Abstract
Biotin-thiamine responsive basal ganglia disease (BTBGD) is a rare metabolic condition caused by mutations in the SLC19A3 gene. BTBGD presents with encephalopathy and significant disease progression when not treated with biotin and/or thiamine. We present a patient of Mexican and European ancestry diagnosed with BTBGD found to have compound heterozygous frameshift mutations, one novel. Our report adds to the genotype-phenotype correlation, highlighting the clinical importance of considering SLC19A3 gene defects as part of the differential diagnosis for Leigh syndrome.
Keywords: Biotin thiamine responsive basal ganglia disease, BTBGD, Leigh syndrome, SLC19A3, Basal ganglia, Thiamine transporter-2
1. Introduction
Biotin-thiamine responsive basal ganglia disease (BTBGD) also referred to as thiamine metabolism dysfunction syndrome 2, is a rare, likely under diagnosed, autosomal recessive neurometabolic condition (OMIM 607483). It was first described by Ozand et al. in 1998 as biotin responsive basal ganglia disease (BBGD) in patients with subacute encephalopathy; progressing to dystonia, quadriparesis and death without treatment of biotin [1]. The disease was mapped to 2q36.3 and linked to mutations in the thiamine transporter-2 gene, SLC19A3, thus making BTBGD a more appropriate name [2], [3]. The majority of patients described in the literature are Saudi Arabian; however individuals of Portuguese, Spanish, Canadian, Moroccan, Yemeni, Indian, Lebanese, Japanese and Syrian ancestry have also been reported [4], [5], [6], [7], [8]. Four different phenotypes have been described; infantile lactic acidosis with encephalopathy [9], infantile epileptic spasms [10], early childhood encephalopathy triggered by illness or trauma [1], and Wernicke-like encephalopathy in the second decade [8]. Typical MRI findings include cortical, subcortical white matter lesions, and bilateral abnormal signal intensity in the basal ganglia with less frequent involvement of thalami, brain stem, cerebellum and cervical spine [1], [3], [5], [11]. Due to the similarity of clinical, biochemical, and MRI findings, BTBGD can be misdiagnosed as a mitochondrial disease [4].
2. Case report
Our patient was the second daughter of healthy non-consanguineous mixed ancestry parents (Mexican and Irish/Scottish/English). The perinatal course was uncomplicated. By 1 month, the patient had developed a social smile, head control, and appeared to turn towards sound and follow visually.
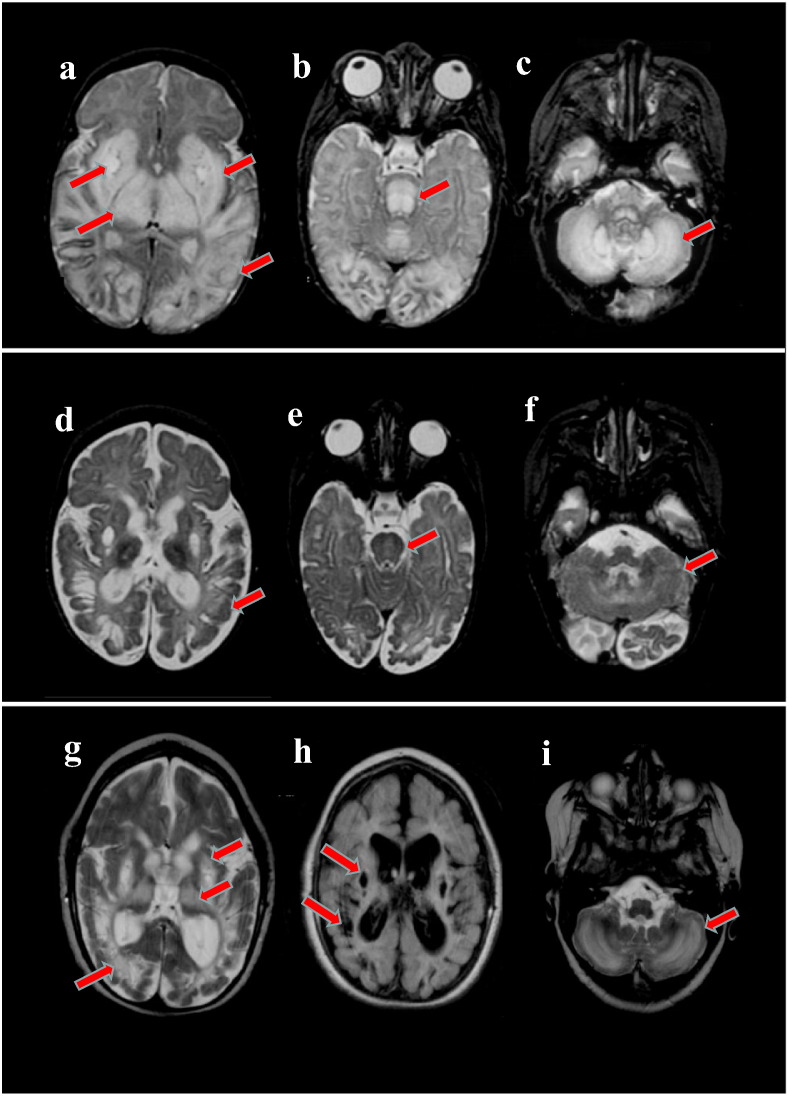
At 6 weeks of age, regression of developmental milestones was noted and the patient was admitted to the hospital with lethargy, hypotonia, and poor feedings. Additionally, the exam showed a weak cry, horizontal nystagmus, and generalized brisk hyperreflexia. She had an episode of apnea, requiring intubation and mechanical ventilation. MRI of the brain revealed diffuse bilateral T2 signal abnormalities and cystic-like lesions in the globus pallidi (Fig. 1, a-c). Increased levels of lactate were seen on MRS.
Fig. 1.
Patient MRI Finding. a-c: Initial MRI obtained at 6 weeks of age revealed diffuse bilateral abnormal T2 signal in the basal ganglia, thalami and parieto-occipital white matter. Bilateral, well-circumscribed cyst-like lesions in the globus pallidi was observed (a). Abnormal T2 signal extended to the brain stem (b) and cerebellum (c). d-f: A repeat MRI obtained at 6 months of age demonstrated improvement of the T2 signal hyperintensities in the white matter of the brain (d), brainstem (e), and cerebellum (f). Diffuse brain atrophy was present. g-i: The final MRI, completed at 2.5 years of age, showed worsening of the white matter, basal ganglia, and thalamic abnormalities (g). Increased cystic lesions and atrophy are shown in Flair (h). Worsening of the cerebellar white matter hyperintensities in T2 was also noted (i).
Although there were no clinical seizures, an EEG showed generalized burst suppression pattern and multifocal epileptiform discharges, initially treated with phenobarbital. Infectious diseases were ruled out. Biochemical analysis revealed elevated blood lactate at 3.8 mmol/L (nl 0.6-2.2) but CSF lactic acid was within normal limits at 1.6 mmol/L. Otherwise, the remainder of metabolic testing was negative. Specifically, quantitative urine organic acids did not show any abnormalities suggestive of mitochondrial disease, with alpha-ketoglutarate measured at 279 mmol/mol Cr (nl 29–636). Muscle histology and ETC. activities were normal.
The patient was started on thiamine (10 mg/day), pyridoxine, riboflavin, coenzyme Q10, and levocarnitine on the second day of admission, almost 1 week after symptoms were first noted. Six days later, the thiamine dose was increased to 40 mg/day (~ 10 mg/kg/day). Within 5 days of the increased thiamine dose, there was return of spontaneous activity and respiration; and patient was extubated. She was discharged at 9 weeks of age on the vitamin cocktail; tolerating oral feedings and without clinical seizure activity.
During a 3 month follow-up, acquired microcephaly and increased muscle tone were noted and the patient began to have myoclonic-like seizures, increasing in frequency around 6 months of age. A video-EEG demonstrated infantile spasms and hypsarrhythmia, with an electrode decrement pattern associated with the clinical spasms. She underwent 2 courses of ACTH due to infantile spasm recurrence which evolved into Lennox-Gastaut syndrome with multiple seizure types that were not responsive to medications. MRI at 6 months of age revealed brain atrophy, however there was improvement of the T2 signal hyperintensity (Fig. 1, d-f). She temporarily regained head control; but regressed by 1 year of age.
The patient continued to have multiple admissions for intercurrent illnesses and seizures, requiring a tracheotomy and gastric feeding tube placement at 2 years of age. A repeat brain MRI showed worsening of the previous lesions (Fig. 1, g-i). By then, the dose of thiamine of 40 mg/day was equivalent to 4.6 mg/kg/day.
Extensive laboratory investigations, including multiple molecular testing for mitochondrial disease, as well as pyruvate dehydrogenase complex activity, failed to identify the etiology of our patient’s disease. Vitamin cocktail doses varied over time, with her thiamine ultimately decreasing to 25 mg/day. Over the years, she continued to be awake, but had minimal awareness of her surroundings, until her death at 12 years of age due to respiratory illness.
Whole exome sequencing (WES) was performed at Baylor College of Medicine, Medical Genetics Laboratory and confirmed compound heterozygous frameshift mutations in the SLC19A3 gene in trans; c.74dup (p.S26fs), previously reported, and c.81_82dup (p.M28fs), which is novel [7], [11]. Both mutations result in the insertion of a premature termination codon, suggesting that any mRNA that is synthesized will be degraded via nonsense-mediated RNA decay, unlikely to produce a functional protein product.
3. Discussion and conclusions
BTBGD is a relatively new entity with four different phenotypes described. We report a new patient, of Mexican and European ancestry, with clinical and radiographic manifestations consistent with the early-infantile form of the disease [10].
Kevelam et al. categorized the brain MRI findings of the early infantile subgroup into: acute, post-acute, intermediate, and end stage phases [5]. Our patient’s initial MRI abnormalities are similar to those reported in the acute phase. However, cystic lesions, which are described in later stages, were already present in our patient at 6 weeks of age (Fig. 1, a-c) [5]. These striking abnormalities suggest a more aggressive course than previously reported and is consistent with the predicted severity of the mutations. Both molecular defects identified in our patient are frameshift, and likely cause a non-functional or absent protein product. The c.81_82dup mutation is novel, whereas the c.74dup mutation has been reported, along with an intronic mutation, in patients with a late onset presentation [7], [11].
Historically, patients with BTBGD presenting with the early childhood encephalopathy phenotype have shown clinical improvements with treatment of biotin (2–10 mg/kg/day) with or without the supplementation of thiamine (100–300 mg/day) [1], [4], [8]. This treatment strategy is supported by in vitro studies suggesting that high doses of thiamine and biotin restore insufficient thiamine transport by overexpression of SLC19A3 gene [6]. However, there are still limited reports in the literature on the efficacy of biotin and/or thiamine supplementation in patients with the early infantile form. Yamada et al. reported 4 patients who presented in early infancy with epileptic spasms, psychomotor retardation, along with characteristic brain MRI findings of progressive brain atrophy, bilateral thalami and basal ganglia lesions. The effect of treatment could not be determined in this report as only 1 patient in the cohort was treated, and that was with biotin (5 mg/kg/day) only [10]. Kevelam et al. described 7 patients with irritability, progressive spasticity, significant MRI findings, and death before 2 years of age. The only two patients reported to be treated in that group received a mitochondrial cocktail that included biotin but not thiamine, and did not show signs of improvement [5]. In contrast, Perez-Duenas, reported a patient with early infantile phenotype who improved clinically on treatment with thiamine (100 mg/day) and biotin (10 mg/day), and remained in good metabolic control for 6 months after biotin was discontinued [9]. Our patient’s initial improvement was similar to the latter patient and could therefore be attributed to the thiamine treatment. However, the dose prescribed in our case was lower than recommended and further decreased overtime in relation to the patient’s weight. This low dose was likely not enough for a sustained clinical improvement, although it may have contributed to her longer life span compared to other patients described with the early infantile form [5], [10].
In summary, we expand upon the clinical and molecular description of BTBGD by presenting a new patient of a different ethnic background than previously reported, with two severe mutations including one novel, and striking early onset MRI findings. Our case highlights the clinical importance of including SLC19A3 gene defects as part of the differential diagnosis for mitochondrial disease, given that early supplementation with biotin and/or thiamine can improve the devastating progression of this condition and allow for appropriate genetic counseling.
Acknowledgments
The authors wish to thank the family of our patient for their cooperation and support.
Web resources:
Online Mendelian Inheritance in Man (OMIM), http://omim.org/entry/607483 (for THMD2/BBGD)
References
- 1.Ozand P.T., Gascon G.G., Al Essa M., Joshi S., Al Jishi E., Bakheet S., Al Watban J., Al-Kawi M.Z., Dabbagh O. Biotin-responsive basal ganglia disease: a novel entity. Brain. 1998;121(Pt. 7):1267–1279. doi: 10.1093/brain/121.7.1267. [DOI] [PubMed] [Google Scholar]
- 2.Zeng W.-Q., Al-Yamani E., Acierno J.S., Slaugenhaupt S., Gillis T., MacDonald M.E., Ozand P.T., Gusella J.F. Biotin-Responsive Basal Ganglia Disease Maps to 2q36.3 and Is Due to Mutations in SLC19A3. Am. J. Hum. Genet. 2005;77:16–26. doi: 10.1086/431216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfadhel M., Almuntashri M., Jadah R.H., Bashiri F.A., Al Rifai M., Al Shalaan H., Al Balwi M., Al Rumayan A., Eyaid W., Al-Twaijri W. Biotin-responsive basal ganglia disease should be renamed biotin-thiamine-responsive basal ganglia disease: a retrospective review of the clinical, radiological and molecular findings of 18 new cases. Orphanet J. Rare Dis. 2013;8:83. doi: 10.1186/1750-1172-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabarki B., Al-Shafi S., Al-Shahwan S., Azmat Z., Al-Hashem A., Al-Adwani N., Biary N., Al-Zawahmah M., Khan S., Zuccoli G. Biotin-responsive basal ganglia disease revisited: clinical, radiologic, and genetic findings. Neurology. 2013;80:261–267. doi: 10.1212/WNL.0b013e31827deb4c. [DOI] [PubMed] [Google Scholar]
- 5.Kevelam S.H., Bugiani M., Salomons G.S., Feigenbaum A., Blaser S., Prasad C., Haberle J., Baric I., Bakker I.M.C., Postma N.L., Kanhai W.A., Wolf N.I., Abbink T.E.M., Waisfisz Q., Heutink P., van der Knaap M.S. Exome sequencing reveals mutated SLC19A3 in patients with an early-infantile, lethal encephalopathy. Brain. 2013;136:1534–1543. doi: 10.1093/brain/awt054. [DOI] [PubMed] [Google Scholar]
- 6.Gerards M., Kamps R., van Oevelen J., Boesten I., Jongen E., de Koning B., Scholte H.R., de Angst I., Schoonderwoerd K., Sefiani A., Ratbi I., Coppieters W., Karim L., de Coo R., van den Bosch B., Smeets H. Exome sequencing reveals a novel Moroccan founder mutation in SLC19A3 as a new cause of early-childhood fatal Leigh syndrome. Brain. 2013;136:882–890. doi: 10.1093/brain/awt013. [DOI] [PubMed] [Google Scholar]
- 7.Serrano M., Rebollo M., Depienne C., Rastetter A., Fernández-Álvarez E., Muchart J., Martorell L., Artuch R., Obeso J.A., Pérez-Dueñas B. Reversible generalized dystonia and encephalopathy from thiamine transporter 2 deficiency. Mov. Disord. 2012;27:1295–1298. doi: 10.1002/mds.25008. [DOI] [PubMed] [Google Scholar]
- 8.Kono S., Miyajima H., Yoshida K., Togawa A., Shirakawa K., Suzuki H. Mutations in a Thiamine-Transporter Gene and Wernicke's-like Encephalopathy. N. Engl. J. Med. 2009;360:1792–1794. doi: 10.1056/NEJMc0809100. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Duenas B., Serrano M., Rebollo M., Muchart J., Gargallo E., Dupuits C., Artuch R. Reversible Lactic Acidosis in a Newborn With Thiamine Transporter-2 Deficiency. Pediatrics. 2013;131:e1670. doi: 10.1542/peds.2012-2988. [DOI] [PubMed] [Google Scholar]
- 10.Yamada K., Miura K., Hara K., Suzuki M., Nakanishi K., Kumagai T., Ishihara N., Yamada Y., Kuwano R., Tsuji S., Wakamatsu N. A wide spectrum of clinical and brain MRI findings in patients with SLC19A3 mutations. BMC Med. Genet. 2010;11:171. doi: 10.1186/1471-2350-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debs R. Biotin-Responsive Basal Ganglia Disease in Ethnic Europeans With Novel SLC19A3 MutationsBBGD in Ethnic Europeans With SCL19A3 Mutations. Arch. Neurol. 2010;67:126. doi: 10.1001/archneurol.2009.293. [DOI] [PubMed] [Google Scholar]