Abstract
A 1-year-old girl born to consanguineous parents presented with unexplained liver failure, leading to transplantation at 19 months. Subsequent partial splenectomy for persistent cytopenia showed the presence of foamy cells, and Gaucher disease was confirmed by homozygosity for the p.Leu483Pro mutation in the GBA gene. She was treated by enzyme replacement therapy (ERT). Clinical follow-up showed mild developmental delay, strabismus, nystagmus and oculomotor apraxia. Biochemical studies revealed multiple respiratory chain deficiencies and a mosaic pattern of deficient complex IV immunostaining in liver and fibroblast. Molecular analysis identified a mtDNA depletion syndrome due to the homozygous p.Pro98Leu mutation in MPV17. A younger sister unaffected by mtDNA depletion, presented with pancytopenia and hepatosplenomegaly. ERT for Gaucher disease resulted in visceral normalization without any neurological symptom. A third sister, affected by both conditions, had marked developmental delay, strabismus and ophthalmoplegia but no liver cirrhosis. In conclusion, intrafamilal variability occurs in MPV17-related disease. The combined pathological effect of Gaucher and mitochondrial diseases can negatively impact neurological and liver functions and influence the outcome in consanguineous families. The immunocytochemical staining of OXPHOS protein in tissues and cultured cells is a powerful tool revealing mosaic pattern of deficiency pointing to mtDNA-related mitochondrial disorders.
Abbreviations: ERT, enzyme replacement therapy; mtDNA, mitochondrial DNA
Keywords: Mitochondrial disease, mtDNA depletion, Gaucher disease, Neurohepatic
1. Introduction
The clinical evaluation of children presenting with a progressive liver disease can be a challenging task. Once common causes have been ruled out, mitochondrial hepatopathy is frequently considered. However, mitochondrial enzyme activities in damaged tissues can be difficult to interpret or can be misleading [1], [2]. In this paper, we report on the complex diagnostic process in a family in which three sisters presented with liver disease and neurological symptoms. Due to consanguinity, mitochondrial DNA depletion and Gaucher disease occurred in the same sibship. Because of the presence of clinical inconsistencies regarding each diagnostic hypothesis, both diagnoses were sequentially considered, before molecular analyses confirmed their co-occurrence.
Liver mitochondrial DNA depletion syndrome (MIM 203700, 251880, 271245, 256810) is a group of autosomal recessive disorders characterized by progressive liver disease and encephalopathy. It is a heterogeneous condition that can be caused by mutations in the POLG, DGUOK, PEO1 and MPV17 genes [3]. In Gaucher disease (MIM 230800), visceral involvement predominates in the spleen, but liver infiltration by Gaucher cells is usually responsible for hepatomegaly and mild liver cytolysis. However, progressive hepatopathy has been described in Gaucher disease, eventually leading to cirrhosis [4], [5]. In addition, type 3 Gaucher disease (MIM231000) is also responsible for neurological involvement including developmental delay and supranuclear gaze palsy [6]. Thus, mitochondrial DNA depletion and Gaucher have both a neuro-hepatic pathologic pattern. In this report, we discuss the contribution of each disease to the differential clinical involvement of three sisters.
2. Methods
2.1. Biochemical analyses
Respiratory chain complex enzyme activities were measured in frozen liver (patients 1, 2 and 3), frozen muscle (patients 1 and 3) and cultured fibroblasts (patient 1) by spectrophotometry, as previously described [7].
2.2. Cytological and histological analyses
The immunocytochemical staining of cultured skin fibroblasts and paraffin-embedded liver was carried out as published [8], the latter adjusted for detection with the Envision G/2 AP permanent red System (Dako, Glostrup, Denmark) according to the manufacturer's specifications. The concentration of primary antibodies against complex I (MTND6) and complex IV (MTCO1) was 2 μg/ml (Life Technologies, Carlsbad, CA, USA). For immunofluorescent staining, cells were incubated with 25 ng/ml Mitotracker Red CMXRos (Life Technologies) after which they were fixed in 3.5% paraformaldehyde and permeabilized in ice-cold acetone. Subsequently, slides were incubated with monoclonal antibodies directed against complex IV (MTCO1) and AlexaFluor488-labeled secondary antibody (Life Technologies). Slides were mounted with vectashield containing DAPI to counterstain cell nuclei (Vector Laboratories, Burlingame, CA, USA).
2.3. Molecular studies
DNA was extracted from muscle and blood according to standard purification protocols (Chemagen, Perkin Elmer, Zaventem, Belgium). All coding exons and the relevant flanking intronic nucleotides of the MPV17 gene were Sanger sequenced using the BigDye Terminator Cycle Sequencing kit (v.3.1) according to the manufacturer's instructions on a ABI3130XL capillary electrophoresis device (Life Technologies, Merelbeke, Belgium). DNA sequences were aligned with the reference sequence NM_002437.4.
The mitochondrial genome content in muscle tissue was determined by real time quantitative polymerase chain reaction using specific primers for the mitochondrial transfer RNA leucine (MTTL1) and the nuclear single copy beta-2-microglobulin gene (TaqMan probe and primers available on request). The PCR reactions were done in triplicate using the TaqMan Universal PCR Master Mix system (Life Technologies) on an Applied Biosystems 7500 real-time Sequence Detection System. The relative quantification of mtDNA/nuclear DNA ratio was determined by the comparative threshold cycle (CT) method. The depletion of mtDNA was determined by comparing the patient's mtDNA amounts with that of tissue and age matched controls [9].
2.4. Case reports
2.4.1. Patient 1
A 10-year-old girl, born from consanguineous Moroccan parents, presented since the age 3 months with progressive hepatosplenomegaly and mild liver cytolysis. The common causes for hepatitis were ruled out and metabolic investigations showed mild but persistent excretion of 3-methylglutaconate, 3-methylglutarate and ethylmalonate, suggesting possible mitochondrial dysfunction. Biopsies revealed liver cirrhosis with cholestasis and microvesicular steatosis. Respiratory chain studies measured in a liver biopsy sample showed a deficiency of complex I, complex III and complex IV (Table 1). Progressive liver failure and cirrhosis led to liver transplantation performed at the age of 19 months.
Table 1.
OXPHOS activities in different tissues from the patients measured by spectrophotometric analysis.
| Tissue | Patient | Complex I/CS | Complex II/CS | Complex II + III/CS | Complex III/CS | Complex IV/CS | Citrate synthase⁎ |
|---|---|---|---|---|---|---|---|
| Skeletal muscle | P1 | 0.53 (− 1,83) | 0.61 (− 1.75) | 0.59 (− 2.25) | 0.78 (− 1.57) | 0.89 (− 1.83) | 270 |
| P3 | 0.63 (− 0.17) | 0.69 (0.25) | 0.61 (− 1.75) | 0.79 (− 1.43) | 0.89 (− 1.83) | 190 | |
| Controls (n = 30) | 0.64 ± 0.06 | 0.68 ± 0.04 | 0.68 ± 0.04 | 0.89 ± 0.07 | 1.00 ± 0.06 | 174 ± 70 | |
| Liver | P1 | 0.26 (− 3.46) | 0.89 (− 1.73) | 0.39 (− 5.25) | 0.46 (− 6.00) | 0.57 (− 3.92) | 219 |
| P2 | 0.78 (0.54) | 1.10 (0.18) | 0.84 (0.37) | 1.00 (0.00) | 1.04 (0.00) | 126 | |
| P3 | 0.53 (− 1.38) | 0.93 (− 1.36) | 0.56 (− 3.13) | 0.72 (− 3.11) | 0.71 (− 2.75) | 165 | |
| Controls (n = 22) | 0.71 ± 0.13 | 1.08 ± 0.11 | 0.81 ± 0.08 | 1.00 ± 0.09 | 1.04 ± 0.12 | 79 ± 32 | |
| Cultured skin fibroblasts | P1 | ND | 0.64 (0.60) | 0.63 (− 0.43) | 0.72 (− 2.14) | 0.83 (− 2.60) | 120 |
| Controls (n = 30) | ND | 0.61 ± 0.05 | 0.66 ± 0.07 | 0.87 ± 0.07 | 0.96 ± 0.05 | 82 ± 15 |
Activities are considered deficient when the Z-score is <− 3.0 and are shown in bold.
Abbreviations: CS: citrate synthase; ND: not done.
Specific activity is expressed as nanomoles of substrate per minute per milligram of protein. All other data are expressed as the logarithm of OXPHOS activity divided by the logarithm of citrate synthase activity. Control sample ratios, shown in italics, are given as mean ± SD. The Z-scores, values inside parentheses, are calculated as the activity ratio for the patient sample minus the mean activity ratio for the control samples divided by the SD for the control samples.
During the year following the liver transplantation, splenomegaly became more obvious and accompanied by severe anemia and thrombocytopenia. Bone marrow examination, performed twice, was normal. At 3 years of age, partial splenectomy was performed and histological analysis revealed the presence of foamy cells considered as consistent with Gaucher's cells (Fig. 1). Gaucher disease was confirmed by low glucocerebrosidase activity and presence of the homozygous c.1448 T > C (p.Leu483Pro) mutation in the GBA gene, usually associated with type 3 Gaucher disease. Enzyme replacement therapy (ERT) was started by imiglucerase at the dose of 60 U/kg, followed by normalization of hematological parameters. Clinical follow-up showed failure to thrive, developmental delay and mild cerebellar ataxia. Unaided walk was achieved at the age of 3 years. Fine motor control was impaired by clumsiness. She also presented with nystagmus, strabismus, paucity of facial movement and progressive external ophthalmoplegia. Nevertheless, she made progress, and at age 10 years, she was able to attend normal school with support.
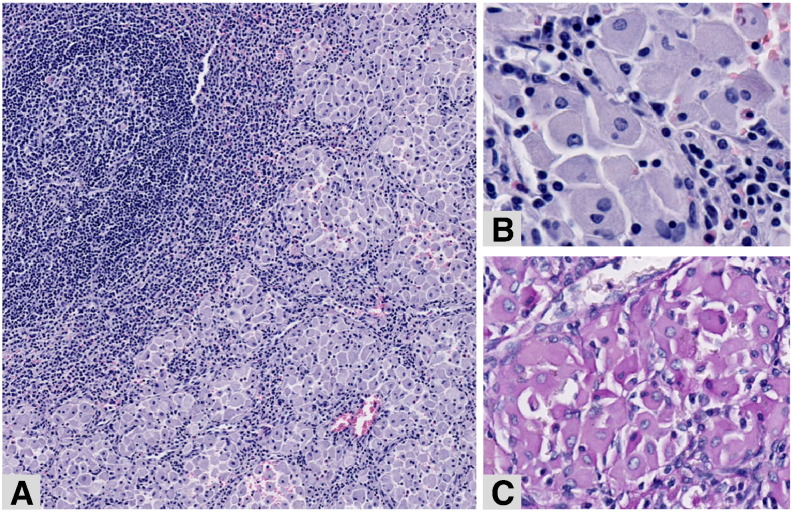
Fig. 1.
Spleen resection—Patient 1.
The spleen's sinusoids are filled with groups of macrophages (A, hematoxylin–eosin, original magnification × 8). At higher magnification (B, hematoxylin-eosin, original magnification × 30 and C, PAS staining, original magnification × 20), the macrophages have a greyish fibrillary cytoplasm, weakly positive for PAS.
2.4.2. Patient 2
The young sister of patient 1 was born at the time of the patient 1 transplantation. At the age of two, she was admitted for an acute episode of abdominal pain. She had major hepatosplenomegaly and pancytopenia. The diagnosis of Gaucher disease was confirmed by enzyme assay and molecular analyses. She was treated by ERT (imiglucerase), allowing rapid and complete normalization of hematological abnormalities and progressive disappearance of visceral storage. Psychomotor development was completely normal. Aged of eight, ataxia, strabismus and ophthalmoplegia were not present.
2.4.3. Patient 3
A third sister was systematically screened at age 3 months. She had mild hepatomegaly without splenomegaly. Gaucher's disease was rapidly confirmed by molecular analysis and ERT started before severe hematological abnormalities occurred. Despite early treatment, she had mild persistent hepatomegaly and mild chronic liver cytolysis. She also was found to be hypotonic with psychomotor delay, strabismus, intermittent nystagmus and ophthalmoplegia. Metabolic screening was normal, including urinary organic acid chromatography and blood lactate in basal condition. An oral glucose tolerance test was performed at age 1 year which showed normal profile, without hyperlactatemia. Liver biopsies showed steatosis, an oncocytic appearance of some hepatocytes in addition to the presence of Gaucher cells (Fig. 2). Currently aged 3.5 years, she has speech delay and severe mental and motor delay (unable to walk unaided). Neurologic examination shows persistent axial hypotonia, moderate lower limb stiffness, paucity of facial movement with trismus and near complete ophthalmoplegia. She has no clinical signs of cirrhosis, and liver ultrasound examination does not reveal liver heterogeneity or portal hypertension.
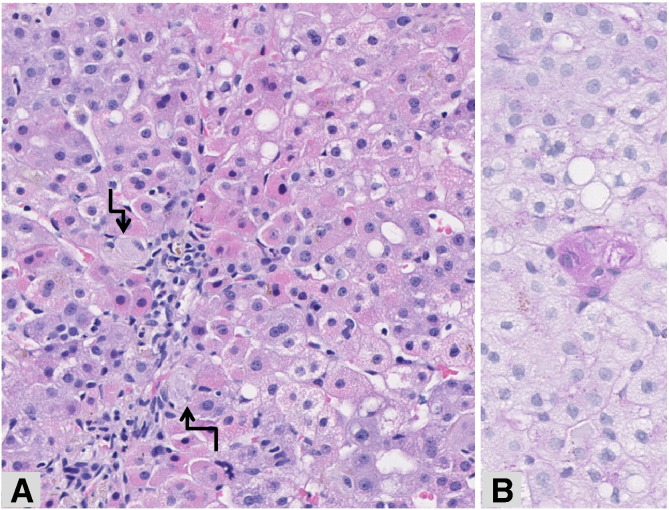
Fig. 2.
Liver biopsy—Patient 3.
The liver parenchyma shows steatosis together with granular red, oncocytic hepatocytes in periportal areas, suggestive of mitochondriopathy. Small aggregates of macrophages characteristic of Gaucher disease (arrows) are also recognizable within the sinusoids (A, hematoxylin–eosin, original magnification × 10), better underlined after PAS staining (B, PAS staining, original magnification × 26).
Tissue biopsies and molecular studies were performed with informed consent of the parents.
3. Results
3.1. Biochemical analyses
Respiratory chain analysis in liver biopsy samples from patients 1 and 3 showed a combined deficiency of the respiratory chain complex I, complex III and complex IV. Respiratory chain analyses in skeletal muscle and fibroblasts were normal (Table 1).
3.2. Cytological and histological analyses
Immunocytological examination in cultured skin fibroblasts from patient 1 revealed a mosaic pattern for complex IV, while staining of the other complexes was normal. This has been described to represent a valuable diagnostic clue for an underlying mtDNA defect [10]. Immunofluorescent double staining showed a reduction of the mitotracker signal (Fig. 3A) accompanied by heterogeneous immunodetection of complex IV, i.e., cells staining negative alongside positive cells with varying staining intensities (Fig. 3B). Also, as clearly seen in the composed image (Fig. 3C), red and green staining is segregated in some cells, pointing to the presence of mitochondria lacking mitochondrial membrane potential build-up.
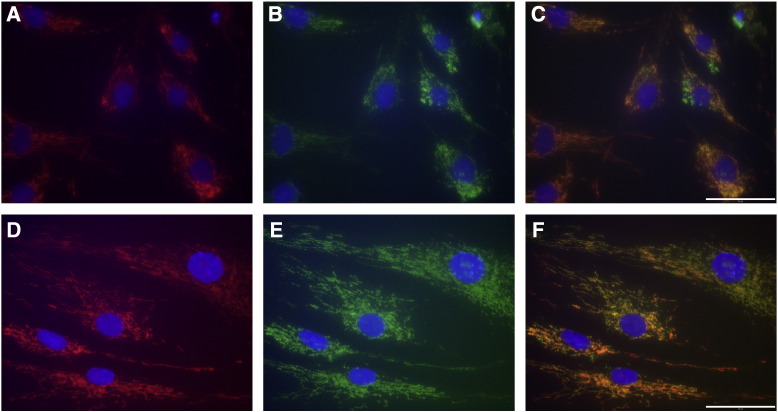
Fig. 3.
Immunofluorescent studies in fibroblasts.
Cultured skin fibroblasts from patient 1 (A–C) and an age-matched healthy control (D–F). Mitotracker in red shows reduced staining in the patient (A). Complex IV immunostaining in green shows reduction and heterogeneity in the patient's cells (B). Panel C shows segregation of staining: the presence of complex IV positive mitochondria (green) that lack mitochondrial membrane potential sensitive Mitotracker staining. Cell nuclei were stained with DAPI (blue in all panels). Panels D to F show normal staining in a control cell line. Scale bars = 50 μm.
Immunostaining on liver biopsies revealed a mosaic pattern of deficient complex IV staining in patient 1 (Figs. 4A, C), consistent with the mtDNA depletion. By contrast, immunostaining in patient 2, unaffected by the mtDNA depletion syndrome, was normal (Figs. 4B, D).
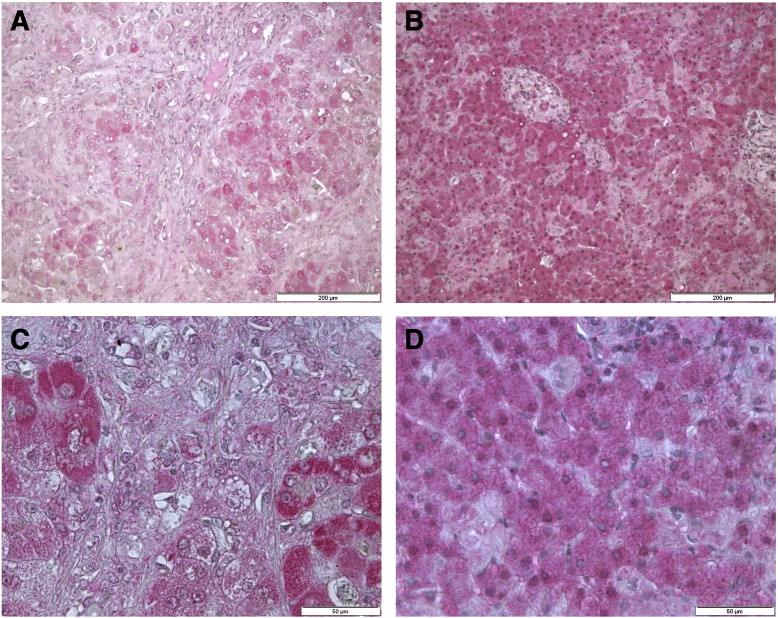
Fig. 4.
Immunohistochemical staining for complex IV MTCO1 in paraffin-embedded liver tissues.
Immunostaining of complex IV in the liver of patient 1 (A, C) and patient 2 (B, D), visualized with permanent red (pink), nuclei were counterstained with hematoxylin (blue). Liver of patient 1 displays a mosaic staining pattern (left panels), while normal staining is observed in patient 2 (right panels). Scale bars = 200 μm (A–B) 50 μm (C–D).
3.3. Molecular studies
A severe mtDNA depletion was identified in liver of patient 1, as the residual mtDNA content in this depleted tissue was only 16%. Muscle and liver tissues of the sisters were not available for molecular assay. Liver mtDNA depletion was a strong indication for an underlying molecular defect in the POLG, DGUOK or MPV17 genes. Analyses of both POLG and DGUOK genes in patient 1 showed no pathogenic mutation. Conversely, sequence of MPV17 gene revealed a homozygous c.293C > T mutation (p.Pro98Leu). Patient 3 was also homozygous for this mutation whereas both parents and patient 2 were heterozygotes.
4. Discussion
The final accurate diagnosis was difficult to establish in this family because of multiple pitfalls. First, although the results of enzymes activity measurement in the liver biopsy pointed to a mitochondrial disorder, the subsequent diagnosis of Gaucher disease raised question about a possible non specific secondary respiratory chain dysfunction in the cirrhotic liver, and molecular investigations for mitochondrial diseases were not performed at this time. Conversely, before the finding of foamy cells, splenomegaly could have been interpreted as a manifestation of portal hypertension, without consideration for a storage disorder. In addition, bone marrow examinations were not informative, and Gaucher cells were eventually identified only in the spleen. Finally, progressive neurological impairment led to reevaluation for a mitochondrial disorder, even though the neurological disease could be explained by type 3 Gaucher disease. Immunocytological staining was performed at this stage. The finding of a mosaic pattern of complex IV deficiency in cultured skin fibroblasts and liver tissue sustained the possibility of a mtDNA-related disease, and the quantification of mtDNA in the explanted liver confirmed depletion, eventually leading to specific molecular analyses and final diagnosis. A mosaic mtDNA depletion in cultured skin fibroblasts has been recently reported in MPV17 disease [11]. For the first time, we show that this mosaic pattern of deficiency can be also observed by immunostaining for respiratory chain complexes in fibroblasts and liver. This case highlights the need for exhaustive investigations in complex multisystemic disorders.
Patient 1 presented with severe liver disease, leading to transplantation. Progressive liver disease and cirrhosis can occur in Gaucher disease [4], [5], although uncommonly. The precise pathogenesis of hepatic dysfunction in Gaucher disease is unknown. Gaucher disease is predominantly a disease of macrophages, and the liver contains the largest organ bulk of tissue macrophages. Disturbances of sphingolipid metabolism in hepatic Gaucher cells may induce dysregulation of cellular inflammatory pathways, aberrant cytokine production and secondary fibrosis and hepatic dysfunction. In this proband, hepatopathy was most likely caused by mtDNA depletion, as MPV17-related disorders are usually characterized by infantile-onset liver dysfunction that typically progresses to liver failure [11], [12], [13]. This hypothesis was even reinforced by the fact that the second sister, presenting Gaucher disease without mtDNA depletion, had no liver disease. However, one can speculate that concomitant Gaucher disease caused a cumulative injury in the liver already affected by mitochondrial hepatopathy, eventually leading to fibrosis and cirrhosis. Indeed, untreated Gaucher disease in patient 1 could have had a negative impact because she presented liver failure at age 18 months, whereas the youngest sister, treated by ERT since the age of 4 months, did not develop progressive liver disease, at least until the current age of 3.5 years. Liver transplantation is the only therapeutic option in case of MPV17-related liver failure. However, it is associated with a significant mortality rate and does not prevent evolution of neurologic symptoms [12], [13]. The current favorable outcome of patient 1 suggests that some patients remain candidate for liver transplantation. Regarding Gaucher disease, liver transplantation has been reported in exceptional cases presenting end-stage liver disease and was associated with an excellent outcome [14]. However, all patients received ERT following transplantation. Nevertheless, it was hypothesized that glucocerebrosidase activity within donor macrophages of the transplanted liver may give a beneficial effect within the transplanted organ itself [14]. Interestingly, the presence of donor cells has been demonstrated in the skin, intestine, bone marrow and lymph node of Gaucher disease patients 26 months post-transplantation, indicating postallograft microchimerism [15].
The p.Pro98Leu mutation in MPV17 identified in our patients has been previously reported in combination with another mutation in a 2.5-year-old girl, presenting with typical neuro-hepatic disease [16]. The same mutation has also been reported in the homozygous state in a 21-year-old man presenting with neuropathy and leukoencephalopathy [17] and in a 25-year-old woman presenting with secondary amenorrhea, megaloblastic anemia, lactic acidosis, leukoencephalopathy, peripheral neuropathy and liver cirrhosis [18]. These late-onset cases suggest that this mutation could be a milder allele responsible for attenuated or, at least, delayed phenotypes. Perhaps this accounts for the absence of early progressive liver disease in the youngest sister, and the currently relatively favorable outcome in the oldest transplanted girl.
The most difficult question is to delineate the respective implications of Gaucher disease and mtDNA depletion syndrome in the neurological involvement of these patients. On one hand, except in the Navajo population of the southwestern United States, MPV17 is usually responsible for marked developmental delay, infantile hypotonia, muscle weakness and peripheral neuropathy, leading to severe encephalomyopathy. However, as discussed previously, the specific homozygote mutation identified in our patients has been reported with later onset cases, albeit in only two patients [17], [18]. On the other hand, the p.Leu483Pro mutation in the homozygote state is the most common genotype associated with the subacute neuropathic form (type 3b) of Gaucher disease [19], [20]. In that case, patients usually also present with motor and mental delay. In addition, saccadic initiation delay, oculomotor apraxia and early onset horizontal gaze palsy typically occur in type 3 Gaucher disease [6], [21]. Pyramidal tract signs, trismus and bulbar signs can be present, including stridor, swallowing difficulties and paucity of facial movement [6]. Rotary nystagmus and opsoclonus are typical manifestations of hepatic mtDNA depletion syndrome caused by DGUOK mutations [22], but oculomotor abnormalities were not reported in MPV17-related disorders.
Overall, the neurological phenotype of patients 1 and 3 is well explained by type 3b Gaucher, which is consistent with their GBA genotype. However, patient 2 who is only affected by Gaucher disease has a completely normal motor and mental development and her neurological examination is strictly normal. Despite her GBA genotype, the phenotype of patient 2 looks like type 1, raising question about genotype–phenotype correlations in Gaucher disease. It is also possible that ERT started early in infancy may have slowed down progression of neurological lesions in this patient. However, the effects of ERT on neurological involvement in Gaucher disease has not been firmly established [23].
In conclusion, a wide clinical variability occurs in the neurohepatic involvement observed in this family. This variability is tentatively explained by the combined effects of two autosomal recessive metabolic disorders. The differential effect of each disease on the patient's phenotypes is difficult to determine and a cumulative negative impact of mitochondrial and lysosomal diseases is hypothesized. However, variability remains incompletely understood. Multiple recessive disorders should be considered in consanguineous families with complex phenotypes.
References
- 1.Chretien D. Pitfalls and tips in measuring and interpreting mitochondrial enzyme activities. J. Inherit. Metab. Dis. 2003;26:189–198. doi: 10.1023/a:1024437201166. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Garcia J. Skeletal muscle mitochondrial defects in nonspecific neurologic disorders. Pediatr. Neurol. 1999;21:538–542. doi: 10.1016/s0887-8994(99)00038-7. [DOI] [PubMed] [Google Scholar]
- 3.El-Hattab A.W., Scaglia F. Mitochondrial DNA Depletion syndromes: review and updates of genetic basis, manifestations, and therapeutic options. Neurotherapeutics. 2013;10:186–198. doi: 10.1007/s13311-013-0177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lachmann R.H. Massive hepatic fibrosis in Gaucher's disease: clinicopathological and radiological features. QJM. 2000;93(4):237–244. doi: 10.1093/qjmed/93.4.237. [DOI] [PubMed] [Google Scholar]
- 5.Perel Y. Gaucher's disease and fatal hepatic fibrosis despite prolonged enzyme replacement therapy. Pediatrics. 2002;109:1170–1173. doi: 10.1542/peds.109.6.1170. [DOI] [PubMed] [Google Scholar]
- 6.Tylki-Szymańska A., Vellodi A., El-Beshlawy A., Cole J.A., Kolodny E. Neuronopathic Gaucher disease: demographic and clinical features of 131 patients enrolled in the International Collaborative Gaucher Group Neurological Outcomes Subregistry. J. Inherit. Metab. Dis. 2010 Aug;33(4):339–346. doi: 10.1007/s10545-009-9009-6. [DOI] [PubMed] [Google Scholar]
- 7.Trounce I.A., Kim Y.L., Jun A.S., Wallace D.C. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts and transmitochondrial cell lines. Methods Enzymol. 1996;264:484–509. doi: 10.1016/s0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- 8.De Paepe B., De Beecker J.L., Van Coster R. Histochemical methods for the diagnosis of mitochondrial diseases. Curr. Protoc. Hum. Genet. 2009;63:19.2.1–19.2.19. doi: 10.1002/0471142905.hg1902s63. (Chapter 19: unit 19:2) [DOI] [PubMed] [Google Scholar]
- 9.Bai R.-K., Wong L.-J. Simultaneous detection and quantification of mitochondrial DNA deletion(s), depletion, and over-replication in patients with mitochondrial disease. J. Mol. Diagn. 2005;7(5):613–622. doi: 10.1016/S1525-1578(10)60595-8. (PubMed: 16258160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Paepe Smet J., Leroy J.G. Diagnostic value of immunostaining in cultured skin fibroblasts from patients with oxidative phosphorylation defects. Pediatr. Res. 2006;59(no.1):2–6. doi: 10.1203/01.pdr.0000191294.34122.ab. [DOI] [PubMed] [Google Scholar]
- 11.Uusimaa J., Evans J., Smith C., Butterworth A., Craig K., Ashley N., Liao C., Carver J., Diot A., Macleod L., Hargreaves I., Al-Hussaini A., Faqeih E., Asery A., Al Balwi M., Eyaid W., Al-Sunaid A., Kelly D., van Mourik I., Ball S., Jarvis J., Mulay A., Hadzic N., Samyn M., Baker A., Rahman S., Stewart H., Morris A.A., Seller A., Fratter C., Taylor R.W., Poulton J. Clinical, biochemical, cellular and molecular characterization of mitochondrial DNA depletion syndrome due to novel mutations in the MPV17 gene. Eur. J. Hum. Genet. 2013 doi: 10.1038/ejhg.2013.112. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong L.J., Brunetti-Pierri N., Zhang Q., Yazigi N., Bove K.E., Dahms B.B., Puchowicz M.A., Gonzalez-Gomez I., Schmitt E.S., Truong C.K., Hoppel C.L., Chou P.C., Wang J., Baldwin E.E., Adams D., Leslie N., Boles R.G., Kerr D.S., Craigen W.J. Mutations in the MPV17 gene are responsible for rapidly progressive liver failure in infancy. Hepatology. 2007;46:1218–1227. doi: 10.1002/hep.21799. [DOI] [PubMed] [Google Scholar]
- 13.El-Hattab A.W., Scaglia F., Craigen W.J., Wong L.J. MPV17-Related Hepatocerebral Mitochondrial DNA Depletion Syndrome. 2012 May 17. In: Pagon R.A., Adam M.P., Bird T.D., editors. GeneReviews™. University of Washington, Seattle; Seattle (WA): 2012. (1993–2013. Available from: http://www.ncbi.nlm.nih.gov/books/NBK92947/) [Google Scholar]
- 14.Ayto R.M., Hughes D.A., Jeevaratnam P., Rolles K., Burroughs A.K., Mistry P.K., Mehta A.B., Pastores G.M. Long-term outcomes of liver transplantation in type 1 Gaucher disease. Am. J. Transplant. 2010;10:1934–1939. doi: 10.1111/j.1600-6143.2010.03168.x. [DOI] [PubMed] [Google Scholar]
- 15.Starzl T.E., Demetris A.J., Trucco M., Ricordi C., Ildstad S., Terasaki P.I., Murase N., Kendall R.S., Kocova M., Rudert W.A. Chimerism after liver transplantation for type IV glycogen storage disease and type 1 Gaucher's disease. N. Engl. J. Med. 1993;328:745–749. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Hattab A.W., Li F.Y., Schmitt E., Zhang S., Craigen W.J., Wong L.J. MPV17-associated hepatocerebral mitochondrial DNA depletion syndrome: new patients and novel mutations. Mol. Genet. Metab. 2010;99:300–308. doi: 10.1016/j.ymgme.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Blakely E.L., Butterworth A., Hadden R.D., Bodi I., He L., McFarland R., Taylor R.W. MPV17 mutation causes neuropathy and leukoencephalopathy with multiple mtDNA deletions in muscle. Neuromuscul. Disord. 2012;22:587–591. doi: 10.1016/j.nmd.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendelsohn B.A., Mehta N., Hameed B., Pekmezci M., Packman S., Ralph J. Adult-Onset Fatal Neurohepatopathy in a Woman Caused by MPV17 Mutation. JIMD Rep. 2013 doi: 10.1007/8904_2013_267. (Epub ahead of print] PMID:24190800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koprivica V., Stone D.L., Park J.K., Callahan M., Frisch A., Cohen I.J., Tayebi N., Sidransky E. Analysis and classification of 304 mutant alleles in patients with Type 1 and Type 3 Gaucher disease. Am. J. Hum. Genet. 2000;66:1777–1786. doi: 10.1086/302925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hrusk K.S., LaMarca M.E., Sidransky E. Gaucher disease: molecular biology and genotype-phenotype correlations. In: Futerman A.H., Zimran A., editors. Gaucher disease. Taylor & Francis; Boca Raton, FL: 2006. pp. 13–48. [Google Scholar]
- 21.Harris C.M., Taylor D.S., Vellodi A. Ocular motor abnormalities in Gaucher disease. Neuropediatrics. 1999;30:289–293. doi: 10.1055/s-2007-973507. [DOI] [PubMed] [Google Scholar]
- 22.Dimmock D.P., Zhang Q., Dionisi-Vici C., Carrozzo R., Shieh J., Tang L.Y., Truong C., Schmitt E., Sifry-Platt M., Lucioli S., Santorelli F.M., Ficicioglu C.H., Rodriguez M., Wierenga K., Enns G.M., Longo N., Lipson M.H., Vallance H., Craigen W.J., Scaglia F., Wong L.J. Clinical and molecular features of mitochondrial DNA depletion due to mutations in deoxyguanosine kinase. Hum. Mutat. 2008;29:330–331. doi: 10.1002/humu.9519. [DOI] [PubMed] [Google Scholar]
- 23.Vellodi A., Tylki-Szymanska A., Davies E.H., Kolodny E., Bembi B., Collin-Histed T., Mengel E., Erikson A., Schiffmann R., European Working Group on Gaucher Disease Management of neuronopathic Gaucher disease: revised recommendations. J. Inherit. Metab. Dis. 2009;32:660–664. doi: 10.1007/s10545-009-1164-2. [DOI] [PubMed] [Google Scholar]