Abstract
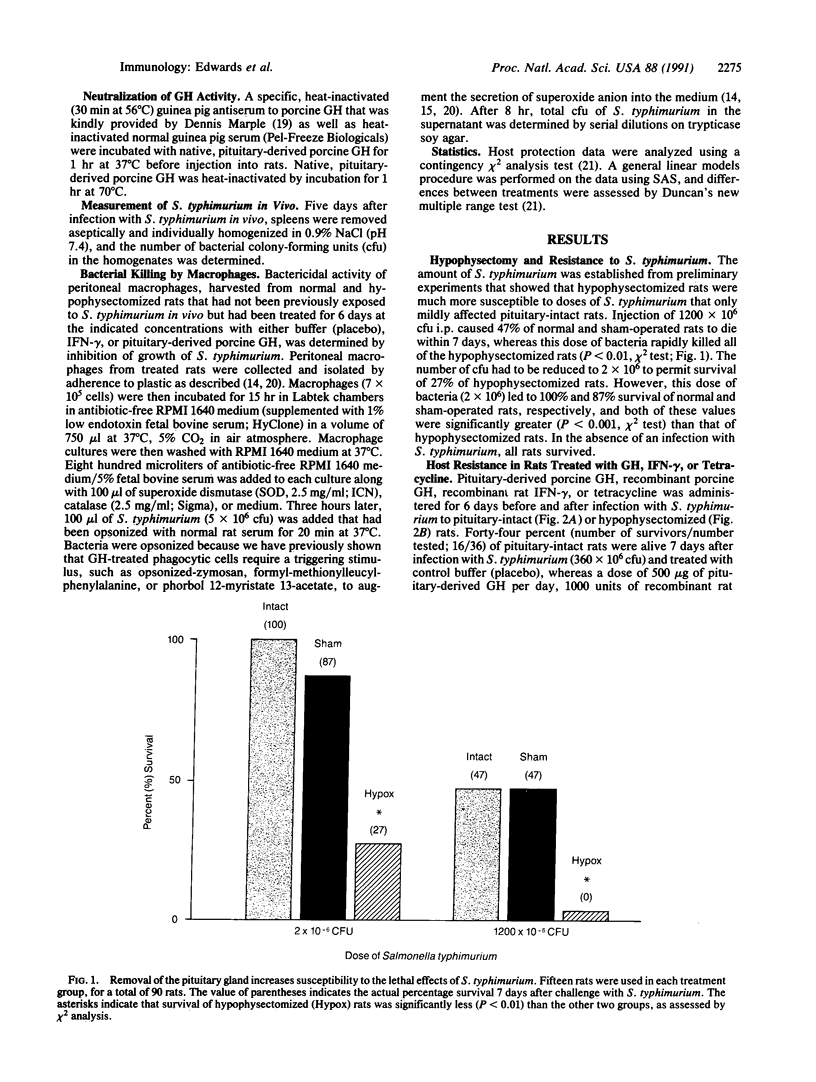
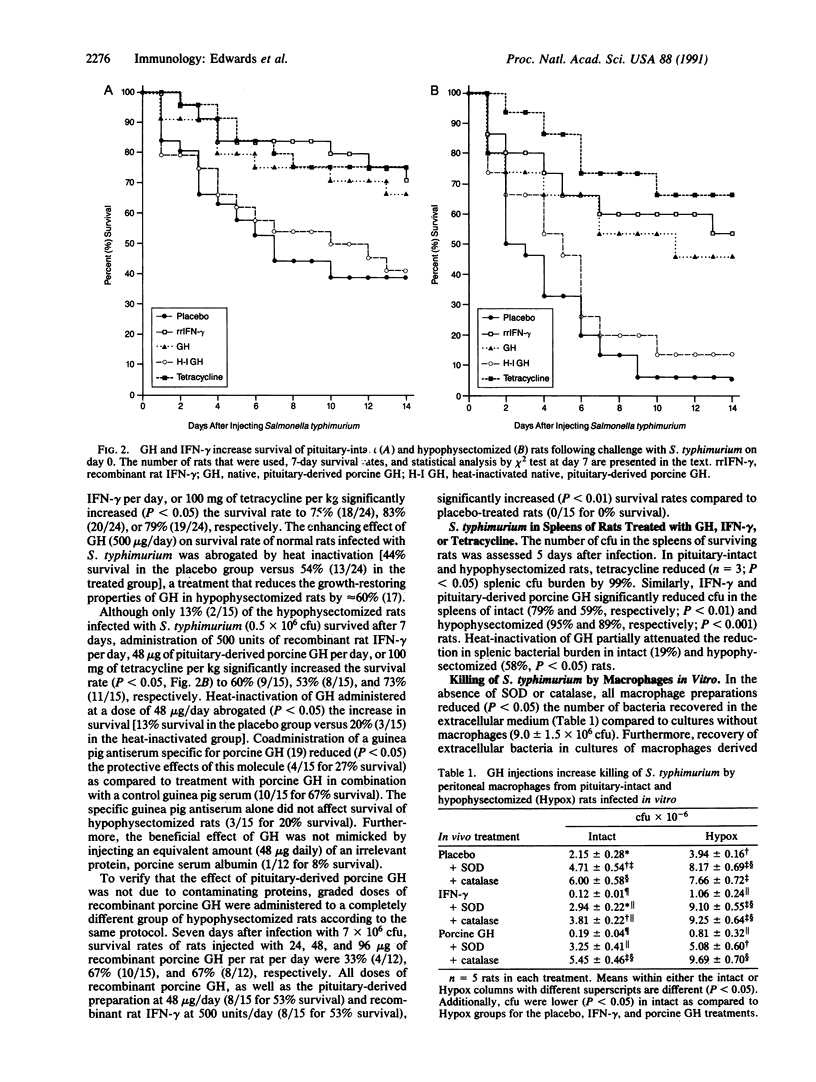
One-half of pituitary-intact or sham-operated rats survive infection with 10(9) colony-forming units of Salmonella typhimurium, whereas rats without a pituitary gland all die within a few days. When the dose of S. typhimurium is reduced 600-fold, 15-25% of the hypophysectomized rats survive, and the survival rate is significantly enhanced by administration of tetracycline, recombinant interferon gamma (IFN-gamma), or recombinant growth hormone (GH). The protective effect of GH is abolished by heat inactivation or with an antibody to GH. Spleens from normal and hypophysectomized rats treated with tetracycline, IFN-gamma, or GH have 59-99% fewer bacteria 5 days after infection as compared to control rats. Peritoneal macrophages from hypophysectomized rats that are infected in vitro with S. typhimurium kill half as many extracellular bacteria as compared to pituitary-intact rats, and this bactericidal capacity is significantly augmented 75-95% by either GH or IFN-gamma. These data establish that the pituitary gland is essential for homeostasis during an infectious episode and that GH plays an important role in host resistance by augmenting the ability of macrophages to kill S. typhimurium.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. Molecular transductional mechanisms by which IFN gamma and other signals regulate macrophage development. Immunol Rev. 1987 Jun;97:5–27. doi: 10.1111/j.1600-065x.1987.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Berczi I. Immunoregulation by neuroendocrine factors. Dev Comp Immunol. 1989 Fall;13(4):329–341. doi: 10.1016/0145-305x(89)90042-6. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Meltzer M. S., Holaday J. W. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science. 1988 Jan 22;239(4838):401–404. doi: 10.1126/science.3122324. [DOI] [PubMed] [Google Scholar]
- Blalock J. E. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol Rev. 1989 Jan;69(1):1–32. doi: 10.1152/physrev.1989.69.1.1. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Davila D. R., Brief S., Simon J., Hammer R. E., Brinster R. L., Kelley K. W. Role of growth hormone in regulating T-dependent immune events in aged, nude, and transgenic rodents. J Neurosci Res. 1987;18(1):108–116. doi: 10.1002/jnr.490180118. [DOI] [PubMed] [Google Scholar]
- Davila D. R., Edwards C. K., 3rd, Arkins S., Simon J., Kelley K. W. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. FASEB J. 1990 Aug;4(11):2906–2911. doi: 10.1096/fasebj.4.11.2165948. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Ghiasuddin S. M., Schepper J. M., Yunger L. M., Kelley K. W. A newly defined property of somatotropin: priming of macrophages for production of superoxide anion. Science. 1988 Feb 12;239(4841 Pt 1):769–771. doi: 10.1126/science.2829357. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Lorence R. M., Dunham D. M., Arkins S., Yunger L. M., Greager J. A., Walter R. J., Dantzer R., Kelley K. W. Hypophysectomy inhibits the synthesis of tumor necrosis factor alpha by rat macrophages: partial restoration by exogenous growth hormone or interferon gamma. Endocrinology. 1991 Feb;128(2):989–986. doi: 10.1210/endo-128-2-989. [DOI] [PubMed] [Google Scholar]
- Fu Y. K., Arkins S., Wang B. S., Kelley K. W. A novel role of growth hormone and insulin-like growth factor-I. Priming neutrophils for superoxide anion secretion. J Immunol. 1991 Mar 1;146(5):1602–1608. [PubMed] [Google Scholar]
- Juskevich J. C., Guyer C. G. Bovine growth hormone: human food safety evaluation. Science. 1990 Aug 24;249(4971):875–884. doi: 10.1126/science.2203142. [DOI] [PubMed] [Google Scholar]
- Kelley K. W., Brief S., Westly H. J., Novakofski J., Bechtel P. J., Simon J., Walker E. B. GH3 pituitary adenoma cells can reverse thymic aging in rats. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5663–5667. doi: 10.1073/pnas.83.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley K. W. Growth hormone, lymphocytes and macrophages. Biochem Pharmacol. 1989 Mar 1;38(5):705–713. doi: 10.1016/0006-2952(89)90222-0. [DOI] [PubMed] [Google Scholar]
- Langermans J. A., van der Hulst M. E., Nibbering P. H., van Furth R. Activation of mouse peritoneal macrophages during infection with Salmonella typhimurium does not result in enhanced intracellular killing. J Immunol. 1990 Jun 1;144(11):4340–4346. [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- Marple D. N., Aberle E. D. Porcine plasma growth hormone levels: radioimmunoassay technique and its application. J Anim Sci. 1972 Feb;34(2):261–266. doi: 10.2527/jas1972.342261x. [DOI] [PubMed] [Google Scholar]
- Mason D., MacPhee I., Antoni F. The role of the neuroendocrine system in determining genetic susceptibility to experimental allergic encephalomyelitis in the rat. Immunology. 1990 May;70(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Matsumura H., Onozuka K., Terada Y., Nakano Y., Nakano M. Effect of murine recombinant interferon-gamma in the protection of mice against Salmonella. Int J Immunopharmacol. 1990;12(1):49–56. doi: 10.1016/0192-0561(90)90067-w. [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Charrier K. GM-CSF administration augments the survival of ity-resistant A/J mice, but not ity-susceptible C57BL/6 mice, to a lethal challenge with Salmonella typhimurium. J Immunol. 1990 Jan 15;144(2):557–561. [PubMed] [Google Scholar]
- Nakano Y., Onozuka K., Terada Y., Shinomiya H., Nakano M. Protective effect of recombinant tumor necrosis factor-alpha in murine salmonellosis. J Immunol. 1990 Mar 1;144(5):1935–1941. [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. G., Nathan C. F., Pihl D. L., Rodricks P., Shanebeck K., Conlon P. J., Grabstein K. H. Recombinant granulocyte/macrophage colony-stimulating factor activates macrophages to inhibit Trypanosoma cruzi and release hydrogen peroxide. Comparison with interferon gamma. J Exp Med. 1987 Dec 1;166(6):1734–1746. doi: 10.1084/jem.166.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman D., Feller A. G., Nagraj H. S., Gergans G. A., Lalitha P. Y., Goldberg A. F., Schlenker R. A., Cohn L., Rudman I. W., Mattson D. E. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990 Jul 5;323(1):1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- Saxena Q. B., Saxena R. K., Adler W. H. Regulation of natural killer activity in vivo. III. Effect of hypophysectomy and growth hormone treatment on the natural killer activity of the mouse spleen cell population. Int Arch Allergy Appl Immunol. 1982;67(2):169–174. [PubMed] [Google Scholar]
- Schauenstein K., Fässler R., Dietrich H., Schwarz S., Krömer G., Wick G. Disturbed immune-endocrine communication in autoimmune disease. Lack of corticosterone response to immune signals in obese strain chickens with spontaneous autoimmune thyroiditis. J Immunol. 1987 Sep 15;139(6):1830–1833. [PubMed] [Google Scholar]
- Sternberg E. M., Hill J. M., Chrousos G. P., Kamilaris T., Listwak S. J., Gold P. W., Wilder R. L. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]