Abstract
Adipose triglyceride lipase (ATGL) deficiency manifesting neutral lipid storage disease with myopathy/triglyceride deposit cardiomyovasculopathy presents distinct fat-containing vacuoles known as Jordans' anomaly in peripheral leucocytes. To develop an automatic notification system for Jordans' anomaly in ATGL-deficient patients, we analyzed circulatory leukocyte scattergrams on automated hematology analyzer XE-5000. The BASO-WX and BASO-WY values were found to be significantly higher in patients than those in non-affected subjects. The two parameters measured by automated hematology analyzer may be expected to provide an important diagnostic clue for homozygous ATGL deficiency.
Abbreviations: ATGL, adipose triglyceride lipase; NLSD-M, Neutral lipid storage disease with myopathy (NLSD-M); TGCV, triglyceride deposit cardiomyovasculopathy
Keywords: Adipose triglyceride lipase deficiency, Automated hematology analyzer, BASO-WX and BASO-WY, Circulatory neutrophils, Jordans' anomaly, Triglyceride deposit cardiomyovasculopathy
1. Introduction
Adipose triglyceride lipase (ATGL, EC 3.1.1.3) deficiency is caused by mutations in ATGL gene, also called PNPLA2 [1], [2], [3]. It presents profound lipid accumulation mainly in skeletal and cardiac muscles, manifesting neutral lipid storage disease with myopathy (NLSD-M)/triglyceride deposit cardiomyovasculopathy (TGCV) [3], [4], [5], [6], [7]. Only up to 40 patients have been reported globally [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]. Most of the reported cases were diagnosed in adulthood except for one case each in childhood and adolescence [12], [13]. In adulthood, the myopathy and cardiomyopathy can be severe and rapidly progressive, and refractory to various therapies. Affected patients with ATGL deficiency exclusively exhibit persistent lipid droplets in the cytoplasm of circulatory neutrophils known as Jordans' anomaly (Fig. 1A) [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]. In earlier life, clinical symptoms seem to be absent or minimal in most cases, however Jordans' anomaly has been documented in subclinical or preclinical adolescents with ATGL deficiency [12], [13]. Blood smear examination with May-Giemsa staining has been used for the detection of vacuoles in leucocytes. This report concerns a simple, easy and feasible laboratory test using a routine automated hematological analyzer that detects leukocyte abnormalities in patients with myopathy or cardiomyovasculopathy and possibly leads to a diagnosis of homozygous ATGL deficiency.
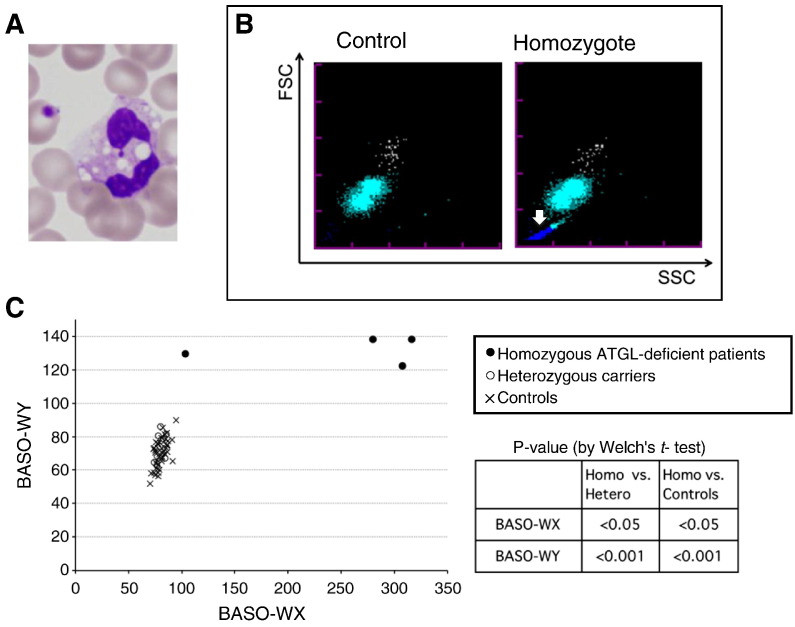
Fig. 1.
(A) A representative image of May-Giemsa staining of blood smears from ATGL-deficient patients. Lipid droplets in peripheral leucocytes known as Jordans' anomaly were found in the neutrophils from all the ATGL-deficient patients. (B) Typical BASO scattergrams of control's blood (left) or homozygote's blood (right). Horizontal (X) and vertical (Y) axes indicate side and forward scattered light intensity, respectively. Colors indicate basophils (white dots), degenerated white blood cells (pale blue dots), and the other small particles (blue dots, white arrow). In these samples, BASO-WX and BASO-WY values are 79.5 and 60.3 (left, healthy), or 362.9 and 139.5 (right, patients), respectively. (C) Scatter plot of BASO-WX and BASO-WY. BASO-WX/BASO-WY values for ATGL-deficient patients (●), heterozygous ATGL carriers (○), controls (X). BASO-WX/BASO-WY values for ATGL-deficient patients were significantly higher than in other groups in Welch's t-test.
2. Methods
2.1. Subjects and specimens
Four homozygous ATGL-deficient patients (3 males and 1 female, 45–60 years of age) (Table 1) and nine heterozygous family members (4 males and 5 females, 17–83 years), lacking ATGL deficiency-associated symptoms, were enrolled. The diagnosis of ATGL deficiency was based on gene analyses together with clinical manifestations of myopathy, including easy fatigability, reduced exercise capability and limb weakness, and cardiomyopathy. Forty-three healthy subjects (14 males and 29 females, 32–84 years) lacking the mutations in ATGL gene and having no abnormality under the physical examination were also enrolled as controls. The peripheral blood specimens were collected with EDTA. Written informed consent was obtained from the enrolled subjects before study initiation.
Table 1.
Backgrounds of four adipose triglyceride lipase-deficient patients.
| Case | Sex | Gene mutations | Reference | Present age | Cardiac function | Skeletal myopathy | Age at diagnosisa (Jordan's anomaly) |
|---|---|---|---|---|---|---|---|
| 1 | M | c.865C>T | 6 | 50 | NYHA4 | Mild | 41 |
| 2 | M | c.696+1G>C | 8 | 47 | NYHA4 | Mild | 33 |
| 3 | F | 477_478dupCTCC | 4 | 45 | NYHA1 | Severe | 31 |
| 4 | M | c.576delC | 11 | 60 | NYHA3 | Mild | 58 |
All patients showed Jordans' anomaly in their blood smears at the diagnoses.
2.2. Sample analysis
Blood specimens were analyzed by the XE-5000 automated hematology analyzer (Sysmex, Kobe, Japan) and investigated all the parameters including WBC/BASO channel of the XE-5000 to screen for Jordans' anomaly. In the WBC/BASO channel, its hemolyzing reagent, Stromatolyzer FB (Sysmex), lyses plasma membranes of cells other than basophils in the specimen and, as a result, the cytosolic components of non-basophils are released from the cells. Lipid droplets released from ATGL-deficient leukocytes which retain their shape in aqueous environment due to their own lipid monolayer membranes can be detected as smaller particles [14]. Thus, in the WBC/BASO scattergram, almost intact basophils, nucleus of non-basophil leukocytes, and relatively large cytosolic components including lipid droplets or debris are detected as particles (Fig. 1B). Parameters named BASO-WX and BASO-WY, which stand for the spread of particle distribution in side and forward scattered light, respectively, are calculated in the WBC/BASO channel.
2.3. Statistical analysis
An analysis of Welch's t-test was performed to compare differences in BASO-WX and BASO-WY values between homozygous and heterozygous or controls. p < 0.05 was set to be statistically significant.
3. Results and discussion
After confirming that all the specimens from the four ATGL-deficient patients had Jordans' anomaly by examining their blood smears stained with May-Giemsa (Fig. 1A), we investigated all the parameters of the XE-5000 automated hematology analyzer to find any change corresponding to Jordans' anomaly. The WBC/BASO scattergram revealed an increased number of small particles in the homozygous patients, typically shown as the blue dots in Fig. 1B (white arrow in the right panel). Such change was not observed in the controls (Fig. 1B left) and in the heterozygote carriers (data not shown). The observed small particles are supposed to be the lipid droplets released from the patients' leukocytes, because lipid droplets can be expected to retain their spherical forms with neutral lipid core and phospholipid monolayer surface in this aqueous environment [14]. The BASO-WX and BASO-WY values obtained from the WBC/BASO channel of XE-5000 were significantly higher in the ATGL-deficient patients than those in the non-affected heterozygotes and the controls (Fig. 1C): 251.8 ± 100/132.0 ± 7.7 (BASO-WX/BASO-WY, mean ± SD) for the ATGL-deficient patients, 80.2 ± 3.8/74.8 ± 8.2 for the heterozygous carriers, and 80.4 ± 5.5/70.3 ± 8.2 for the controls.
We, therefore, anticipate that detection of the leucocyte abnormality in a routine automated hematological analysis may be a first step toward the diagnosis of homozygous ATGL deficiency. We further suggest that detected positive subjects should undergo more detailed analyses, including ATGL gene analyses, to establish the diagnosis in the clinical practice.
In homozygous ATGL deficiency, once the clinical presentations of myopathy and cardiomyopathy occur, it has been difficult so far to regress or resolve symptoms through any conventional treatments [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. We reported two patients with severe cardiomyovasculopathy and heart failure requiring cardiac transplantation [6], [8]. We recently provided data indicating that up-regulation of peroxisome proliferated activated receptor-γ and the related genes may promote triglyceride accumulation in the skeletal and cardiac muscles in ATGL deficiency [8]. We believe that the development of an easy method to detect cellular triglyceride accumulation is desired. It is quite likely that the change in the leukocyte emerges before the development of myopathy or cardiomyovasculopathy, as it does in other congenital lipid storage diseases such as Gaucher's and Niemann–Pick disease, which show distinct lipid storage in circulatory and bone marrow macrophages [15].
In our settings, heterozygous carriers could not be differentiated from control subjects, even though Jordans' anomaly has been reported in some heterozygous ATGL deficiency [16]. We speculate that one of the reasons for this may be that heterozygous leukocytes may have enough ATGL enzymatic activity to reduce the number and/or size of intracellular lipid droplets, so that BASO-WX and BASO-WY parameters were not different between heterozygous and control subjects.
It has been known that Jordans' anomaly in leukocytes is present not only in ATGL deficiency but also in Chanarin–Dorfman syndrome also called NLSD with ichthyosis, which is caused by deficiency of the protein CGI-58, an activator of the ATGL enzyme [17], [18]. Further, in carnitine palmitoyltransferase deficiency type 1, a fatty acid beta-oxidation disorder engendering hypoglycemia and acidosis, this anomaly can sometimes be found in blood smears [19]. It would be of interest to know whether the present system can detect leucocyte abnormalities in these disorders, even we did not have the chance to test the possibility because of the disease rarity.
The sensitivity and specificity of BASO-WX and BASO-WY for ATGL deficiency remains to be investigated, however we believe that this automatic detection of changes in leukocytes with an automated hematology analyzer may provide an earlier diagnostic clue for ATGL deficiency to clinicians, who encounter patients with neuromuscular and cardiovascular disorders, whose causes are unknown.
In order to increase information on the natural history and pathophysiology in NLSD/TGCV patients, we have started an international registry system on the web (http://www.tgcv.org/r/home.html).
4. Conclusions
The BASO-WX and BASO-WY values obtained from automated hematology analyzer XE-5000 could help to detect Jordans' anomaly. A notification system using an automated hematology analyzer may prompt the earlier and easier diagnosis of homozygous ATGL deficiency.
Acknowledgments
The authors would like to thank Dr. Toshimitsu Hamasaki for giving us valuable advice on statistical analysis. This work is supported by a research grant for rare and intractable diseases from the Ministry of Health, Labour, and Welfare of Japan. Y.O., A.W. and Y.S. are employees of Sysmex Corporation.
Contributor Information
Hitoshi Chiba, Email: chibahit@med.hokudai.ac.jp.
Ken-ichi Hirano, Email: khirano@cnt-osaka.com.
References
- 1.Bruno C., Dimauro S. Lipid storage myopathies. Curr. Opin. Neurol. 2008;21:601–606. doi: 10.1097/WCO.0b013e32830dd5a6. [DOI] [PubMed] [Google Scholar]
- 2.Laforêt P., Vianey-Saban C. Disorders of muscle lipid metabolism: diagnostic and therapeutic challenges. Neuromuscul. Disord. 2010;20:693–700. doi: 10.1016/j.nmd.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Fischer J., Lefèvre C., Morava E., Mussini J.M., Laforêt P., Negre-Salvayre A., Lathrop M., Salvayre R. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat. Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama M., Sakai K., Ogawa M., McMillan J.R., Sawamura D., Shimizu H. Novel duplication mutation in the patatin domain of adipose triglyceride lipase (PNPLA2) in neutral lipid storage disease with severe myopathy. Muscle Nerve. 2007;36:856–859. doi: 10.1002/mus.20869. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi K., Inoguchi T., Maeda Y., Nakashima N., Kuwano A., Eto E., Ueno N., Sasaki S., Sawada F., Fujii M., Matoba Y., Sumiyoshi S., Kawate H., Takayanagi R. The lack of the C-terminal domain of adipose triglyceride lipase causes neutral lipid storage disease through impaired interactions with lipid droplets. J. Clin. Endocrinol. Metab. 2008;93:2877–2884. doi: 10.1210/jc.2007-2247. [DOI] [PubMed] [Google Scholar]
- 6.Hirano K., Ikeda Y., Zaima N., Sakata Y., Matsumiya G. Triglyceride deposit cardiomyovasculopathy. N. Engl. J. Med. 2008;359:2396–2398. doi: 10.1056/NEJMc0805305. [DOI] [PubMed] [Google Scholar]
- 7.Hirano K. A novel clinical entity: triglyceride deposit cardiomyovasculopathy. J. Atheroscler. Thromb. 2009;16:702–705. doi: 10.5551/jat.1669. [DOI] [PubMed] [Google Scholar]
- 8.Hirano K., Tanaka T., Ikeda Y., Yamaguchi S., Zaima N., Kobayashi K., Suzuki A., Sakata Y., Sakata Y., Kobayashi K., Toda T., Fukushima N., Ishibashi-Ueda H., Tavian D., Nagasaka H., Hui S.P., Chiba H., Sawa Y., Hori M. Genetic mutations in adipose triglyceride lipase and myocardial up-regulation of peroxisome proliferated activated receptor-gamma in patients with triglyceride deposit cardiomyovasculopathy. Biochem. Biophys. Res. Commun. 2014;443:574–579. doi: 10.1016/j.bbrc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Reilich P., Horvath R., Krause S., Schramm N., Turnbull D.M., Trenell M., Hollingsworth K.G., Gorman G.S., Hans V.H., Reimann J., MacMillan A., Turner L., Schollen A., Witte G., Czermin B., Holinski-Feder E., Walter M.C., Schoser B., Lochmüller H. The phenotypic spectrum of neutral lipid storage myopathy due to mutations in the PNPLA2 gene. J. Neurol. 2011;258:1987–1997. doi: 10.1007/s00415-011-6055-4. [DOI] [PubMed] [Google Scholar]
- 10.Tavian D., Missaglia S., Redaelli C., Pennisi E.M., Invernici G., Wessalowski R., Maiwald R., Arca M., Coleman R.A. Contribution of novel ATGL missense mutations to the clinical phenotype of NLSD-M: a strikingly low amount of lipase activity may preserve cardiac function. Hum. Mol. Genet. 2012;21:5318–5328. doi: 10.1093/hmg/dds388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.K. Kaneko, H. Kuroda, R. Izumi, M. Tateyama, M. Kato, K. Sugimura, Y. Sakata, Y. Ikeda, K. Hirano, M. Aoki, A novel mutation in PNPLA2 causes neutral lipid storage disease with myopathy and triglyceride deposit cardiomyovasculopathy: a case report and literature review. Neuromuscul. Disord. (in press). http://dx.doi.org/10.1016/j.nmd.2014.04.001. [DOI] [PubMed]
- 12.Akman H.O., Davidzon G., Tanji K., Macdermott E.J., Larsen L., Davidson M.M., Haller R.G., Szczepaniak L.S., Lehman T.J., Hirano M., DiMauro S. Neutral lipid storage disease with subclinical myopathy due to a retrotransposal insertion in the PNPLA2 gene. Neuromuscul. Disord. 2010;20:397–402. doi: 10.1016/j.nmd.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrin L., Féasson L., Furby A., Laforêt P., Petit F.M., Gautheron V., Chabrier S. PNPLA2 mutation: a paediatric case with early onset but indolent course. Neuromuscul. Disord. 2013;23:986–991. doi: 10.1016/j.nmd.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Tauchi-Sato K., Ozeki S., Houjou T., Taguchi R., Fujimoto T. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J. Biol. Chem. 2002;277:44507–44512. doi: 10.1074/jbc.M207712200. [DOI] [PubMed] [Google Scholar]
- 15.Wang R.Y., Bodamer O.A., Watson M.S., Wilcox W.R. ACMG Work Group on Diagnostic Confirmation of Lysosomal Storage Diseases: diagnostic confirmation and management of presymptomatic individuals. Genet. Med. 2011;13:457–484. doi: 10.1097/GIM.0b013e318211a7e1. [DOI] [PubMed] [Google Scholar]
- 16.Janssen M.C., van Engelen B., Kapusta L., Lammens M., van Dijk M., Fischer J., van der Graaf M., Wevers R.A., Fahrleitner M., Zimmermann R., Morava E. Symptomatic lipid storage in carriers for the PNPLA2 gene. Eur. J. Hum. Genet. 2013;21:807–815. doi: 10.1038/ejhg.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefèvre C., Jobard F., Caux F., Bouadjar B., Karaduman A., Heilig R., Lakhdar H., Wollenberg A., Verret J.L., Weissenbach J., Ozguc M., Lathrop M., Prud'homme J.F., Fischer J. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin–Dorfman syndrome. Am. J. Hum. Genet. 2001;69:1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruno C., Bertini E., Di Rocco M., Cassandrini D., Ruffa G., De Toni T., Seri M., Spada M., Li Volti G., D'Amico A., Trucco F., Arca M., Casali C., Angelini C., Dimauro S., Minetti C. Clinical and genetic characterization of Chanarin–Dorfman syndrome. Biochem. Biophys. Res. Commun. 2008;369:1125–1128. doi: 10.1016/j.bbrc.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Bonnefont J.P., Demaugre F., Prip-Buus C., Saudubray J.M., Brivet M., Abadi N., Thuillier L. Carnitine palmitoyltransferase deficiencies. Mol. Genet. Metab. 1999;68:424–440. doi: 10.1006/mgme.1999.2938. [DOI] [PubMed] [Google Scholar]