Abstract
Defects in two subunits of succinate-CoA ligase encoded by the genes SUCLG1 and SUCLA2 have been identified in mitochondrial DNA (mtDNA) depletion syndromes. Patients generally present with encephalomyopathy and mild methylmalonic acidemia (MMA), however mutations in SUCLG1 normally appear to result in a more severe clinical phenotype. In this report, we describe a patient with fatal infantile lactic acidosis and multiple congenital anomalies (MCAs) including renal and cardiac defects. Molecular studies showed a defective electron transport chain (ETC), mtDNA depletion, and a novel homozygous mutation in the SUCLG1 gene. Although our patient's clinical biochemical phenotype is consistent with a SUCLG1 mutation, it is unclear whether the MCAs observed in our patient are a result of the SUCLG1 mutation or alterations in a second gene. An increasing number of reports have described MCAs associated with mitochondrial disorders and SUCLG1 specifically. Additional studies such as whole exome sequencing will further define whether additional genes are responsible for the observed MCAs.
Abbreviations: mtDNA, mitochondrial DNA; SUCL, succinate-CoA ligase; ETC, electron transport chain; MMA, methylmalonic acidemia; MCAs, multiple congenital anomalies; RC, respiratory chain; ERT, enzyme replacement therapy; PEO, progressive external ophthalmoplegia
Keywords: Mitochondria, Succinate-CoA ligase, Methylmalonic acidemia, mtDNA depletion
1. Introduction
Succinate-CoA ligase (SUCL), also known as succinyl-CoA synthase, is a mitochondrial enzyme in the Krebs cycle that converts succinyl-CoA to succinate and free CoA. This enzyme consists of two subunits, the alpha subunit is encoded by the SUCLG1 gene, whereas the beta subunit is encoded by SUCLA2 or SUCLG2 depending on whether the substrate is ADP or GDP respectively. SUCLG1 is ubiquitously expressed, whereas SUCLA2 is primarily present in heart, skeletal muscle and brain and SUCLG2 in liver and kidney [1], [2].
Mutations in the SUCLG1 and SUCLA2 genes have been previously reported [3], [4], [5], [6], [7], [8] whereas bona fide mutations in SUCLG2 have yet to be identified. These mutations are inherited in an autosomal recessive fashion. Defects in SUCLA2 have been shown to result in Leigh disease or a Leigh-like syndrome, with severe muscular atrophy, hypotonia, mild methylmalonic acidemia, and mtDNA depletion. To date, at least a dozen patients have been described with mutations in SUCLG1. The clinical phenotype of these patients ranges from severe lactic acidosis and death during the first days of life [6], [8] to encephalomyopathy with mild methylmalonic acidemia and mtDNA depletion similar to SUCLA2 deficiency [3], [9]. The life expectancy is short, with death generally occurring prior to the age of three years, although one patient was reported to still be alive at the age of 20 [10]. It has been proposed that the severity of the phenotype caused by mutations in SUCLG1 is a result of decreased amounts of SUCLG1 protein [9].
Multiple congenital anomalies (MCAs) have been noted in multiple patients with SUCLG1 deficiency [7], [8]. Rivera et al. [7] reported two patients with MCAs in addition to severe lactic acidosis and elevated methylmalonic acid. One patient was a child with horseshoe kidney, renal fusion abnormalities and hypospadias who died at 3.5 days of life with multiple organ failure. The second child had persistent ductus arteriosus with a permanent left–right shut, right ventricular hypertrophy, and an interrupted aortic arch and died at 72 h of life. Randolph et al. [8] described a patient with asymmetrical intrauterine growth restriction, a right single palmar crease, and craniofacial dysmorphisms as well as high levels of MMA with lactic acidosis, who died at ten months of age. Here we describe the clinical and biochemical characteristics of an additional child presenting with severe lactic acidosis, elevated MMA, mtDNA depletion, and MCAs, found to be homozygous for a SUCLG1 mutation.
2. Case presentation
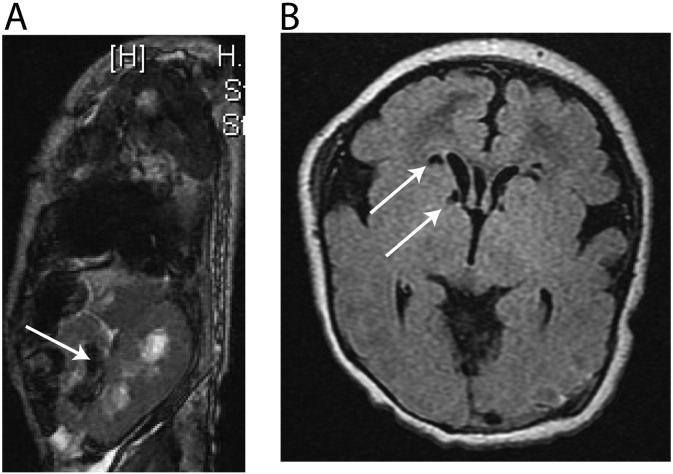
The patient was a four week old male delivered to a 33 year old mother. Family history included one healthy female child, a spontaneous abortion at ten weeks of gestation and death of a male child at one day of life. No consanguinity was reported. Prenatal ultrasound revealed abnormalities including superior vena cava dilation, bilateral short femurs and humeri, as well as left renal pelviectasis. Labor and delivery were uncomplicated. At the time of delivery, the child was noted to have cleft lip with clefting of the anterior hard palate. An echocardiogram performed in the newborn nursery revealed a coarctation of the aorta. At 12 h of life, the patient became mottled and cyanotic, and was severely acidotic (pH 6.883, bicarbonate 5, base excess of − 27, lactate > 20). A full sepsis workup was negative and the child remained acidotic (lactate often > 20) despite numerous fluid boluses and administration of sodium bicarbonate. In addition to the cleft lip and palate, physical exam revealed a dysmorphic right nare. The liver edge was appreciably 1 cm below the right costal margin, the left femur was shorter than the right, and humeri were short bilaterally. A repeat echocardiogram showed good biventricular function, a seven millimeter patent ductus arteriosus (PDA) with bidirectional shunting, aortic arch hypoplasia between the left carotid and left subclavian artery, and a patent foramen ovale (PFO) with left to right shut. An MRI of the abdomen showed dilation of the left collecting system and accessory left kidney with possible cross-fused ectopia (Fig. 1A). Cranial ultrasound revealed cystic changes of the bilateral ventricles and a small cyst at the right caudothalamic groove. A brain MRI showed multifocal areas of acute ischemia, most pronounced in the left occipitoparietal periphery, with a central component of acute hemorrhage (Fig. 1B). Laboratory testing revealed an elevated lactate:pyruvate ratio (> 200), abnormal urinary methylmalonic acid (579 mmol/Mol creatinine), 3-hydroxypropionic acid (99 mmol/Mol creatinine) and 3-hydroxyisovaleric acid (853 mmol/Mol creatinine). A plasma acylcarnitine profile showed elevated C3 (10.4 mcM) and C5 (1.13 mcM), elevated free carnitine (62 mcM) and an elevated C3:C2 ratio (0.20). A muscle biopsy showed increased intra-myofiber lipid content and mitochondrial ultrastructural abnormalities. Collectively, the patient's clinical phenotype, laboratory results, and muscle biopsy suggested generalized mitochondrial dysfunction. The child showed no improvement over four weeks despite intense interventions. Life support was withdrawn, and the child expired shortly thereafter.
Fig. 1.
MRI images of abdomen and brain. A.) MRI of the abdomen showed dilation of the left collecting system and an accessory left kidney with possible cross-fused ectopia. B.) MRI of the brain showed multifocal areas of acute ischemia, most pronounced in the left occipitoparietal periphery, with a central component of acute hemorrhage.
2.1. Biochemical and molecular studies
A karyotype and an oligonucleotide array were reported normal, and no large genomic changes were detected. Electron transport chain (ETC) analysis on the skeletal muscle biopsy revealed severe deficiencies in rotenone sensitive complex I + III and complex IV (Table 1). The activity of complex III was also reduced. However complex II activity was preserved, suggesting a generalized mtDNA depletion. Subsequent quantification of mtDNA in the muscle sample demonstrated an mtDNA content of approximately 27% of the mean value of age and tissue matched controls, confirming a mtDNA depletion in our patient. The mild elevation of MMA and mtDNA depletion suggested the involvement of succinate-CoA ligase. Sequence analysis of the SUCLA2 gene was negative. However, analysis of the SUCLG1 gene identified an apparently homozygous c.749A > G (p.E263G) mutation. Both of the individual's parents were therefore tested and determined to be carriers of the c.749A > G (p.E263G) change, confirming that the proband was truly homozygous. Although no consanguinity was reported in the family, a SNP array (Affymetrix CytoScanHD) was performed on our patient to determine the extent of genomic homozygosity. Blocks of absence of heterozygosity (AOH) greater than 5 Mb suggestive of consanguinity were not observed. Nine blocks of AOH between 2 and 5 Mb were detected, consistent with what is observed in the general population. The c.749A > G (p.E263G) lies within one of these blocks of AOH. Although it is possible that a second homozygous mutation in an alternate autosomal recessive gene is present in one of these blocks of AOH, it is also possible that the MCAs are due to a single mutation in an autosomal dominant disorder with incomplete penetrance or a mutation that arose de novo. While a whole exome analysis may provide additional information, since the patient passed away and a number of molecular studies were performed on the only available sample, there was not sufficient sample left to perform whole exome sequencing.
Table 1.
Electron transport chain enzyme activity in patient's muscle specimen.
| ETC complexes | Activity (μmol/min/g) |
||
|---|---|---|---|
| Patient |
Controls (n = 49) |
%Patient/mean | |
| Mean ± SD | Mean ± SD | ||
| I + III NADH-cyt. c reductase (rotenone sensitive) | 0.04 | 1.2 ± 1.1 | 4% |
| I NADH-FeCN reductase | 35.7 | 29.9 ± 12.9 | 119% |
| II + III Succinate-cyt. c reductase (antimycin sensitive) | 1.2 | 2.1 ± 1.2 | 56% |
| II Succinate dehydrogenase | 1.1 | 0.8 ± 0.4 | 141% |
| III Decylubiquinol-cyt. c reductase | 6.8 | 15.2 ± 6.8 | 44% |
| IV Cytochrome c oxidase | 19.9 | 148.9 ± 67.2 | 13% |
| Citrate synthase | 16.6 | 18.6 ± 4.7 | 89% |
3. Discussion
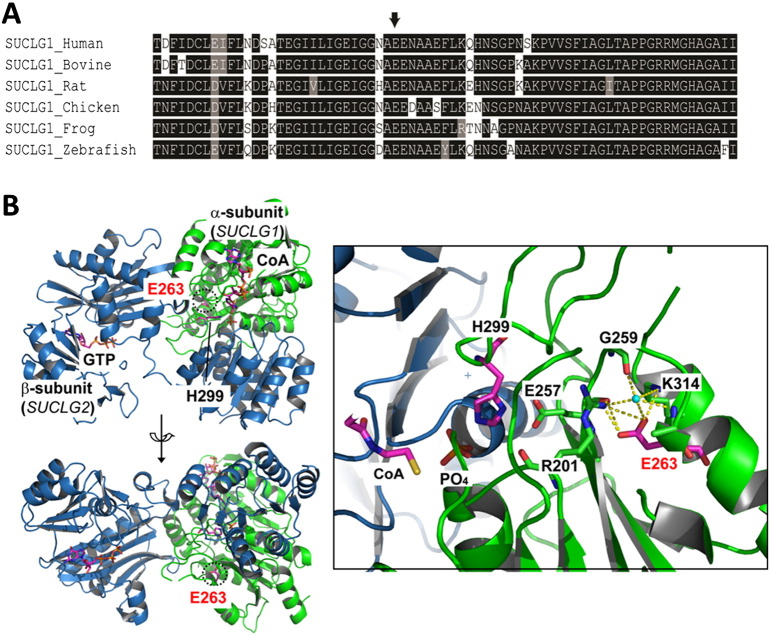
Multiple lines of evidence support c.749A > G (p.E263G) as a pathogenic mutation in the SUCLG1 gene leading to mtDNA depletion. Our patient's clinical and biochemical phenotype is consistent with a loss of SUCLG1 function. The glutamic acid at position 263 of the SUCLG1 protein lies within the CoA ligase domain and is conserved from zebrafish to human (Fig. 2A). Computer-based algorithms SIFT and PolyPhen-2 predict p.E263G to be deleterious, and this change has not been observed in over 1000 chromosomes previously tested in our laboratory. The p.E263G mutation lies within the active site of the SUCLG1 protein (Fig. 2B). Changing of the glutamic acid to a glycine residue abolishes the extensive hydrogen bonding, resulting in the possible inability to bind the water molecule, which is crucial in the ligase reaction. This change also destabilizes the interaction of the p.E257 with the catalytic residue p.H299 and interferes with normal catalysis. In addition, we have detected the c.749A > G (p.E263G) mutation in a compound heterozygous state with a splice site mutation, c.58 + 1delG, in a different patient with infantile mitochondrial DNA depletion syndrome ([14], unpublished data). That patient presented with mtDNA depletion, developmental delay, hypotonia, lactic acidosis, and elevated MMA. At the time of the publication [14], the genetic cause was not determined. However, subsequent sequence analysis in our laboratory identified the c.749A > G (p.E263G) and c.58 + 1delG mutations in SUCLG1 (unpublished data).
Fig. 2.
The c.749A > G (p.E263G) mutation lies within a conserved region of the SUCLG1 protein. A.) Evolutionary conservation of the p.E263 from zebrafish to human. B.) Ribbon representation of human succinyl-CoA synthetase active site modeled with GTP and CoA. The α-subunit encoded by SUCLG1 gene is colored in green and the β-subunit encoded by SUCLG2 gene in light blue. The molecule of GTP, CoA, p.E263 and p.H299 is represented by a ball-stick model. The p.E263 residue is annotated in red font and its position is circled with a dashed line. The hydrogen bonding network is illustrated by the yellow dashed lines surrounding p.E263 and the water molecule colored in cyan. The p.E257 residue interacts directly with the catalytic residue p.H299, and the main chain oxygen atom of p.E257 is hydrogen bonded to the side chain of p.E263 and the water molecule.
The mechanism by which SUCLG1 mutations lead to mitochondria depletion is as yet unclear. Previous studies have suggested that SUCL forms a complex with the mitochondrial nucleoside diphosphate kinase (NDPK-D) [15]. NDPK isoenzymes function to maintain the pool of nucleotides within the cell by catalyzing the reversible transfer of a terminal phosphoryl group between di- and tri-phosphonucleosides [16]. Miller et al. [17] recently showed that fibroblasts derived from patients with SUCLA2 deficiency have normal amounts of mtDNA, normal or only slightly decreased COX activity and normal NDPK activity. However, knockdown of SUCLG2 in both patient and control fibroblasts resulted in a decrease of mtDNA, COX activity, and NDPK activity. These data suggest that SUCLG2 is able to compensate for a loss of SUCLA2 but not the inverse, and that the loss of SUCLG2 correlates with a loss of NDPK activity. As previously noted, to date no disease causing mutations have been identified in SUCLG2 suggesting that loss of SUCLG2 function may not be compatible with life. Since SUCLG1 and SUCLG2 form a complex, mutations in SUCLG1 may also affect the interaction with NDPK leading to the defects observed in patients with SUCLG1 mutations.
The causal relationship of SUCLG1 mutation in our patient and the MCAs is also unclear. Since mitochondria are a major source of energy for a majority of cellular processes, it is not surprising that defects in mitochondrial function may lead to developmental malformations. For example, a retrospective study of patients with proven postnatal RC defects found that intrauterine growth retardation and developmental anomalies in multiple organs were not uncommon [18]. More recently, an infant with MCAs, severe myopathy and prolonged paralysis was reported to have mtDNA depletion syndrome, however no mutations in any of the genes known to cause mtDNA depletion were identified [19]. As previously noted, this is the fourth child to present with SUCLG1 deficiency and MCAs. However, the reported MCAs vary by organ systems.
With whole exome sequencing becoming more common, an increasing number of patients with multiple disease etiologies have been identified. Yang et al. reported that four out of the first 250 clinical exome patients had clinical phenotypes that could only be explained by two different disorders. These patients all had a combination of an autosomal recessive disorder in addition to an autosomal dominant or X-linked disorder [12]. Craigen et al. reported a patient who had been diagnosed with a mitochondrial disease for many years when in fact he appeared to have two different disorders, Spinocerebellar Ataxia, Autosomal Recessive 1 (SCAR1) and Lowe syndrome, caused by mutations in the SEXT and ORCL genes respectively. These mutations, when presenting together, only appeared to be mitochondrial in nature. The patient did not have a mitochondrial disease, but a genocopy caused by mutations in two different gene loci [13].
Although the SNP array analysis did not provide evidence of consanguinity in our case, the odds of a patient having two concurrent autosomal recessive disorders increase with consanguinity. Recently consanguineous siblings with both mtDNA depletion and Gaucher disease were reported [11]. The family had three children diagnosed with Gaucher disease, which was confirmed biochemically as well as by the detection of a homozygous mutation in the GBA gene. However, even with enzyme replacement therapy (ERT) the oldest and youngest siblings had failure to thrive, developmental delay, cerebellar ataxia, nystagmus, strabismus, and progressive external ophthalmoplegia (PEO). Therefore, even though the siblings were given a diagnosis of type 3 Gaucher disease, an additional workup for mitochondrial disease was performed. RC defects were detected in liver biopsy samples from both siblings one and three, and mtDNA depletion was identified in sibling one. An analysis of genes that are mutated in cases of mtDNA depletion detected a homozygous mutation in the MPV17 gene. Both the oldest and youngest siblings were homozygous for the mutation; the middle sibling who did not have the psychomotor issues was heterozygous. These data indicate that the additional clinical presentation not contributed by Gaucher disease was due to mtDNA depletion caused by a mutation in the MPV17 gene, and highlight the need for additional studies when a patient's full clinical phenotype does not fit their genotype. However, to our knowledge, there have been no published reports of a non-consanguineous individual with two separate autosomal recessive diseases caused by mutations in different genes.
In conclusion, we present the fourth SUCLG1 mutation case with MCAs and mtDNA depletion syndrome. It remains unclear whether the MCAs in patients with SUCLG1 mtDNA depletion are due to RC deficiency or other unidentified genes. While we were unable to perform exome sequencing on our patient, this case highlights the importance of considering comprehensive exome analysis in order to identify additional genetic cause of complex clinical presentation. Additional studies on patients with mutations in SUCLG1 with MCAs may shed light on whether the MCAs are caused by defects in the SUCL protein or another disorder entirely.
Disclosure
MLL, VWZ, and LJCW are employed by the Medical Genetics Laboratory at Baylor College of Medicine where the molecular testing was performed. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from the molecular diagnostic tests offered by the Medical Genetics Laboratory.
Acknowledgments
We would like to thank the family involved in this article as well as the technicians in the laboratory that performed the testing.
References
- 1.Johnson J.D., Mehus J.G., Tews K., Milavetz B.I., Lambeth D.O. Genetic evidence for the expression of ATP- and GTP-specific succinyl-CoA synthetases in multicellular eucaryotes. J. Biol. Chem. 1998;273:27580–27586. doi: 10.1074/jbc.273.42.27580. [DOI] [PubMed] [Google Scholar]
- 2.Lambeth D.O., Tews K.N., Adkins S., Frohlich D., Milavetz B.I. Expression of two succinyl-CoA synthetases with different nucleotide specificities in mammalian tissues. J. Biol. Chem. 2004;279:36621–36624. doi: 10.1074/jbc.M406884200. [DOI] [PubMed] [Google Scholar]
- 3.Van Hove J.L., Saenz M.S., Thomas J.A., Gallagher R.C., Lovell M.A., Fenton L.Z., Shanske S., Myers S.M., Wanders R.J., Ruiter J., Turkenburg M., Waterham H.R. Succinyl-CoA ligase deficiency: a mitochondrial hepatoencephalomyopathy. Pediatr. Res. 2010;68:159–164. doi: 10.1203/PDR.0b013e3181e5c3a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostergaard E., Schwartz M., Batbayli M., Christensen E., Hjalmarson O., Kollberg G., Holme E. A novel missense mutation in SUCLG1 associated with mitochondrial DNA depletion, encephalomyopathic form, with methylmalonic aciduria. Eur. J. Pediatr. 2010;169:201–205. doi: 10.1007/s00431-009-1007-z. [DOI] [PubMed] [Google Scholar]
- 5.Ostergaard E., Hansen F.J., Sorensen N., Duno M., Vissing J., Larsen P.L., Faeroe O., Thorgrimsson S., Wibrand F., Christensen E., Schwartz M. Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain. 2007;130:853–861. doi: 10.1093/brain/awl383. [DOI] [PubMed] [Google Scholar]
- 6.Ostergaard E., Christensen E., Kristensen E., Mogensen B., Duno M., Shoubridge E.A., Wibrand F. Deficiency of the alpha subunit of succinate-coenzyme A ligase causes fatal infantile lactic acidosis with mitochondrial DNA depletion. Am. J. Hum. Genet. 2007;81:383–387. doi: 10.1086/519222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera H., Merinero B., Martinez-Pardo M., Arroyo I., Ruiz-Sala P., Bornstein B., Serra-Suhe C., Gallardo E., Marti R., Moran M.J., Ugalde C., Perez-Jurado L.A., Andreu A.L., Garesse R., Ugarte M., Arenas J., Martin M.A. Marked mitochondrial DNA depletion associated with a novel SUCLG1 gene mutation resulting in lethal neonatal acidosis, multi-organ failure, and interrupted aortic arch. Mitochondrion. 2010;10:362–368. doi: 10.1016/j.mito.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Randolph L.M., Jackson H.A., Wang J., Shimada H., Sanchez-Lara P.A., Wong D.A., Wong L.J., Boles R.G. Fatal infantile lactic acidosis and a novel homozygous mutation in the SUCLG1 gene: a mitochondrial DNA depletion disorder. Mol. Genet. Metab. 2011;102:149–152. doi: 10.1016/j.ymgme.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Rouzier C., Le Guedard-Mereuze S., Fragaki K., Serre V., Miro J., Tuffery-Giraud S., Chaussenot A., Bannwarth S., Caruba C., Ostergaard E., Pellissier J.F., Richelme C., Espil C., Chabrol B., Paquis-Flucklinger V. The severity of phenotype linked to SUCLG1 mutations could be correlated with residual amount of SUCLG1 protein. J. Med. Genet. 2010;47:670–676. doi: 10.1136/jmg.2009.073445. [DOI] [PubMed] [Google Scholar]
- 10.Navarro-Sastre A., Tort F., Garcia-Villoria J., Pons M.R., Nascimento A., Colomer J., Campistol J., Yoldi M.E., Lopez-Gallardo E., Montoya J., Unceta M., Martinez M.J., Briones P., Ribes A. Mitochondrial DNA depletion syndrome: new descriptions and the use of citrate synthase as a helpful tool to better characterise the patients. Mol. Genet. Metab. 2012;107:409–415. doi: 10.1016/j.ymgme.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Harvengt J., Wantyb C., De Paepec B., Sempouxd C., Revencue N., Joél Smetc J., Van Costerc R., Lissensf W., Senecaf S., Weekersa L., Sokalb E., Debray F. Clinical variability in neurohepatic syndrome due to combined mitochondrial DNA depletion and Gaucher disease. Mol. Genet. Metab. Rep. 2014;1:223–231. doi: 10.1016/j.ymgmr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y., Muzny D.M., Reid J.G., Bainbridge M.N., Willis A., Ward P.A., Braxton A., Beuten J., Xia F., Niu Z., Hardison M., Person R., Bekheirnia M.R., Leduc M.S., Kirby A., Pham P., Scull J., Wang M., Ding Y., Plon S.E., Lupski J.R., Beaudet A.L., Gibbs R.A., Eng C.M. Clinical whole-exome sequencing for the diagnosis of Mendelian disorders. N. Engl. J. Med. 2013;369:1502–1511. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craigen W.J., Graham B.H., Wong L.J., Scaglia F., Lewis R.A., Bonnen P.E. Exome sequencing of a patient with suspected mitochondrial disease reveals a likely multigenic etiology. BMC Med. Genet. 2013;14:83. doi: 10.1186/1471-2350-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yano S., Li L., Le T.P., Moseley K., Guedalia A., Lee J., Gonzalez I., Boles R.G. Infantile mitochondrial DNA depletion syndrome associated with methylmalonic aciduria and 3-methylcrotonyl-CoA and propionyl-CoA carboxylase deficiencies in two unrelated patients: a new phenotype of mtDNA depletion syndrome. J. Inherit. Metab. Dis. 2003;26:481–488. doi: 10.1023/a:1025125427868. [DOI] [PubMed] [Google Scholar]
- 15.Kowluru A., Tannous M., Chen H.Q. Localization and characterization of the mitochondrial isoform of the nucleoside diphosphate kinase in the pancreatic beta cell: evidence for its complexation with mitochondrial succinyl-CoA synthetase. Arch. Biochem. Biophys. 2002;398:160–169. doi: 10.1006/abbi.2001.2710. [DOI] [PubMed] [Google Scholar]
- 16.Lascu I., Gonin P. The catalytic mechanism of nucleoside diphosphate kinases. J. Bioenerg. Biomembr. 2000;32:237–246. doi: 10.1023/a:1005532912212. [DOI] [PubMed] [Google Scholar]
- 17.Miller C., Wang L., Ostergaard E., Dan P., Saada A. The interplay between SUCLA2, SUCLG2, and mitochondrial DNA depletion. Biochim. Biophys. Acta. 2011;1812:625–629. doi: 10.1016/j.bbadis.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 18.von Kleist-Retzow J.C., Cormier-Daire V., Viot G., Goldenberg A., Mardach B., Amiel J., Saada P., Dumez Y., Brunelle F., Saudubray J.M., Chretien D., Rotig A., Rustin P., Munnich A., De Lonlay P. Antenatal manifestations of mitochondrial respiratory chain deficiency. J. Pediatr. 2003;143:208–212. doi: 10.1067/S0022-3476(03)00130-6. [DOI] [PubMed] [Google Scholar]
- 19.Thomas M., Salpietro V., Canham N., Ruggieri M., Phadke R., Kinali M. Mitochondria DNA depletion syndrome in a infant with multiple congenital malformations, severe myopathy, and prolonged postoperative paralysis. J. Child Neurol. 2014 doi: 10.1177/0883073814532546. (April 30, Epub ahead of print) [DOI] [PubMed] [Google Scholar]