Abstract
The developmental origins of health and disease (DOHaD) is a paradigm for understanding metabolic diseases of modern humans. Vulnerability to disease is linked to perturbations in development during critical time periods in fetal and neonatal life. These perturbations are caused by environmental signals, often generated or transduced by the mother. The regulation of mammalian development depends to a large extent on maternal biochemical signals to her offspring. We argue that this adaptation is ancient, and originated with the evolution of lactation. Lactation evolved earlier than live birth and before the extensive placental development of modern eutherian mammals. Milk contains a host of signaling molecules including nutrients, immunoglobulins, growth factors and metabolic hormones. As evidenced by marsupials, lactation originally served to supply the biochemical factors for growth and development for what is essentially a fetus to a weanling transitioning to independent existence. In placental mammals maternal signaling in earliest life is accomplished through the maternal–placental–fetal connection, with more of development shifted to in utero life. However, significant development occurs postpartum, supported by milk. Mothers of all taxa provide biochemical signals to their offspring, but for non-mammalian mothers the time window is short. Developing mammals receive maternal biochemical signals over an extended period. These signals serve to guide normal development, but also can vary in response to environmental conditions. The ancient adaptation of lactation resulted in a lineage (mammals) in which maternal regulation of offspring development evolved to a heightened degree, with the ability to modify development at multiple time points. Modern metabolic diseases may arise due to a mismatch between maternal regulation and eventual circumstances of the offspring, and due to a large proportion of mothers that exceed past evolutionary norms in body fat and pregnancy weight gain such that maternal signals may no longer be within the adaptive range.
Keywords: Lactation, Milk, Evolution
Highlights
-
•
Mammals display extensive maternal control of offspring development.
-
•
Milk contains bioactive substances that affect infant development.
-
•
Milk continues oral biochemical signaling that starts in utero via amniotic fluid.
-
•
Milk preadapted mammalian fetuses to receive signaling via amniotic fluid.
1. Introduction
The developmental origins of health and disease (DOHaD) is a fundamental paradigm for investigating and understanding the etiology of many of the metabolic diseases of modern humans (Barker, 1998, Barker, 2004, Gluckman and Hanson, 2005, Gluckman and Hanson, 2006). Briefly, environmental signals have the potential to alter the developmental pathways of a young organism resulting in eventual adult physiology and metabolism that is strongly affected by those early life signals. Thus, the physiological health of an adult is shaped to a great extent by the circumstances of early life, from in utero through early childhood. Obesity, diabetes, hypertension, cardiovascular disease, asthma, allergies, and other conditions all have potential origins in early life, both pre and postpartum.
Often, the environmental signal that affects development originates from the mother. Mammalian mothers are signaling biochemically to their offspring from the moment of implantation until weaning. The maternal response to environmental challenges modulates her signaling to her offspring, which in turn modulates offspring development. The evidence for in utero effects on adult physiology and disease risk in mammals is substantial, from the early epidemiological work of Forsdahl (1977) and Barker, 1990, Barker, 1993, to a host of experimental studies on laboratory animals. There is also a growing body of evidence for environmental effects in early postnatal life on later disease risk, both direct and due to maternal effects (e.g. Gluckman et al., 2007).
Milk is a complex biochemical fluid with essential function for the growth and development of mammalian young. Milk is the sole food for all mammals for some length of time after birth, and thus must provide the essential nutrients required for early growth and development. However, milk provides much more than nutrients. It has been long known that maternal immunoglobulins (e.g. secretory IgA) are transferred via milk, priming the neonatal immune system (Hanson and Winberg, 1972, Cruz et al., 1982, Hanson et al., 1985). Recent evidence demonstrates that milk also contains physiological concentrations of growth factors and metabolic hormones, such as epidermal growth factor (EGF), leptin, and adiponectin (Savino et al., 2011). Milk appears to have important developmental effects on neonatal intestinal health and development; for example, giving breast milk to preterm infants reduces the incidence of necrotizing enterocolitis (Sisk et al., 2007, Henderson et al., 2009, Arslanoglu et al., 2010). Other hormones in milk (e.g. relaxin, leptin, adiponectin, and insulin-like growth factors) may have developmental functions in the neonate, affecting multiple organ systems from the gut to the brain. The term lactocrine has been proposed for this maternal signaling to offspring via milk (Bartol et al., 2008).
In essence, aspects of mammalian development, both pre and postnatal, are strongly influenced by biochemical signals from the mother. We suggest that the importance of maternal biochemical signaling in guiding offspring development is an ancient adaptation of mammals, dating back to the origin of lactation, and becoming enhanced with the evolution of the placenta.
1.1. Environmental effects on development
That the environment has significant effects on growth and development of organisms is a truism. At the least the environment must be permissive of development. However, in many cases the environment guides development. An example is temperature dependent sex determination, such as found in crocodilians and many other reptiles. In the context of DOHaD in humans (and other mammals) environmental conditions result in variable phenotypic changes that have later effects on physiology and metabolism, which alter the risk of adult onset disease. These environmental signals can be direct, but more often are considered to originate from or be transduced through the mother (maternal effects).
The range of developmental outcomes arising from environmental effects has different implications for the evolution of these changes by selection. A developing organism that is energy or nutrient restricted to an extent that still allows survival but results in a stunted individual may simply represent the best outcome possible given the environmental constraint. The environment constrains more than guides development in this instance. However, selection undoubtedly still has acted on the developmental program such that under constraint certain organ systems are spared at the expense of others. Some deficits of function will have greater adaptive consequences than will others, and selection would act to favor deficits with lesser fitness consequences over ones which reduce fitness to a greater extent. For example, in intrauterine growth restriction (IUGR) fetuses redistribute blood flow such that the brain, heart and adrenals are less affected than other organs. The greater head to abdominal circumference observed in IUGR infants is largely explained by a greater impairment in liver growth leading to a lower abdominal circumference (Nathanielsz, 2006). The organ sparing effect is relative; depending on the severity of the IUGR brain and heart will also be affected, but to a lesser extent than other organs.
However, the theory behind the development of adult disease due to early life in humans suggests a subtler and more adaptive process. If circumstances during early life are predictive of challenges to be faced in later life than developmental changes in response to early life circumstances could be adaptive for later life, and not merely the best of a bad situation. In this scenario maternal circumstances (e.g. plane of nutrition, disease history, social status) result in biochemical signals to the developing offspring, both in utero through the maternal–placental–fetal connection and after birth via milk, which are reliable indicators of later life circumstances. The offspring's development is affected to achieve an eventual physiology that is appropriate to its expected later life condition.
This developmental programming is considered adaptive in the short to medium term (relative to the reproductive life span) but can result in later disease either because the resulting physiology is not as robust as other outcomes (e.g. allows survival and reproduction but results in frail or unhealthy physiology in late adult life), or because the later life circumstances do not match the predicted and the resulting mismatch in physiology and circumstances results in poor health and increased metabolic disease.
1.2. Maternal biochemical signaling to offspring
Mothers of all taxa signal biochemically to their offspring. Females of oviparous taxa (e.g. birds and most reptiles) deposit substantial resources into the egg, and from those resources the embryo will develop until hatching. Obviously these maternal resources include signaling molecules as well as the basic nutrients and other building blocks of life. For example, maternally derived steroid hormones are deposited into the egg, and the amounts of these hormones can vary, presumably due to maternal circumstances. Experimental manipulations of steroid levels in eggs (by injecting into the egg) have demonstrated that developmental characteristics of the offspring can be affected. For example, bird egg androgen levels are associated with the length of time to hatching, growth rate of embryos, mortality, immune function, and begging behavior after hatching (Eising et al., 2001, Eising et al., 2003, Navara et al., 2005, Schwabl, 1996). One difference between an oviparous species and taxa with live birth, such as most mammals, is that the time period during which the maternal biochemical signals can be directed at the offspring is severely constrained in oviparous species. The female cannot later alter what she has put into the egg.
In a placental mammal, such as humans, maternal biochemical signaling is a continuous process over an extended period of maternal dependence. It starts at implantation via the maternal–placental–fetal axis, and continues postpartum via milk. An extensive amount of development of mammalian fetuses and neonates depends upon signals that originate in the mother. These signals change over time, not only in a programmed way that matches the changing developmental circumstances as the offspring age and mature, but also as maternal circumstances change. Mammalian mothers may be able to alter their developmental signals depending on the maternal environment, or in some cases may have no choice, as the signaling depends upon maternal circumstances. Again, this is possible for an egg laying vertebrate such as a bird as well, but the time frame for mammals is much broader. For most bird species, the maternal circumstances at the time the egg is produced essentially determine the biochemical composition of the egg, and hence the signals transmitted to the developing embryo. For placental mammals the maternal circumstances at the time of ovulation may have very little to do with the eventual path of development for those processes that can be influenced by maternal signaling. There are certainly essential time periods during which development of species characteristics can be modified, but for any given trait these time periods may be at any point of gestation or even after birth.
Thus, for a mammal, a perturbation of maternal circumstances at any point during the extensive period of offspring dependence could have later life consequences for the offspring. In an experimental example, rat pups injected with leptin shortly after birth have altered expression of hepatic 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2). However, the direction of the change depends upon maternal circumstances during gestation. Pups from well nourished dams showed an increase in 11β-HSD2 expression while pups from dams that were food restricted by 30% showed a decrease in expression (Gluckman et al., 2007). The physiological reaction to a manipulation was dependent upon maternal circumstances, presumably due to the altered transfer of resources and signals from food-restricted dams to their fetuses.
We argue that the ancient adaptation of lactation was the origin of the mammalian pattern of substantial maternal regulation of offspring development and that mammals derive from a lineage for which maternal regulation of offspring development was enhanced and more flexible than other vertebrate taxa. In effect, maternal regulation of offspring development over an extended period of offspring maternal dependence is a significant adaptive feature of mammalian biology that probably predates the existence of mammals.
2. Placenta
The chorio-allantoic placenta is a more recent adaptation of mammals compared to lactation, originating perhaps as recently as 100–110 million years ago (Murphy et al., 2001). Although a relative novice compared to milk, the evolution of a chorio-allantoic placenta increased the extent and sophistication of maternal–fetal biochemical signaling in mammals. The human placenta connects a mother and her baby physically, metabolically and immunologically. Made from fetal cells, it is the first organ any mammal ever makes. The evolution of a placenta has influenced multiple aspects of mammalian biology, especially for biological regulation, from metabolism to the genome. The placenta allows biochemical signaling to go from offspring to mother, as well as the reverse. Mothers and babies are “speaking” to each other at a fundamental biological level from the moment of implantation.
The placenta is, fundamentally, a regulatory organ, producing a wide array of hormones, growth factors, cytokines, immune function molecules and so forth (Petraglia et al., 2005). These molecules act locally as well as at a distance to affect the mother, the fetus and the placenta itself, in essence exchanging information among these compartments in order to coordinate the necessary physiological and metabolic processes required to produce a viable neonate. The intrauterine environment has been shown to have a strong influence over growth and development, and to have significant effects on offspring future health and wellbeing long after birth. The placenta coordinates maternal physiology with fetal development, and even plays a role in stimulating maternal changes that prime the female for caregiving behaviors after birth (Numan et al., 2006). The placental connection that evolved in the extant eutherian mammal lineage increased the duration, amount and flexibility of the maternal–fetal exchange.
Placental influence extended to the evolution of genetic mechanisms as well. The existence of this intimate connection increased the salience and selective pressures inherent in the inevitable to and fro of maternal–fetal and maternal–paternal genetic cooperation and conflict regarding fitness imperatives. Male and female mammals are fundamentally different in their reproductive biology, and this difference has profound evolutionary implications. The placenta is a primary arena where maternal and paternal genes interact, in both cooperation and conflict. The placenta appears to have allowed or even driven changes in aspects of placental mammal genetic regulation so that they differ fundamentally from that of other vertebrates such as birds or amphibians. For example, imprinted genes appear to be a therian mammal adaptation within vertebrates, with many imprinted genes in placental and marsupial mammal species, but none so far found in reptiles or even in the monotremes (Renfree et al., 2013). Imprinted genes are important to placental development and function; and the placenta as a reproductive adaptation was a selective pressure for the evolution of gene imprinting (Reik and Lewis, 2005). The placenta appears to have influenced our biology at all levels.
3. The continuity of maternal signaling pre and postpartum
Not surprisingly, maternal signaling pre and postpartum often shows continuity. For example, EGF and TGF-β are found in amniotic fluid (Underwood et al., 2005), which is swallowed by the developing fetus. Thus, in utero the developing gut is exposed to these (and other molecules) of maternal origin which likely have regulatory and developmental function and are necessary for the appropriate development of the gut. After birth the signaling continues, now via milk. It is instructive to consider that milk was the original source of most potential maternal signals in early mammals. In marsupials it remains the prime source. Thus, delivery of maternal signals to offspring orally is an ancient adaptation in mammals. After the evolution of the chorio-allantoic placenta, with the fetus enclosed within the amniotic sac for a much longer period of development, fetal swallowing of amniotic fluid allowed this signaling to continue. The adaptation of lactation may have served as a preadaptation to signaling via amniotic fluid.
4. Lactation
Lactation probably arose more than 250 million years ago in a synapsid lineage from which mammals are the sole surviving descendants. The original function of lactation is uncertain, though suggestions generally revolve around a protective effect on eggs, either to reduce desiccation and/or protect against microbial/fungal diseases (Oftedal, 2002, Oftedal, 2012). The molecular evidence from milk proteins indicate that some form of complex lactation was in existence a quarter of a billion years ago. For example, α-lactalbumin, which functions to convert galactosyltransferase to lactose synthase, derives from a duplication of the lysozyme c gene. The original duplication event may have preceded the divergence of the synapsid and diapsid (ancestors of reptiles and birds) lineages (Prager and Wilson, 1988, Oftedal, 2012). Casein proteins derive from duplications of genes that code for secretory calcium-binding phosphoproteins (Oftedal, 2012). These are unfolded proteins that are associated with mineralized tissue, and are involved in processes such as mineralization of tooth enamel. The casein proteins in milk provide not only amino acids to the offspring, but also most of the calcium and phosphorus. All three types of casein genes (α-, β-, and κ-caseins) are found in monotremes, marsupials and placental mammals, which puts their origin prior to 170 million years ago, roughly coincident with the loss of vitellogenin genes in mammals (Brawand et al., 2008), which have nutrient transport function in reptile and bird egg yolk similar to that of the caseins in milk.
Lactation as a reproductive adaptation has fundamentally affected mammalian biology. It began the strong asymmetry in reproductive effort between female and male mammals, placing the main reproductive burden on mothers. It allowed the reduction of maternal resources deposited into the egg, which has culminated in the extremely nutrient-poor eggs of placental mammals that implant and develop a chorio-allantoic placenta which nourishes the fetus by transferring nutrients and gases from maternal circulation to the fetus. After birth this intimate transfer of maternal resources to her offspring continues via milk. The resources transferred include more than just nutrients. Both milk and the placenta also allow mammalian mothers to signal biochemically to their offspring over an extended period, guiding the development of their young. Milk needs to be examined from the perspective of regulatory and developmental biology; to look beyond its nutritional importance and begin to investigate milk as a regulatory mechanism.
In the ancestral mammalian lineage, milk was the earliest mechanism through which mothers signaled biochemically to their offspring. In the marsupials it is still the primary mechanism, with almost all of the resources and regulatory signaling delivered to offspring via milk from a fetal stage through weaning. Functions performed by the placenta are accomplished through milk in marsupials. In the placental mammals the placenta now plays a major role, but milk remains important. The neonatal and infant period is one of substantial growth and development, especially in humans. For example, a human infant's brain will more than double in size during the time when, in our evolutionary past the infant would have been completely reliant on mother's milk for nutrition. Milk certainly provides the nutritional building blocks for this growth and development. But milk is providing much more than nutrition. Modern research has found that milk may provide much of the regulatory signaling that directs infant growth and development as well.
For example, important aspects of uterine wall development occur in the first few days after birth in female piglets (gilts), and these morphogenetic changes are estrogen receptor dependent and estrogen sensitive (Chen et al., 2010a). Gilts fed milk replacer from birth differed from gilts that nursed by having no detectable expression of estrogen receptor-α or vascular endothelial growth factor (VEGF) in uterine tissue (Chen et al., 2011). Exogenous relaxin enhanced estrogen receptor-α gene expression in nursed gilts but had no effect on formula fed gilts, though it enhanced VEGF expression in both. Bioactive factors in colostrum (earliest milk) appear to be necessary for normal gene expression in gilt uterine tissue (Bartol et al., 2008, Chen et al., 2011). Thus, the epigenetic programming of female piglet uterine tissue development is regulated by maternal signals via milk.
4.1. Immune function molecules in milk
The first evidence of maternal effects on offspring via milk was the existence of maternal immunoglobulins in milk and their immunoprotective effect on her offspring (Fitzsimmons et al., 1994). In humans and other species with hemochorial placentas immunoglobulins also are transferred across the placenta during gestation. For humans this is primarily IgG, with secretory IgA (sIgA) being transferred via milk. Other placental types (epithelialchorial and endothelialchorial placentas) apparently cannot pass immunoglobulins. In species with those placenta types (e.g. horses, dogs, prosimian primates) little or no maternal immunoglobulin is transferred during gestation, and milk provides the only avenue for transmission of maternal immunity to offspring; in the milks of these species there are significant concentrations of IgG as well as sIgA (Van de Perre, 2003). Regardless, mothers are the source of infant immunity to a variety of diseases.
Importantly, breast milk transfers not only passive immunity from mother to offspring, but also contains proteins that direct the development of the infant's immune system (Petherick, 2010). Human breast milk contains immunomodulatory molecules, cytokines, and hormones that coordinate the development of gut-associated lymphoid tissue and the development of the gut's barrier function and innate defenses. The gut is an important immune organ. The skin, lungs and gut are the organs that are constantly exposed to the external environment, and thus are necessarily first lines of defense.
Of course not all things transmitted to an infant via milk are good. Some pathogens can also be transmitted. Two recent case studies concluded likely transmission of yellow fever live virus from maternal vaccination to her infant, probably via breast milk (Centers for Disease Control and Prevention, 2010, Kuhn et al., 2011). West Nile virus also appears able to be passed through breast milk (Centers for Disease Control, Prevention, 2002), though the incidence of such occurrences is rare (Hinckley et al., 2007). Of most importance to public health considerations, about 10% of maternal–child transmission of the HIV virus occurs via breast milk (Dunn et al., 1992, Nduati and John, 2000). This leads to a public health dilemma in the developing world, where breastfeeding is an important factor protecting against diarrhea and other diseases.
The efficiency of HIV viral transfer via breast milk is low. Without antiviral drugs about 10–15% of breastfed infants will contract HIV (Dunn et al., 1992, Coutsoudis et al., 2004). However, infants who are exclusively breastfed are at lower risk of acquiring HIV than are infants who are fed a mix of formula and breast milk or breast milk and supplemental foods (Coovadia et al., 2007). There appear to be factors in milk that inhibit HIV transfer. For example, HIV-infected women with above average amounts of oligosaccharides in their milk were less likely to transmit the virus, especially if the oligosaccharides were also low in 3′-sialyllactose (Bode et al., 2012). With highly active antiretroviral therapy (HAART) less than 3% of breastfed infants will contract HIV. In a study of 102 HIV-infected mothers undergoing HAART in Uganda, no infants were diagnosed as HIV positive and the risk of infant death was six-fold higher among infants breastfed for less than 6 months (Homsy et al., 2010). In developing countries breastfeeding in conjunction with HAART may be preferable to formula feeding despite the non-zero risk of HIV transmission.
4.2. Growth factors and metabolic hormones in milk
Breast milk contains a wide array of bioactive molecules (Table 1) that likely play a role in shaping growth and metabolism in the neonate (Savino et al., 2010). These include growth factors (e.g. the insulin-like growth factors (IGFs), epidermal growth factor (EGF) and transforming growth factor β (TGF-β)) and metabolic hormones (e.g. ghrelin, leptin and adiponectin) that directly impact infant physiology, particularly the developing gastrointestinal tract (Rautava and Walker, 2009). A broad spectrum of evidence suggests that these signaling molecules have important effects on growth and development of neonates. Studies using in vitro and animal model approaches show that milk bioactive factors such as IGF-I, TGF-ß, EGF, leptin, ghrelin, and adiponectin are involved in epithelial proliferation, gut differentiation, and suppression of gut inflammation, and shape growth and metabolism in the neonate (Wagner, 2002). Feeding preterm infants with human breast milk reduces their risk of developing necrotizing enterocolitis, a major source of morbidity in preterm babies (Sullivan et al., 2010). The specific signaling molecules in breast milk that act on the infant's intestinal tract are not known, although EGF and TGF-β-2 are logical candidates. Both of these growth factors have significant effects on intestinal epithelial cell proliferation and maturation (Coursodon and Dvorak, 2012). Many other metabolic hormones found in milk (e.g. leptin, adiponectin) have been suggested to play a role in the development of infant metabolism, and to be potential risk factors for early onset of obesity and type-2 diabetes (Savino et al., 2009, Savino et al., 2011).
Table 1.
A partial list of signaling molecules detected in milk, most of which have also been detected in amniotic fluid.
| Detected in amniotic fluid | |
|---|---|
| Epidermal growth factor (EGF) | Yes |
| Hepatic growth factor (HGF) | Yes |
| Vascular endothelial growth factor (VEGF) | Yes |
| Basic fibroblast growth factor (b-FGF) | Yes |
| Insulin-like growth factor-I (IGF-I) | |
| Platelet-derived growth factor (PDGF) | |
| Transforming growth factor-β1 (TGF-β1) | |
| Transforming growth factor-β2 (TGF-β2) | Yes |
| Interleukin 4 (IL-4) | |
| Interleukin 5 (IL-5) | |
| Interleukin 6 (IL-6) | Yes |
| Interleukin 10 (IL-10) | Yes |
| Interleukin 13 (IL-13) | |
| Interferon-gamma (IFN-γ) | Yes |
| Relaxin | Yes |
| Leptin | Yes |
| Ghrelin | Yes |
| Adiponectin | Yes |
| Insulin | Yes |
| Cortisol | Yes |
Evidence from in vivo studies of premature infants, who experience enhanced gut maturation upon ingesting mother's milk which contains higher levels of trophic factors further supports the critical role of these bioactives in mediating neonatal and infant gut development. The immature neonatal gut is poor at restricting the passage of intact proteins into circulation, a factor suspected to be a major cause of asthma and various food allergies. Breast milk is protective against these risks (Van Odijk et al., 2003, Scholtens et al., 2009). Breast milk also may be protective against childhood obesity (Savino et al., 2009) and may play an important role in the development of glucose regulatory physiology probably via milk-borne hormones such as leptin, ghrelin and adiponectin (Savino et al., 2011).
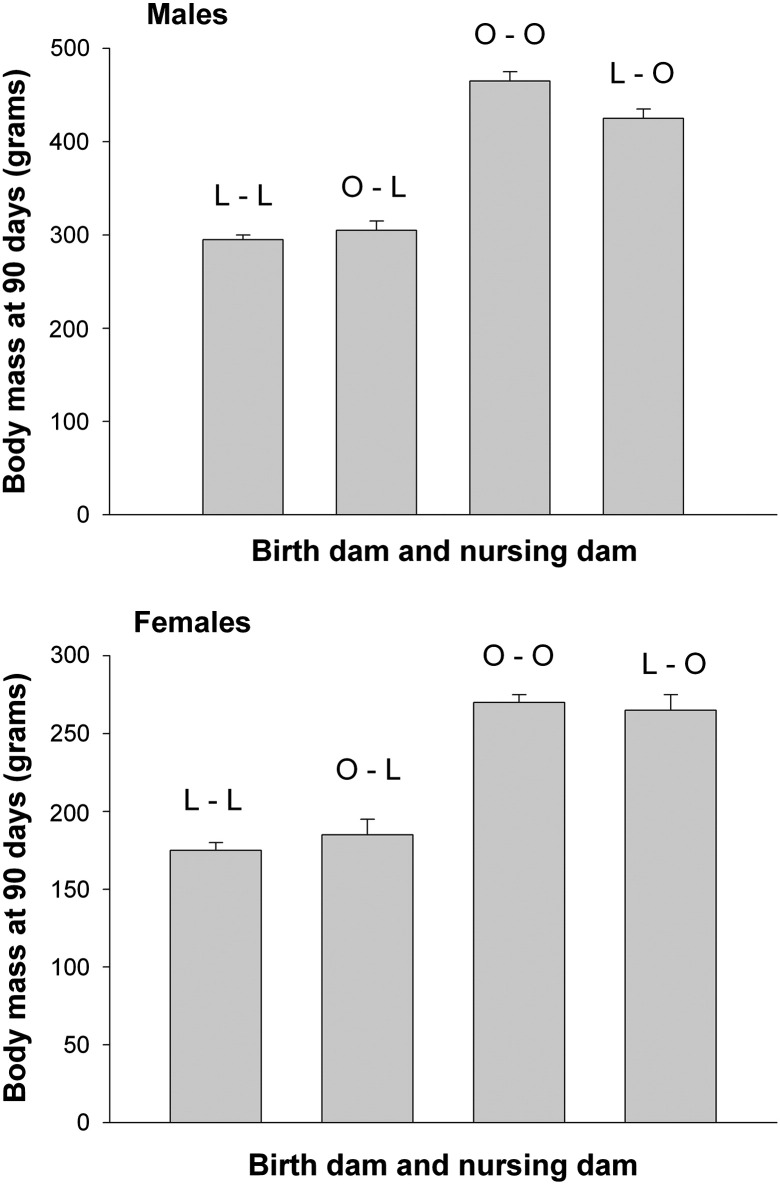
Recent evidence suggests that mothers may pass on their metabolic history to their infants. Maternal obesity predisposes offspring to obesity and metabolic dysfunction in rodent models. Cross fostering mouse pups from lean dams to obese dams during lactation results in those pups becoming vulnerable to obesity and metabolic disease (Gorski et al., 2006, Oben et al., 2010). Cross fostering mouse pups that are predisposed to obesity onto lean dams lowers the extent of obesity in the pups (Reifsnyder et al., 2000) and improves insulin sensitivity (Gorski et al., 2006). Obesity-prone and obesity-resistant rat pups displayed a phenotype consistent with the foster (nursing) dam's genotype prior to weaning rather than to their own genotype (Fig. 1), although after weaning their food intake and weight gain reflected their own genotype (Schroeder et al., 2010). There was an effect of sex, as male obesity-prone pups fostered to obesity-resistant dams, although still becoming obese post-weaning, had significantly lower body weight and fat mass than obesity-prone male pups nursed by obesity prone dams (Fig. 1; Schroeder et al., 2010). Important aspects of early postnatal development appear to be regulated to a significant extent via milk, though what bioactive factors are most important are not yet known.
Fig. 1.
Body mass at 90 days for rat pups born to either a lean genotype dam (L) or an obese phenotype dam (O) and cross fostered to either a lean or obese genotype dam. L–L = lean–lean; O–L = lean–obese; O–O = obese–obese; L–O = lean–obese. Pup phenotype matched the nursing dam genotype, although O–O males were significantly larger than O–L males.
Data from Schroeder et al., 2010.
Human studies to date have shown limited evidence for milk bioactive factors affecting growth and adiposity. Over the first six months of lactation milk adiponectin concentration was shown to be negatively associated with weight-for-length and weight-for-age, but not with length-for-age, implying lower adiposity in infants drinking breast milk with high adiponectin (Woo et al., 2009). However, those infants experienced catch-up growth in the second year of life, with an increase in adiposity (Woo et al., 2012). Higher concentration of IL-6 in breast milk was associated with lower weight gain and fat mass in infants (Fields and Demarath, 2012). Small for gestational age (SGA) infants who are breast fed gain less fat mass than formula fed SGA infants and maintain a healthier metabolic profile in terms of circulating IGF-1 and high-molecular-weight adiponectin (de Zegher et al., 2013). Previous studies using enriched formula have shown that SGA infants that have greater catch-up growth have higher fat mass (Singhal et al., 2010) and higher blood pressure at 6 to 8 years of age (Singhal et al., 2007). The increased risk of cardiovascular disease in later life for SGA infants may in part be due to well-intentioned but possibly biologically unsound efforts to enhance weight gain. Whether the slower growth and better metabolic profiles of breast fed SGA infants is due solely to nutritional factors or whether signaling molecules in breast milk contribute is unknown.
There are also environmental contaminants in breast milk due to pollution and the modern industrial world. Many of these compounds have endocrine action or act as endocrine disruptors (e.g. PCBs, dioxins). The concentrations and diversity of these contaminants varies markedly between countries and within regions within a country. For example, breast milk samples from Denmark and Finland could be accurately distinguished by the relative concentrations of different dioxins (Krysiak-Baltyn et al., 2009). These bioactive milk contaminants have the potential to affect development. For example, phthalates are chemicals known to alter Leydig cell differentiation and function in rodent models, and are now ubiquitous in the modern environment. They are found in breast milk with wide variations in concentrations among different regions. The concentration of different phthalates in breast milk was associated with alterations in reproductive hormones in three month old infants in Denmark and Finland, with higher sex-hormone binding globulin, luteinizing hormone, and lower free testosterone in infants of mothers with high breast-milk phthalate concentrations (Main et al., 2006).
4.3. microRNA
Recent studies have detected significant quantities of microRNAs (miRNA), small regulatory RNA molecules, in human breast milk (Kosaka et al., 2010, Zhou et al., 2012) and cow milk (Chen et al., 2010b). Breast milk contained the highest concentration of total RNA of 12 body fluids tested, almost three times higher than seminal fluid which had the second highest concentration, and more than 80 times the concentration found in amniotic fluid (Weber et al., 2010). The number of miRNAs with known function detected in breast milk ranged from 429 (Weber et al., 2010) to more than 600 (Zhou et al., 2012). Among the twenty most common miRNAs detected in breast milk, four were found only in breast milk and not in the other body fluids tested, and six were also found in amniotic fluid (Table 2).
Table 2.
The 20 most abundant miRNAs detected in either breast milk or amniotic fluid in order of abundance. miRNAs in bold are among the 20 most abundant for both breast milk and amniotic fluid; miRNAs in brackets were only found in either breast milk or amniotic fluid, and not in plasma, tears, urine, seminal fluid, saliva, bronchial lavage, cerebrospinal fluid, pleural fluid, or peritoneal fluid.
| Breast milk | Amniotic fluid |
|---|---|
| miRNA-335* | miRNA-518e |
| miRNA-26a-2* | miRNA-335* |
| miRNA-181d | [miRNA-302c] |
| miRNA-509-5p | miRNA-515-3p |
| miRNA-524-5p | [miRNA-452] |
| miRNA-137 | miRNA-892a |
| miRNA-26a-1* | miRNA-671-5p |
| [miRNA-595] | miRNA-515-5p |
| miRNA-580 | miRNA-137 |
| miRNA-130a | [miRNA-593*] |
| miRNA-515-3p | miRNA-590-3p |
| [miRNA-513c] | miRNA-873 |
| miRNA-671-5p | miRNA-410 |
| [miRNA-490-5p] | miRNA-509-5p |
| miRNA-367 | [miRNA-548d-5p] |
| miRNA-181b | miRNA-223* |
| miRNA-598 | miRNA-616* |
| miRNA-515-5p | [miRNA-148b*] |
| [miRNA-578] | miRNA-590-5p |
| miRNA-487b | miRNA-302d |
Data from Weber et al., 2010.
Many of the miRNAs found in milk have immune-related function, regulating T-cells and inducing B-cell differentiation, and thus have the potential to activate and potentiate immune function. Immune cell-related miRNAs comprised the majority of miRNAs found in cow milk (Chen et al., 2010b). Two-thirds of the well-characterized human immune-related pre-miRNAs were detected in breast milk (Zhou et al., 2012). However, the existence of many human miRNAs in breast milk that are not immune-related suggests that milk miRNAs may regulate physiological processes outside of the immune system as well.
These milk miRNAs appear resistant to degradation by acid and RNase digestion, suggesting they could survive the acidic stomach and digestive enzymes of the small intestine (Kosaka et al., 2010). This resistance to degradation is likely due to the miRNAs being contained within exosomes, tiny endosome-derived membrane vesicles that are released by cells into the extracellular environment (Zhou et al., 2012). Microvesicles (likely exosomes) containing miRNA have also been found in cow milk (Hata et al., 2010). Breast milk miRNAs likely survive intact into the small intestine, where they can be taken up by lumen epithelium. They may represent another regulatory mechanism allowing the maternal genome to influence development of her offspring.
4.4. Epigenetics, imprinted genes and lactation
The mammary gland undergoes extensive remodeling during the end of pregnancy in order to become a functioning lactating gland, and then at the end of lactation during involution to return to the non-lactating state. Not surprisingly, evidence is accumulating that the dramatic changes in peptide production and secretion by the mammary gland during lactation is accomplished at least in part through epigenetic changes (reviewed in Rijnekls et al., 2010). There is evidence of hypomethylation of DNA in epithelial cells from lactating mammary glands at various lactation-related genes such as those for casein proteins (Johnson et al., 1983, Thompson and Nakhasi, 1985) and whey acidic protein (Dandekar et al., 1982). The promoter region for the bovine αS1-casein gene is hypomethylated during lactation, but becomes remythylated during mastitis (Vanselow et al., 2006) and during involution (Singh et al., 2008). Prolactin appears to exert some of its lactogenic effects by interacting with glucocorticoids to regulate gene expression (Rosen et al., 1999), in part by creating a more open chromatin structure (Rijnkels et al., 2010).
Lactational performance of dairy cows can be affected by early life nutritional manipulations, with a program of initial energy restriction followed by replenishment in young heifers resulting in approximately 10% greater milk yield as adults (Park, 2005). Whether that change is passed on to daughters is uncertain, with studies giving contradictory results (reviewed in Singh et al., 2012).
Genetic imprinting also appears important in the mammary gland. In mouse mammary epithelial cells the maternal X chromosome is non-randomly silenced (Jiao et al., 2012). In humans monoallelic expression of Igf2 by mammary epithelium is the norm (Yun et al., 1999). The insulin gene was monoallelically expressed in mammary tissue of the marsupial, the tamar wallaby (Stringer et al., 2012). The mammary gland appears to be a potential hot spot for imprinted genes, along with the brain and placenta.
4.5. Gut biome
Breast milk is a major avenue and facilitator for infant gut microbe colonization. Not only does breast milk contain microorganisms, but some of the oligosaccharides in milk appear to act as prebiotics, with certain commensal gut microbes (e.g. Bifidobacterium infantis) able to metabolize certain human milk oligosaccharides while many pathogenic strains cannot (Ward et al., 2006, Sela and Mills, 2010). Thus breast milk both provides a source of microbes and a source of energy for specific microbial strains.
The gut microbial populations of breastfed infants differ from that of bottle fed infants (Bezirtzoglou et al., 2011), and generally reflect their mother's gut biome. Breast milk contains staphylococci, streptococci, bifidobacteria and lactic acid bacteria, and the same strains that are found in a mother's milk are usually found in her infant's feces (Martín et al., 2012). Many of the bacteria found in breast milk are also found in the maternal gut, and are considered to be commensal organisms (Martín et al., 2004). This suggests that breast milk may be a vehicle by which mothers colonize their babies' guts with maternal gut microbes.
The origin of what appear to be maternal gut microbes in breast milk is somewhat controversial. The standard theory was that bacteria in milk were the result of contamination from maternal skin or the infant's oral cavity. And both these surfaces likely do contribute some microbes to the breast and into the milk. Recently, the existence of an enteric–mammary transport system that encapsulates bacteria from the maternal gut and transports it to the mammary gland has been suggested (Martín et al., 2012, Fernández et al., 2013). Dendritic cells have been shown to be able to penetrate the gut epithelium and take up bacteria from the lumen (Rescigno et al., 2001). Intestinal dendritic cells can retain small numbers of live bacteria for several days (Macpherson and Uhr, 2004). Macrophages may also be able to take up, retain and then disperse live bacteria. Transfer of live gut bacteria to mesenteric lymph nodes and to the mammary gland occurs during late pregnancy and lactation in mice (Perez et al., 2007). The feces of infants born by cesarean section to women given oral lactobacilli during gestation contain that strain of lactobacilli, even though they were not exposed to the vaginal environment (Schultz et al., 2004). Milk is the likely colonizing source, implying that live lactobacilli in the maternal gut can reach the mammary glands.
The gut biome contains at least two orders of magnitude more expressed DNA than does the human host genome, and it produces a large array of biologically active products that can affect other gut residents, gut epithelium, and even be absorbed into circulation. The gut biome has recently been shown to play a large role in physiological processes. For example, malnutrition has been linked to differences in the gut biome in a study of twins in Malawi. There was evidence that the gut biome differences not only potentially affected digestive and absorptive efficiency, but were actually related to changes in regulation of aspects of the Krebs cycle in these children (Smith et al., 2013).
Thus, it appears that mothers can inoculate their infants with maternal gut microbes via milk, with potential physiological consequences for the infants. An aberrant gut biome is associated with increased disease risk in infants (Isolauri, 2012). Interestingly, obese women produce milk with a less diverse set of microbes, and women who gain excessive weight produce milk with different microbes from those in milk of women who had normal weight gain during pregnancy (Cabrera-Rubio et al., 2012). Obesity in adults is associated with an altered gut microbial population. Obese mothers may pass on their obesogenic gut biome to their breast fed infants.
5. Adaptive consequences of maternal control of development
Maternal control of development presents advantages and challenges to both mother and offspring. Ceding control of certain developmental processes to the mother reduces fetal endogenous requirements by taking more from the maternal system. This might even be required in mammalian embryonic life, as the fetal organs and regulatory systems may not be mature enough and the resources initially transferred into the egg are so minimal that endogenous regulation may not be possible. Although it extends the time period during which mothers transfer resources to offspring, it reduces the daily extent of the resource transfer, spreading the cost over time. Most likely a strong maternal influence on fetal and neonatal development is usually a win–win for both mother and offspring; but it does provide grounds for maternal–offspring conflict. For example, greater the maternal control allows a withdrawal of resources due to maternal circumstances, in extreme cases aborting; adaptive for the mother, but not for the fetus. Thus, maternal–offspring conflict has become potentially heightened in mammals, especially as the fetus has acquired a greater ability to influence its mother via placental signaling.
The greatly extended time frame over which mammalian mothers can influence offspring development increases the flexibility of phenotype. The genotype–environment interaction is transduced through the mother to a large extent; and changes in maternal circumstances can potentially affect offspring development at many time points from implantation through weaning. Unfortunately in the modern human environment maternal circumstances may not be as predictive of the infant's post-weaning nutritional plane as it was in our past. In developing countries this can lead to a mismatch between an impoverished maternal circumstances but a consistent higher-calorie intake by her offspring. In developed countries, maternal circumstances have exceeded, in terms of fat mass and plane of nutrition, the evolutionary norm. In the past very few babies were born to mothers with body mass indices above 30 kg/m2 or who gained more than 40 lb during gestation. Gestational diabetes was very rare. The signals coming to infants from their mothers may be well outside of the normative ranges under which our species evolved. The infant's metabolism and physiology will still adjust in response to those signals, but the adjustments may no longer represent an adaptive response; or at least may not result in long-term good health. The mammalian adaptation of extensive and long term maternal influence on development that we hypothesize began with the evolution of lactation may underlie many modern human metabolic diseases. The modern human pathologies associated with DOHaD may represent the failure of an adaptive response due to an inappropriate environment.
6. Conclusions
-
1.
The mammalian lineage has adapted a strategy of significant maternal control of offspring development from embryo to weanling. We hypothesize that the origin of that adaptation was the evolution of lactation.
-
2.
Milk contains a host of bioactive substances (e.g. immuno, regulatory, and microbial) many of which likely survive the acid stomach and enter the infant's small intestine. These substances can affect intestinal epithelial cells, be absorbed into circulation, or, in the case of microbes, affect the infant's gut biome. And all can affect development and metabolism.
-
3.
Milk continues oral biochemical signaling that in the fetus occurs via swallowing amniotic fluid. Milk is the more ancient adaptation for oral maternal biochemical signaling to offspring, and preadapted mammalian fetuses to receive signaling via amniotic fluid.
-
4.
The evolution of the chorio-allantoic placenta increased the complexity of maternal–fetal signaling, and has enhanced the variation in development and metabolism that arises from maternal signals.
-
5.
We hypothesize that the developmental diseases that plague modern humans arise from mismatches between this evolved, adaptive maternal regulation of development and the modern environment.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Arslanoglu S., Ziegler E.E., Moro E.E. WAPM Working Group on Nutrition. Donor human milk in preterm infant feeding: evidence and recommendations. Journal of Perinatal Medicine. 2010;38:347–351. doi: 10.1515/jpm.2010.064. [DOI] [PubMed] [Google Scholar]
- Barker D.J.P. The fetal and infant origins of adult disease. British Medical Journal. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J.P. BMJ London; UK: 1993. Fetal and Infant Origins of Adult Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J.P. In utero programming of chronic disease. Clinical Science. 1998;95:115–128. [PubMed] [Google Scholar]
- Barker D.J.P. The developmental origins of adult disease. Journal of the American College of Nutrition. 2004;23:588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- Bartol F.F., Wiley A.A., Bagnell C.A. Epigentic programming of porcine endometrial function and the lactocrine hypothesis. Reproduction in Domestic Animals. 2008;43(Suppl. 2):273–279. doi: 10.1111/j.1439-0531.2008.01174.x. [DOI] [PubMed] [Google Scholar]
- Bezirtzoglou E., Tsiotsias A., Welling G.W. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH) Anaerobe. 2011;17:468–482. doi: 10.1016/j.anaerobe.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Bode L., Kuhn L., Kim H.-Y., Hsiao L., Nissan C., Sinkala M., Kankasa C., Mwiya M., Thea D.M., Aldrovandi G.M. Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding. American Journal of Clinical Nutrition. 2012;96:831–839. doi: 10.3945/ajcn.112.039503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawand D., Wahli W., Kaessmann H. Loss of egg yolk genes in mammals and the origin of lactation and placentation. PLoS Biology. 2008;6(3):e63. doi: 10.1371/journal.pbio.0060063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Rubio R., Collado M.C., Laitinen K., Salminen S., Isolauri E., Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. American Journal of Clinical Nutrition. 2012;96:544–551. doi: 10.3945/ajcn.112.037382. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Possible West Nile virus transmission to an infant through breast-feeding: Michigan, 2002. MMWR. Morbidity and Mortality Weekly Report. 2002;51:877–878. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Transfusion-related transmission of yellow fever vaccine virus: California, 2009. MMWR. Morbidity and Mortality Weekly Report. 2010;59:34–37. [PubMed] [Google Scholar]
- Chen J.C., Wiley A.A., Ho T.-Y., Frankshun A.-L., Hord K.M., Bartol F.F., Bagnell C.A. Transient estrogen exposure from birth affects uterine expression of developmental markers in neonatal gilts with lasting consequences in pregnant adults. Reproduction. 2010;139:623–630. doi: 10.1530/REP-09-0454. [DOI] [PubMed] [Google Scholar]
- Chen X., Gao C., Li H., Huang L., Sun Q., Dong Y., Tian C., Gao S., Dong H., Guan D., Hu X., Zhao S., Li L., Zhu L., Yan Q., Zhang J., Zen K., Zhang C.-Y. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Research. 2010;20:1128–1137. doi: 10.1038/cr.2010.80. [DOI] [PubMed] [Google Scholar]
- Chen J.C., Frankshun A.-L., Wiley A.A., Miller D.J., Welch K.A., Ho T.-Y., Bartol F.F., Bagnell C.A. Milk-borne lactocrine-acting factors affect gene expression patterns in the developing neonatal porcine uterus. Reproduction. 2011;141:675–683. doi: 10.1530/REP-10-0320. [DOI] [PubMed] [Google Scholar]
- Coovadia H.M., Rollins N.C., Bland R.M., Little K., Coutsoudis A., Bennish M.L., Newell M.-L. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369:1107–1116. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- Coursodon C.F., Dvorak B. Epidermal growth factor and necrotizing enterocolitis. Current Opinion in Pediatrics. 2012;24:160–164. doi: 10.1097/MOP.0b013e3283504ddb. [DOI] [PubMed] [Google Scholar]
- Coutsoudis A., Dabis F., Fawzi W. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient meta-analysis. Journal of Infectious Diseases. 2004;189:2154–2166. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- Cruz J.R., Carlsson B., Garcia B., Gebre-Medhin M., Hofvander Y., Urrutia J.J., Hanson L.A. Studies on human milk III. Secretory IgA quantity and antibody levels against Escherichia coli in colostrum and milk from underprivileged and privileged mothers. Pediatric Research. 1982;16:272–276. doi: 10.1203/00006450-198204000-00004. [DOI] [PubMed] [Google Scholar]
- Dandekar A.M., Robinson E.A., Appella E., Qasba P.K. Complete sequence analysis of cDNA clones encoding rat whey phosphoprotein: homology to a protease inhibitor. Proceedings of the National Academy of Science. 1982;79:3987–3991. doi: 10.1073/pnas.79.13.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zegher F., Sabastiani G., Diaz M., Gómez-Roig M.D., López-Bermejo A., Ibáñez L. Breast-feeding vs formula-feeding for infants born small-for-gestational-age: divergent effects on fat mass and on circulating IGF-I and high-molecular-weight adiponenctin in late infancy. Journal of Clinical Endocrinology and Metabolism. 2013;98:1242–1247. doi: 10.1210/jc.2012-3480. [DOI] [PubMed] [Google Scholar]
- Dunn D.T., Newell M.L., Ades A.E., Peckham C.S. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet. 1992;340:585–588. doi: 10.1016/0140-6736(92)92115-v. [DOI] [PubMed] [Google Scholar]
- Eising C.M., Eikenaar C., Schwabl H., Groothuis T.G. Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proceedings of the Royal Society of London. 2001;268:839–846. doi: 10.1098/rspb.2001.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eising C.M., Müller W., Dijkstra C., Groothuis T.G. Maternal androgens in egg yolks: relation with sex, incubation time and embryonic growth. General and Comparative Endocrinology. 2003;132:241–247. doi: 10.1016/s0016-6480(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Fernández L., Langa S., Martín V., Maldonado A., Jiménez E., Martín R., Rodríguez J.M. The human milk microbiota: origin and potential roles in health and disease. Pharmacological Research. 2013;69:1–10. doi: 10.1016/j.phrs.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Fields D.A., Demerath E.W. Relationship of insulin, glucose, leptin, IL-6 and TNF-α in human breast milk with infant growth and body composition. Pediatric Obesity. 2012;7:304–312. doi: 10.1111/j.2047-6310.2012.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons S.P., Evans M.K., Pearce C.L., Sheridan M.J., Wientzen R., Cole M.F. Immunoglobulin A subclasses in infants' saliva and in saliva and milk from their mothers. Journal of Pediatrics. 1994;124:566–573. doi: 10.1016/s0022-3476(05)83135-x. [DOI] [PubMed] [Google Scholar]
- Forsdahl A. Are poor living conditions in childhood and adolescence important risk factors for arteiosclerotic heart disease? British Journal of Preventive & Social Medicine. 1977;31:91–95. doi: 10.1136/jech.31.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A. Maternal constraint of fetal growth and its consequences. Seminars in Fetal and Neonatal Medicine. 2005;9:419–425. doi: 10.1016/j.siny.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A. Oxford University Press; Oxford: 2006. Mismatch; How our World no Longer Fits our Bodies. [Google Scholar]
- Gluckman P.D., Hanson M.A., Beedle A.S. Non-genomic transgenerational inheritance of disease risk. Bioessays. 2007;29:145–154. doi: 10.1002/bies.20522. [DOI] [PubMed] [Google Scholar]
- Gorski J.N., Dunn-Meynell A.A., Hartman T.G., Levin B.E. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. American Journal of Physiology — Regulatory, Integrative and Comparative Physiology. 2006;291:R768–R778. doi: 10.1152/ajpregu.00138.2006. [DOI] [PubMed] [Google Scholar]
- Hanson L.A., Winberg J. Breast milk and defence against infection in the newborn. Archives of Disease in Childhood. 1972;47:845. doi: 10.1136/adc.47.256.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson L.A., Ahlstedt S., Andersson B., Carlsson B., Fällström S.P., Mellander L., Edén C.S. Protective factors in milk and the development of the immune system. Pediatrics. 1985;75:172–176. [PubMed] [Google Scholar]
- Hata T., Murakami K., Nakatani H., Yamamoto Y., Matsuda T., Aoki N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochemical and Biophysical Research Communications. 2010;396:528–533. doi: 10.1016/j.bbrc.2010.04.135. [DOI] [PubMed] [Google Scholar]
- Henderson G., Craig S., Brocklehurst P., McGuire W. Enteral feeding regimens and necrotizing enterocolitis in preterm infants: a multicentre case–control study. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2009;94:F120–F123. doi: 10.1136/adc.2007.119560. [DOI] [PubMed] [Google Scholar]
- Hinckley A.F., O'Leary D.R., Hayes E.B. Transmission of West Nile virus through human breast milk seems to be rare. Pediatrics. 2007;119:e666–e671. doi: 10.1542/peds.2006-2107. [DOI] [PubMed] [Google Scholar]
- Homsy J., Moore D., Barasa A., Were W., Likichiho C., Waiswa B., Downing R., Malamba S., Tappero J., Mermin J. Breastfeeding, mother-to-child HIV transmission, and mortality among infants born to HIV-infected women on highly active antiretroviral therapy in rural Uganda. Journal of Acquired Immune Deficiency Syndromes. 2010;53:28–35. doi: 10.1097/QAI.0b013e3181bdf65a. [DOI] [PubMed] [Google Scholar]
- Isolauri E. Development of healthy gut microbiota early in life. Journal of Paediatrics and Child Health. 2012;48(Suppl. 3):1–6. doi: 10.1111/j.1440-1754.2012.02489.x. [DOI] [PubMed] [Google Scholar]
- Jiao B., Ma H., Shokhirev M.N., Drung A., Yang Q., Sin J.D., Lu S., Byron M., Kalantry S., Mercurio A.M., Lawrence J.B., Hoffmann A., Bach I. Paternal RLIM/Rnf12 is a survival factor for milk-producing alveolar cells. Cell. 2012;149:630–641. doi: 10.1016/j.cell.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.L., Levy J., Supowit S.C., Yu-Lee L.-Y., Rosen J.M. Tissue- and cell-specific casein gene expression. II. Relationship to site-specific DNA methylation. Journal of Biological Chemistry. 1983;258:10805–10811. [PubMed] [Google Scholar]
- Kosaka N., Izumi H., Sekine K., Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence. 2010;1(7) doi: 10.1186/1758-907X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysiak-Baltyn K., Toppari J., Skakkebaek N.E., Jensen T.S., Virtanen H.E., Schramm K.-W., Shen H., Vartianen T., Kiviranta H., Taboureau O., Brunak S., Main K.M. Country-specific chemical signatures of persistent environmental compounds in breast milk. International Journal of Andrology. 2009;32:1–9. doi: 10.1111/j.1365-2605.2009.00996.x. [DOI] [PubMed] [Google Scholar]
- Kuhn S., Twele-Montecinos L., MacDonald J., Webster P., Law B. Case report: probable transmission of vaccine strain of yellow fever virus to an infant via breast milk. Canadian Medical Association Journal. 2011;183:E243–E245. doi: 10.1503/cmaj.100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson A.J., Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- Main K.M., Mortensen G.K., Kaleva M.M., Boisen K.A., Damgaard I.N., Chellakooty M., Schmidt I.M., Suomi A.-M., Virtanen H.E., Petersen J.H., Andersson A.-M., Toppari J., Skakkebæk N.E. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environmental Health Perspectives. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín R., Langa S., Reviriego C., Jiménez E., Marín M.L., Olivares M., Rodríguez J.M. The commensal microflora of human milk: new perspectives for food bacteriotherapy and probiotics. Trends in Food Science & Technology. 2004;15:121–127. [Google Scholar]
- Martín V., Maldonado-Barragán A., Moles L., Rodriguez-Baños M., del Campo R., Fernández L., Rodríguez J.M., Jiménez E. Sharing bacterial strains between breast milk and infant feces. Journal of Human Lactation. 2012;28:36–44. doi: 10.1177/0890334411424729. [DOI] [PubMed] [Google Scholar]
- Murphy W.J., Eizirik E., O'Brien S.J., Madsen O., Scally M., Douady C.J., Teeling E., Ryder O.A., Stanhope M.J., de Jong W.W., Springer M.S. Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science. 2001;294:2348–2352. doi: 10.1126/science.1067179. [DOI] [PubMed] [Google Scholar]
- Nathanielsz P.W. Animal models that elucidate basic principles of the developmental origins of adult diseases. International League of Associations for Rheumatology Journal. 2006;47:73–82. doi: 10.1093/ilar.47.1.73. [DOI] [PubMed] [Google Scholar]
- Navara K.J., Hill G.E., Mendonça M.T. Variable effects of yolk androgens on growth, survival, and immunity in Eastern Bluebird nestlings. Physiological and Biochemical Zoology. 2005;78:57–578. doi: 10.1086/430689. [DOI] [PubMed] [Google Scholar]
- Nduati R., John G., Mbori-Ngacha D., Richardson B., Overbaugh J., Mwatha A., Ndinya-Achola J., Onyango F.E., Hughes J., Kreiss J. Effect of breastfeeding and formula feeding on transmission of HIV-1. Journal of the American Medical Association. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- Numan M., Fleming A.S., Levy F. Third ed. Elsevier; St. Louis: 2006. Maternal Behavior. In Knobil and Neill's Physiology of Reproduction, Ed. JD Neill, 1921–1993. [Google Scholar]
- Oben J.A., Mouralidarane A., Samuelsson A.-M., Matthews P.J., Morgan M.L., Mckee C., Soeda J., Fernandez-Twinn D.S., Martin-Gronert M.S., Osanne S.E., Sigala B., Novelli M., Poston L., Taylor P.D. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. Journal of Hepatology. 2010;52:913–920. doi: 10.1016/j.jhep.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Oftedal O.T. The origin of lactation as a water source for parchment shelled eggs. Journal of Mammal Gland Biology and Neoplasia. 2002;2:253–266. doi: 10.1023/a:1022848632125. [DOI] [PubMed] [Google Scholar]
- Oftedal O.T. The evolution of milk secretion and its ancient origins. Animal. 2012;6:355–368. doi: 10.1017/S1751731111001935. [DOI] [PubMed] [Google Scholar]
- Park C.S. Role of compensatory mammary growth in epigenetic control of gene expression. The FASEB Journal. 2005;19:1586–1591. doi: 10.1096/fj.05-3816hyp. [DOI] [PubMed] [Google Scholar]
- Perez P.F., Doré J., Leclerc M., Levenez F., Benyacoub J., Serrant P., Segura-Roggero I., Schiffrin E.J., Donnet-Hughes A. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics. 2007;119:e724–e732. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- Petherick A.A. Development: mother's milk: a rich opportunity. Nature. 2010;468:S5–S7. doi: 10.1038/468S5a. [DOI] [PubMed] [Google Scholar]
- Petraglia F., Florio P., Vale W.W. Placental expression of neurohormones and other neuroactive molecules in human pregnancy. In: Power M.L., Schulkin J., editors. Birth, Distress and Disease. 2005. pp. 16–73. [Google Scholar]
- Prager E.M., Wilson A.C. Ancient origin of lactalbumin from lysozyme: analysis of DNA and amino acid sequences. Journal of Molecular Evolution. 1988;27:326–335. doi: 10.1007/BF02101195. [DOI] [PubMed] [Google Scholar]
- Rautava S., Walker W.A. Breastfeeding — an extrauterine link between mother and child. Breastfeeding Medicine. 2009;4:3–10. doi: 10.1089/bfm.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifsnyder P.C., Churchill G., Leiter E.H. Maternal environment and genotype interact to establish diabesity in mice. Genome Research. 2000;10:1568–1578. doi: 10.1101/gr.147000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W., Lewis A. Co-evolution of X-chromosome inactivation and imprinting in mammals. Nature Reviews Genetics. 2005;6:403–410. doi: 10.1038/nrg1602. [DOI] [PubMed] [Google Scholar]
- Renfree M.B., Suzuki S., Kaneko-Ishino T. The origin and evolution of genomic imprinting and vivipauty in mammals. Philosophical Transactions of the Royal Society B. 2013;368:20120151. doi: 10.1098/rstb.2012.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M., Rotta G., Valzasina B., Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunology. 2001;204:572–581. doi: 10.1078/0171-2985-00094. [DOI] [PubMed] [Google Scholar]
- Rijnkels M., Kabotyanski E., Montazer-Torbati M.B., Beauvais C.H., Vassetsky Y., Rosen J.M., Devinoy E. The epigenetic landscape of mammary gland development and functional differentiation. Journal of Mammary Gland Biology and Neoplasia. 2010;15:85–100. doi: 10.1007/s10911-010-9170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J.M., Wyszomierski S.L., Hadsell D. Regulation of milk protein gene expression. Annual Review of Nutrition. 1999;19:407–436. doi: 10.1146/annurev.nutr.19.1.407. [DOI] [PubMed] [Google Scholar]
- Savino F., Liguori S.A., Fissore M.F., Oggero R. Breast milk hormones and their protective effect on obesity. International Journal of Pediatric Endocrinology. 2009 doi: 10.1155/2009/327505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino F., Liguori S.A., Lupica M.M. Adipokines in breast milk and preterm infants. Early Human Development. 2010;86:77–80. doi: 10.1016/j.earlhumdev.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Savino F., Liguori S.A., Sorrenti M., Fissore M.F., Oggero R. Breast milk hormones and regulation of glucose homeostasis. International Journal of Pediatric Endocrinology. 2011 doi: 10.1155/2011/803985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtens S., Wijga A.H., Brunekreef B., Kerkhof M., Hoekstra M.O., Gerritsen J., Aalberse R., de Jongste J.C., Smit H.A. Thorax. 2009;64:604–609. doi: 10.1136/thx.2007.094938. [DOI] [PubMed] [Google Scholar]
- Schroeder M., Shbiro L., Moran T.H., Weller A. Maternal environmental contribution to adult sensitivity and resistance to obesity in Long Evans rats. PLoS One. 2010;5(11):e13825. doi: 10.1371/journal.pone.0013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz M., Göttl C., Young R.J., Iwen P., Vanderhoof J.A. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. Journal of Pediatric Gastroenterology and Nutrition. 2004;38:293–297. doi: 10.1097/00005176-200403000-00012. [DOI] [PubMed] [Google Scholar]
- Schwabl H. Environment modifies he testosterone levels of a female bird and its eggs. The Journal of Experimental Zoology. 1996;276:157–163. doi: 10.1002/(SICI)1097-010X(19961001)276:2<157::AID-JEZ9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Sela D.A., Mills D.A. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends in Microbiology. 2010;18:298–307. doi: 10.1016/j.tim.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Swanson K., Couldrey C., Seyfert H.-M., Stelwagen K. Suppression of bovine αS1-casein gene expression during involution of the mammary gland is associated with increased DNA methylation at a STAT5-binding site in the αS1-casein promoter. Journal of Dairy Science. 2008;91:378. [Google Scholar]
- Singh K., Molenaar A.J., Swanson K.M., Gudex B., Arias J.A., Erdman R.A., Stelwagen K. Epigenetics: a possible role in acute and transgenerational regulation of dairy cow milk production. Animal. 2012;6:375–381. doi: 10.1017/S1751731111002564. [DOI] [PubMed] [Google Scholar]
- Singhal A., Cole T.J., Fewtrell M., Kennedy K., Stephenson T., Elias-Jones A., Lucas A. Promotion of faster weight gain in infants born small for gestational age: is there an adverse effect on later blood pressure? Circulation. 2007;115:213–220. doi: 10.1161/CIRCULATIONAHA.106.617811. [DOI] [PubMed] [Google Scholar]
- Singhal A., Kennedy K., Lanigan J., Fewtrell M., Cole T.J., Stephenson T., Elias-Jones A., Weaver L.T., Ibhanesebhor S., MacDonald P.D., Bindels J., Lucas A. Nutrition in infancy and long-term risk of obesity: evidence from 2 randomized controlled trials. American Journal of Clinical Nutrition. 2010;92:1133–1144. doi: 10.3945/ajcn.2010.29302. [DOI] [PubMed] [Google Scholar]
- Sisk P.M., Lovelady C.A., Dillard R.G., Gruber K.J., O'Shea T.M. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. Journal of Perinatology. 2007;27:428–433. doi: 10.1038/sj.jp.7211758. [DOI] [PubMed] [Google Scholar]
- Smith M.I., Yatsunenko T., Manary M.J., Trehan I., Mkakosya R., Cheng J., Kau A.L., Rich S.S., Concannon P., Mychaleckyj J.C., Liu J., Houpt E., Li J.V., Holmes E., Nicholson J., Knights D., Ursell L.K., Knight R., Gordon J.I. Gut microbes of Malawain twin pairs discordant for Kwashiorkor. Science. 2013;339:548–554. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer J.M., Suzuki S., Pask A.J., Shaw G., Renfree M.B. Selected imprinting of INS in the marsupial. Epigenetics & Chromatin. 2012;5:14. doi: 10.1186/1756-8935-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S., Schanler R.J., Kim J.H., Patel A.L., Trawöger R., Kiechl-Kohlendorfer U., Chan G.M., Blanco C.L., Abrams S., Cotton C.M., Laroia N., Ehrenkranz R.A., Dudell G., Cristofalo E.A., Meier P., Lee M.L., Rechtman D.J., Lucas A. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocoloitis than a diet of human milk and bovine milk-based products. Journal of Pediatrics. 2010;156:562–567.e1. doi: 10.1016/j.jpeds.2009.10.040. [DOI] [PubMed] [Google Scholar]
- Thompson M.D., Nakhasi H.L. Methylation and expression of rat κ-casein gene in normal and neoplastic rat mammary gland. Cancer Research. 1985;45:1291–1295. [PubMed] [Google Scholar]
- Underwood M.A., Gilbert W.M., Sherman M.P. Amniotic fluid: not just fetal urine anymore. Journal of Perinatology. 2005;25:341–348. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- Van de Perre P. Transfer of antibody via mother's milk. Vaccine. 2003;21:3374–3376. doi: 10.1016/s0264-410x(03)00336-0. [DOI] [PubMed] [Google Scholar]
- Van Odijk J., Kull I., Borres M.P., Brandtzaeg P., Edberg U., Hanson L.A., Høst A., Kuitunen M., Olsen S.F., Skerfving S., Sundell J., Wille S. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1996–2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy. 2003;58:833–843. doi: 10.1034/j.1398-9995.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- Vanselow J., Yang W., Herrmann J., Zerbe H., Schuberth H.-J., Petzi W., Tomek W., Seyfert H.-M. DNA-remethylation around a STAT5-binding enhancer in the αS1-casein promoter is associated with abrupt shutdown of αS1-casein synthesis during acute mastitis. Journal of Molecular Endocrinology. 2006;37:463–477. doi: 10.1677/jme.1.02131. [DOI] [PubMed] [Google Scholar]
- Wagner Carol L. Amniotic fluid and human milk: a continuum of effect? Journal of Pediatric Gastroenterology and Nutrition. 2002;34:513–514. doi: 10.1097/00005176-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Ward R.E., Niñonuevo M., Mills D.A., Lebrilla C.B., German J.B. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Applied and Environmental Microbiology. 2006;72:4497–4499. doi: 10.1128/AEM.02515-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J.A., Baxter D.H., Zhang S., Huang D.Y., Huang K.H., Lee M.J., Galas D.J., Wang K. The microRNA spectrum in 12 body fluids. Clinical Chemistry. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J.G., Guerrero M.L., Altaye M., Ruiz-Palacios G.M., Martin L.J., Dubert-Ferrandon A., Newburg D.S., Morrow A.L. Human milk adiponectin is associated with infant growth in two independent cohorts. Breastfeeding Medicine. 2009;4:101–109. doi: 10.1089/bfm.2008.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J.G., Guerrero M.L., Guo F., Martin L.J., Davidson B.S., Ortega H., Ruiz-Palacios G.M., Morrow A.L. Human milk adiponectin affects infant weight trajectory during the second year of life. Journal of Pediatric Gastroenterology and Nutrition. 2012;54:532–539. doi: 10.1097/MPG.0b013e31823fde04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun K., Soejima H., Merrie A.E.H., McCall J.L., Reeve A.E. Analysis of IGF2 gene imprinting in breast and colorectal cancer by allele specific-PCR. The Journal of Pathology. 1999;187:518–522. doi: 10.1002/(SICI)1096-9896(199904)187:5<518::AID-PATH276>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Li M., Wang X., Li Q., Wang T., Zhu Q., Li X. Immune-related microRNAs are abundant in breast milk exosomes. International Journal of Biological Sciences. 2012;8:118–123. doi: 10.7150/ijbs.8.118. [DOI] [PMC free article] [PubMed] [Google Scholar]