Abstract
The metabolic syndrome is a cluster of metabolic disorders characterized by insulin resistance and hyperinsulinaemia, and its presence can increase the risk of cardiovascular disease significantly. The metabolic syndrome is associated with increased circulating androgen levels in women, which may originate from the ovaries and adrenal glands. Adipocytes are also able to synthesise steroid hormones, and this output has been hypothesised to increase with elevated insulin plasma concentrations. However, the contribution of the adipocytes to the circulating androgen levels in women with metabolic syndrome is limited and the effects of insulin are not fully understood. The aim of this study was to investigate the presence of steroid precursors and synthetic enzymes in human adipocyte biopsies as markers of possible adipocyte androgen synthesis. We examined pre and mature adipocytes taken from tissue biopsies of abdominal subcutaneous adipose tissue of participating women from the Department of Obstetrics and Gynaecology, of the Royal Derby Hospital. The results showed the potential for localised adipocyte androgen synthesis through the presence of the androgen precursor progesterone, as well as the steroid-converting enzyme 17α-hydroxylase. Furthermore, we found the controlled secretion of androstenedione in vitro and that insulin treatment caused levels to increase. Continued examination of a localised source of androgen production is therefore of clinical relevance due to its influence on adipocyte metabolism, its negative impact on female steroidogenic homeostasis, and the possible aggravation this may have when associated to obesity and obesity related metabolic abnormalities such as hyperinsulinaemia.
Keywords: Adipocyte, Steroid hormones, Androgen, Insulin, Steroidogenic enzymes, 17α-Hydroxylase
1. Introduction
Adipose tissue plays an important role in the regulation of levels and bioactivity of sex steroids [2], [7], [13]. Examination into the adipocyte steroidogenic pathway involved in oestrogen conversion has shown androstenedione conversion by aromatase to estrone (E1), and that this is cytochrome P450-dependent. Changes in localised levels of these hormones may also be involved in gender-related fat deposition. Studies have detected the presence of enzymes including aromatase, 3-beta-hydroxysteroid dehydrogenase (HSD), 3-alpha-HSD, 1,1-beta-HSD, 17-beta-HSD, 7-alpha-hydroxylase, 5-alpha-reductase and UDP-glucuronosyltransferase 2B15, in adipose cells and that a correlation exists towards obesity, central fat accumulation, and the metabolic syndrome [8], [9]. With the importance of obesity and the association this has with metabolic abnormalities such as insulin resistance [1], hyperinsulinemia and subsequent disorders of the cardiovascular system, examination into adipocyte function and their response to hormonal messengers is of clinical importance. Furthermore associations have been made between obesity and androgen regulation with androgens being shown to decrease plasma adiponectin, which may subsequently decrease in insulin sensitivity [32].
It is well recognised that androgens are produced in sex organs [5] and the adrenal glands [37], [39], [42]. However, de novo synthesis of sex hormones also occurs within sub-cutaneous adipocytes, which have been shown to be more steroidogenically active than visceral adipocytes. However limited studies have examined the capabilities of peripheral tissues such as skin and adipose tissue to synthesise weaker circulatory androgens. Currently 15 steroidogenic enzymes are recognised to exist within adipose tissue including aromatase, 3β-hydroxysteroid dehydrogenase [6] type 1 [8], 11β-hydroxysteroid dehydrogenase types 1 and 2 [9], 5α-reductase [10] and 17β-HSD types 2, 3 and 5. It is also recognised that adipocytes contain components necessary for transport and metabolism of cholesterol which is essential for the initial steps of steroid synthesis. Furthermore CYP11A1 has the capacity to produce pregnenolone, the precursor to progesterone and the foundation to androgen steroidogenesis. This has been demonstrated through the mitochondrial product 27-hydroxycholesterol (27HC) and by the action of CYP27A1 also now known to be present in adipocytes [28]. Based on these findings, the capacity for peripheral tissue to synthesise and inactivate androgens is feasible.
Examination of a localised source of androgen production may therefore be of clinical relevance due to its general influence on adipocyte metabolism, its negative impact in female steroidogenic homeostasis and the possible aggravation this may have when associated to obesity and obesity related metabolic abnormalities [11]. In the present study we hypothesised that the precursors and enzymes involved in the aromatisation and production of steroids within adipose tissue were suggestive of localised androgen synthesis. More specifically we attempted to support the controversial presence of CYP17 as described by Puche et al. [35] and MacKenzie et al. [29] by showing the expression of the steroid-converting enzyme 17α-hydroxylase [6], [29], [35], the progesterone precursor, and the controlled secretion of androstenedione in vitro. Our rationale was based on the androgen synthetic pathway found within ovarian steroidogenesis [17], whereby the presence of known precursors and enzymes necessary for thecal steroid metabolism in adipocytes may indicate the presence of androgen synthesis. Subcutaneous adipocyte samples were chosen based on reports of increased expression of steroidogenic enzymes compared to visceral adipocyte samples, which may suggest higher hormonal output.
2. Materials and methods
2.1. Tissue collection
Adipose tissue biopsies (~ 5 g) were taken from subcutaneous adipose tissue of the abdominal wall of participating women either during a planned surgical procedure or under local anaesthesia in the outpatient clinic [4] in the Department of Obstetrics and Gynaecology, of the Royal Derby Hospital. Inclusion criteria for the study consisted of women with regular menstrual cycles (28 day cycle), normal serum levels of androstenedione (0.2–2.9 nmol/l) and fasting insulin (17.8–173 pmol/l). All women were of childbearing age, ranging from 20 to 45 years with BMI < 35 kg/m2. Exclusion criteria consisted of any metabolic or endocrine disease, such as diabetes mellitus and thyroid disease and concurrent treatment with: hormonal therapy such as hormonal contraception, progestogen therapy, thyroxin hormone or corticosteroids, metformin treatment or cholesterol lowering agents. The study was approved by the Derbyshire Ethics Committee with all patients providing written, informed consent prior to the surgical procedure. Biopsies were collected in Hanks Balanced 1% penicillin (100 U/ml), streptomycin (100 U/ml), HEPES (15 mM) and transported to the lab. Adipose tissue was minced then digested in DMEM containing 1 mg/ml collagenase (Sigma Aldrich, UK) for 1 h at 37 °C. The digested material was filtered through a double-layered cotton mesh and seeded into T25 flasks for culture incubation at 37 °C 5% O2/95% CO2. Tissue not intended for primary culture processing was snap frozen in nitrogen and stored at − 80 °C for later use in Western blotting.
2.2. Primary human subcutaneous adipocyte culture
Isolated cells were centrifuged at 250 ×g for 10 min and the resulting pellet was washed in HBSS prior to re-suspension in high glucose (4500 mg/ml) DMEM (Sigma Aldrich) supplemented with 10% fetal bovine serum.
Briefly, minced adipose tissue was digested in DMEM containing collagenase (1 mg/ml) for 1 h at 37 °C. The digested material was filtered through a double-layered cotton mesh. The isolated cells were then centrifuged at 250 ×g for 10 min and the resulting pellet was washed with HBSS and re suspended in DMEM including 10% FBS and the cells were seeded at a density of 5 × 103 cells/ml in a T25 flask. Cells were maintained in a humidified incubator at 37 °C 5% O2/95% CO2, and grown in DMEM and 10% FBS. Cultures were used at passage 2 when preadipocytes were clearly distinguishable, and cultured until 80% confluent for differentiation.
2.3. Differentiation of pre adipocyte cultures
Preadipocytes were cultured in maintenance medium that included DMEM high glucose + 10% FBS to 80–90% confluence, allowing for cell stability [43]. At day 14, media were removed and exchanged for differentiation medium containing 50 nM insulin, 100 nM dexamethasone, and 0.25 mM 3-isobutyl-1-methylxanthine for culturing cells for the first 3 days. The medium was then replaced with DMEM and 10% fetal bovine serum containing 50 nM insulin and 100 nM dexamethasone and changed every 2 days until accumulation of lipid droplets (d14–21) as described [24] was visible. Oil red O was used to determine droplet formation as stated by Chen et al. [15].
Preadipocytes were examined following differentiation within in vitro conditions and maintained in a high glucose media (Sigma DMEM). Subcutaneous adipose biopsies were used in primary culture as they were previously determined to be more steroidogenically active than visceral samples [25], [36]. Subcutaneous biopsies were also used due to their role in abdominal obesity and their association to metabolic dysfunction [16], [20], [38].
2.4. Assay formation
Following passage of primary cultured cells at passages of 3–5, the viability of the cells was determined using 1:1 v/v trypan blue and cells suspended in media then observed using a haemocytometer. Approximately 5 × 103 cells were plated in 200 μl of adipocyte maintenance media in triplicate 96 well plates and exposed to treatments of either insulin (0, 1, 10, 100 ng.ml), LY292004 (PI3-K inhibitor) + insulin (0, 1, 10, 100 ng.ml), or no-treatment; and incubated for 24, 48 or 96 h. At the relevant time point, cells were harvested, removed from conditioned media, and examined for levels of progesterone and androstenedione.
2.5. Western blot
For Western blotting, cells were carefully washed with HBSS and 100 ml of trypsin/EDTA was added for 2 min before the cell suspension was removed from the well. Cells were counted using a haemocytometer and washed further in HBSS. Cells were then centrifuged at 250 ×g for 10 min and the supernatant was discarded. The cells were then lysed using lysis buffer consisting of Tris base pH 7.5 25 mM, sucrose 300 nM, monothyioglycerol 10 mM, EDTA 1 mM, 1% (v/v) igepal, protease inhibitor cocktail 100 l in 100 ml, phosphatse inhibitor II (100 l in 100 ml). Lysates were stored at − 80 °C for use in Western blotting.
Western blotting: proteins were resolved by 10% PAGE. Following protein quantification using BCA gel electrophoresis was undertaken for examination of CYP17 (Santa Cruz), anti-leptin (antiOB, R&D systems), anti-β-Actin (Sigma). Protein concentration was adjusted to 10 mg/ml using Laemmli buffer (Sigma). The samples of interest were then denatured at 95 °C, before electrophoresis. Each lane was loaded with 100 μg of protein. In addition, the first lane was loaded with 10 μl of Kaleidoscope pre-stained molecular weight markers (Sigma) to facilitate protein size determination. Electrophoresis was run in buffer consisting of Tris (25 mM), Glycine (192 mM) and ddH2O for 1 h at 40 mA [25]. The proteins were then electro-blotted onto nitrocellulose membranes in ice cold transfer buffer (tris base (24 mM), glycine (80 mM), 20% (v/v) methanol) within a BioRad Mini-PROTEAN blotting system consisting of gel transference to nitrocellulose via a charge of 100 V [25] run for ~ 2 h at 4 °C to transfer.
The nitrocellulose membranes were then blocked by immersion into a bath of 5% Marvel milk and TBS (Tris (12.11 g), NaCl (146.1 g), dH2O) for 1 h. The relevant antibody was then added, and incubated overnight at 4 °C then washed with TBST (× 6) for 10 min followed by TBS washes (× 3) for a further 10 min each. The blots were incubated with alkaline phosphatase (AP) conjugated secondary antibodies (BioRad) in 3% marvel for 2 h at room temperature with gentle agitation. The membrane was washed again as described above and incubated for 10 min using enhancer and substrate solution (non-isotopic chemiluminescent detection system Immun-Star-Alkaline phosphotase kit (AP:Biorad)). Visualisation was enabled, by using 50 μl of enhancer added to 2.5 ml substrate evenly coating the nitrocellulose blot. Blots were stripped using 15 g glycine, 1 g SDS and 10 ml Tween 20 pH 2.2. The nitrocellulose was then re-probed using Beta-Actin antibody control.
Results were analysed by densitometry and were normalised against the reference protein (β-Actin control) using the Chemi Doc (vers 4.2.1) imaging system [41]. All Western blotting experiments were repeated at least three times for each patient sample.
2.6. Immunofluorescence
Preadipocyte cells were seeded at 5 × 103 in 24 well plates and grown to required confluence (1–2 days) before careful removal of media and washing in ice cold PBS (× 2). The Chinese hamster ovarian (CHO) cell line was cultured for use as a positive control for CYP17. Leucocyte cultures were purified from blood and used as a negative control against all our primary cells.
Chilled acetone:methanol (1:1) (v/v) was added to each well and left for 10 min before careful removal and further washes in ice cold PBS (× 2). Fixed cells were blocked using 20% goat serum in PBS and left for 30 min at RT. Following incubation, blocking solution was removed and the antibody of interest was diluted in PBS as required and added to the wells. Cells were incubated at 4 °C. All procedures were done alongside negative and positive control cells utilising the same protocol. Further negative controls were prepared using fixed cells omitting the primary antibody and stored in PBS overnight at 4 °C. Primary antibodies CYP17 (Santa Cruz), anti-leptin (antiOB, R&D systems), and anti-β-Actin (Sigma) were carefully removed and all cells washed for 5 min in ice cold PBS (× 4). A single isomer fluorescin isothiocyanate [26] tag was used bound to the chosen secondary antibody. Following PBS washes, secondary antibody with FITC tag (Sigma SAB3700246/3700265) was added to all cells and incubated at room temperature for 1 h, wrapped in foil to prevent direct light bleaching or fluorophore excitation. Cells were then washed in ice cold PBS for 5 min (× 7). Cells in PBS were then viewed in a dark room using Carl Zeiss Axiovert 25 Scope with Cell^F imaging software (Build 1131). Images were viewed and edited in a Ziess LSM image browser V4.2.0.121.
2.7. ELISA
Adipocytes were cultured to maturity (see primary and differentiation) and assayed for 3 days before media harvests were examined for progesterone and androstenedione following manufacturers instructions and against calibration curve with ranges (Ridgeway Science, Genway Science). Theca and osteoclast controls were used throughout to support findings, as was the same ELISA kit. Levels of progesterone and androstenedione were determined by ELISA.
The progesterone ELISA (Ridgeway) allowed for measurement of samples to a sensitivity of 0.025 ng/ml–0.39 pmol/1000 cells using a standard range of known progesterone samples as supplied. The androstenedione ELISA (GenWay Science) allowed for a sensitivity of 0.019 ng/ml–0.03 pmol/1000 cells and absorbance was measured (570 nm) to create a standard curve. All results were obtained following manufacturers instruction.
2.8. Data and statistical analysis
Normality was tested throughout using Shapiro–Wilk/Kruskal–Wallis normality test using the prism software (vers. 5.0b). Multiple means for unpaired samples were compared using a two way analysis of variance (ANOVA) (non-parametric) with Bonferroni post-hoc test while paired observations were analysed using a two tailed Student's t-test (parametric). P-values < 0.05 allowed for rejection of the null hypothesis.
3. Results
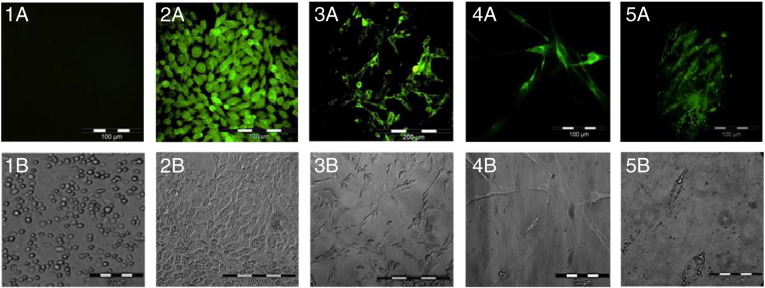
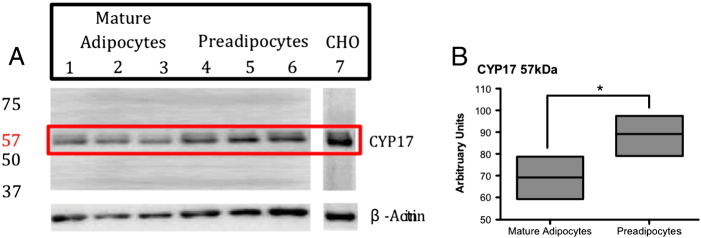
Negative controls (Fig. 1A) showed no fluorescence in contrast to positive immunofluorescence detected in CHO cells for CYP17 (Fig. 1B). β-actin probing of preadipocytes was used to show fluorescence supporting the protocol used (Fig. 1C). Examination of the presence of CYP17 within pre and mature adipocytes of our human cultures showed that CYP17 was predominantly expressed within the cytoplasm with abundant perinuclear localisation (Figs. 1D/E.). Furthermore westernblott analysis of CYP17 in both pre and mature adipocytes showed significant increased expression in preadipocytes samples (see Fig. 2).
Fig. 1.
1A/B–5A/B matched bright fields columns from left to right. 1A/B) Immunofluorescence of leucocytes using CYP17 antibody. Human leucocytes were probed with CYP17 primary antibody acting as a negative control. Results show no CYP17 fluorescence was present. 2A/B) Immunofluorescence of Chinese hamster ovary cells (CHO). CHO cells were used as a positive control allowing the expression of CYP17 to be seen within the cytosol. 3A/B) Fluorescence shows preadipocytes probed with β-actin antibody acted as positive control of IF protocol. 4A/B) Immunofluorescence of human preadipocytes using CYP17 antibody. Fluorescence can be seen within the cytososl. 5A/B) Immunofluorescence of mature human adipocytes using CYP17 antibody. Fluorescence can also be seen within the cytososl.
Fig. 2.
A) Shows Western blot CYP17 expression of human mature adipocyte (lanes 1–3) and preadipocyte (lanes 4–6) lysates. Exposure at 640 s shows bands at 57 kDa, which were more predominant in preadipocyte samples. The blot was then stripped and re-probed using β-actin to allow for loading control. B) Summarizing the densitometry representative of average expression of CYP17 in pre and mature human samples. Using β-actin normalisation to compare levels of band intensity in Western blot CYP17 expression (see panel A) densitometry graphs were created. A significant increase was seen in expression of CYP17 in pre adipocyte lysates (n = 3,*P < 0.05).
Progesterone was measured from mature adipocyte media following 3 days maintenance (mean 5.5 ± 1.29 pmol/1000 cells). A significant increase was seen in adipocyte media against osteoclast samples (P = 0.018). Ovarian theca cell media was also examined as a positive marker of progesterone secretion as well as an intra assay control throughout all the ELISA techniques performed. Again 3 day untreated media was removed from theca cultures and results were normalised to pmol/1000 cells. Results showed progesterone secretion to be ≈ 3 times that found within the adipocyte media (mean 16.37 ± 0.44 pmol/1000).
Fig. 3 shows ELISA measurements of steroid hormone androstenedione. Examination against positive (theca cells) and negative controls (osteoclasts) showed significant levels of secretion against both 0 treatment and negative control (2.2 pmol/1000 cells).
Fig. 3.
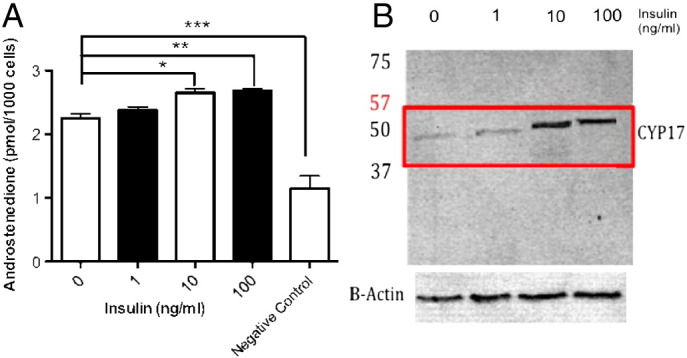
A) Androstenedione secretion (pmol/1000 cells) by mature adipocytes after varied insulin treatment. Mature adipocyte cultures (n = 12) were grown after varied insulin treatments (1, 10, and 100 ng/ml) for 24, 48 and 86 h 3 days. Androstenedione levels were measured in conditioned media using ELISA. All measurements were compared to negative osteoclast controls and androstenedione levels found to be increased significantly (***P < 0.001) in all untreated and treated adipocyte cultures. Significance was also seen against the untreated cultures at 10 ng/ml insulin (*P < 0.05) and 100 ng/ml (**P < 0.01). B) Western blot CYP17 expression in mature adipocyte after varied insulin. Mature adipocytes were treated with varied doses of insulin in-vitro (insulin 0–100 ng/ml). Lanes were probed using CYP17a1 antibody. Exposure at 640 s shows band intensity at 59 kDa with increases in insulin. Increased CYP17 expression can be seen in adipocytes treated with 10–100 ng/ml insulin. The blot was then stripped and re probed using β-actin to control for protein loading. All blots were probed against leucocyte negative controls (not shown).
Androstenedione secretory levels were examined under insulin treatment (0–10 and 10–100 ng/ml) (n = 9) and showed no variation across insulin treatment range (see Fig. 3). CYP17 expression was also examined under the same insulin treatments. CYP17 was seen to increase at around 10 ng/ml treatments with no variation in expression with further increases to 100 ng/ml.
With the possible influence insulin may therefore play in androgen synthesis we examined a possible signalling pathway through inhibition of PI3-K, shown to be involved in ovarian steroidogenesis [31]. Fig. 4. shows that no variation in mature adipocyte androgen secretion was seen when comparing insulin stimulation of androgen secretion and inhibition of PI3-K pathway.
Fig. 4.
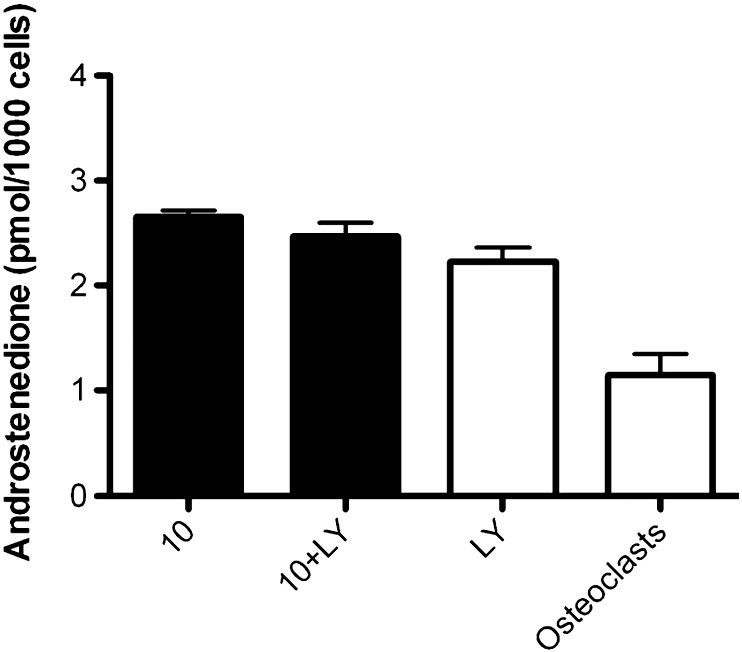
Comparison of mature adipocyte androstenedione secretion with and without inhibition of the insulin-signalling pathway. Mature adipocytes (n = 12) were treated with insulin with and without the PI3-K inhibitor LY292004 and androstenedione secretion measured. No significant variation was found between treated and untreated cultures.
4. Discussion
Synthesis and secretion from adipocytes may contribute to the elevated circulating levels of androgens in women with obesity and metabolic syndrome. Androgen secretion from adipocytes may become elevated through the action of insulin in the hyperinsulinaemic state. We have examined the ability of human primary pre and mature adipocytes to independently produce and secrete androstenedione and the effects insulin has on this synthesis. In doing so we may determine a significant contribution to circulatory androgen levels directly associated to obesity and therefore related to conditions such as insulin resistance, hyperinsulinemia and subsequent disorders of the cardiovascular system, and metabolic syndrome.
Immunofluorescence and comparative phase contrast, showed clear cell morphology and fluorescence representative of CYP17 expression in the cytoplasm of our primary human pre and mature SC adipocyte cultures. Blouin et al. [11] compared human omental and subcutaneous samples for steroidogenic activity at both preadipocyte and mature adipocyte stages [11], [12]. They found increased activity in SC cultures with a greater expression of the steroidogenic enzymes AKR1C3, RDH5, AKR1C2, P-450 aromatase, steroid sulfatase, ERα and 17β-HSD-3, following maturation. We however, found perinuculear staining for CYP17 within our preadipocyte cultures, which pointed to active protein synthesis at this stage of adipocyte development. This coupled with increased expression against our mature adipocyte cultures shown by our Western blot analysis suggests an increase of this particular steroidogenic enzyme during the preadipocyte phase. Interestingly, Blouin also reports CYP19 increases following differentiation contradictory to reports by Mceternan et al., [44] and Diuedonne et al. [45] who both report CYP19 mRNA reduction in mature or differentiated SC cultures [19], [30]. A decrease in CYP19 expression following differentiation may occur in order to reduce oestrogen synthesis. With adipocytes unable to proliferate at this phase of development, a reduction in both androgen and oestrogen syntheses at maturity may therefore be associated and also explain the down regulation of CYP17.
By showing the existence of the steroidogenic enzyme CYP17 and its cellular localisation within both pre and mature adipocytes evidence exists towards localised adipocyte androgen synthesis. Currently adipocytes are known to take part in steroidogenic activity through oestrogen and androgen modification [12], [22], [40]. This requires circulatory precursors and the existence of an androgen synthetic pathway although theorised, has not been reported [12]. The presence of androgen receptors (AR) as determined by Pedersen et al. [33], in both pre and mature adipocytes may also support the presence of an androgen pathway [3], [14], [21], [33]. Furthermore a feedback mechanism may be occurring with reports of decreases in adipocyte proliferation and adipogenesis when exposed to androstenedione [14]. It has also been shown that ARs are more abundant within preadipocytes more so in human and animal model comparison [18], which supports a greater involvement of androgen at the preadipocyte stage of development. This is suggested by our finding of significantly greater levels of CYP17 expression within human preadipocytes. By showing the existence of CYP17 both pre/post differentiation, we support the important regulatory role androgen may have and therefore require localised synthesis.
Progesterone plays a vital role as a precursor within ovarian androstenedione synthesis. By determining its presence in adipocyte secretion we have shown the requisites necessary for this steroidogenic pathway. It is understood that progesterone is synthesised by the ovary, testis, adrenal glands and the placenta during pregnancy. Although it is yet to be established, it is also believed that neurons also have the ability to locally produce progesterone. If this were viable it may occur through P450scc/desmolase action on the cholesterol precursor and further converted to pregnenolone for conversion by dehydrogenase [23], [34]. With adipocytes shown to have the necessary proteins and related precursors necessary to also locally biosynthesise progesterone the possibility remains feasible and supports our findings of progesterone presence in isolated adipocyte cultures. Although, progesterone can also be stored at any stage of preadipocyte development this was accounted for through passage of our cultures.
With the discovery of the progesterone receptor by Sherman et al. [46] more evidence pointed to progesterone's involvement in adipocyte metabolism. Secretion from localised production may allow self-regulation and research has shown that progesterone may be involved in the regulation of lipogenesis through stimulation of transcription factors such as ADD1/SREBP1c [27].
Our continued examination of steroid secretion showed androstenedione present within our media harvest. We ran the same protocol under varied insulin treatments to determine if any influence on levels of secretion existed. In doing so we hoped to examine one of the key influences to stimulating maturation and effects at the more biologically activity stage of development. The addition of our insulin treatment range showed an increase in androstenedione secretion at 10–100 ng/ml significant against the negative osteoclast control and with no variation between these treatments. From this we suggest that insulin influences androstenedione metabolism in mature adipocytes at this treatment level causing an increase in secretion over 3 days. We took these results further and examined a possible pathway in which insulin may affect androstenedione synthesis similar to that seen in ovarian androgen synthesis. The protocol used was similar to that used by Munir et al. [34] and used a PI3-K inhibitor (LY294002) in combination with insulin treatment levels previously shown to increase androstenedione levels [31]. This allowed us to examine the effects insulin had on levels of secretion. The results showed that no variation occurred between inhibited and un-treated samples. This would suggest that if insulin were to be stimulating androstenedione secretion it is not working through the PI3-K pathway. Our further examination into the progesterone precursor required for localised androstenedione synthesis, also showed supportive evidence of a hormonal influence and the pathway involved in androgen synthesis. Although no significant difference in progesterone level was found under hormonal treatments, the results (not shown) showed a possible trend towards decreased progesterone synthesis at 10 ng/ml insulin. This is the same level required to cause androstenedione levels to increase. If a pathway utilising progesterone existed in the conversion to androgen then a possible reduction may be seen at this point due to demand.
If we were to determine that progesterone was active in an adipocyte androgen synthesis pathway and that adipocyte cells are also producing increased levels of androgen at the preadipocyte phase then the increased levels associated to insulin fluctuations required for maturation would support the increase in androgen secretion. From a clinical perspective this may be linked with conditions found in metabolic syndrome and diabetes with symptomatic hyperinsulineamia acting to increase androstenedione, which is required for aromatisation in oestrogen production. With oestrogen shown to stimulate proliferation and adipogenesis these conditions may have strong correlations to obesity.
Acknowledgments
The authors thank Dr. Christopher Towlson for his guidance and support throughout this research and Dr. Robert Barrington for his knowledgeable input in the final paper.
References
- 1.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Ahima S., Flier S. Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 2000;11(8):327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 3.Anderson L.A., McTernan P.G., Barnett A.H., Kumar S. The effects of androgens and estrogens on preadipocyte proliferation in human adipose tissue: influence of gender and site. J. Clin. Endocrinol. Metab. 2001;86(10):5045–5051. doi: 10.1210/jcem.86.10.7955. [DOI] [PubMed] [Google Scholar]
- 4.Aprath-Husmann I.R., Gottschling-Zeller H., Skurk T., Scriba D., Birgel M., Hauner H. Effects of leptin on the differentiation and metabolism of human adipocytes. Int. J. Obes. Relat. Metab. Disord. 2001;25(10):1465–1470. doi: 10.1038/sj.ijo.0801737. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong D.T., Dorrington J.H. Estrogen biosynthesis in the ovaries and testes. Adv. Sex Horm. Res. 1977;3:217–258. [PubMed] [Google Scholar]
- 6.Attia G.R., Rainey W.E., Carr B.R. Metformin directly inhibits androgen production in human thecal cells. Fertil. Steril. 2001;76(3):517–524. doi: 10.1016/s0015-0282(01)01975-6. [DOI] [PubMed] [Google Scholar]
- 7.Basdevant A., Raison J., De Lignieres B., Guy-Grand B. Metabolism of sex hormones and adipose tissue. J. Gynecol. Obstet. Biol.Reprod. (Paris) 1986;15(2):147–152. [PubMed] [Google Scholar]
- 8.Belanger C., Hould F.S., Lebel S., Biron S., Brochu G., Tchernof A. Omental and subcutaneous adipose tissue steroid levels in obese men. Steroids. 2006;71(8):674–682. doi: 10.1016/j.steroids.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Belanger C., Luu V., Dupont P., Tchernof A. Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm. Metab. Res. 2002;34(11–12):737–745. doi: 10.1055/s-2002-38265. [DOI] [PubMed] [Google Scholar]
- 10.Blanchette S., Blouin K., Richard C., Dupont P., Luu-The V., Tchernof A. Expression and activity of 20alpha-hydroxysteroid dehydrogenase (AKR1C1) in abdominal subcutaneous and omental adipose tissue in women. J. Clin. Endocrinol. Metab. 2005;90(1):264–270. doi: 10.1210/jc.2004-0583. [DOI] [PubMed] [Google Scholar]
- 11.Blouin K., Nadeau M., Mailloux J., Daris M., Lebel S., Luu-The V., Tchernof A. Pathways of adipose tissue androgen metabolism in women: depot differences and modulation by adipogenesis. Am. J. Physiol. Endocrinol. Metab. 2009;296(2):E244–E255. doi: 10.1152/ajpendo.00039.2008. [DOI] [PubMed] [Google Scholar]
- 12.Blouin K., Veilleux A., Luu-The V., Tchernof A. Androgen metabolism in adipose tissue: recent advances. Mol. Cell. Endocrinol. 2009;301(1–2):97–103. doi: 10.1016/j.mce.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Boulton K.L., Hudson D.U., Coppack S.W., Frayn K.N. Steroid hormone interconversions in human adipose tissue in vivo. Metabolism. 1992;41(5):556–559. doi: 10.1016/0026-0495(92)90219-z. [DOI] [PubMed] [Google Scholar]
- 14.Chazenbalk G., Singh P., Irge D., Shah A., Abbott D.H., Dumesic D.A. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids. 2013;78(9):920–926. doi: 10.1016/j.steroids.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X.W., Jiang P., Gao J.H., Liao Y.J., Han Z. Experimental study of human adipocyte dedifferentiation for adipose tissue engineering. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29(4):606–610. [PubMed] [Google Scholar]
- 16.Despres J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 17.Diamanti-Kandarakis E., Argyrakopoulou G., Economou F., Kandaraki E., Koutsilieris M. Defects in insulin signaling pathways in ovarian steroidogenesis and other tissues in polycystic ovary syndrome (PCOS) J. Steroid. Biochemi. Mol. Biol. 2008;109(3–5):242–246. doi: 10.1016/j.jsbmb.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Dieudonne M.N., Pecquery R., Boumediene A., Leneveu M.C., Giudicelli Y. Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am. J. Physiol. 1998;274(6 Pt 1):C1645–C1652. doi: 10.1152/ajpcell.1998.274.6.C1645. [DOI] [PubMed] [Google Scholar]
- 19.Dieudonne M.N., Pecquery R., Leneveu M.C., Giudicelli Y. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma2. Endocrinology. 2000;141(2):649–656. doi: 10.1210/endo.141.2.7293. [DOI] [PubMed] [Google Scholar]
- 20.Fujioka S., Matsuzawa Y., Tokunaga K., Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36(1):54–59. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 21.Garcia E., Lacasa M., Agli B., Giudicelli Y., Lacasa D. Modulation of rat preadipocyte adipose conversion by androgenic status: involvement of C/EBPs transcription factors. J. Endocrinol. 1999;161(1):89–97. doi: 10.1677/joe.0.1610089. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh S., Choudary A., Musi N., Hu Y., Li R. IKKbeta mediates cell shape-induced aromatase expression and estrogen biosynthesis in adipose stromal cells. Mol. Endocrinol. 2009;23(5):662–670. doi: 10.1210/me.2008-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanukoglu J. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. Steroid. Biochem. Mol. Biol. 1992;43:779–804. doi: 10.1016/0960-0760(92)90307-5. [DOI] [PubMed] [Google Scholar]
- 24.Hauner H. Secretory factors from human adipose tissue and their functional role. Proc. Nutr. Soc. 2005;64(2):163–169. doi: 10.1079/pns2005428. [DOI] [PubMed] [Google Scholar]
- 25.Ionescu-Tirgoviste C., Valentin Matei Ioan, Gubceac E., Militaru M., Gutu D., Lixandru D. A cytomorphometric analysis of adipocytes from the omental and abdominal subcutaneous adipose tissue. Proc. Rom. Acad., Series B. 2011;3:212–236. [Google Scholar]
- 26.King J.D., Jr., Fitch A.C., Lee J.K., McCane J.E., Mak D.O.n, Foskett J.K., Hallows K.R. AMP-activated protein kinase phosphorylation of the R domain inhibits PKA stimulation of CFTR. Am. J. Physiol. Cell Physiol. 2009;297(1):C94–C101. doi: 10.1152/ajpcell.00677.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacasa D. Le, Liepvre X., Ferre P., Dugail I. Progesterone stimulates adipocyte determination and differentiation 1/sterol regulatory element-binding protein 1c gene expression. potential mechanism for the lipogenic effect of progesterone in adipose tissue. J. Biol. Chem. 2001;276(15):11512–11516. doi: 10.1074/jbc.M008556200. [DOI] [PubMed] [Google Scholar]
- 28.Li J., Daly E., Campioli E., Wabitsch M., Papadopoulos V. De novo synthesis of steroids and oxysterols in adipocytes. J. Biol. Chem. 2013 doi: 10.1074/jbc.M113.534172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacKenzie S.M., Huda S.S., Sattar N., Fraser R., Connell J.M., Davies E. Depot-specific steroidogenic gene transcription in human adipose tissue. Clin. Endocrinol. (Oxf.) 2008;69(6):848–854. doi: 10.1111/j.1365-2265.2008.03262.x. [DOI] [PubMed] [Google Scholar]
- 30.McTernan P.G., Anderson L.A., Anwar A.J., Eggo M.C., Crocker J., Barnett A.H., Stewart P.M., Kumar S. Glucocorticoid regulation of p450 aromatase activity in human adipose tissue: gender and site differences. J. Clin. Endocrinol. Metab. 2002;87(3):1327–1336. doi: 10.1210/jcem.87.3.8288. [DOI] [PubMed] [Google Scholar]
- 31.Munir I., Yen H.W., Geller D.H., Torbati D., Bierden R.M., Weitsman S.R., Agarwal S.K., Magoffin D.A. Insulin augmentation of 17{alpha}-hydroxylase activity is mediated by phosphatidyl inositol 3-kinase but not extracellular signal-regulated kinase-1/2 in human ovarian theca cells. Endocrinology. 2004;145(1):175–183. doi: 10.1210/en.2003-0329. [DOI] [PubMed] [Google Scholar]
- 32.Nishizawa H., Shimomura I., Kishida K., Maeda N., Kuriyama H., Nagaretani H., Matsuda M., Kondo H., Furuyama N., Kihara S., Nakamura T., Tochino Y., Funahashi T., Matsuzawa Y. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51(9):2734–2741. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen S.B., Fuglsig S., Sjogren P., Richelsen B. Identification of steroid receptors in human adipose tissue. Eur. J. Clin. Invest. 1996;26(12):1051–1056. doi: 10.1046/j.1365-2362.1996.380603.x. [DOI] [PubMed] [Google Scholar]
- 34.Pikuleva I. Cytochrome P450s and cholesterol homeostasis. Pharmacol. Ther. 2006;112(3):761–773. doi: 10.1016/j.pharmthera.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Puche C., Jose M., Cabero A., Meseguer A. Expression and enzymatic activity of the P450c17 gene in human adipose tissue. Eur. J. Endocrinol. 2002;146(2):223–229. doi: 10.1530/eje.0.1460223. [DOI] [PubMed] [Google Scholar]
- 36.Quinkler M., Sinha B., Tomlinson J.W., Bujalska I.J., Stewart P.M., Arlt W. Androgen generation in adipose tissue in women with simple obesity — a site-specific role for 17beta-hydroxysteroid dehydrogenase type 5. J. Endocrinol. 2004;183(2):331–342. doi: 10.1677/joe.1.05762. [DOI] [PubMed] [Google Scholar]
- 37.Acien P., Quereda F., Matallin P., Villarroya E., Lopez-Fernandez J.A., Acien M., Mauri M., Alfayate R. Insulin, androgens, and obesity in women with and without polycystic ovary syndrome: a heterogeneous group of disorders. Fertil. Steril. 1999;72(1):32–40. doi: 10.1016/s0015-0282(99)00184-3. [DOI] [PubMed] [Google Scholar]
- 38.Ritchie S.A., Connell J.M. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2007;17(4):319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Rosenfield L. Ovarian and adrenal function in polycystic ovary syndrome. Endocrinol. Metab. Clin. North Am. 1999;28 doi: 10.1016/s0889-8529(05)70070-0. [DOI] [PubMed] [Google Scholar]
- 40.Simpson E., Rubin G., Clyne C., Robertson K., O'Donnell L., Davis S., Jones M. Local estrogen biosynthesis in males and females. Endocr. Relat. Cancer. 1999;6(2):131–137. doi: 10.1677/erc.0.0060131. [DOI] [PubMed] [Google Scholar]
- 41.Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Goeke N.M., Olson B.J., Klenk D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 42.Thompson D.L., Horton N., Rittmaster R.S. Androsterone glucuronide is a marker of adrenal hyperandrogenism in hirsute women. Clin. Endocrinol. (Oxf.) 1990;32(3):283–292. doi: 10.1111/j.1365-2265.1990.tb00868.x. [DOI] [PubMed] [Google Scholar]
- 43.Bujalska I.J., Kumar S., Hewison M., Stewart P.M. Differentiation of adipose stromal cells: the roles of glucocorticoids and 11beta-hydroxysteroid dehydrogenase. Endocrinol. 1999;140(7):3188–3196. doi: 10.1210/endo.140.7.6868. [DOI] [PubMed] [Google Scholar]
- 44.McTernan P.G., Anderson L.A., Anwar A.J., Eggo M.C., Crocker J., Barnett A.H., Stewart P.M., Kumar S. Glucocorticoid regulation of p450 aromatase activity in human adipose tissue: gender and site Differences. J. Clin. Endocrinol. Metab. 2002;87(3):1327–1336. doi: 10.1210/jcem.87.3.8288. [DOI] [PubMed] [Google Scholar]
- 45.Dieudonne M.N., Pecquery R., Leneveu M.C., Giudicelli Y. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma-2. Endocrinology. 2000;141(2):649–656. doi: 10.1210/endo.141.2.7293. [DOI] [PubMed] [Google Scholar]
- 46.Sherman M.R., Corvol P.L., O'Malley B.W. Progesterone-binding components of chick oviduct. I. Preliminary characterization of cytoplasmic components. J. Biol. Chem. 1970;245(22):6085–6096. [PubMed] [Google Scholar]