Abstract
Approximately 35–40% of patients with classic infantile Pompe disease treated with enzyme replacement therapy (ERT) develop high, sustained antibody titers against the therapeutic enzyme alglucosidase alfa, which abrogates the treatment efficacy. Induction of antigen-specific immune tolerance would greatly enhance ERT for these patients. Here we show that a short-course treatment with non-depleting anti-CD4 monoclonal antibody successfully induced long-term ERT-specific immune tolerance in Pompe disease mice. Our data suggest an effective adjuvant therapy to ERT.
Abbreviations: GAA, acid-α-glucosidase; ERT, enzyme replacement therapy; IPD, infantile Pompe disease; CRIM, cross-reacting immunologic material; ITI, immune tolerance induction; MTX, methotrexate
Keywords: Pompe disease, Anti-CD4 antibody, Antigen-specific immune tolerance, Enzyme replacement therapy
Highlights
-
•
The authors described an antigen-specific approach to induce immune tolerance to ERT for Pompe disease.
-
•
A short-term treatment with anti-CD4 mAb reduced anti-hGAA antibody in Pompe disease mice.
-
•
his study suggests an effective adjuvant therapy to ERT for patients with Pompe disease.
1. Introduction
Classic infantile Pompe disease (IPD) is the most severe form of Pompe disease (OMIM 232300; glycogen storage disease type II, acid maltase deficiency). Without treatment, death secondary to cardiorespiratory failure typically occurs within the first 1–2 years of life [1], [2], [3]. Enzyme replacement therapy (ERT) with recombinant human GAA (rhGAA, alglucosidase alfa, Myozyme®) has increased the lifespan for many patients with IPD [4], [5], [6]. However, the development of high sustained antibody titers to rhGAA occurs in 35–40% of patients including the majority of cross-reactive immunologic material negative (CRIM−) patients and a subset of CRIM + patients. This negatively impacts the therapeutic outcome of ERT [7], [8].
Immune tolerance induction (ITI) would be a feasible approach to improve the efficacy of ERT in these patients. Several clinical studies have demonstrated benefits of ITI using a combination of immunosuppressive drugs like rituximab, methotrexate and intravenous immunoglobulin [9], [10], [11]. Some challenges remain including the potential long-term effects of such treatment, the requirement of repeated drug administration in some instances, and the more proximal risk of infection due to immune suppression in these fragile babies. A short-term antigen-specific approach is needed to induce long-term immune tolerance to rhGAA [12].
Extensive preclinical studies in rodents and nonhuman primates demonstrated that co-administration of a short-course of non-depleting anti-CD4 monoclonal antibodies (mAbs) with a desired antigen induced long-term antigen-specific immune tolerance while the normal immune responses to other (3rd party) antigens were preserved [13], [14], [15]. In this study we sought to determine whether this strategy could induce long-term tolerance to rhGAA in a mouse model of Pompe disease.
2. Materials and methods
2.1. Mice
6neo/6neo GAA knockout (GAA-KO) mice used in this study were generated by Raben and colleagues [16] by targeted disruption of the GAA gene in 129/Sv RW4 embryonic stem cells. All institutional and national guidelines for the care and use of laboratory animals were followed.
2.2. Drugs
Clinical grade rhGAA (alglucosidase alfa; Myozyme®) was provided by Genzyme Corporation (Cambridge, MA, USA). Non-depleting anti-CD4 mAb (YTS177) was obtained from Tolerx Inc. (Cambridge, MA, USA). Methotrexate was purchased from Calbiochem (San Diego, CA). Diphenhydramine was purchased from Baxter Healthcare Corporation (Deerfield, IL).
2.3. rhGAA injection
All mice received weekly ERT with 20 mg/kg of rhGAA, the equivalent dose for treatment of human patients, via tail-vein injection starting from two months of age. For each mouse, pretreatment with 15–25 mg/kg diphenhydramine by intraperitoneal (i.p.) injection was performed 10–15 min prior to rhGAA administration to prevent anaphylactic reactions [17].
2.4. Experimental groups
Initial experiments included three groups: 1) The control group were treated with intravenous (IV) sterile 0.9% saline, 2) The MTX group received IV methotrexate (10 mg/kg) at 0, 24 and 48 h following the first 3 weekly rhGAA treatments [17], and 3) The anti-CD4x3 group received 3 doses of anti-CD4 mAb (IV, 50 mg/kg) on Day − 1, + 6, and + 13 relative to the first rhGAA treatment. Each group included 10 mice and all three groups were followed for up to 28 weeks on ERT. The weekly rhGAA treatment was stopped after 28 weeks and then resumed at 40 weeks to determine whether re-challenge of rhGAA would boost anti-rhGAA antibody levels.
A second experiment was carried out to evaluate different dosing regimens of anti-CD4 mAb using two additional groups: 1) The anti-CD4x2 group (n = 10) received two doses of anti-CD4 mAb (IV, 50 mg/kg) on Day − 1 and + 6 with respect to the first dose of rhGAA, and 2) The anti-CD4x1 group (n = 10) received only one dose of anti-CD4 mAb (IV, 50 mg/kg) on Day − 1 with respect to the first dose of rhGAA. Both groups were followed for up to 22 weeks.
2.5. Anti-rhGAA antibody quantification
Mice were bled every two weeks one day before the rhGAA administration. Anti-rhGAA IgG antibody levels were measured by ELISA as described [18].
2.6. Statistical analyses
Data were presented as mean ± standard deviation. The significance of differences was assessed using two-tailed, equal variance student t-test. p < 0.05 was considered significant.
3. Results
The initial experiment showed that mice in the control group generated high levels of anti-rhGAA antibody that started increasing from 2 weeks, peaked at 16 weeks ((absorbance = 0.225) and then declined remaining consistently above 0.13 level after 24 weeks (Fig. 1A). Both the methotrexate (MTX group) and 3-dose anti-CD4 mAb (anti-CD4x3 group) treated groups showed significantly (p < 0.05) decreased anti-rhGAA antibody levels after 4 weeks of rhGAA treatment when compared to the control group (Fig. 1A). The overall antibody levels for the anti-CD4x3 group remained lower than those of the MTX group throughout the 28-week treatment with rhGAA. Comparing the area under the curve, the anti-CD4x3 group and the MTX group showed a 94.3% and 84.5% reduction of antibody levels, respectively, as compared to the control group (Fig. 1A). Rechallenge with rhGAA at 40 weeks did not significantly change the antibody levels in any of the 3 groups (Fig. 1A). The serum anti-CD4 mAb levels in the anti-CD4x3 group continuously decreased after administration and remained undetectable after 23 weeks (data not shown).
Fig. 1.
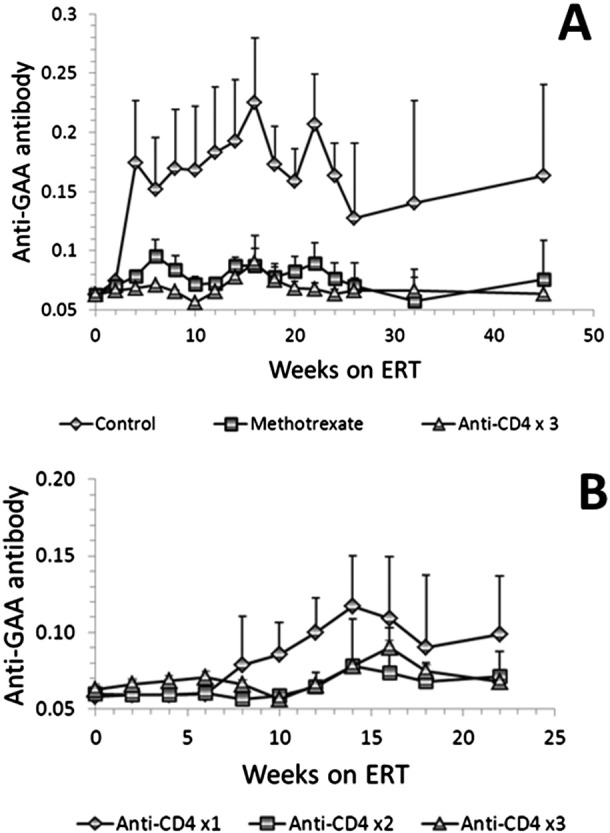
ELISA for anti-hGAA IgG antibody in GAA-KO mice under weekly ERT with 20 mg/kg of rhGAA. The antibody levels were presented by the absorbance for 1:200 dilution of plasma from the indicated groups of mice. Mean plus standard deviation is shown, n = 10 for each group. (A) Both the 3-dose anti-CD4 mAb (anti-CD4x3 group) and methotrexate treatments significantly (p < 0.05) reduced rhGAA antibody levels in the GAA-KO mice after 4 weeks of rhGAA treatment in comparison with the control mice (ERT only). (B) A 2-dose treatment (anti-CD4x2 group) was similarly effective in inhibiting anti-rhGAA antibody formation as the 3-dose regimen (anti-CD4x3 group), but a single-dose treatment (anti-CD4x1 group) failed to significantly (p > 0.05) reduce the anti-rhGAA antibody levels, in comparison to the control group.
The second experiment evaluated the minimum number of doses of anti-CD4 mAb needed for ITI. A 2-dose treatment (anti-CD4x2 group) was similarly effective in inhibiting anti-rhGAA antibody formation as the 3-dose regimen (anti-CD4x3 group), but a single-dose treatment (anti-CD4x1 group) failed to significantly (p > 0.05) reduce the anti-rhGAA antibody levels, in comparison to the control group (Fig. 1B).
4. Discussion
The formation of sustained high-titer anti-rhGAA antibody in some IPD patients has been correlated with poor clinical outcomes of ERT [7], [8], [19], [20]. Several ITI protocols have been attempted in these patients using immunosuppressive agents like rituximab to deplete B cells and/or MTX to target the antigen-activated T and B cells [9], [10], [21], [22]. These protocols have limitations including generalized immunosuppression, side effects, and in some cases, variable responses. Antigen specific approaches such as gene therapy are highly favored, but may take weeks to months to induce durable tolerance, a time frame that may not be appropriate for the rapid requirement for treatment of Pompe patients with cardiac and respiratory compromise. Thus, protocols and agents that favor the shortest duration of more antigen-specific immune suppression are needed.
The utility of a non-depleting anti-CD4 antibody in reduction of antigen-specific antibodies was first reported by Qin et al. [14] with many other subsequent examples e.g. with co-administration of factor VIII or IX in mice [15]. Since then, a humanized anti-CD4 monoclonal antibody (TRX1) has been constructed and was found to successfully induce long-term, antigen-specific tolerance in non-human primates without compromising normal immune function or depleting CD4 lymphocytes [23]. Data from a Phase 1 clinical trial showed that TRX1 was well tolerated and did not deplete T cells [24]. Proposed mechanisms of action of non-depleting anti-CD4 include down-modulation of the CD4 receptor on T cells, and induction of regulatory T-cells [25], [26], [27]. In this study we showed that a short-course treatment with an anti-CD4 mAb successfully induced long-term ERT-specific immune tolerance in Pompe disease mice, implying that this may prove a favorable prophylactic ITI protocol for infants.
IPD serves as a particularly good study model because of its rapid progression and well-defined clinical endpoints (e.g., survival, ventilator status, motor function). The anti-CD4 mAb treatment could be given prophylactically with induction of ERT treatment, thus allowing for a greater potential for successful tolerance induction in these cases. Our data suggest an effective adjuvant therapy to ERT.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This project was supported by a grant from Tolerx Inc. GAA-KO mice were provided courtesy of Dr. Nina Raben at the National Institutes of Health (Bethesda, MD). We thank Dr. Dwight D. Koeberl and Songtao Li for their technical assistance in carrying out the experiments.
Contributor Information
Baodong Sun, Email: baodong.sun@duke.edu.
Priya S. Kishnani, Email: kishn001@mc.duke.edu.
References
- 1.Hirschhorn R., Reuser A.J.J. Glycogen storage disease type II: acid a-glucosidase (acid maltase) deficiency. In: Valle D., Scriver C.R., editors. Scriver's OMMBID the Online Metabolic & Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2009. [Google Scholar]
- 2.Kishnani P.S., Hwu W.L., Mandel H., Nicolino M., Yong F., Corzo D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J. Pediatr. 2006;148:671–676. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 3.van den Hout H.M., Hop W., van Diggelen O.P., Smeitink J.A., Smit G.P., Poll-The B.T., Bakker H.D., Loonen M.C., de Klerk J.B., Reuser A.J., van der Ploeg A.T. The natural course of infantile Pompe's disease: 20 original cases compared with 133 cases from the literature. Pediatrics. 2003;112:332–340. doi: 10.1542/peds.112.2.332. [DOI] [PubMed] [Google Scholar]
- 4.Kishnani P.S., Corzo D., Leslie N.D., Gruskin D., Van der Ploeg A., Clancy J.P., Parini R., Morin G., Beck M., Bauer M.S., Jokic M., Tsai C.E., Tsai B.W., Morgan C., O'Meara T., Richards S., Tsao E.C., Mandel H. Early treatment with alglucosidase alpha prolongs long-term survival of infants with Pompe disease. Pediatr. Res. 2009;66:329–335. doi: 10.1203/PDR.0b013e3181b24e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishnani P.S., Corzo D., Nicolino M., Byrne B., Mandel H., Hwu W.L., Leslie N., Levine J., Spencer C., McDonald M., Li J., Dumontier J., Halberthal M., Chien Y.H., Hopkin R., Vijayaraghavan S., Gruskin D., Bartholomew D., van der Ploeg A., Clancy J.P., Parini R., Morin G., Beck M., De la Gastine G.S., Jokic M., Thurberg B., Richards S., Bali D., Davison M., Worden M.A., Chen Y.T., Wraith J.E. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- 6.Nicolino M., Byrne B., Wraith J.E., Leslie N., Mandel H., Freyer D.R., Arnold G.L., Pivnick E.K., Ottinger C.J., Robinson P.H., Loo J.C., Smitka M., Jardine P., Tato L., Chabrol B., McCandless S., Kimura S., Mehta L., Bali D., Skrinar A., Morgan C., Rangachari L., Corzo D., Kishnani P.S. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet. Med. 2009;11:210–219. doi: 10.1097/GIM.0b013e31819d0996. [DOI] [PubMed] [Google Scholar]
- 7.Banugaria S.G., Prater S.N., Ng Y.K., Kobori J.A., Finkel R.S., Ladda R.L., Chen Y.T., Rosenberg A.S., Kishnani P.S. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet. Med. 2011;13:729–736. doi: 10.1097/GIM.0b013e3182174703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishnani P.S., Goldenberg P.C., DeArmey S.L., Heller J., Benjamin D., Young S., Bali D., Smith S.A., Li J.S., Mandel H., Koeberl D., Rosenberg A., Chen Y.T. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol. Genet. Metab. 2010;99:26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendelsohn N.J., Messinger Y.H., Rosenberg A.S., Kishnani P.S. Elimination of antibodies to recombinant enzyme in Pompe's disease. N. Engl. J. Med. 2009;360:194–195. doi: 10.1056/NEJMc0806809. [DOI] [PubMed] [Google Scholar]
- 10.Messinger Y.H., Mendelsohn N.J., Rhead W., Dimmock D., Hershkovitz E., Champion M., Jones S.A., Olson R., White A., Wells C., Bali D., Case L.E., Young S.P., Rosenberg A.S., Kishnani P.S. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet. Med. 2012;14:135–142. doi: 10.1038/gim.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elder M.E., Nayak S., Collins S.W., Lawson L.A., Kelley J.S., Herzog R.W., Modica R.F., Lew J., Lawrence R.M., Byrne B.J. B-cell depletion and immunomodulation before initiation of enzyme replacement therapy blocks the immune response to acid alpha-glucosidase in infantile-onset Pompe disease. J. Pediatr. 2013;163:847–854. doi: 10.1016/j.jpeds.2013.03.002. (e841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banugaria S.G., Patel T.T., Kishnani P.S. Immune modulation in Pompe disease treated with enzyme replacement therapy. Expert. Rev. Clin. Immunol. 2012;8:497–499. doi: 10.1586/eci.12.40. [DOI] [PubMed] [Google Scholar]
- 13.Waldmann H. Manipulation of T-cell responses with monoclonal antibodies. Annu. Rev. Immunol. 1989;7:407–444. doi: 10.1146/annurev.iy.07.040189.002203. [DOI] [PubMed] [Google Scholar]
- 14.Qin S.X., Wise M., Cobbold S.P., Leong L., Kong Y.C., Parnes J.R., Waldmann H. Induction of tolerance in peripheral T cells with monoclonal antibodies. Eur. J. Immunol. 1990;20:2737–2745. doi: 10.1002/eji.1830201231. [DOI] [PubMed] [Google Scholar]
- 15.Salooja N., Kemball-Cook G., Tuddenham E.G., Dyson J. Use of a non-depleting anti-CD4 antibody to modulate the immune response to coagulation factors VIII and IX. Br. J. Haematol. 2002;118:839–842. doi: 10.1046/j.1365-2141.2002.03666.x. [DOI] [PubMed] [Google Scholar]
- 16.Raben N., Nagaraju K., Lee E., Kessler P., Byrne B., Lee L., LaMarca M., King C., Ward J., Sauer B., Plotz P. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J. Biol. Chem. 1998;273:19086–19092. doi: 10.1074/jbc.273.30.19086. [DOI] [PubMed] [Google Scholar]
- 17.Joseph A., Munroe K., Housman M., Garman R., Richards S. Immune tolerance induction to enzyme-replacement therapy by co-administration of short-term, low-dose methotrexate in a murine Pompe disease model. Clin. Exp. Immunol. 2008;152:138–146. doi: 10.1111/j.1365-2249.2008.03602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun B., Bird A., Young S.P., Kishnani P.S., Chen Y.T., Koeberl D.D. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am. J. Hum. Genet. 2007;81:1042–1049. doi: 10.1086/522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amalfitano A., Bengur A.R., Morse R.P., Majure J.M., Case L.E., Veerling D.L., Mackey J., Kishnani P., Smith W., McVie-Wylie A., Sullivan J.A., Hoganson G.E., Phillips J.A., III, Schaefer G.B., Charrow J., Ware R.E., Bossen E.H., Chen Y.T. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet. Med. 2001;3:132–138. doi: 10.109700125817-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Abbott M.A., Prater S.N., Banugaria S.G., Richards S.M., Young S.P., Rosenberg A.S., Kishnani P.S. Atypical immunologic response in a patient with CRIM-negative Pompe disease. Mol. Genet. Metab. 2011;104:583–586. doi: 10.1016/j.ymgme.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunley T.E., Corzo D., Dudek M., Kishnani P., Amalfitano A., Chen Y.T., Richards S.M., Phillips J.A., III, Fogo A.B., Tiller G.E. Nephrotic syndrome complicating alpha-glucosidase replacement therapy for Pompe disease. Pediatrics. 2004;114:e532–e535. doi: 10.1542/peds.2003-0988-L. [DOI] [PubMed] [Google Scholar]
- 22.Banugaria S.G., Patel T.T., Mackey J., Das S., Amalfitano A., Rosenberg A.S., Charrow J., Chen Y.T., Kishnani P.S. Persistence of high sustained antibodies to enzyme replacement therapy despite extensive immunomodulatory therapy in an infant with Pompe disease: need for agents to target antibody-secreting plasma cells. Mol. Genet. Metab. 2012;105:677–680. doi: 10.1016/j.ymgme.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winsor-Hines D., Merrill C., O'Mahony M., Rao P.E., Cobbold S.P., Waldmann H., Ringler D.J., Ponath P.D. Induction of immunological tolerance/hyporesponsiveness in baboons with a nondepleting CD4 antibody. J. Immunol. 2004;173:4715–4723. doi: 10.4049/jimmunol.173.7.4715. [DOI] [PubMed] [Google Scholar]
- 24.Ng C.M., Stefanich E., Anand B.S., Fielder P.J., Vaickus L. Pharmacokinetics/pharmacodynamics of nondepleting anti-CD4 monoclonal antibody (TRX1) in healthy human volunteers. Pharm. Res. 2006;23:95–103. doi: 10.1007/s11095-005-8814-3. [DOI] [PubMed] [Google Scholar]
- 25.Gottlieb A.B., Lebwohl M., Shirin S., Sherr A., Gilleaudeau P., Singer G., Solodkina G., Grossman R., Gisoldi E., Phillips S., Neisler H.M., Krueger J.G. Anti-CD4 monoclonal antibody treatment of moderate to severe psoriasis vulgaris: results of a pilot, multicenter, multiple-dose, placebo-controlled study. J. Am. Acad. Dermatol. 2000;43:595–604. doi: 10.1067/mjd.2000.107945. [DOI] [PubMed] [Google Scholar]
- 26.Qin S., Cobbold S.P., Pope H., Elliott J., Kioussis D., Davies J., Waldmann H. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira V.G., Agua-Doce A., Curotto de Lafaille M.A., Lafaille J.J., Graca L. Adjuvant facilitates tolerance induction to factor VIII in hemophilic mice through a Foxp3-independent mechanism that relies on IL-10. Blood. 2013;121:3936–3945. doi: 10.1182/blood-2012-09-457135. (S3931) [DOI] [PubMed] [Google Scholar]


