Abstract
Hydroxypropyl-β-cyclodextrin (HPBCD) is an attractive drug candidate against Niemann–Pick Type C (NPC) disease. However, the safety of HPBCD treatment for NPC patients remains to be elucidated. In this study, we examined the acute toxicity of HPBCD in Npc1-deficient mice. When treated with HPBCD (20,000 mg/kg, subcutaneously), over half of the wild-type (Npc1+/+) or Npc1+/− mice died by 72 h after the injection. In contrast, all of the Npc1−/− mice survived. Marked pathophysiological changes, such as an elevation in serum transaminase and creatinine levels, hepatocellular necrosis, renal tubular damage, interstitial thickening, and hemorrhages in lungs, were induced by the HPBCD treatment in Npc1+/+ or Npc1+/− mice. However, these pathophysiological changes were significantly alleviated in Npc1−/− mice. In addition, in vitro analysis showed that the Npc1 gene deficiency and treatment with U18666A, an Npc1 inhibitor, remarkably attenuated the cytotoxicity of HPBCD in Chinese hamster ovary cells. These results suggest that the NPC1 genotype exacerbates the cytotoxicity of HPBCD and Npc1−/− mice have substantial resistance to the lethality and the organ injury induced by HPBCD injection compared with Npc1+/+ or Npc1+/− mice. We suggest that the Npc1 genotype should be considered in the safety evaluation of HPBCD using experimental animals and cells.
Abbreviations: NPC, Niemann–Pick Type C disease; HPBCD, Hydroxypropyl-β-cyclodextrin; ALT, Alanine aminotransferase; CHO, Chinese hamster ovary
Keywords: Niemann–Pick Type C, Hydroxypropyl-β-cyclodextrin, Npc1-deficient mice, Lysosomal storage disease, Autosomal recessive disorder, U18666A
1. Introduction
Niemann–Pick Type C (NPC) disease, an autosomal recessive disorder caused by mutations in either of the Npc1 or Npc2 genes, is characterized by progressive neurological deterioration and death during childhood [1]. Marked lysosomal accumulation of unesterified cholesterol and shortage of esterified cholesterol in cellular compartments are observed in NPC disease, and cholesterol sequestration may be a key factor in developing the disease. Recently, some reports have shown that hydroxypropyl-β-cyclodextrin (HPBCD), a cyclic oligosaccharide derivative that has a solubilizing ability on lipophilic compounds, including cholesterol, attenuated cholesterol sequestration in systemic cells and prolonged the lifespan in Npc1 null mice [2], [3], [4]. In addition, Matsuo et al. [5] reported that treatment with HPBCD improved hepatosplenomegaly and central nervous system dysfunction in two patients with NPC disease.
HPBCD has been used as a pharmaceutical additive with high aqueous solubility and extremely low toxicity and has been used clinically with cardinal remedies in parenteral formulations [6]. Based on these facts, HPBCD is compassionately used to treat patients with NPC disease. In our previous study, patients were administered high-dose HPBCD (2000–2500 mg/kg) infusions twice or more per week without severe adverse events [5]. However, Chien et al. [7] reported that chronic HPBCD infusion induced the pneumonia in healthy pigs and suggested the risk of lung toxicity by HPBCD treatment for NPC disease. In addition, some reports have demonstrated that HPBCD caused organ injury, such as renal and liver dysfunction, in animals [8], [9]. Therefore, the safety of HPBCD treatment for NPC patients remains to be elucidated.
Based on these facts, this study was conducted to evaluate the acute toxicity of HPBCD in NPC disease. We examined the toxic effects of HPBCD on mice as determined by survival rate, changes in serum biochemical parameters, and histological analysis in wild-type or homozygous and heterozygous Npc1 mutant mice. In addition, to evaluate the effects of NPC disease on cellular injury induced by HPBCD, we examined the effects of NPC1 inhibition by gene deletion and pharmacological inhibition using U18666A on the HPBCD-induced cell injury in in vitro cultured cells.
2. Material and methods
2.1. Reagents
HPBCD was kindly donated by Nihon Shokuhin Kako Co., Ltd. (Tokyo, Japan). Mayer's hematoxylin, 1% eosin alcohol solution, and mounting medium for histological examination (malinol) were from MUTO Pure Chemicals (Tokyo, Japan). Dulbecco's modified Eagle's medium and F-12 medium were obtained from Gibco-Life Technologies (Life Technologies Japan, Tokyo, Japan). HyClone™ fetal bovine serum (FBS) was purchased from Thermo Scientific (Logan, UT, USA). The cell counting kit and Cellstain® Double Staining Kit were obtained from Dojindo Laboratories (Kumamoto, Japan). All other reagents and solvents were of reagent grade. De-ionized and distilled bio-pure grade water was used throughout the study.
2.2. Animal experiments
Age-matched (9–11 weeks) male wild-type (Npc1+/+) mice and homozygous (Npc1−/−) and heterozygous (Npc1+/−) mutant (BALB/cNctr-Npc1m1N) mice [10] were used. A total of 75 mice were used in this study, 35 and 40 mice were used for survival study and for biological and histological analysis, respectively. The mutant mice were bred and kept in specific pathogen-free conditions in the Center for Animal Resources and Development (CARD), Kumamoto University. Animals were housed in cages in a room under controlled conditions at 24 °C with a 12-h light cycle and given free access to food and water. All experimental procedures conformed to the animal use guidelines of the Committee for Ethics on Animal Experiments of Kumamoto University (approval numbers M24-367).
In our preliminary study, we examined the effects of 4000 to 10,000 mg/kg of HPBCD on the parameters used in this study, such as survival rate, serum biochemical parameters, and histological changes in Npc1 mutant mice. However, significant changes were not observed at these doses. Therefore, we chose a dose of 20,000 mg/kg of HPBCD in this study. In the saline-treated groups, saline was subcutaneous injected instead of HPBCD solution. In the survival study, mice were divided into the following groups: (1) HPBCD-treated Npc1+/+ group (n = 12) (2) HPBCD-treated Npc1+/− group (n = 12), and (3) HPBCD-treated Npc1−/− group (n = 11) and were monitored for 72 h after the injection. In the biological and histological analysis, mice were divided into the following groups: (1) saline-treated Npc1+/+ group (n = 7); (2) HPBCD-treated Npc1+/+ group (n = 7); (3) saline-treated Npc1+/− group (n = 7); (4) HPBCD-treated Npc1+/− group (n = 7); (5) saline-treated Npc1−/− group (n = 5); and (6) HPBCD-treated Npc1−/− group (n = 7). In the HPBCD-treated groups, HPBCD was dissolved in water and adjusted to pH 7.4 and administered by a subcutaneous injection through the back of the neck in mice at a dose of 20,000 mg/kg. For measurements of biochemical parameters and histological analysis, mice were euthanized 8 h after the injection, and blood and organ samples were collected.
2.3. Measurement of serum biochemical parameters
Blood samples, collected from the inferior vena cava at 8 h after the HPBCD injection, were immediately centrifuged at 4000 ×g at 4 °C for 10 min, and sera were collected. Alanine aminotransferase (ALT) and creatinine were measured using a bio-analyzer (SPOTCHEM EZ SP-4430; ARKRAY, Inc., Kyoto, Japan).
2.4. Histological analysis
Tissue samples were fixed in 10% neutral buffered formalin and then embedded in paraffin before being cut into 4-mm-thick sections. For histological examination of the liver and lung, sections were stained first with Mayer's hematoxylin and then with 1% eosin alcohol solution. For histological examination of the kidney, sections were stained with periodic acid-Schiff stain. Samples were mounted with malinol and inspected using a light microscope (Biorevo; Keyence Co., Osaka, Japan). Lung injury score was determined macroscopically by an observer unaware of the treatment the mice had received. According to a previously reported method [11], the lung injury score was scored as follows: 0 (no damage) to 4 + (maximal damage) according to the combined assessments of alveolar congestion, hemorrhage, infiltration/aggregation of inflammatory cells in the airspace or vessel wall, and thickness of the alveolar wall. Histological analysis, including the lung injury score, was determined by light microscopy by three independent observers. The assignment of study groups was blinded to the observers.
2.5. Cell culture and measurement of cytotoxicity
Wild-type and Npc1 null Chinese hamster ovary (CHO) cells that we previously developed [12] were used in this study. The cells were grown in culture medium consisting of a 1:1 mixture of DMEM/F12 supplemented with 10% FBS. Cells were maintained at 37 °C in a saturated humidity atmosphere of 95% air and 5% CO2.
To evaluate the cytotoxic effects of HPBCD, assays to measure cell viability and cell death were performed. HPBCD-induced cell injury was evaluated by a cell viability assay using mitochondrial dehydrogenase activity and by a calcein-acetomethoxy and propidium iodide (calcein-AM and PI stain viable and dead cells, respectively) dual-staining assay. Mitochondrial dehydrogenase activity was measured using a modified MTT assay, namely the water-soluble tetrazolium salt (WST-8) assay, using a Cell Counting Kit according to the manufacturer's protocol. Calcein-AM/PI co-staining was performed using the Cellstain® Double Staining Kit. CHO cells were incubated in 96-well plates (1 × 104 cells/well) in culture medium at 37 °C for 24 h. After 24 h to allow cells to adhere, the medium was replaced with fresh medium containing HPBCD (0–80 mM) without FBS for 3 h and then incubated with the WST-8 solution for 1.5 h at 37 °C. The maximum absorption of the WST-8 formazan (450 nm) was measured using a micro plate reader (Tecan Co., Ltd, Männedorf, Switzerland). Cell viability was expressed as a percentage of the viable cells relative to the untreated controls. Cells were incubated with calcein-AM and 0.4 mmol/L PI in phosphate-buffered saline for 15 min. Cell death was observed by measuring the fluorescence of calcein-AM and PI at excitation/emission wavelengths of 490/510 nm and 530/580 nm, respectively, using a fluorescence microscope (Biorevo; Keyence, Osaka, Japan).
2.6. Statistical analysis
Statistical analysis was performed using GraphPad Prism ver. 5.01 (GraphPad Software, San Diego, CA, USA). Analysis of the histological score was also performed. Survival data were analyzed using the Kaplan–Meier method, and the log-rank test was used to compare statistical significances. Multiple comparisons were conducted to examine the statistical significance of the results. When uniform variance of the result was identified by Bartlett's analysis (p < 0.05), one-way analysis of variance was used to test for significant differences. When significant differences (p < 0.05) were identified, the results were further analyzed by the Dunnett's or Tukey's multiple range test for significant differences among the values. If uniform variance of the result was not identified, non-parametric multiple comparisons were made. After confirming significant differences (p < 0.05) using the Kruskal–Wallis analysis, the differences were then examined using the Dunnett's test. Analysis of histological score was also performed using these nonparametric multiple comparison tests.
3. Results
3.1. Survival rate of wild type and Npc1 mutant mice treated with a toxic dose of HPBCD
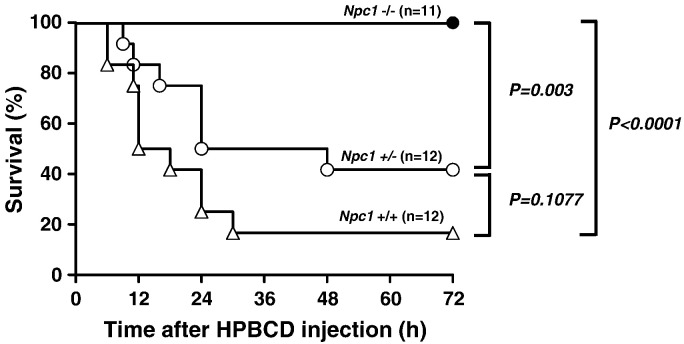
We examined the effects of subcutaneously injected 20,000 mg/kg of HPBCD on survival in Npc1 mutant mice. Over half of the mice of the Npc1+/+ or Npc1+/− groups were dead within 72 h by an administration of HPBCD (Fig. 1). Stress responses, such as anorexia and fluffing and withering of the fur, were observed in the surviving mice of the wild-type and Npc1+/− groups. In contrast, all of the Npc1−/− mice survived, and stress responses exhibited by the Npc1+/− mice were not observed. In the Kaplan–Meier analysis, significant differences were observed in the Npc1−/− group compared with the Npc1+/+ and Npc1+/− groups. Although statistical significances were not observed between the Npc1+/+ and Npc1+/− groups, the survival of the Npc1+/+ group tended to be lower than that of the Npc1+/− groups (p = 0.108 in log-rank test).
Fig. 1.
Effect of HPBCD injection on survival rate of Npc1 mutant mice.
Npc1+/+ (n = 12), Npc1+/− (n = 12) and Npc1−/− (n = 11) mice were administered HPBCD (20,000 mg/kg) subcutaneously and monitored for 72 h.
3.2. Biochemical and histological analysis of Npc1 mutant mice treated with a toxic dose of HPBCD
3.2.1. Liver
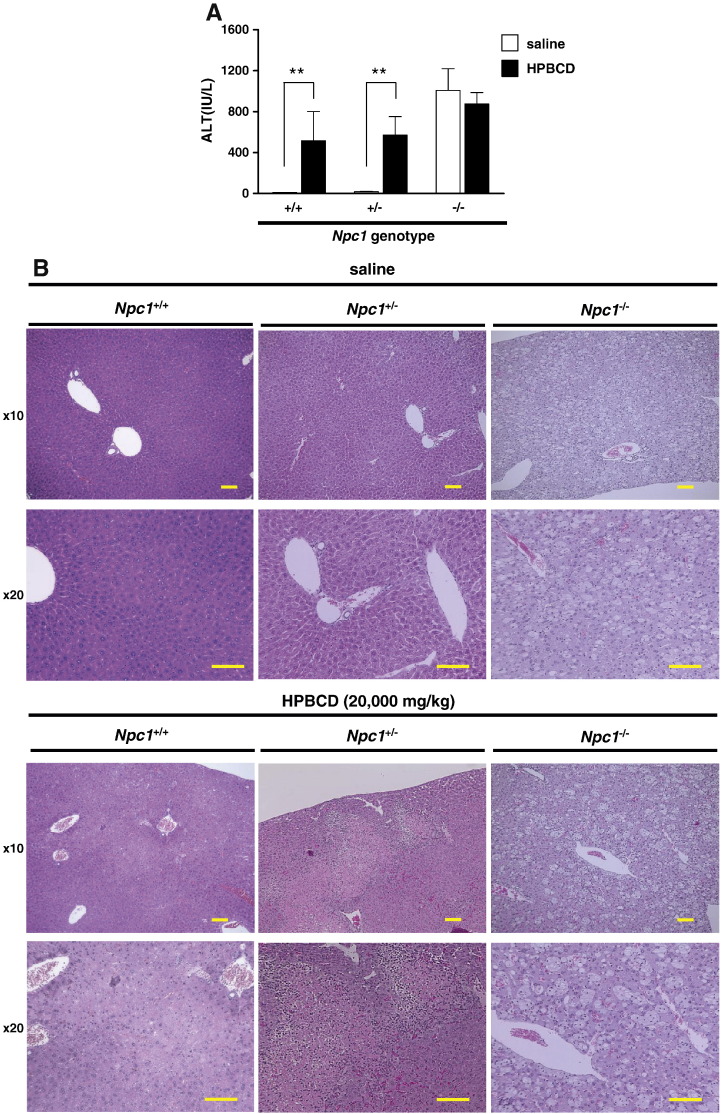
The measurements of serum ALT levels and histological analysis were performed 8 h after subcutaneous administration of HPBCD (20,000 mg/kg) in the Npc1+/+ or Npc1 mutant mice. As shown in Fig. 2A, serum ALT levels were significantly increased in the HPBCD-treated groups compared with the saline-treated groups of both Npc1+/+ and Npc1+/− mice. Although significant differences were not observed between saline- and HPBCD-treated groups in Npc1−/− mice, the Npc1−/− mice showed high serum ALT levels (approximately 1000 IU/L) in both the saline- and HPBCD-treated groups. The histological section of the HPBCD-treated Npc1+/+ and Npc1+/− mice showed extensive hepatocellular necrosis with congestion and infiltration of inflammatory cells, such as lymphocytes. In contrast, these pathological changes were not observed in the saline-treated Npc1+/+ and Npc1+/− mice (Fig. 2B). Many vacuolated hepatocytes and Kupffer cells in the histological section and hepatomegaly and fatty liver-like morphology were observed in both the saline- and HPBCD-treated groups in Npc1−/− mice. However, hepatocellular necrosis as shown in the HPBCD-treated Npc1+/+ and Npc1+/− mice was not observed in Npc1−/− mouse groups.
Fig. 2.
Hepatic biochemical and histological analysis of wild-type and Npc1 mutant mice treated with a toxic dose of HPBCD.
Serum ALT levels (A) and representative hepatic sections (hematoxylin eosin stained) (B) 8 h after saline or HPBCD (20,000 mg/kg) subcutaneous injection. Values are the mean ± S.E.M., (n = 5–7). ** P < 0.01; n.s., not significant. Scale bar = 400 μm.
3.2.2. Kidney
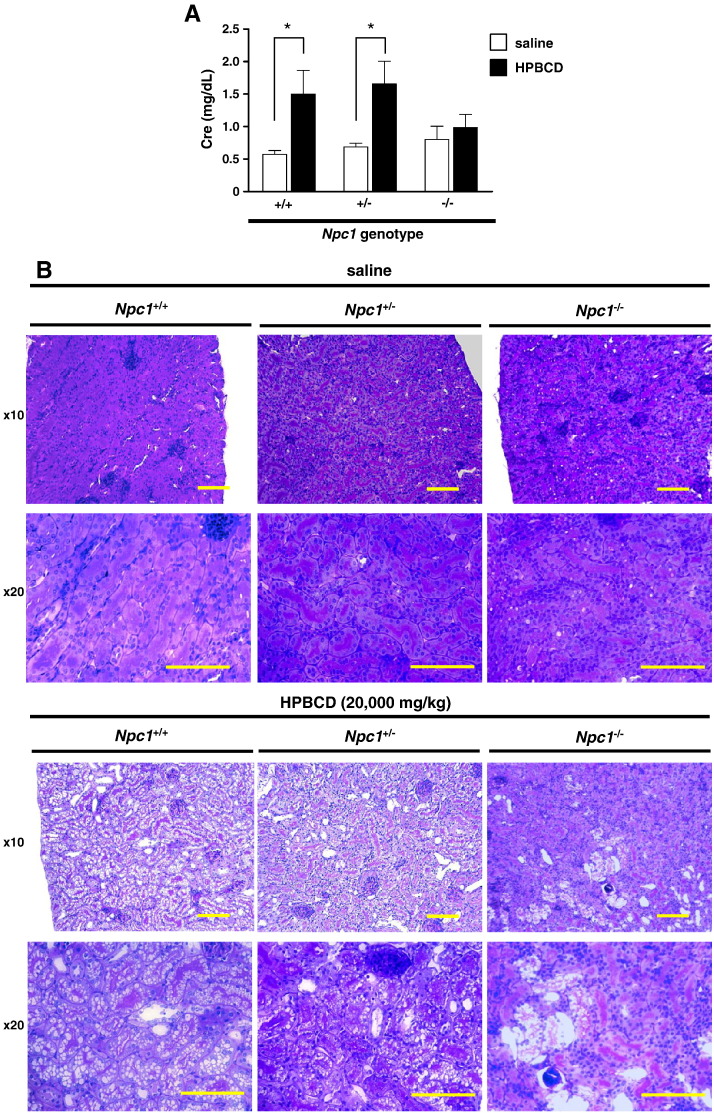
To evaluate renal toxicity, measurements of serum creatinine concentration and histological analysis were performed 8 h after subcutaneous administration of HPBCD (20,000 mg/kg) in Npc1 mutant mice. As shown in Fig. 3A, serum creatinine levels were significantly increased in the HPBCD-treated groups compared with the saline-treated groups of both Npc1+/+ and Npc1+/− mice. In contrast, little difference in the serum creatinine level was observed between the saline- and HPBCD-treated groups in Npc1−/− mice. Although urinary hemorrhage was observed in the HPBCD-treated Npc1+/+ and Npc1+/− mouse groups, no changes were observed in the HPBCD-treated Npc1−/− group. In histological analysis, significant vacuolization of the tubular epithelium and hyperplasia of the Bowman capsule were observed in the HPBCD-treated Npc1+/+ and Npc1+/− mouse groups (Fig. 3B). However, a slight degree of histological change induced by HPBCD was observed in the Npc1−/− mouse groups.
Fig. 3.
Renal biochemical and histological analysis of wild-type and Npc1 mutant mice treated with a toxic dose of HPBCD.
Serum creatinine levels (A) and representative renal sections (periodic acid-Schiff stained) (B) 8 h after saline or HPBCD (20,000 mg/kg) subcutaneous injection. Values are the mean ± S.E.M., (n = 5–7). * P < 0.05; n.s., not significant. Scale bar = 400 μm.
3.2.3. Lung
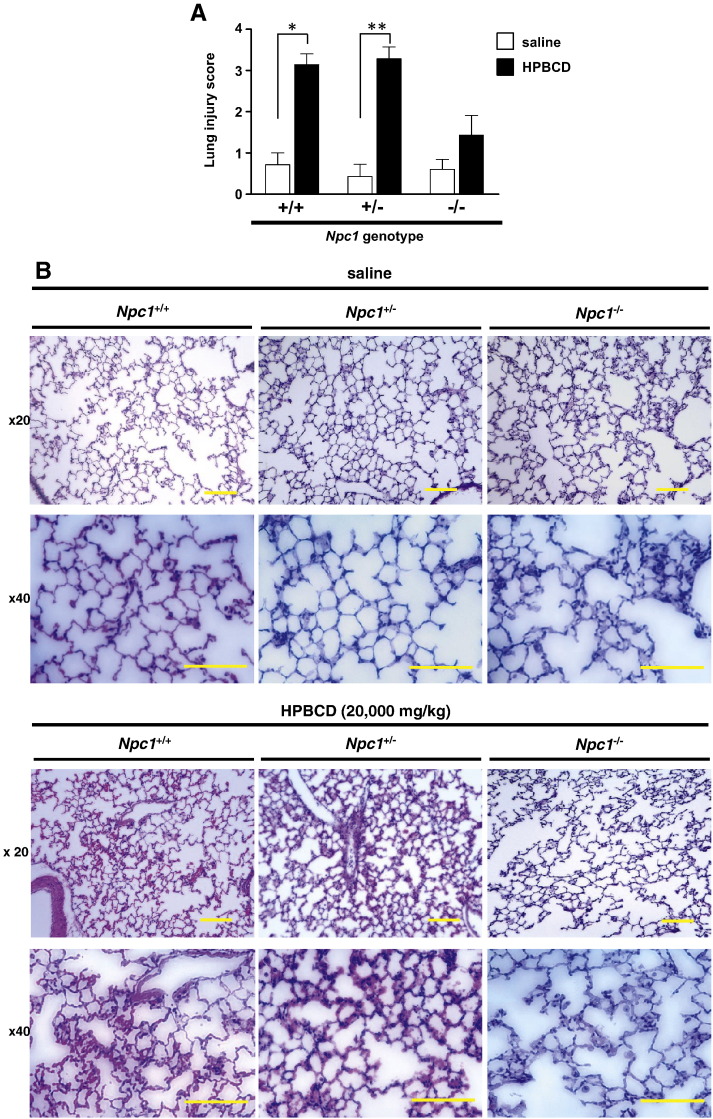
Lung histological sections of the saline-treated Npc1+/+ and Npc1+/− mouse groups exhibited normal morphology. In contrast, severe hemorrhage, infiltration of inflammatory cells, and thickened alveolar septum were observed in the HPBCD-treated Npc1+/+ and Npc1+/− mouse groups (Fig. 4A). Many vacuolated alveolar macrophages were found in both the saline- and HPBCD-treated Npc1−/− mouse groups. Minor lung pathological changes that were exhibited in the HPBCD-treated Npc1+/+ and Npc1+/− mouse groups were also observed in the HPBCD-treated Npc1−/− mouse group. As shown in Fig. 4B, the histopathological scores in the HPBCD-treated groups were significantly higher than in the saline-treated Npc1+/+ and Npc1+/− mouse groups. In contrast, significant differences were not observed between the HPBCD- and saline-treated groups of Npc1−/− mice.
Fig. 4.
Pulmonary histological analysis of wild-type and Npc1 mutant mice treated with a toxic dose of HPBCD.
Representative lung sections (hematoxylin eosin stained) (A) and lung injury scores (B) 8 h after saline or HPBCD (20,000 mg/kg) subcutaneous injection. Lung injury score was measured as described in the Material and Methods. Values are the mean ± S.E.M., (n = 5–7). * P < 0.05, ** P < 0.01; n.s., not significant. Scale bar = 400 μm.
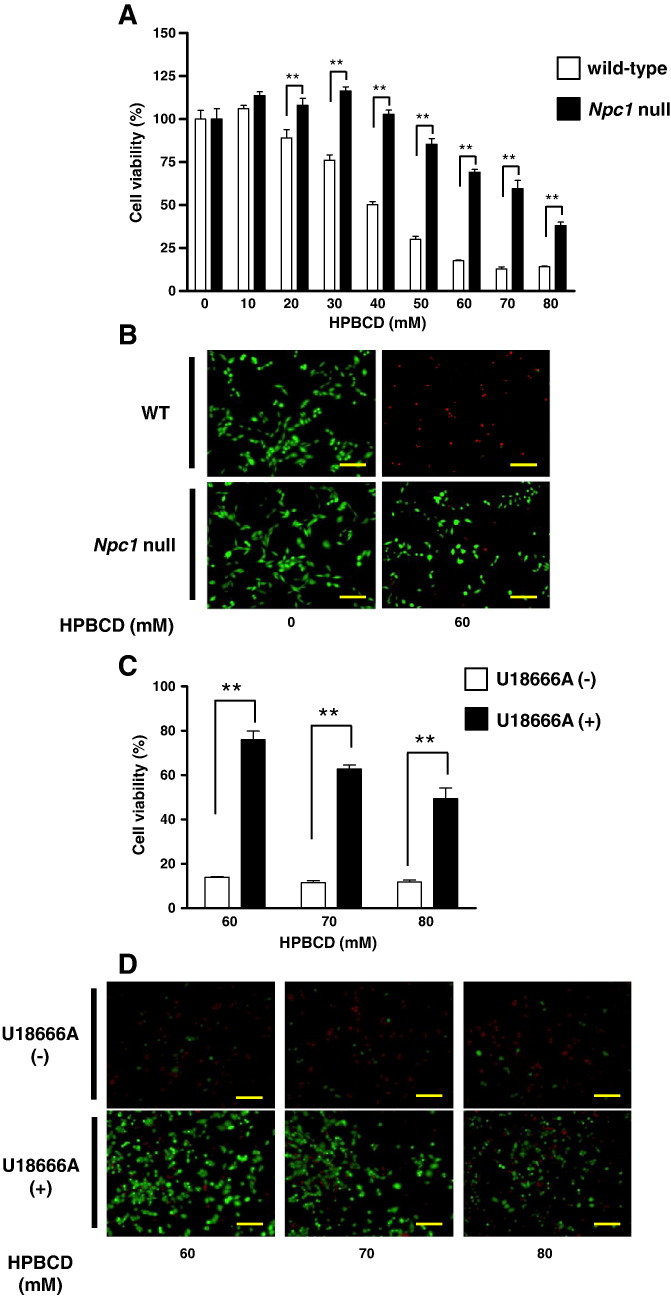
3.3. In vitro analysis of HPBCD-induced cell injury
As shown in Fig. 5A, treatment with HPBCD induced a decrease in cell viability in wild-type CHO cells in a dose-dependent manner. Significant decreases were observed when cells were treated with 30 mM or higher HPBCD. In Npc1 null CHO cells, statistically significant decreases in cell viability were observed when cells were treated with 60 mM or higher HPBCD. There were significant differences in the decrease in cell viability induced by HPBCD between wild-type and Npc1 null cells. Representative fluorescence images of calcein-AM and PI co-stained cells are shown in Fig. 5B. Although a significant number of PI-stained cells (red) were observed when wild-type cells were treated with HPBCD, many cells were stained with calcein-AM (green) in Npc1 null cells after HPBCD treatment. In addition, the decreases in cell viability induced by HPBCD (60–80 mM) exposure were significantly attenuated by pretreatment with U18666A (1 μM), an Npc1 inhibitor, in wild-type CHO cells (Fig. 5C). The increase in PI-stained cells (red) and decrease in calcein-AM-stained cells (green) induced by HPBCD exposure in wild-type cells were reduced by treatment with U18666A (Fig. 5D).
Fig. 5.
Effect of gene deletion or pharmacological inhibition of NPC1 against HPBCD cytotoxicity.
Cell viability was measured 3 h after HPBCD treatment using the WST-8 assay (A and C) and calcein-AM and PI co-staining (B and D). HPBCD-induced cytotoxicity in wild-type or Npc1 null CHO cells (A and B) and U18666A (1 μM) pretreated CHO cells (C and D). Values are the mean ± S.E.M., (n = 3–4). ** P < 0.01; * P < 0.05. Scale bar = 100 μm.
4. Discussion
In this study, we demonstrated that a subcutaneous injection of 20,000 mg/kg HPBCD induced over 50% death within 72 h and severe injury of principal organs, such as the liver, kidneys, and lungs, at 8 h after injection in Npc1+/+ and Npc1+/− mice. In contrast, we demonstrated that the lethality and organ injury attributed to an injection of HPBCD were alleviated in Npc1−/− mice. These results suggest that Npc1−/− mice have substantial resistance to the lethality and organ injury induced by HPBCD injection compared with Npc1+/+ and Npc1+/− mice. In addition, the in vitro data demonstrated that HPBCD-induced cell injury was mild in Npc1 null cells compared with wild-type cells and suggests that the Npc1 genotype influences the cytotoxicity of HPBCD.
The data on survival in Npc1 mutant mice suggest that a subcutaneous injection of 20,000 mg/kg of HPBCD may approximate a sublethal dose (50% to 80% lethal doses) in Npc1+/+ and Npc1+/− mice, respectively. Although the exact cause of the lethality is unknown, acute multiple organ injury seems to be involved. Severe hepatocellular necrosis with accumulation of inflammatory cells, significant vacuolization of the renal tubular epithelium, an increase in serum creatinine level, severe pulmonary hemorrhage, and inflammation were induced by this dose of HPBCD in both Npc1+/+ and Npc1+/− mice at 8 h after injection. In contrast, less lethality and less severe organ injuries by HPBCD in Npc1−/− mice compared with Npc1+/+ and Npc1+/− mice indicate that the Npc1 genotype affects cell death sensitivity of HPBCD in mice. This suggestion is also supported by our in vitro data on the cytotoxicity induced by HPBCD in wild-type and Npc1 null CHO cells. In addition, Appelqvist et al. [13], [14] demonstrated that the NPC1 mutant and U18666A-treated cells show a higher resistance to cellular injury induced by hydrogen peroxide and O-methyl-serine dodecylamide hydrochloride, a lysosomotropic apoptosis inducer. Their reports seem to be concordant with our in vivo and in vitro results of HPBCD toxicity. Based on these facts, we suggest that the Npc1 mutation plays an important role in cellular/organ injury induced by high doses of HPBCD.
The results of our present study may be valuable to evaluate the safety of HPBCD therapy for patients with NPC disease. Thus far, HPBCD is the only attractive drug candidate to treat NPC disease and offers a small ray of hope for an effective cure for these patients. However, some reports strike a note of warning for the use of HPBCD in NPC patients [7], [8], [9]. Chien et al. [7] showed the possibility of HPBCD-induced pneumonia in young pigs and advocated that the pulmonary toxicity of HPBCD should not be neglected. However, the Npc1 genotype (or function) was not considered in their study. In the present study, we also observed lung injury induced with an injection of a sublethal dose of HPBCD. Additionally, the susceptibility to HPBCD was different between Npc1+/+ and Npc1+/−, and Npc1−/− mice. Muralidhar et al. [15] and Ramirez et al. [4] reported that treatment of Npc1−/− mice with HPBCD had little or no effect on the development of progressive pulmonary disease. Therefore, when evaluating the safety of HPBCD therapy for NPC disease, the difference of NPC1 genotype should be considered.
Significantly elevated ALT, hepatomegaly, and fatty liver-like morphology are a recognized hepatic Npc1−/− phenotype [16]. This phenotype seems to obfuscate the hepatotoxicity of HPBCD treatment. In the present study, both saline- and HPBCD-treated Npc1−/− mouse groups as well as the HPBCD-treated Npc1+/− mouse group exhibited high ALT levels. Significant hepatocellular necrosis was not observed in the HPBCD-treated Npc1−/− mouse group compared with the HPBCD-treated Npc1+/− mouse group. We considered that the HPBCD-induced hepatotoxicity was not exerted in Npc1−/− mice compared with Npc1+/− mice.
Although we demonstrated the high tolerability of Npc1 null CHO cells against HPBCD toxicity compared with wild-type CHO cells, the precise mechanisms of the tolerability are unclear. In our previous study, we demonstrated that there was a positive correlation between in vitro hemolytic activity and cholesterol-solubilizing activity of cyclodextrin derivatives and therefore suggested that the cellular injury induced by cyclodextrins was involved in cholesterol extraction from the cell surface membrane [17], [18]. Some reports have indicated that Npc1 null cells or U18666A-treated cells showed a lower cholesterol content in the plasma membrane and a higher content in the lysosome compared with the plasma membrane of non-treated wild-type cells [19], [20], [21]. Therefore, we considered that the lower cholesterol portion on the plasma membrane in Npc1 null cells or U18666A-treated cells may be related to lower toxicity by HPBCD. In addition, Appelqvist et al. demonstrated that Npc1 null cells or U18666A-treated cells significantly attenuated cell injury induced by O-methyl-serine dodecylamide hydrochloride, an apoptosis inducer, and hydrogen peroxide, an oxidative stress inducer, and suggested that the Npc1 mutation and inhibition can modulate lysosomal function through an increase in cholesterol content in the lysosome and thereby influence cell death sensitivity [14]. Therefore, complicated mechanisms of tolerability of the Npc1-deficient cells against cellular injury seem to be involved. To clarify the precise molecular mechanisms of tolerability to HPBCD toxicity, further study will be necessary.
Although we provided evidence of low susceptibility to acute HPBCD toxicity in Npc1−/− mice and CHO cells, further basic and clinical studies are warranted to establish the safety of HPBCD therapy for NPC patients. We examined the acute toxicity of a single high-dose HPBCD treatment in mice in this study. Some previous reports have demonstrated that the usual dosage of HPBCD, which can attenuate cholesterol sequestration in organs and prolong lifespan in Npc1−/− mice, was 4000 mg/kg, and it was subcutaneously injected once a week for life [2], [3], [4]. Therefore, data of “sub-acute or chronic” safety of “multiple administration” of HPBCD in Npc1−/− mice are needed. In addition, information regarding appropriate dosage regimen and blood concentrations of HPBCD, which ensure both efficacy and safety in Npc1−/− mice and NPC patients, is insufficient. Therefore, both pharmacokinetic and pharmacodynamic studies of HPBCD in Npc1−/− mice and NPC patients are warranted.
In our previous study, NPC patients were administered HPBCD (2000–2500 mg/kg) infusions twice or more per week [5]. Despite administration of high- and multiple-doses of HPBCD, severe adverse reactions were not observed during the therapy periods. In contrast, fever and transient diffuse pulmonary cloudiness on chest X-ray following HPBCD infusion were observed in an NPC patient at 23 months after the start of the HPBCD therapy [5]. The patient also suffered from aspiration pneumonia when the episode of fever and pulmonary cloudiness was observed. Since then, same symptoms have not been observed; however, the possibility of an adverse reaction of HPBCD cannot be denied. Therefore, we considered that further clinical studies are warranted to establish the long-term safety of HPBCD therapy for NPC patients.
5. Conclusion
We demonstrated that the lethality and organ injury induced by an injection of HPBCD, which was observed in Npc1+/− mice, were attenuated in Npc1−/− mice. In addition, HPBCD-induced cell injury was alleviated in Npc1 null CHO cells, and the cell injury was also attenuated by U18666A, an Npc1 inhibitor, in wild-type CHO cells. These results suggest that the Npc1 genotype affects the cytotoxicity of HPBCD, and therefore, Npc1−/− mice have substantial resistance to the lethality and organ injury induced by HPBCD injection compared with Npc1+/− mice. Up to the present, healthy (wild-type) animals have been used to evaluate the safety of HPBCD as a therapeutic agent for NPC disease. We suggest that the Npc1 genotype should be considered when performing a safety evaluation of HPBCD therapy for NPC disease in animal or cellular experiments.
Competing interests
The authors declare that they have no competing interests. This work was supported by JSPS KAKENHI Grant Number 23590642.
Acknowledgments
We are grateful to Yuka Horikoshi, Shiori Takeuji, Yumiko Hirose, Koki Shiraishi, Makiko Taguchi and all of the staff of division of reproductive engineering, center for animal resources and development (CARD), Kumamoto University for breeding mice. We also gratefully acknowledge the financial support from the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number 23590642).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Vanier M.T. Niemann-Pick disease type C. Orphanet J. Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu B., Turley S.D., Burns D.K., Miller A.M., Repa J.J., Dietschy J.M. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1 −/− mouse. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2377–2382. doi: 10.1073/pnas.0810895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidson C.D., Ali N.F., Micsenyi M.C., Stephney G., Renault S., Dobrenis K., Ory D.S., Vanier M.T., Walkley S.U. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One. 2009;4:e6951. doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramirez C.M., Liu B., Taylor A.M., Repa J.J., Burns D.K., Weinberg A.G., Turley S.D., Dietschy J.M. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr. Res. 2010;68:309–315. doi: 10.1203/PDR.0b013e3181ee4dd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuo M., Togawa M., Hirabaru K., Mochinaga S., Narita A., Adachi M., Egashira M., Irie T., Ohno K. Effects of cyclodextrin in two patients with Niemann-Pick Type C disease. Mol. Genet. Metab. 2013;108:76–81. doi: 10.1016/j.ymgme.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Brewster M.E., Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007;59:645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Chien Y.H., Shieh Y.D., Yang C.Y., Lee N.C., Hwu W.L. Lung toxicity of hydroxypropyl-beta-cyclodextrin infusion. Mol. Genet. Metab. 2013;109:231–232. doi: 10.1016/j.ymgme.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Thackaberry E.A., Kopytek S., Sherratt P., Trouba K., McIntyre B. Comprehensive investigation of hydroxypropyl methylcellulose, propylene glycol, polysorbate 80, and hydroxypropyl-beta-cyclodextrin for use in general toxicology studies. Toxicol. Sci. 2010;117:485–492. doi: 10.1093/toxsci/kfq207. [DOI] [PubMed] [Google Scholar]
- 9.Rosseels M.L., Delaunois A.G., Hanon E., Guillaume P.J., Martin F.D., van den Dobbelsteen D.J. Hydroxypropyl-beta-cyclodextrin impacts renal and systemic hemodynamics in the anesthetized dog. Regul. Toxicol. Pharmacol. 2013;67:351–359. doi: 10.1016/j.yrtph.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Loftus S.K. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science. 1997;277:232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- 11.Furue S., Kuwabara K., Mikawa K., Nishina K., Shiga M., Maekawa N., Ueno M., Chikazawa Y., Ono T., Hori Y., Matsukawa A., Yoshinaga M., Obara H. Crucial role of group IIA phospholipase A(2) in oleic acid-induced acute lung injury in rabbits. Am. J. Respir. Crit. Care Med. 1999;160:1292–1302. doi: 10.1164/ajrccm.160.4.9812042. [DOI] [PubMed] [Google Scholar]
- 12.Higaki K., Ninomiya H., Sugimoto Y., Suzuki T., Taniguchi M., Niwa H., Pentchev P.G., Vanier M.T., Ohno K. Isolation of NPC1-deficient Chinese hamster ovary cell mutants by gene trap mutagenesis. J. Biochem. 2001;129:875–880. doi: 10.1093/oxfordjournals.jbchem.a002932. [DOI] [PubMed] [Google Scholar]
- 13.Appelqvist H., Nilsson C., Garner B., Brown A.J., Kagedal K., Ollinger K. Attenuation of the lysosomal death pathway by lysosomal cholesterol accumulation. Am. J. Pathol. 2011;178:629–639. doi: 10.1016/j.ajpath.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appelqvist H., Sandin L., Bjornstrom K., Saftig P., Garner B., Ollinger K., Kagedal K. Sensitivity to lysosome-dependent cell death is directly regulated by lysosomal cholesterol content. PLoS One. 2012;7:e50262. doi: 10.1371/journal.pone.0050262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muralidhar A., Borbon I.A., Esharif D.M., Ke W., Manacheril R., Daines M., Erickson R.P. Pulmonary function and pathology in hydroxypropyl-beta-cyclodextin-treated and untreated Npc1(−)/(−) mice. Mol. Genet. Metab. 2011;103:142–147. doi: 10.1016/j.ymgme.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltroy E.P., Richardson J.A., Horton J.D., Turley S.D., Dietschy J.M. Cholesterol accumulation and liver cell death in mice with Niemann-Pick type C disease. Hepatology. 2005;42:886–893. doi: 10.1002/hep.20868. [DOI] [PubMed] [Google Scholar]
- 17.Irie T., Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997;86:147–162. doi: 10.1021/js960213f. [DOI] [PubMed] [Google Scholar]
- 18.Wojtanik K.M., Liscum L. The transport of low density lipoprotein-derived cholesterol to the plasma membrane is defective in NPC1 cells. J. Biol. Chem. 2003;278:14850–14856. doi: 10.1074/jbc.M300488200. [DOI] [PubMed] [Google Scholar]
- 19.Kilsdonk E.P., Yancey P.G., Stoudt G.W., Bangerter F.W., Johnson W.J., Phillips M.C., Rothblat G.H. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- 20.Lange Y., Ye J., Rigney M., Steck T. Cholesterol movement in Niemann-Pick type C cells and in cells treated with amphiphiles. J. Biol. Chem. 2000;275:17468–17475. doi: 10.1074/jbc.M000875200. [DOI] [PubMed] [Google Scholar]
- 21.Tashiro Y., Yamazaki T., Shimada Y., Ohno-Iwashita Y., Okamoto K. Axon-dominant localization of cell-surface cholesterol in cultured hippocampal neurons and its disappearance in Niemann-Pick type C model cells. Eur. J. Neurosci. 2004;20:2015–2021. doi: 10.1111/j.1460-9568.2004.03677.x. [DOI] [PubMed] [Google Scholar]