Abstract
Hyperhomocysteinemia due to cystathionine beta synthase deficiency confers diverse clinical manifestations. It is characterized by elevated plasma homocysteine levels, a common amino acid metabolized by remethylation to methionine or transsulfuration to cysteine. We recently found a relationship between hepatic Dyrk1A protein expression, a serine/threonine kinase involved in signal transduction in biological processes, hepatic S-adenosylhomocysteine activity, and plasma homocysteine levels. We aimed to study whether there is also a relationship between Dyrk1a and cystathionine beta synthase activity. We used different murine models carrying altered gene coy numbers for Dyrk1a, and found a decreased cystathionine beta synthase activity in the liver of mice under-expressing Dyrk1a, and an increased in liver of mice over-expressing Dyrk1a. For each model, a positive correlation was found between cystathionine beta synthase activity and Dyrk1a protein expression in the liver of mice, which was confirmed in a non-modified genetic context. The positive correlation found between liver Dyrk1a protein expression and CBS activity in modified and non-modified genetic context strengthens the role of this kinase in one carbon metabolism.
Abbreviations: CBS, cystathionine beta synthase; DS, Down syndrome; DYRK, dual-specificity tyrosine-(Y)-phosphorylation regulated kinase; EGCG, epigallocatechin-gallate; GABA, gamma-amino-butyric-acid; GK, Goto-Kakizaki; hcy, homocysteine; NQO1, NAD(P)H:quinone oxidoreductase; PLP, pyridoxal phosphate; PTZ, pentylenetetrazole; SAH, S-adenosylhomocysteine; SAHH, SAH hydrolase
Keywords: Dyrk1a, Homocysteine, Cystathionine beta synthase, Liver, Murine model
1. Introduction
Homocysteine (hcy) is an intermediate in the sulfur amino acid metabolism. Once hcy is formed, it may be metabolized by remethylation to methionine or transsulfuration to cysteine. Cystathionine beta synthase (CBS), which gene is localized on human chromosome 21, is the first enzyme involved in the transsulfuration pathway and catalyzes the condensation of hcy with serine to form cystathionine. Hcy can also turn back to S-adenosylhomocysteine (SAH) via reversal of the SAH hydrolase (SAHH) reaction [1]. Homocystinuria or severe hyperhomocysteinemia, defined by elevated plasma hcy level, is a metabolic disorder with defect in genes encoding for methionine metabolism enzymes. The clinical features consist in ophthalmic, neurologic, orthopedic and vascular manifestations. Homocystinuria is mainly due to CBS deficiency. It is the second metabolic encephalopathy in order of frequency [2]. The disorder is also associated with cognitive dysfunctions such as intellectual disability, cerebral atrophy, and seizures [2], [3]. Further, elevated hcy level has also been associated with neurological disorders such as epilepsy [4], [5].
The identification of intragenic DYRK1A rearrangements or 21q22 micro-deletions including only DYRK1A in patients with intellectual disability, microcephaly, seizures and epilepsy hypothesized the role of DYRK1A for such a phenotype [6], [7], [8], [9], [10], [11]. Dyrk1A, which gene is localized on human chromosome 21, is a protein kinase that belongs to an evolutionarily conserved family of proteins known as DYRK (dual-specificity tyrosine-(Y)-phosphorylation regulated kinase) that might be responsible for intellectual disability in Down's syndrome (DS) patients [12]. We recently analyzed the expression of Dyrk1A in the liver of CBS-deficient mice, a murine model of hyperhomocysteinemia, and found a reduced protein level, concomitant with a decreased hepatic SAHH activity [13], [14], [15]. On the contrary, an over-expression of Dyrk1A decreased plasma hcy level and increased the hepatic SAHH activity by a mechanism dependent of NAD(P)H:quinone oxidoreductase (NQO1) activity [15]. We therefore have established a link between hepatic Dyrk1a expression, hepatic SAHH activity and plasma hcy levels. However, the key question asked now is to study whether there is also a relationship between Dyrk1a and CBS. To analyze further the relation between Dyrk1a and CBS, we used two mice models to demonstrate the effect of the over-expression and the under-expression of Dyrk1a on hepatic CBS activity. We also used a spontaneous nonobese model of type 2 diabetes with lower plasma hcy level and increased liver CBS activity [16] to confirm the link established in modified genetic context.
2. Materials and methods
2.1. Animals
All Animal care was conducted in accordance with internal guidelines of the French Agriculture Ministry for animal handling (authorization number 75–369). Mice and rats were housed in a controlled environment (temperature = 20 ± 1 °C; humidity = 60%) with unlimited access to food and water on 12-h light/dark cycle. A number of mice and rats and suffering were minimized as possible. Mice carrying the mBACtgDYRK1A construct were maintained on a C57BL/6 J background and genotyped as described [17]. Dyrk1a (+/−) mice were maintained on a CD1 background and genotyped as described [18]. The Goto-Kakizaki (GK) line was established by repeated inbreeding from Wistar rats selected at the upper limit of normal distribution for glucose tolerance [19], [20]. All experiments were conducted on age-matched control animals.
2.2. Sample preparation, tissue collection, and plasma total hcy assay
Following euthanization of mice and rats, blood samples were obtained by retro-orbital sinus sampling with heparinized capillaries, collected into tubes containing a 1/10 volume of 3.8% sodium citrate, and immediately placed on ice. Plasma was isolated by centrifugation at 2500 ×g for 15 min at 4 °C. The liver was rapidly removed and snap-frozen in liquid nitrogen before being stored at − 80 °C until use. Plasma total hcy, defined as the total concentration of hcy after quantitative reductive cleavage of all disulfide bonds, was assayed using the fluorimetric high-performance liquid chromatography (HPLC) method described by Fortin and Genest [21]. The inter- and intra-assay coefficients of variation for mean total hcy level were 4.2% and 6.3% respectively and the linearity was from 1 to 100 μM [22].
2.3. Dyrk1a protein analysis by slot blot
Protein samples were prepared by homogenizing the liver in 500 μL phosphate-buffered saline with a cocktail of proteases inhibitors. Protein concentrations were detected with the Bio-Rad Protein Assay reagent (Bio-Rad). To assess the relative amount of Dyrk1a, we used a slot blot method previously developed [23]. Protein preparations were blotted on Hybond-C Extra membrane (GE Healthcare Europe GmbH) using Bio-Dot SF Microfiltration Apparatus (Bio-Rad). After transfer, membranes were saturated by incubation in 5% w/v nonfat milk powder in Tris-saline buffer (1.5 mM Tris base, pH 8; 5 mM NaCl; 0.1% Tween-20), and incubated overnight at 4 °C with an antibody directed against DYRK1A (1/500) (Abnova corporation, Tebu, France). Binding of the primary antibody was detected by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody using the Western Blotting Luminol Reagent (Santa Cruz Biotechnology, Tebu, France). Ponceau-S coloration (Sigma-Aldrich, France) was used as an internal control. Digitized images of the immunoblots obtained using an LAS-3000 imaging system (Fuji Photo Film Co., Ltd.) were used for densitometric measurements with an image analyzer (UnScan It software, Silk Scientific Inc.).
2.4. CBS enzyme activity assays
Determination of CBS activity was assayed on 400 μg of total proteins obtained from liver samples, as described [24]. Proteins were incubated for 1 h at 37 °C with 1 mM of propargylglycine, 0.2 mM of pyridoxal phosphate (PLP), 10 mM of l-serine, 10 mM of DL-Hcy, 0.8 mM of SAM, using DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)) based-assay. All the chemical products were obtained from Sigma (Sigma-Aldrich, France).
2.5. Data analysis
Statistical analysis was performed with one-way analysis of variance (ANOVA) followed by Fisher's post-hoc test using Statview software. The results are expressed as medians with interquartile ranges. Data were considered significant when p ≤ 0.05. Correlations between Dyrk1a protein level and CBS activity were determined by using Spearman's rank correlation, as data were not normally distributed according to Shapiro–Wilk test.
3. Results
3.1. CBS activity is decreased in liver of mice under-expressing Dyrk1a
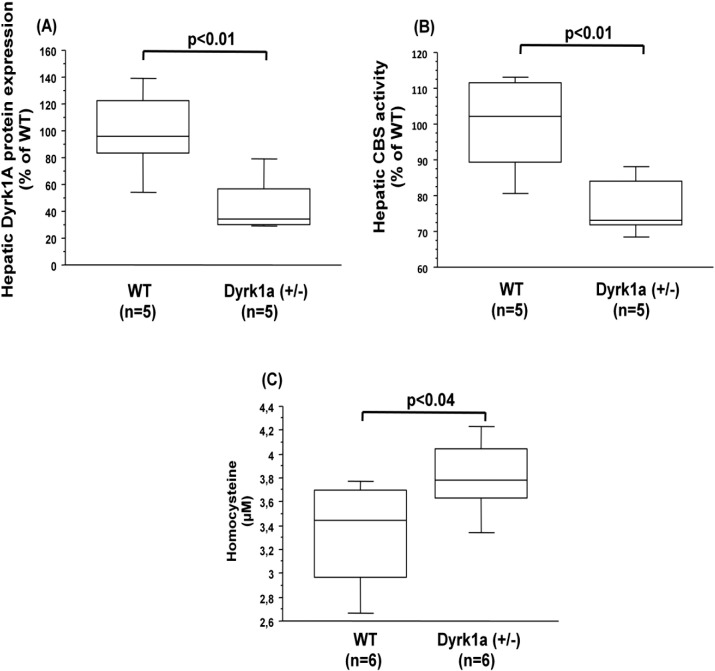
We first conducted the analysis of CBS activity in the liver of mice carrying only one copy of Dyrk1A, the Dyrk1a (+/−) mice [18]. The decreased protein expression of Dyrk1a (Fig. 1A), analyzed by slot blot method previously validated [23], was associated with a decreased CBS activity in liver of Dyrk1a (+/−) mice compared to control (wild type) mice (Fig. 1B). We observed a significant positive correlation between the liver CBS activity and liver Dyrk1A protein expression (ρ = 0.867, p < 0.009). Commensurate with the decreased hepatic CBS activity, plasma hcy levels were increased in Dyrk1a (+/−) mice compared to control (wild type) mice (Fig. 1C).
Fig. 1.
Hepatic dyrk1A protein expression, CBS activity, and plasma hcy levels in mice under-expressing Dyrk1a. (A) Dyrk1A protein expression was analyzed by slot blotting in the liver of wild type (WT) and mice under-expressing Dyrk1a (Dyrk1a (+/−)). (B) CBS activity in the liver of wild type (WT) and mice over-expressing Dyrk1a (Dyrk1a (+/−)). Data were normalized to the mean of wild-type (WT) mice. (C) Plasma hcy level. Data correspond to the medians with interquartile ranges. n = number of mice.
3.2. CBS activity is increased in liver of mice over-expressing Dyrk1a
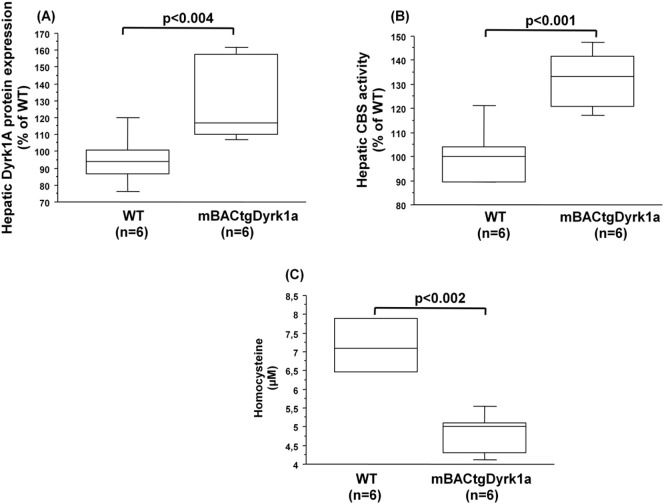
We also conducted the analysis of CBS activity in the liver of mice carrying the murine BAC containing one copy of the entire murine Dyrk1a gene, the mBACtgDyrk1a mice [17]. The increased protein expression of Dyrk1a (Fig. 2A) was associated with an increased CBS activity in the liver of mBACtgDyrk1a mice compared to control (wild type) mice (Fig. 2B). We also observed a significant positive correlation between the liver Dyrk1A protein expression and CBS activity (ρ = 0.603, p < 0.006). As previously described [15], plasma hcy levels were decreased in mBACtgDyrk1a mice compared to control (wild type) mice (Fig. 2C).
Fig. 2.
Hepatic dyrk1A protein expression, CBS activity, and plasma hcy levels in mice over-expressing Dyrk1a. (A) Dyrk1A protein expression was analyzed by slot blotting in the liver of wild type (WT) and mice over-expressing Dyrk1a (mBACtgDyrk1a). (B) CBS activity in the liver of wild type (WT) and mice over-expressing Dyrk1a (mBACtgDyrk1a). Data were normalized to the mean of wild-type (WT) mice. (C) Plasma hcy level. Data correspond to the medians with interquartile ranges. n = number of mice.
3.3. Dyrk1a protein expression is increased in liver of GK rats with increased CBS activity
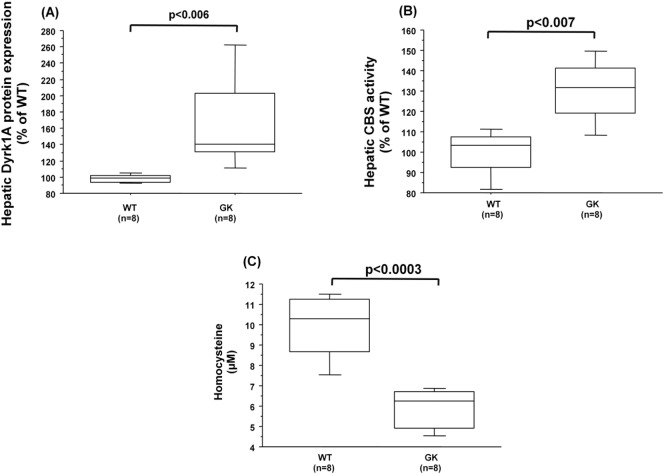
We previously found a lower plasma level of hcy concomitantly with an increased liver activity of CBS in GK rats, a spontaneous nonobese model of type 2 diabetes [16]. We therefore used this rat model in order to analyze the hepatic Dyrk1a protein expression in a non-modified genetic context. The increased protein expression of Dyrk1a (Fig. 3A) was associated with an increased CBS activity in the liver of GK rats compared to nondiabetic Wistar (wild type) rats (Fig. 3B). We also observed a significant positive correlation between liver Dyrk1A protein expression and CBS activity (ρ = 0.625, p < 0.02). As previously described [15], plasma hcy levels were decreased in GK rats compared to Wistar (wild type) rats (Fig. 3C).
Fig. 3.
Hepatic dyrk1A protein expression, CBS activity, and plasma hcy levels in GK and Wistar rats. (A) Dyrk1A protein expression was analyzed by slot blotting in the liver of Wistar (WT) and Goto-Kakizaki (GK) rats. (B) CBS activity in the liver of Wistar (WT) and Goto-Kakizaki (GK) rats. Data were normalized to the mean of Wistar (WT) rats. (C) Plasma hcy level. Data correspond to the medians with interquartile ranges. n = number of rats.
4. Discussion
Dyrk1a encodes a dual-specificity tyrosine-phosphorylation-regulated kinase, which has been shown to play an important role in signaling transduction in biological processes. Along with quantifying the link between CBS activity and Dyrk1a, we used two different models carrying altered gene copy numbers for Dyrk1a: a mBACtgDyrk1a model with three copies of the murine Dyrk1a gene and a Dyrk1a (+/−) model with only one copy. For each model, a positive correlation was found between the liver CBS activity and liver Dyrk1a protein expression. This correlation was confirmed in a non-modified genetic context, a spontaneous nonobese rat model of type 2 diabetes. We previously reported a reduction of Dyrk1a protein level in the liver of CBS-deficient mice [14], suggesting a link between Dyrk1a related pathways and the hcy cycle. We also previously showed that the over-expression of Dyrk1a diminishes the plasma hcy level [15], which is confirmed in this present study with the murine mBACtgDyrk1a model. This result was obtained with different murine models which contain the human (a YAC transgenic for a human chromosome 21 fragment carrying five genes including PIGP, TTC3, DSCR9, DSCR3 and Dyrk1a) or the murine gene (the mBACtgDyrk1a model) and correlates with an increased hepatic SAHH activity [15]. However, unlike the present study, we found no effect on hepatic CBS activity in YAC transgenic mice in agreement with the hepatic SAM levels (an allosteric activator of CBS) [15]. Even if the difference does not share statistical differences, hepatic levels of SAM and SAM/SAH ratio were increased in mBACtgDyrk1a mice. CBS catalyzes the PLP-dependent condensation of serine with hcy. We recently found that an adenoviral construct designed to restrict expression of Dyrk1a to hepatocytes decreased plasma hcy levels in CBS deficient mice [25]. This result was associated with a significant increase in hepatic PLP, hepatic SAM/SAH ratio, hepatic CBS activity and SAHH activity [25]. Taken together, our results reinforce the role of Dyrk1a in one carbon metabolism.
Minimal deletions in the region where human DYRK1A maps have been described in partial monosomy 21 patients [26], who present microcephaly, intellectual disability, intrauterine and postnatal growth retardation, characteristic physical features, seizures, epilepsy and mild to severe MRI alterations resembling brain atrophy, and idiopathic thrombocytopenia [8]. Dyrk1a (+/−) mice present reductions in brain size, possible intrauterine growth retardation, and postnatal growth retardation. The striking phenotypic similarities between Dyrk1a (+/−) mice and partial monosomy 21 patients suggest that some of the characteristic features of partial monosomy 21 could be directly related to the dose reduction of Dyrk1a [18]. Truncation mutations of DYRK1A result in a severe clinical phenotype including microcephaly [6]. A microdeletion in DYRK1A induces similar phenotypes [9]. On clinical reexamination at 13 years of age, one patient had pectus excavatum, kyphosis, and slight scoliosis, and his skin was very thin [6]. Some phenotypes seen in these patients were compatible with the phenotype of CBS deficient patients. The major clinical manifestations of CBS deficient patients include intellectual disability, developmental retardation, seizures, behavioral or psychiatric disorder, dislocation of the optic lens, skeletal abnormalities, and early thromboembolic disorder and decreased platelet survival time [2], [27]. Up to 20% of CBS deficient patients have seizures [28]. Elevated CSF hcy levels have been shown to compromise blood–brain barrier integrity and reduce seizure threshold in rodents [29], [30], [31]. Epilepsy is primarily caused by the inhibition of gamma-amino-butyric-acid (GABA) receptor, an inhibitory neurotransmitter in the neuronal synapse. Epileptogenesis induced by pentylenetetrazole (PTZ) caused a significant increase in serum hcy in comparison to control rats [32]. Unlüçerçi and colleagues [33] also reported increase of serum hcy levels in epilepsy. Elevated hcy levels were found to inhibit GABA by competing with it for attachment to its receptors [34]. Indeed, mBACtgDyrk1a mice with increased dosage of Dyrk1a and decreased plasma hcy levels are protected against PTZ-induced seizures [34]. Conversely, Dyrk1a (+/−) mice with increased plasma hcy levels, display more convulsions following PTZ administration [35]. Increased Dyrk1a expression and low plasma hcy levels have been associated with DS, the most common genetic cause of significant cognitive disability [36], [37]. However until now, plasma hcy levels were not analyzed in partial monosomy 21 patients.
By identifying metabolic alterations associated with cognitive impairment, it may be possible to develop medical or dietary interventions to ameliorate cognitive disabilities in persons with DS. Thus, among the genes from the common duplicated region and deleted regions, Dyrk1a is the best candidate gene for cognitive impairment phenotypes. There is evidence that CBS enzyme activity is increased in persons with DS [38]. If CBS, which gene is on chromosome 21, also contributes to the cognitive disability seen in persons with DS, then treatments that decrease the enzyme activity would also be desirable. De la Torre and colleagues recently demonstrated that green tea flavonol epigallocatechin-gallate (EGCG), an inhibitor of Dyrk1a, reverses the cognitive deficits in a pilot study in DS individuals with effects on memory recognition, working memory and quality of life [39]. In light of the present results, if EGCG will be a promising therapeutic tool for cognitive enhancement in DS, it would be interesting to analyze its effect targeting abnormalities of one carbon metabolism.
5. Conclusions
A positive correlation was found between the liver Dyrk1a protein expression and CBS activity in modified and non-modified genetic context, which reinforces the role of this kinase in one carbon metabolism.
Acknowledgments
This work was supported by the Fondation Jérôme Lejeune (30CA1140087) and the Association Gaspard Félix (L'AGAFE) (30S1B07). We acknowledge the platform accommodation and animal testing of the animal house at the Institute Jacques-Monod (University Paris Diderot).
References
- 1.Selhub J. Homocysteine metabolism. Annu. Rev. Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Mudd S.H., Skovby F., Levy H.L., Pettigrew K.D., Wilcken B., Pyeritz R.E. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am. J. Hum. Genet. 1985;37:1–31. [PMC free article] [PubMed] [Google Scholar]
- 3.Sachdev P.S., Valenzuela M., Wang X.L., Looi J.C., Brodaty H. Relationship between plasma homocysteine levels and brain atrophy in healthy elderly individuals. Neurology. 2002;58:1539–1541. doi: 10.1212/wnl.58.10.1539. [DOI] [PubMed] [Google Scholar]
- 4.Sachdev P.S. Homocysteine and brain atrophy. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005:1152–1161. doi: 10.1016/j.pnpbp.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Obeid R., McCaddon A., Herrmann W. The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. Clin. Chem. Lab. Med. 2007;45:1590–1606. doi: 10.1515/CCLM.2007.356. [DOI] [PubMed] [Google Scholar]
- 6.Moller R.S., Kubart S., Hoeltzenbein M., Heye B., Vogel I., Hansen C.P., Menzel C., Ullmann R., Tommerup N., Ropers H.H., Tumer Z., Kalscheuer V.M. Truncation of the Down syndrome candidate gene DYRK1A in two unrelated patients with microcephaly. Am. J. Hum. Genet. 2008:1165–1170. doi: 10.1016/j.ajhg.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto T., Shimolima K., Nishizawa T., Matsuo M., Ito M., Imai K.T. Clinical manifestations of the deletion of Down syndrome critical region including DYRK1A and KCNJ6. Am. J. Med. Genet. A. 2011;155:113–119. doi: 10.1002/ajmg.a.33735. [DOI] [PubMed] [Google Scholar]
- 8.Oegema R., de Klein A., Verkerk A.J., Schot R., Dumee B., Douben H., Eussen B., Dubbel L., Poddighe P.J., van der Laar I., Dobyns W.B., van der Spek P.J., Lequin M.H., de Coo I.F., de Wit M.C., Wessels M.W., Mancini G.M. Distinctive phenotypic abnormalities associated with submicroscopic 21q22 deletion including DYRK1A. Mol. Syndromol. 2010;1:113–120. doi: 10.1159/000320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Bon B.W., Hoischen A., Hehir-Kwa J., de Brouwer A.P., Ruivenkamp C., Gijsbers A.C., Marcelis C.L., de Leeuw N., Veltman J.A., Brunner H.G., de Vries B.B. Intragenic deletion in DYRK1A leads to mental retardation and primary microcephaly. Clin. Genet. 2011;79:296–299. doi: 10.1111/j.1399-0004.2010.01544.x. [DOI] [PubMed] [Google Scholar]
- 10.Courcet J.B., Faivre L., Malzac P., Masurel-Paulet A., Lopez E., Callier P., Lambert L., Lemesle M., Thevenon J., Gigot N., Duplomb L., Ragon C., Marle N., Mosca-Boidron A.L., Huet F., Philippe C., Moncla A., Thauvin-Robinet C. The DYRK1A gene is a cause of syndromic intellectual disability with severe microcephaly and epilepsy. J. Med. Genet. 2012;49:731–736. doi: 10.1136/jmedgenet-2012-101251. [DOI] [PubMed] [Google Scholar]
- 11.Valetto A., Orsini A., Bertini V., Toschi B., Bonuccelli A., Simi F., Sammartino I., Taddeucci G., Simi P., Saggese G. Molecular cytogenetic characterization of an interstitial deletion of chromosome 21 (21q22.13q22.3) in a patient with dysmorphic features, intellectual disability and severe generalized epilepsy. Eur. J. Med. Genet. 2012;55:362–366. doi: 10.1016/j.ejmg.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Ryu Y.S., Park S.Y., Jung M.S., Yoon S.H., Kwen M.Y., Lee S.Y., Choi S.H., Radnaabazar C., Kim M.K., Kim H., Kim K., Song W.J., Chung S.H. Dyrk1A-mediated phosphorylation of Presilin 1: a functional link between Down syndrome and Alzheimer's disease. J. Neurochem. 2010;115:574–584. doi: 10.1111/j.1471-4159.2010.06769.x. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe M., Osada J., Aratani Y., Kluckman K., Reddick R., Malinow M.R., Maeda N. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc. Natl. Acad. Sci. U. S. A. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamelet J., Noll C., Ripoll C., Paul J.L., Janel N., Delabar J.M. Effect of hyperhomocysteinemia on the protein DYRK1A in liver of mice. Biochem. Biophys. Res. Commun. 2009;378:673–677. doi: 10.1016/j.bbrc.2008.11.126. [DOI] [PubMed] [Google Scholar]
- 15.Noll C., Planque C., Ripoll C., Guedj F., Diez A., Ducros V., Belin N., Duchon A., Paul J.L., Badel A., Freminville B., Grattau Y., Bléhaut H., Herault Y., Janel N., Delabar J.M. DYRK1A, a novel determinant of the methionine–homocysteine cycle in different mouse models overexpressing this Down-syndrome-associated kinase. PLoS ONE. 2009;4:e7540. doi: 10.1371/journal.pone.0007540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noll C., Lacraz G., Ehses J., Coulaud J., Bailbe D., Paul J.L., Portha B., Homo-Delarche F., Janel N. Early reduction of circulating homocysteine levels in Goto-Kakizaki rat, a spontaneous nonobese model of type 2 diabetes. Biochim. Biophys. Acta. 2011;1812:699–702. doi: 10.1016/j.bbadis.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Guedj F., Pereira P.L., Najas S., Barallobre M.J., Chabert C., Souchet B., Sebrie C., Verney C., Herault Y., Arbones M., Delabar J.M. DYRK1A: a master regulatory protein controlling brain growth. Neurobiol. Dis. 2012;46:190–203. doi: 10.1016/j.nbd.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Fotaki V., Dierssen M., Alcantara S., Martinez S., Marti E., Casas C., Visa J., Soriana E., Estivill X., Arbones M.L. Dyrk1A haploinsufficiency affects viability and causes developmental delay and abnormal brain morphology in mice. Mol. Cell. Biol. 2002;22:6636–6647. doi: 10.1128/MCB.22.18.6636-6647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goto Y., Kakizaki M., Masaki N. Production of spontaneous diabetes by repetition of selective breeding. Tohoku J. Exp. Med. 1976;199:85–90. doi: 10.1620/tjem.119.85. [DOI] [PubMed] [Google Scholar]
- 20.Portha B., Lacraz G., Kergoat M., Homo-Delarche F., Giroix M.H., Bailbé D., Gangnerau M.N., Dolz M., Tourrel-Cuzin C., Movassat J. The GK rat beta-cell: a prototype for the diseased human beta-cell in type 2 diabetes? Mol. Cell. Endocrinol. 2009;297:73–85. doi: 10.1016/j.mce.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Fortin L.J., Genest J. Measurement of homocyst(e)ine in the prediction of atherosclerosis. Clin. Biochem. 1995;28:155–162. doi: 10.1016/0009-9120(94)00073-5. [DOI] [PubMed] [Google Scholar]
- 22.Ducros V., Demuth K., Sauvant M.P., Quillard M., Caussé E., Candito M., Read M.H., Drai J., Garcia I., Gerhardt M.F., SFBC Working group on homocysteine. French Society for Clinical Biology Methods for homocysteine analysis and biological relevance of the results. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002;781:207–226. doi: 10.1016/s1570-0232(02)00497-x. [DOI] [PubMed] [Google Scholar]
- 23.Planque C., Dairou J., Noll C., Bui L.C., Ripoll C., Guedj F., Delabar J.M., Janel N. Mice deficient in cystathionine beta synthase display increased Dyrk1A and SAHH activities in brain. J. Mol. Neurosci. 2013;50:1–6. doi: 10.1007/s12031-012-9835-0. [DOI] [PubMed] [Google Scholar]
- 24.Miller J.W., Nadeau M.R., Smith D., Selhub J. Folate-deficiency induced homocysteinaemia in rats: disruption of S-adenosylmethionine's co-ordinate regulation of homocysteine metabolism. Biochem. J. 1994;298:415–419. doi: 10.1042/bj2980415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tlili A., Jacobs F., de Koning L., Mohamed S., Bui L.C., Dairou J., Belin N., Ducros V., Dubois T., Paul J.L., Delabar J.M., De Geest B., Janel N. Hepatocyte-specific Dyrk1a gene transfer rescues plasma apolipoprotein A-I levels and aortic Akt/GSK3 pathways in hyperhomocysteinemic mice. Biochim. Biophys. Acta. 2013;1832:718–728. doi: 10.1016/j.bbadis.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto N., Ohashi H., Tsukahara M., Kim K.C., Soeda E., Niikawa N. Possible narrowed assignment of the loci of monosomy 21- associated microcephaly and intrauterine growth retardation to a 1.2-Mb segment at 21q22.2. Am. J. Hum. Genet. 1997;60:997–999. [PMC free article] [PubMed] [Google Scholar]
- 27.Harker L.A., Slichter S.J., Scott C.R., Ross R. Homocystinemia: vascular injury and arterial thrombosis. N. Engl. J. Med. 1974;291:537–543. doi: 10.1056/NEJM197409122911101. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz S., Zhou G.Z. N-methyl-d-aspartate receptors and CNS symptoms of homocystinuria. Lancet. 1991;337:1226–1227. doi: 10.1016/0140-6736(91)92900-m. [DOI] [PubMed] [Google Scholar]
- 29.Harding C.O., Pillers D.A., Steiner R.D., Bottiglieri T., Rosenblatt D.S., Debley J., Michael Gibson K. Potential for misdiagnosis due to lack of metabolic derangement in combined methylmalonic aciduria/hyperhomocysteinemia (cblC) in the neonate. J. Perinatol. 2003;23:384–386. doi: 10.1038/sj.jp.7210955. [DOI] [PubMed] [Google Scholar]
- 30.Mares P., Folbergrova J., Kubova H. Excitatory amino acids and epileptic seizures in immature brain. Physiol. Res. 2004;53:S115–S124. [PubMed] [Google Scholar]
- 31.Kamath A.F., Chauhan A.K., Kisucka J., Dole V.S., Loscalzo J., Handy D.E., Wagner D.D. Elevated levels of homocysteine compromise blood–brain barrier integrity in mice. Blood. 2006;107:591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haggag B.S., Hasanin A.H., Raafat M.H., Kawy H.S.A. Lamotrigine decreased hippocampal damage and improved vascular risk markers in a rat model of pentylenetetrazole induced kindling seizure. Korean J. Physiol. Pharmacol. 2014;18:269–278. doi: 10.4196/kjpp.2014.18.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unlüçerçi Y., Bekpinar S., Gürdöl F., Seferoğlu G. A study on the relationship between homocysteine and diabetic nephropathy in rats. Pharmacol. Res. 2002;45:249–252. doi: 10.1006/phrs.2001.0942. [DOI] [PubMed] [Google Scholar]
- 34.Lominadze D., Tyagi N., Sen U., Ovechkin A., Tyagi S.C. Homocysteine alters cerebral microvascular integrity and causes remodeling by antagonizing GABA-A receptor. Mol. Cell. Biochem. 2012;371:89–96. doi: 10.1007/s11010-012-1425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Souchet B., Guedj F., Sahún I., Duchon d A., Daubigney F., Badel A., Yanagawa Y., Barallobre M.J., Dierssen M., Yu E., Herault Y., Arbones M., Janel N., Créau N., Delabar J.M. Excitation/inhibition balance and learning are modified by Dyrk1a gene dosage. Neurobiol. Dis. 2014;69:65–75. doi: 10.1016/j.nbd.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Pogribna M., Melnyk S., Pogribny I., Chango A., Yi P., James S.J. Homocysteine metabolism in children with Down syndrome: in vitro modulation. Am. J. Hum. Genet. 2001;69:88–95. doi: 10.1086/321262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Licastro F., Marocchi A., Penco S., Porcellini E., Lio D., Dogliotti G., Corsi M.M. Does Down's syndrome support the homocysteine theory of atherogenesis? Experience in elderly subjects with trisomy 21. Arch. Gerontol. Geriatr. 2006;43:381–387. doi: 10.1016/j.archger.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Chadefaux B., Rethore M.O., Raoul O., Ceballos I., Poissonnier M., Gilgenkranz S., Allard D. Cystathionine beta synthase: gene dosage effect in trisomy 21. Biochem. Biophys. Res. Commun. 1985;128:40–44. doi: 10.1016/0006-291x(85)91641-9. [DOI] [PubMed] [Google Scholar]
- 39.De la Torre R., De Sola S., Pons M., Duchon A., Martınez de Lagran M., Farre M., Fito M., Benejam B., Langohr K., Rodriguez J., Pujadas M., Bizot J.C., Cuenca A., Janel N., Catuara S., Covas M.I., Blehaut H., Herault Y., Delabar J.M., Dierssen M. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol. Nutr. Food Res. 2014;58:278–288. doi: 10.1002/mnfr.201300325. [DOI] [PubMed] [Google Scholar]