysis to avoid potential noise before calculating relative T-RF abundance.
Cloning, Sequencing, and Phylogenetic Analyses
Based on the obtained T-RFLP results, all soil samples at the ripening stage were chosen to establish clone libraries. Libraries for the functional genes mcrA and pmoA were created by ligating PCR products into pEASY-T3 vectors and being transformed into competent cells Escherichia coli JM109 (Takara, Japan) in accordance with the manufacturer’s instructions. Ninety four methanogenic clones and 102 methanotrophic clones were sequenced. All sequences were checked for chimera by using Bellerophon (Huber et al., 2004) before being grouped into operational taxonomic units (OTUs) using the furthest-neighbor clustering algorithm of the DOTUR software with a 96% threshold. In silico digests with TaqI and MspI were undertaken on the sequences to allow the assignment of phylogenetic identity to individual peaks. The closest relatives of each sequence were checked using a BLAST search within GenBank. The representative sequences recovered in this study have been deposited in the GenBank database under accession numbers KU133526-KU133543 (methanogenic mcrA genes) and KU133544-KU133564 (methanotrophic pmoA genes).
Statistical Analysis
Statistical analysis was performed using SPSS 20.0. One-way ANOVA with Tukey’s HSD test was used to test the difference among the treatments at each growth stage. Repeated measures ANOVA were used to determine the effect of climate change factors and plant growth stage on soil properties, CH4 production and oxidization rates, and microbial abundance (log10-transformed mcrA and pmoA gene abundances). Principal component analysis (PCA) of the T-RFLP profiles was performed using Minitab v.15 software based on relative fluorescence intensity of T-RFs. The probability level p < 0.05 was considered to be statistically significant.
Results
Soil Properties
Soil physico-chemical property data of the rice field soil under the simulated climate change conditions are shown in Table 1. Results showed that soil inorganic N (NH4+ and NO3-) did not significantly change under CE, CW, and WA treatments when compared with the control treatment. Soil NH4+ content generally declined with rice growth development across the treatments, ranging from 30.23 mg kg-1 (CW, tillering) to 7.88 mg kg-1 (CW, ripening). However, the content of NO3- was stable without significant changes with the growth stages. Compared to CK, SMBC significantly increased under elevated CO2 levels (CE and CW) at all three growth stages, but the WA treatment significantly increased SMBC only at the heading stage. Repeated measures ANOVA showed that elevated CO2, warming and their combination significantly affected SMBC (p < 0.05), however, the interaction with the growth stage was not significant (Table 2).
Table 1.
Variation in soil properties, CH4 production and oxidation potentials (mg kg-1 dw h-1) of the studied soils under climate change treatments.
| Stage | Treatment | NH4+ (mg kg-1) | NO3- (mg kg-1) | SMBC (mg kg-1) | CH4 production potential | CH4 oxidation potential |
|---|---|---|---|---|---|---|
| Tillering | CK | 17.73 ± 5.46a | 4.87 ± 0.45a | 648.89 ± 38.74c | 168.45 ± 17.22c | 303.34 ± 27.79a |
| CE | 17.22 ± 4.36a | 5.55 ± 0.61a | 883.19 ± 96.67ab | 214.22 ± 14.53ab | 298.23 ± 21.12a | |
| CW | 30.23 ± 7.13a | 4.92 ± 0.15a | 1031.90 ± 81.02a | 225.00 ± 21.63a | 325.78 ± 18.41a | |
| WA | 17.90 ± 7.79a | 4.89 ± 0.49a | 762.81 ± 104.08bc | 177.27 ± 27.07bc | 324.13 ± 51.45a | |
| Heading | CK | 11.56 ± 1.05a | 4.41 ± 0.87ab | 518.87 ± 56.55c | 205.43 ± 22.10b | 191.62 ± 19.39a |
| CE | 14.15 ± 0.63a | 5.40 ± 0.41a | 823.37 ± 51.41ab | 269.10 ± 25.62a | 214.51 ± 19.70a | |
| CW | 11.75 ± 0.29a | 4.10 ± 0.28b | 949.20 ± 33.49a | 288.48 ± 24.21a | 233.83 ± 26.11a | |
| WA | 13.46 ± 2.32a | 4.47 ± 0.39ab | 727.47 ± 137.07b | 197.42 ± 30.26b | 247.08 ± 44.61a | |
| Ripening | CK | 11.12 ± 1.28a | 3.90 ± 1.33a | 608.77 ± 96.99b | 141.14 ± 35.04b | 328.44 ± 30.20b |
| CE | 8.53 ± 3.34a | 4.80 ± 0.57a | 960.04 ± 72.43a | 187.55 ± 12.66a | 359.42 ± 38.30b | |
| CW | 7.88 ± 1.96a | 4.78 ± 0.22a | 930.04 ± 93.37a | 171.10 ± 20.58ab | 437.40 ± 20.71a | |
| WA | 9.54 ± 1.45a | 4.69 ± 0.21a | 694.64 ± 91.14b | 173.87 ± 17.03ab | 473.77 ± 10.55a |
CK, ambient CO2 and ambient temperature; CE, atmosphere CO2 enrichment; CW, atmosphere CO2 enrichment and warming canopy air; WA, warming canopy air; SMBC: soil microbial biomass carbon. Data were presented as means of three replicates ± standard error; different letters within the same column indicate significant differences among treatments within a single growth stage (p < 0.05).
Table 2.
Repeated measures ANOVA for the effects of climate change, growth stage and their interaction on soil properties, CH4 production and oxidation potentials, abundances of mcrA and pmoA in the paddy soils under climate change treatments.
| Stage | NH4+ | NO3- | SMBC | CH4 production | CH4 oxidation | Methanogens abundance | Methanotrophs abundance |
|---|---|---|---|---|---|---|---|
| C | 0.918 | 0.071 | 0.001 | 0.027 | 0.025 | 0.010 | 0.356 |
| T | 0.951 | 0.352 | 0.020 | 0.477 | 0.006 | 0.985 | 0.095 |
| C × T | 0.270 | 0.485 | <0.001 | 0.016 | 0.001 | 0.010 | 0.133 |
| S | 0.008 | 0.320 | 0.072 | <0.001 | <0.001 | <0.001 | <0.001 |
| C × S | 0.407 | 0.938 | 0.385 | 0.540 | 0.973 | 0.048 | 0.642 |
| T × S | 0.674 | 0.642 | 0.562 | 0.353 | 0.004 | 0.837 | 0.063 |
| C × T × S | 0.023 | 0.403 | 0.426 | 0.141 | 0.065 | 0.129 | 0.080 |
C: CO2 concentration; T: temperature; S: stage; SMBC: soil microbial biomass carbon.
CH4 Production and Oxidation Potentials, and CH4 Emissions
The CH4 production potentials ranged from 141.14 (CK, ripening) to 288.48 (CW, heading) mg CH4 kg-1 dw h-1 across the treatments and growth stages (Supplementary Figure S2). The highest values were recorded at the heading stage before a sharp decline at the ripening stage (Table 1). In contrary, CH4 oxidation potential declined at the heading stage before increasing to the highest values at the ripening stage (Supplementary Figure S3). Repeated measures ANOVA showed CO2 enrichment, warming and their interaction resulted in significant effects on CH4 oxidation potential, while elevated CO2 only affected CH4 production potential when crossed with warming (Table 2). Although potential CH4 production and oxidation potentials significantly differed among the growth stages, the effects of CO2 enrichment and warming did not depend on the growth stage. CH4 production potentials significantly increased under CO2 enrichment (CE and CW) treatments at all three growth stages, but no significant changes were observed under WA treatment or for the control. However, CH4 oxidation only increased at the ripening stage under warming treatments (33% for CW and 44% for WA).
The seasonal patterns of CH4 emission profiles were similar between the climate change treatments and the control (Supplementary Figure S1). As the paddy field was waterlogged, CH4 concentration peaks occurred 20–35 days after transplanting. After 35 days, CH4 emissions sharply declined and remained at a low rate until harvest. Elevated CO2 significantly increased CH4 emissions in this paddy field. Compared to the CK treatment, mean CH4 emissions increased by 37% and 25% under CE and CW treatments, respectively.
Abundance of Methanogens and Methanotrophs
Gene abundance data showed that the abundances of mcrA and pmoA genes generally increased with rice growth development, the highest values being attained at the ripening stage (Figure 1). The abundance of mcrA genes under all treatments ranged from 9.13 × 108 (WA, tillering) to 7.73 × 109 (CE, ripening) copies g-1 dw. These results were higher than the abundance of pmoA genes, ranging from 1.31 × 108 (CK, tillering) to 4.55 × 108 (CW, ripening) copies g-1 dw. Repeated measures ANOVA showed that the effects of elevated CO2 and elevated CO2 combined with warming were significant (p < 0.05) on the abundance of mcrA genes, but not on the abundance of pmoA genes (Table 2). In this study, no significant changes in the abundance of mcrA and pmoA genes associated with climate change treatments were observed at the tillering stage. However, the abundance of mcrA genes significantly increased under CE and CW treatments at the heading and ripening stages. Compared to CK, the mean abundance of mcrA genes increased by 63% (CE) and 82% (CW) at the heading stage and 98% (CE) and 78% (CW) at the ripening stage, respectively. In contrast, the pmoA gene copy numbers were more stable without significant changes among the climate change treatments at all three growth stages.
FIGURE 1.
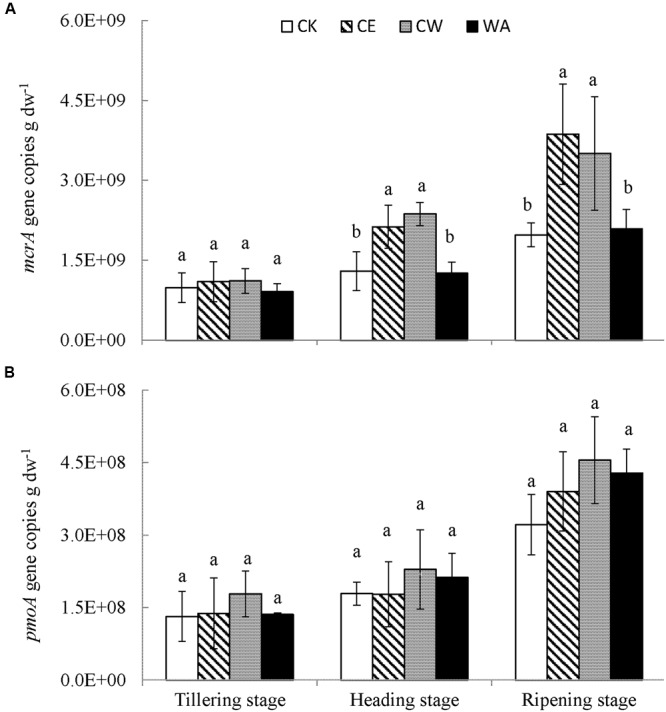
Changes in the mcrA (A) and pmoA (B) gene copy numbers for methanogens and methanotrophs in the studied soils under simulated climate change treatments. Different letters above the columns indicate significant differences among treatments within a single growth stage (p < 0.05).
Structure Composition of Methanogens and Methanotrophs
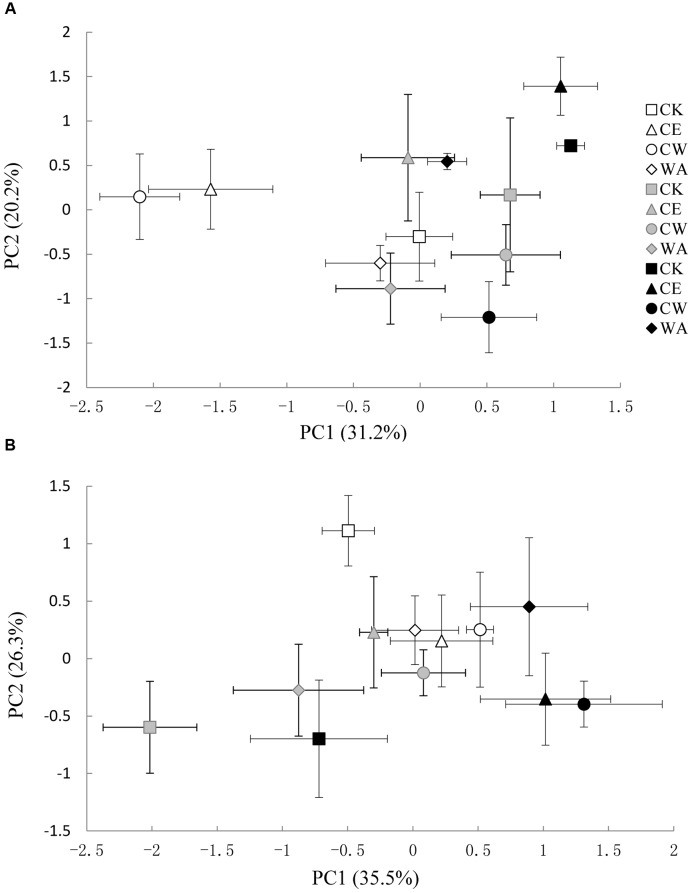
The methanogenic and methanotrophic community structures were analyzed using T-RFLP fingerprints. PCA of the T-RFLP profiles at the three growth stages yielded summaries of data, as 51.4% for mcrA genes and 61.8% for pmoA genes of the total variability was explained by PC1 and PC2 (Figure 2). No clear differences in the methanogenic community structure between the treatments and across the growth stages were highlighted by PCA analysis. Figure 2B shows that the methanotrophic community structure under CE, CW, and WA was distinctively separated from the control at each stage.
FIGURE 2.
Principal component analysis (PCA) of T-RFLP patterns of methanogens (A) and methanotrophs (B) from the studied soils. Tillering stage (white); Heading stage (light gray); Ripening stage (black). The symbols are as follows: ambient CO2 and ambient temperature (CK), squares; atmosphere CO2 enrichment (CE), triangles; atmosphere CO2 enrichment and warming canopy air (CW), circles; warming canopy air (WA), diamonds. The error bars indicate the standard error of the means.
A total of 12 and 8 T-RFs were obtained from the overall samples for mcrA and pmoA genes, respectively. T-RFLP fingerprinting of mcrA genes revealed that the 268bp, 289bp, 306bp, and 460bp TRFs were dominant in all treatments, while T-RFs of 76bp, 245bp, 437bp, and 510bp were dominant in the methanotrophic pmoA T-RFLP profiles (Figure 3). The main T-RFs in the T-RFLP profiles were identified with in silico restriction of clone sequences. T-RFs related to Methanosarcina were the most predominant (35–45%) across all treatments while T-RFs related to Methanocellales and Methanobacteriales ranged from 19–29% and 13–28%, respectively. The effect of simulated climate change scenarios on the relative abundance of mcrA TRFs was insignificant. However, the relative abundance of the 76bp TRF, related to Methylococcus, decreased under CE and CW treatments at the later growth stages, while that of the 437bp TRF related to Methylocystis increased.
FIGURE 3.
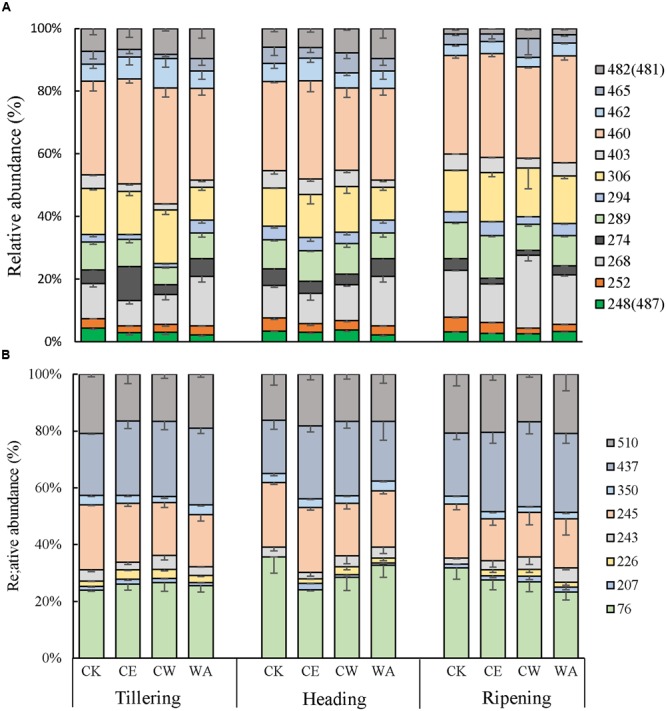
Relative abundance of T-RFs for mcrA (A) and pmoA (B) genes as determined by T-RFLP analysis in the studied soils. CK, ambient CO2 and ambient temperature; CE, atmosphere CO2 enrichment; CW, atmosphere CO2 enrichment and warming canopy air; WA, warming canopy air. Only major T-RFs (reductive abundance >1%) are shown. The error bars indicate the standard error of the means (n = 3).
The clone library analysis of mcrA and pmoA yielded a total of 94 and 102 cloned mcrA and pmoA sequences, respectively. Phylogenetic analyses of the mcrA sequences revealed that the methanogenic community in this paddy soil was dominated by members of Methanosarcina, Methanocellales, Methanobacteriales, and Methanomicrobiales (Figure 4). Twenty-one different OTUs of pmoA sequences were confirmed (Figure 5). The phylogenetic pattern of methanotrophic clones indicated that Methylococcus, Methylocaldum, Methylomonas, Methylosarcina, Methylogaea (Type I), and Methylocystis (Type II) were generally dominant during the rice growth period. As shown in Figure 5, Methylococcus (7 OTUs) and Methylocaldum (4 OTUs) sequences were the most dominant in Type I, representing about 31 and 25% of total clones in the pmoA clone library, respectively. To a smaller extent, Type I sequences were affiliated with Methylomonas (9 sequences), Methylosarcina (6 sequences) and Methylogaea (5 sequences). Methylocystis (3 OTUs, 25 sequences), the only groups of Type II methanotrophs, were identified in the rhizosphere.
FIGURE 4.
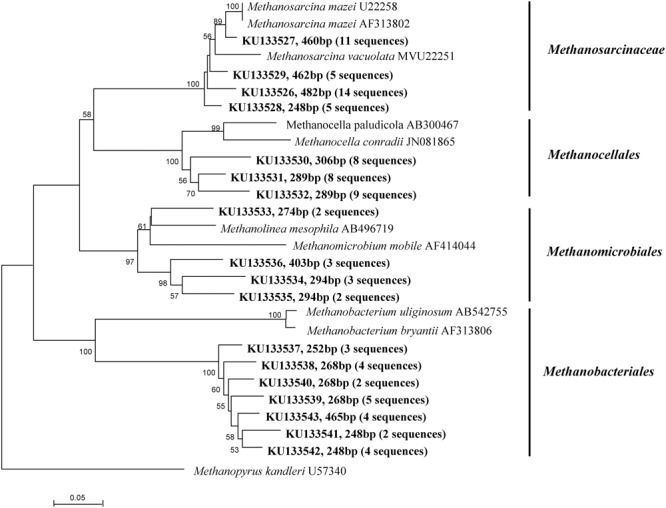
Phylogenetic tree of representative methanogenic sequences retrieved from the rhizosphere samples of paddy soil and reference sequences from GenBank. Bootstrap values of >50% are indicated at branch points. The accession number and terminal restriction fragment (T-RF) sizes digested in silico are shown in bold.
FIGURE 5.
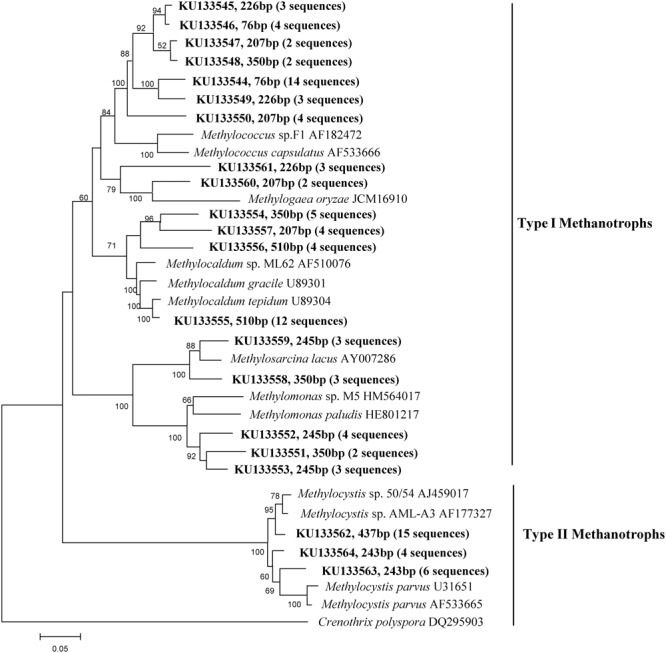
Phylogenetic tree of representative methanotrophic sequences retrieved from the rhizosphere samples of paddy soil and reference sequences from GenBank. Bootstrap values of >50% are indicated at branch points. The accession number and terminal restriction fragment (T-RF) sizes digested in silico are shown in bold.
Discussion
Methane Production Potential and Methanogen Community
Methanogenesis favors anoxic conditions with a low redox potential and a neutral pH in submerged soil (Masscheleyn et al., 1993; Orphan et al., 2001). In a laboratory incubation study, Das and Adhya (2012) found that elevated CO2 significantly increased CH4 production which was highly associated with decreased soil redox potential and pH in paddy soils. In this study, it was observed that CO2 enrichment significantly increased CH4 production potential during the rice growth period, whereas much higher SMBC was found (Table 1). This finding was consistent with results from Das et al. (2011) who observed an increase in SMBC with elevated CO2 concentrations and temperatures in a rice soil incubation experiment. Das and Adhya (2012) noted that an increase of methanogenic population with elevated CO2 levels was the most important reason for enhanced CH4 production under CO2 enrichment. In a free air CO2 enrichment (FACE) experiment, Okubo et al. (2015) also found that the number of copies of the mcrA gene significantly increased when CO2 concentrations were elevated in rice paddy fields. Similarly, our results showed that elevated CO2 concentrations significantly increased the abundance of methanogens in the rice rhizosphere soil (Figure 1). In this experiment, photosynthesis and total biomass of rice increased with elevated CO2 (Cai et al., 2016; Wang et al., 2016), resulting in an increase in root exudates and rhizodeposition in the rhizosphere (Bhattacharyya et al., 2013; Okubo et al., 2014). Furthermore, rhizodeposition is regarded as the primary source of CH4 produced in rice fields (Conrad, 2007). Increased carbon inputs into the rhizosphere soil could also increase microbial biomass and activity (Drissner et al., 2007; French et al., 2009).
Based on T-RFLP and clone sequence analyses, we found that methanogens belonging to Methanosarcina, Methanobacteriales, and Methanocellales (Rice Cluster I) were dominant in this paddy soil. A similar result was found in other studies focusing on methanogenic diversity of paddy soils (Liu et al., 2012; Breidenbach et al., 2015). Methanosarcina, the only species of acetoclastic groups, were present during the rice growth period, suggesting that acetate may be a major substrate for CH4 production in paddy fields. This observation is consistent with previous investigations showing Methanosarcina as a major species in rice fields under both flooded and drained conditions (Watanabe et al., 2009; Itoh et al., 2013). Based on RNA stable isotope probing analysis, Lu and Conrad (2005) indicated that individuals of the species Methanocellales were the most active for metabolizing rice root exudates, and that they play an important role in CH4 production in paddy fields. In this study, elevated CO2 and warming did not significantly alter the relative abundance of methanogenic T-RFs, resulting in minor changes in the composition of the methanogenic community. Thus, it is possible that the increase in CH4 production with elevated atmospheric CO2 is driven by the accumulation of substrate input and an increase in the methanogenic population or cell-specific activity without shifting methanogenic community composition.
In our study, a 2°C increase in air temperature did not change the activity, abundance or community composition of methanogens compared with elevated CO2 treatments. This finding differs from the findings of Das and Adhya (2012); they identified an increase in CH4 production and methanogenic population in trophic paddy soils associated with a temperature increase from 25–45°C, at intervals of ten degrees. Generally, an increase in temperature stimulates the decomposition of organic matter in submerged soils (von Lützow and Kögel-Knabner, 2009; Karhu et al., 2010) which may lead to higher rates of CH4 production under anaerobic conditions (Fey and Conrad, 2000). In this study, the 2°C increase in canopy air temperature resulted in a small increase in soil temperature (<1°C, unpublished data), an increase which is in the range of daily/seasonal fluctuations and heterotrophic microbial communities are insensitive to such temperature increases (Malchair et al., 2010). Moreover, the direct effects of warming on soil microbial communities could be confounded by soil moisture content, substrate availability and plant conditions (Allison and Treseder, 2008; Das and Adhya, 2012).
Methane Oxidation Potential and Methanotroph Community
Previous studies indicated that CH4 oxidation potential increased as soil temperatures increased (Van den Pol-van Dasselaar et al., 1998; Dijkstra et al., 2010). Here, we showed that warming treatments (CW and WA) resulted in significant increases in CH4 oxidation potential at the ripening stage when the paddy field was drained, whereas no change was observed under CE during the rice growth period. In a 13C-labeling study, Kalyuzhanaya et al. (2013) observed that a significantly larger portion of the assimilated carbon (over 62%) was derived from CO2 in the alphaproteobacterial methanotrophs. Autotrophic methanotrophs, potentially utilizing C-1 compounds for their metabolic activity, could be greatly affected by changing environmental conditions. For example, if an increase of temperature results in the soil becoming drier, CH4 oxidation may be enhanced (Dijkstra et al., 2010). Furthermore, as well as temperature, the water-logging regime is the other most important factor for CH4 oxidation in rice fields (Das and Adhya, 2012). In the present study, such an increase of CH4 oxidation potential under warming treatments was greater at the ripening stage (by 33–45%) when the rice fields were drained than at the tillering and heading stages (by 7–28%) when they were flooded. The growth stage of rice could therefore play an important role in the abundance and activity of methanotrophs during the rice growth period (Eller and Frenzel, 2001; Lüke et al., 2010; Lee et al., 2014). In a study of rice paddy soil, Ho et al. (2011) found that the higher methane oxidation potential related well to the cell-specific activity and population of methanotrophs. Though a nitrite-driven anaerobic CH4 oxidation was discovered in wetlands (Hu et al., 2014), aerobic methanotrophs in rice field were predominant (Hu and Lu, 2015).
The composition and distribution of methanotrophs were relatively stable across the simulated climate change treatments. In this study, Methylococcus and Methylocaldum (both Type I), and Methylocystis (Type II), were dominant in the rice rhizosphere (Figures 4 and 5). Similar data have been found in rice field soils from Aichi-ken Anjo Research and Extension Center, central Japan (Jia et al., 2007), Gangetic plain of India (Vishwakarma et al., 2010) and National Rice Research Institute of China (Ma et al., 2010). It is generally assumed that Type I methanotrophs to be highly responsive to high substrate resources (Ho et al., 2013; Chen et al., 2014) while Type II methanotrophs are relatively stable (Eller et al., 2005; Krause et al., 2012). As an indication with a fast growth rate, Type I methanotrophs were predominant in occupying niches with abundant resources (Ho et al., 2013), such as in the rhizosphere and on the roots of rice plants (Wu et al., 2009; Chen et al., 2014). In this study, it is possible that increased labile organic compounds under warming are not large enough to affect the population or composition of the Type I methanotrophs.
Conclusion
In this study, we observed increased CH4 production potential at the three rice stages in response to atmospheric CO2 enrichment. An increase in the methanogenic population is the most likely cause of increased CH4 production under elevated CO2 conditions. Warming treatments resulted in a significant increase in CH4 oxidation potential at the ripening stage, without any change in the abundance and community composition of methanotrophs during the growth period. Our data demonstrate that methanogens and methanotrophs differentially responded to elevated atmospheric CO2 and warming. Results from our investigation will enable a more comprehensive understanding of the future role of microbial processes and related microorganisms in methane emissions. However, future research should also investigate the long-term interactive effects of elevated atmospheric CO2 and warming on the carbon cycle under field conditions.
Author Contributions
GP and LL designed research; YL and XL performed the data analysis. YL wrote the paper. KC, XZ, and JFZ revised and commented on the draft. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (41501304), and the Special Fund for Agro-scientific Research in the Public Interest of China (200903003).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.01895/full#supplementary-material
CH4 flux dynamics during the rice growing season. The symbols are as follows: ambient CO2 and ambient temperature (CK), squares; atmosphere CO2 enrichment (CE), triangles; atmosphere CO2 enrichment and warming canopy air (CW), circles; warming canopy air (WA), diamonds. The error bars indicate the standard error of the mean (n = 3).
CH4 production potentials (mg g-1 dw h-1) during the incubation. Tillering stage (A); Heading stage (B); Ripening stage (C). The symbols are as follows: ambient CO2 and ambient temperature (CK), squares; atmosphere CO2 enrichment (CE), triangles; atmosphere CO2 enrichment and warming canopy air (CW), circles; warming canopy air (WA), diamonds. The error bars indicate the standard error of the mean (n = 3).
CH4 oxidation potentials (mg g-1 dw h-1) during the incubation. Tillering stage (A); Heading stage (B); Ripening stage (C). The symbols are as follows: ambient CO2 and ambient temperature (CK), squares; atmosphere CO2 enrichment (CE), triangles; atmosphere CO2 enrichment and warming canopy air (CW), circles; warming canopy air (WA), diamonds. The error bars indicate the standard error of the mean (n = 3).
References
- Allen L. H., Albrecht S. L., Colon-Guasp W., Covell S. A., Baker J. T., Pan D., et al. (2003). Methane emissions of rice increased by elevated carbon dioxide and temperature. J. Environ. Qual. 32 1978–1991. 10.2134/jeq2003.1978 [DOI] [PubMed] [Google Scholar]
- Allison S. D., Treseder K. K. (2008). Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Change Biol. 142898–2909. 10.1111/j.1365-2486.2008.01716.x [DOI] [Google Scholar]
- Angel R., Kammann C., Claus P., Conrad R. (2012). Effect of long-term free-air CO2 enrichment on the diversity and activity of soil methanogens in a periodically waterlogged grassland. Soil Biol. Biochem. 51 96–103. 10.1016/j.soilbio.2012.04.010 [DOI] [Google Scholar]
- Austin E., Castro H., Sides K., Schadt C., Classen A. (2009). Assessment of 10 years of CO2 fumigation on soil microbial communities and function in a sweetgum plantation. Soil Biol. Biochem. 41 514–520. 10.1016/j.soilbio.2008.12.010 [DOI] [Google Scholar]
- Bhattacharyya P., Roy K., Neogi S., Manna M., Adhya T., Rao K., et al. (2013). Influence of elevated carbon dioxide and temperature on belowground carbon allocation and enzyme activities in tropical flooded soil planted with rice. Environ. Monit. Assess. 185 8659–8671. 10.1007/s10661-013-3202-7 [DOI] [PubMed] [Google Scholar]
- Breidenbach B., Blaser M. B., Klose M., Conrad R. (2015). Crop rotation of flooded rice with upland maize impacts the resident and active methanogenic microbial community. Environ. Microbiol. 18 2868–2885. 10.1111/1462-2920.13041 [DOI] [PubMed] [Google Scholar]
- Breidenbach B., Conrad R. (2015). Seasonal dynamics of bacterial and archaeal methanogenic communities in flooded rice fields and effect of drainage. Front. Microbiol. 5:752 10.3389/fmicb.2014.00752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgham S. D., Cadillo Quiroz H., Keller J., Zhuang Q. (2013). Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Chang. Biol. 19 1325–1346. 10.1111/gcb.12131 [DOI] [PubMed] [Google Scholar]
- Butler J. L., Williams M. A., Bottomley P. J., Myrold D. D. (2003). Microbial community dynamics associated with rhizosphere carbon flow. Appl. Environ. Microbiol. 69 6793–6800. 10.1128/AEM.69.11.6793-6800.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C., Yin X., He S., Jiang W., Si C., Struik P. C., et al. (2016). Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Glob. Chang. Biol. 22 856–874. 10.1111/gcb.13065 [DOI] [PubMed] [Google Scholar]
- Chen R., Wang Y., Wei S., Wang W., Lin X. (2014). Windrow composting mitigated CH4 emissions: characterization of methanogenic and methanotrophic communities in manure management. FEMS Microbiol. Ecol. 90 575–586. 10.1111/1574-6941.12417 [DOI] [PubMed] [Google Scholar]
- Conrad R. (2007). Microbial ecology of methanogens and methanotrophs. Adv. Agron. 96 1–63. 10.1016/S0065-2113(07)96005-8 [DOI] [Google Scholar]
- Conrad R., Klose M. (2006). Dynamics of the methanogenic archaeal community in anoxic rice soil upon addition of straw. Eur. J. Soil Sci. 57 476–484. 10.3389/fmicb.2012.00004 [DOI] [Google Scholar]
- Das S., Adhya T. (2012). Dynamics of methanogenesis and methanotrophy in tropical paddy soils as influenced by elevated CO2 and temperature interaction. Soil Biol. Biochem. 47 36–45. 10.1016/j.soilbio.2011.11.020 [DOI] [Google Scholar]
- Das S., Bhattacharyya P., Adhya T. (2011). Impact of elevated CO2, flooding, and temperature interaction on heterotrophic nitrogen fixation in tropical rice soils. Biol. Fertil. Soils 47 25–30. 10.1007/s00374-010-0496-2 [DOI] [Google Scholar]
- Dijkstra F. A., Morgan J. A., LeCain D. R., Follett R. F. (2010). Microbially mediated CH4 consumption and N2O emission is affected by elevated CO2, soil water content, and composition of semi-arid grassland species. Plant Soil 329 269–281. 10.1007/s11104-009-0152-5 [DOI] [Google Scholar]
- Drissner D., Blum H., Tscherko D., Kandeler E. (2007). Nine years of enriched CO2 changes the function and structural diversity of soil microorganisms in a grassland. Eur. J. Soil Sci. 58 260–269. 10.1111/j.1365-2389.2006.00838.x [DOI] [Google Scholar]
- Dubbs L. L., Whalen S. C. (2010). Reduced net atmospheric CH4 consumption is a sustained response to elevated CO2 in a temperate forest. Biol. Fertil. Soils 46 597–606. 10.1007/s00374-010-0467-7 [DOI] [Google Scholar]
- Eller G., Frenzel P. (2001). Changes in activity and community structure of methane-oxidizing bacteria over the growth period of rice. Appl. Environ. Microbiol. 67 2395–2403. 10.1128/AEM.67.6.2395-2403.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller G., Kruger M., Frenzel P. (2005). Comparing field and microcosm experiments: a case study on methano- and methyl-trophic bacteria in paddy soil. FEMS Microbiol. Ecol. 51 279–291. 10.1016/j.femsec.2004.09.007 [DOI] [PubMed] [Google Scholar]
- Ettwig K. F., Butler M. K., Le Paslier D., Pelletier E., Mangenot S., Kuypers M., et al. (2010). Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464 543–548. 10.1038/nature08883 [DOI] [PubMed] [Google Scholar]
- Fey A., Conrad R. (2000). Effect of temperature on carbon and electron flow and on the archaeal community in methanogenic rice field soil. Appl. Environ. Microbiol. 66 4790–4797. 10.1128/AEM.66.11.4790-4797.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French S., Levy-Booth D., Samarajeewa A., Shannon K., Smith J., Trevors J. (2009). Elevated temperatures and carbon dioxide concentrations: effects on selected microbial activities in temperate agricultural soils. World J. Microbiol. Biotechnol. 25 1887–1900. 10.1007/s11274-009-0107-2 [DOI] [Google Scholar]
- Ho A., Kerckhof F. M., Luke C., Reim A., Krause S., Boon N. (2013). Conceptualizing functional traits and ecological characteristics of methane-oxidizing bacteria as life strategies. Environ. Microbiol. Rep. 5 335–345. 10.1111/j.1758-2229.2012.00370.x [DOI] [PubMed] [Google Scholar]
- Ho A., Luke C., Frenzel P. (2011). Recovery of methanotrophs from disturbances: population dynamics, evenness and functioning. ISME J. 5 750–758. 10.1038/ismej.2010.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høj L., Olsen R. A., Torsvik V. L. (2008). Effects of temperature on the diversity and community structure of known methanogenic groups and other archaea in high Arctic peat. ISME J. 2 37–48. 10.1038/ismej.2007.84 [DOI] [PubMed] [Google Scholar]
- Horz H. P., Yimga M. T., Liesack W. (2001). Detection of methanotroph diversity on roots of submerged rice plants by molecular retrieval of pmoA, mmoX, mxaF, and 16S rRNA and ribosomal DNA, including pmoA-based terminal restriction fragment length polymorphism profiling. Appl. Environ. Microbiol. 67 4177–4185. 10.1128/AEM.67.9.4177-4185.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu A., Lu Y. (2015). The differential effects of ammonium and nitrate on methanotrophs in rice field soil. Soil Biol. Biochem. 85 31–38. 10.1016/j.soilbio.2015.02.033 [DOI] [Google Scholar]
- Hu B. L., Shen L. D., Lian X., Zhu Q., Liu S., Huang Q., et al. (2014). Evidence for nitrite-dependent anaerobic methane oxidation as a previously overlooked microbial methane sink in wetlands. Proc. Natl. Acad. Sci. U.S.A. 111 4495–4500. 10.1073/pnas.1318393111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber T., Faulkner G., Hugenholtz P. (2004). Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20 2317–2319. 10.1093/Bioinformatics/Bth226 [DOI] [PubMed] [Google Scholar]
- Inubushi K., Cheng W., Aonuma S., Hoque M. M., Kobayashi K., Miura S., et al. (2003). Effects of free-air CO2 enrichment (FACE) on CH4 emission from a rice paddy field. Glob. Change Biol. 9 1458–1464. 10.1046/j.1365-2486.2003.00665.x [DOI] [Google Scholar]
- IPCC (2007). Climate Change 2007-the Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the Intergovermental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- Itoh H., Ishii S., Shiratori Y., Oshima K., Otsuka S., Hattori M., et al. (2013). Seasonal transition of active bacterial and archaeal communities in relation to water management in paddy soils. Microbes Environ. 28 370–380. 10.1264/jsme2.ME13030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z., Kikuchi H., Watanabe T., Asakawa S., Kimura M. (2007). Molecular identification of methane oxidizing bacteria in a Japanese rice field soil. Biol. Fertil. Soils 44 121–130. 10.1007/s00374-007-0186-x [DOI] [Google Scholar]
- Joye S. B. (2012). Microbiology: a piece of the methane puzzle. Nature 491 538–539. 10.1038/nature11749 [DOI] [PubMed] [Google Scholar]
- Kalyuzhanaya M. G., Yang S., Matsen J. B., Konopka M., Green-Saxena A., Clubb J., et al. (2013). Global molecular analyses of methane metabolism in methanotrophic Alphaproteobacterium, Methylosinus trichosporium OB3b. Part II. Metabolomics and 13C-labeling study. Front. Microbiol. 4:70 10.3389/fmicb.2013.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhu K., Fritze H., Tuomi M., Vanhala P., Spetz P., Kitunen V., et al. (2010). Temperature sensitivity of organic matter decomposition in two boreal forest soil profiles. Soil Biol. Biochem. 42 72–82. 10.1016/j.soilbio.2009.10.002 [DOI] [Google Scholar]
- Knief C. (2015). Diversity and habitat preferences of cultivated and uncultivated aerobic methanotrophic bacteria evaluated based on pmoA as molecular marker. Front. Microbiol. 6:1346 10.3389/fmicb.2015.01346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch C., Zimmermann U., Blumenberg M., Michaelis W., Pfeiffer E. M. (2008). Methane turnover and temperature response of methane-oxidizing bacteria in permafrost-affected soils of northeast Siberia. Soil Biol. Biochem. 40 3004–3013. 10.1016/j.soilbio.2008.08.020 [DOI] [Google Scholar]
- Krause S., Luke C., Frenzel P. (2012). Methane source strength and energy flow shape methanotrophic communities in oxygen-methane counter-gradients. Environ. Microbiol. Rep. 4 203–208. 10.1111/j.1758-2229.2011.00322.x [DOI] [PubMed] [Google Scholar]
- Lee H. J., Kim S. Y., Kim P. J., Madsen E. L., Jeon C. O. (2014). Methane emission and dynamics of methanotrophic and methanogenic communities in a flooded rice field ecosystem. FEMS Microbiol. Ecol. 88 195–212. 10.1111/1574-6941.12282 [DOI] [PubMed] [Google Scholar]
- Liu G. C., Tokida T., Matsunami T., Nakamura H., Okada M., Sameshima R., et al. (2012). Microbial community composition controls the effects of climate change on methane emission from rice paddies. Environ. Microbiol. Rep. 4 648–654. 10.1111/j.1758-2229.2012.00391.x [DOI] [PubMed] [Google Scholar]
- Liu Y., Li M., Zheng J., Li L., Zhang X., Zheng J., et al. (2014). Short-term responses of microbial community and functioning to experimental CO2 enrichment and warming in a Chinese paddy field. Soil Biol. Biochem. 77 58–68. 10.1016/j.soilbio.2014.06.011 [DOI] [Google Scholar]
- Liu Y., Zhou H., Wang J., Liu X., Cheng K., Li L., et al. (2015). Short-term response of nitrifier communities and potential nitrification activity to elevated CO2 and temperature interaction in a Chinese paddy field. Appl. Soil Ecol. 96 88–98. 10.1016/j.apsoil.2015.06.006 [DOI] [Google Scholar]
- Lu Y., Conrad R. (2005). In situ stable isotope probing of methanogenic archaea in the rice rhizosphere. Science 309 1088–1090. 10.1126/science.1113435 [DOI] [PubMed] [Google Scholar]
- Lüke C., Frenzel P., Ho A., Fiantis D., Schad P., Schneider B., et al. (2014). Macroecology of methane-oxidizing bacteria: the β-diversity of pmoA genotypes in tropical and subtropical rice paddies. Environ. Microbiol. 16 72–83. 10.1111/1462-2920.12190 [DOI] [PubMed] [Google Scholar]
- Lüke C., Krause S., Cavigiolo S., Greppi D., Lupotto E., Frenzel P. (2010). Biogeography of wetland rice methanotrophs. Environ. Microbiol. 12 862–872. 10.1111/j.1462-2920.2009.02131.x [DOI] [PubMed] [Google Scholar]
- Luton P. E., Wayne J. M., Sharp R. J., Riley P. W. (2002). The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology 148 3521–3530. 10.1099/00221287-148-11-3521 [DOI] [PubMed] [Google Scholar]
- Ma K., Qiu Q., Lu Y. (2010). bisque. Glob. Change Biol. 16 3085–3095. [Google Scholar]
- Malchair S., De Boeck H., Lemmens C., Merckx R., Nijs I., Ceulemans R., et al. (2010). Do climate warming and plant species richness affect potential nitrification, basal respiration and ammonia-oxidizing bacteria in experimental grasslands? Soil Biol. Biochem. 42 1944–1951. 10.1016/j.soilbio.2010.07.006 [DOI] [Google Scholar]
- Masscheleyn P. H., DeLaune R. D., Patrick W. H., Jr. (1993). Methane and nitrous oxide emissions from laboratory measurements of rice soil suspension: effect of soil oxidation-reduction status. Chemosphere 26 251–260. 10.1016/0045-6535(93)90426-6 [DOI] [Google Scholar]
- McLain J. E., Ahmann D. M. (2008). Increased moisture and methanogenesis contribute to reduced methane oxidation in elevated CO2 soils. Biol. Fertil. Soils 44 623–631. 10.1007/s00374-007-0246-2 [DOI] [Google Scholar]
- Montzka S. A., Dlugokencky E. J., Butler J. H. (2011). Non-CO2 greenhouse gases and climate change. Nature 476 43–50. 10.1038/nature10322 [DOI] [PubMed] [Google Scholar]
- Nisbet E. G., Dlugokencky E. J., Bousquet P. (2014). Methane on the rise—again. Science 343 493–495. 10.1126/science.1247828 [DOI] [PubMed] [Google Scholar]
- Okubo T., Liu D., Tsurumaru H., Ikeda S., Asakawa S., Tokida T., et al. (2015). Elevated atmospheric CO2 levels affect community structure of rice root-associated bacteria. Front. Microbiol. 6:136 10.3389/fmicb.2015.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T., Tokida T., Ikeda S., Bao Z., Tago K., Hayatsu M., et al. (2014). Effects of elevated carbon dioxide, elevated temperature, and rice growth stage on the community structure of rice root–associated bacteria. Microbes Environ. 29 184–190. 10.1264/jsme2.ME14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op den Camp H. J., Islam T., Stott M. B., Harhangi H. R., Hynes A., Schouten S., et al. (2009). Environmental, genomic and taxonomic perspectives on methanotrophic Verrucomicrobia. Environ. Microbiol. Rep. 1 293–306. 10.1111/j.1758-2229.2009.00022.x [DOI] [PubMed] [Google Scholar]
- Orphan V. J., House C. H., Hinrichs K. U., McKeegan K. D., DeLong E. F. (2001). Methane-consuming archaea revealed by directly coupled isotopic and phylogenetic analysis. Science 293 484–487. 10.1126/science.1061338 [DOI] [PubMed] [Google Scholar]
- Peng J., Lü Z., Rui J., Lu Y. (2008). Dynamics of the methanogenic archaeal community during plant residue decomposition in an anoxic rice field soil. Appl. Environ. Microbiol. 74 2894–2901. 10.1128/AEM.00070-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard S. (2011). Soil organisms and global climate change. Plant Pathol. 60 82–99. 10.1111/j.1365-3059.2010.02405.x [DOI] [Google Scholar]
- Rosenzweig C., Casassa G., Karoly D. J., Imeson A., Liu C., Menzel A., et al. (2007). “Assessment of observed changes and responses in natural and managed systems,” in Proceedings of the Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change: Climate Change 2007: Impacts, Adaptation and Vulnerability eds Parry M. L., Canziani O. F., Palutikof J. P., van der Linden P. J., Hanson C. E. (Cambridge: Cambridge University Press; ) 79–131. [Google Scholar]
- Singh A., Singh R. S., Upadhyay S. N., Joshi C. G., Tripathi A. K., Dubey S. K. (2012). Community structure of methanogenic archaea and methane production associated with compost-treated tropical rice-field soil. FEMS Microbiol. Ecol. 82 118–134. 10.1111/j.1574-6941.2012.01411.x [DOI] [PubMed] [Google Scholar]
- Singh B. K., Bardgett R. D., Smith P., Reay D. S. (2010). Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 8 779–790. 10.1038/nrmicro2439 [DOI] [PubMed] [Google Scholar]
- Tokida T., Fumoto T., Cheng W., Matsunami T., Adachi M., Katayanagi N., et al. (2010). Effects of free-air CO2 enrichment (FACE) and soil warming on CH4 emission from a rice paddy field: impact assessment and stoichiometric evaluation. Biogeoscience 7 2639–2653. 10.5194/bg-7-2639-2010 [DOI] [Google Scholar]
- Van den Pol-van Dasselaar A., Van Beusichem M., Oenema O. (1998). Effects of soil moisture content and temperature on methane uptake by grasslands on sandy soils. Plant Soil 204 213–222. 10.1023/A:1004371309361 [DOI] [Google Scholar]
- van Groenigen K. J., Osenberg C. W., Hungate B. A. (2011). Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 475 214–216. 10.1038/nature10176 [DOI] [PubMed] [Google Scholar]
- Vishwakarma P., Singh M., Dubey S. (2010). Changes in methanotrophic community composition after rice crop harvest in tropical soils. Biol. Fertil. Soils 46 471–479. 10.1007/s00374-010-0454-z [DOI] [Google Scholar]
- von Lützow M., Kögel-Knabner I. (2009). Temperature sensitivity of soil organic matter decomposition—what do we know? Biol. Fertil. Soils 46 1–15. 10.1007/s00374-009-0413-8 [DOI] [Google Scholar]
- Wang J., Liu X., Zhang X., Smith P., Li L., Filley T. R., et al. (2016). Size and variability of crop productivity both impacted by CO2 enrichment and warming—a case study of 4 year field experiment in a Chinese paddy. Agric. Ecosyst. Environ. 221 40–49. 10.1016/j.agee.2016.01.028 [DOI] [Google Scholar]
- Watanabe T., Kimura M., Asakawa S. (2009). Distinct members of a stable methanogenic archaeal community transcribe mcrA genes under flooded and drained conditions in Japanese paddy field soil. Soil Biol. Biochem. 41 276–285. 10.1016/j.soilbio.2008.10.025 [DOI] [Google Scholar]
- Wu J., Joergensen R., Pommerening B., Chaussod R., Brookes P. (1990). Measurement of soil microbial biomass C by fumigation extraction an automated procedure. Soil Biol. Biochem. 22 1167–1169. 10.1016/0038-0717(90)90046-3 [DOI] [Google Scholar]
- Wu L. Q., Ma K., Lu Y. H. (2009). Rice roots select for type I methanotrophs in rice field soil. Syst. Appl. Microbiol. 32 421–428. 10.1016/j.syapm.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Yan X., Akiyama H., Yagi K., Akimoto H. (2009). Global estimations of the inventory and mitigation potential of methane emissions from rice cultivation conducted using the 2006 intergovernmental panel on climate change guidelines. Glob. Biogeochem. Cycles 23 1–15. [Google Scholar]
- Yun J., Yu Z., Li K., Zhang H. (2013). Diversity, abundance and vertical distribution of methane-oxidizing bacteria (methanotrophs) in the sediments of the Xianghai wetland, songnen plain, northeast China. J. Soils Sed. 13 242–252. 10.1007/s11368-012-0610-1 [DOI] [Google Scholar]
- Zou J., Liu S., Qin Y., Pan G., Zhu D. (2009). Sewage irrigation increased methane and nitrous oxide emissions from rice paddies in southeast China. Agric. Ecosyst. Environ. 129 516–522. 10.1016/j.agee.2008.11.006 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CH4 flux dynamics during the rice growing season. The symbols are as follows: ambient CO2 and ambient temperature (CK), squares; atmosphere CO2 enrichment (CE), triangles; atmosphere CO2 enrichment and warming canopy air (CW), circles; warming canopy air (WA), diamonds. The error bars indicate the standard error of the mean (n = 3).
CH4 production potentials (mg g-1 dw h-1) during the incubation. Tillering stage (A); Heading stage (B); Ripening stage (C). The symbols are as follows: ambient CO2 and ambient temperature (CK), squares; atmosphere CO2 enrichment (CE), triangles; atmosphere CO2 enrichment and warming canopy air (CW), circles; warming canopy air (WA), diamonds. The error bars indicate the standard error of the mean (n = 3).
CH4 oxidation potentials (mg g-1 dw h-1) during the incubation. Tillering stage (A); Heading stage (B); Ripening stage (C). The symbols are as follows: ambient CO2 and ambient temperature (CK), squares; atmosphere CO2 enrichment (CE), triangles; atmosphere CO2 enrichment and warming canopy air (CW), circles; warming canopy air (WA), diamonds. The error bars indicate the standard error of the mean (n = 3).