Abstract
Background
False-positive screening results in newborn screening for cystic fibrosis may lead to parental stress, family relationship problems and a changed perception of the child's health.
Aim of the study
To evaluate whether parental anxiety induced by a false positive screening result disappears after six months and to assess whether a special program to inform parents prior and during the screening procedure prevents or diminishes parental anxiety.
Methods
Prospective controlled study assessing the long term effects of false-positive test results of newborn screening for cystic fibrosis (NBSCF) on parental anxiety and stress by means of questionnaires sent to parents of 106 infants with a false positive newborn screening test and 318 randomly selected infants with a true negative screening test. Additionally we interviewed 25 parents of the false-positive group.
Results
Parents showed negative feelings after being informed about the positive screening test result. After confirmation that their child was healthy and not suffering from CF, most parents felt reassured. After six months no difference in anxiety levels between both groups of parents was found. Well-informed parents in the false positive group experienced less stress.
Conclusions
A positive screening test result induces parental anxiety but false positive test results in NBSCF do not seem to cause long-term anxiety. Well-informed parents show lower stress and anxiety levels.
Keywords: Newborn screening, Cystic fibrosis, False positive, Parental knowledge, Parental feelings
1. Introduction
Newborn screening for cystic fibrosis (NBSCF) is implemented in many countries worldwide with a variety of screening programs [1], [2]. False-positive newborn screening results may lead to parental stress, family relationship problems and a changed perception of the child's health [3], [4], [5].
Long-lasting parental stress after false-positive results in newborn screening has been described for various screening programs [3], [5], [6], [7], [8]. Only two of these studies were controlled. In the first study mothers who had received a false-positive result, showed more anxiety and stress than the control group. Children with a false-positive result were also twice as often admitted to the hospital. However, in this study the age of the infants in both groups differed which may have had a substantial effect on the results [8]. Another controlled study found that false-positive results after newborn hearing screening did not cause long-term parental anxiety in the majority of the parents [6]. During a study comparing two novel strategies for NBSCF we investigated whether or not increased parental anxiety induced by a false-positive screening test persisted after six months [9]. A secondary aim was to assess if a special education program to inform health care workers and parents prior and during the screening program could prevent or diminish parental anxiety.
2. Methods
2.1. Screening program
This study was part of a larger study investigating the effectiveness of two novel screening strategies in the Netherlands [9]. In only one of these strategies false-positive screening tests were found. This strategy consisted of a measurement of immunoreactive trypsinogen (IRT) followed by determination of pancreas-associated protein (PAP). The second strategy consisted of IRT analysis, and DNA mutation analysis (35 mutations) when IRT ≥ 60 μg/l followed by DNA sequencing when a single mutation was found.
All newborns with a positive screening test were referred to a Cystic Fibrosis (CF)-centre for a sweat test to confirm or to exclude the diagnosis. The general practitioner (GP), or pediatrician, informed the parents about the positive test and the sweat test appointment (24 to 48 h later). The sweat test was performed at a gestational age of 38 weeks or more and a minimum weight of 2000 g. Sweat test results were given to the parents the same day or the day after, mostly by telephone by one of the staff members of the CF centre.
2.2. Educational material
An information leaflet about NBSCF was developed and pre-tested by eight pregnant women at their appointment with their midwife in a practice in Zoetermeer, the Netherlands. Parents were asked to read the leaflet and comment on its clearness, and whether they missed information or had additional questions. Their knowledge about CF screening was also tested after reading the leaflet.
The leaflet informed parents about CF, screening, genetics, the opting-out procedure, privacy and links to websites for more information. The leaflet was printed in Dutch (also available in nine languages on the internet) and was distributed to parents along with the leaflet of the routine newborn screening program three times: 1) in the third trimester of the pregnancy by their midwife/gynecologist, 2) at the registration of their child's birth at the city hall, and 3) when the heel prick was performed.
Before the screening program started we educated the healthcare workers involved in NBSCF (midwifes/gynecologists/general practitioners/performers of the heel prick/pediatricians). After a positive screening test, the GP received written information about the study, CF and the sweat test. The GP also received written information to give to the parents.
2.3. Study population
All parents of infants with a false-positive screening test result (normal sweat test result; chloride concentration < 30 mmol/l) born in 2008 were invited by letter to complete a questionnaire. Infants with a lethal congenital condition or infants with a positive screening test for another disease were excluded, whereas parents who did not want further participation in the study were not invited.
The three card numbers, following the heel prick card of the infant with a false positive result were used to compose a control group (C) of parents of screen-negative infants.
2.4. Study design
This was a prospective controlled observational study. All parents of babies with a false-positive screening result (FP group) and control (C) group received a questionnaire at home six months after the positive test result. The results were recorded anonymously. Additional semi-structured interviews were held with 25 parents of the FP group.
2.5. Questionnaire
The questionnaire contained items based on a study about the impact of a false-positive result after Medium Chain Acetyl-CoA Dehydrogenase Deficiency (MCADD) screening [10], the existing Hospital Anxiety and Depression Scale (HADS) [11] and items of the TNO-AZL Preschool Children Quality of Life questionnaire (TAPQOL) [12], [13].
First, parents were asked to complete eleven questions about NBS for CF, based on the information leaflet. Scores ranged from 0 (all wrong) to 11 (all answers correct).
These knowledge items were followed by four questions about how parents assessed the risk of their child being affected with CF, their perceived reliability of the test, whether they regretted their participation and if they would participate again. Scores ranged from 1 (negative) to 5 (positive). Parents were asked whether they were informed about NBSCF (yes/no), if they were satisfied with the information by different sources (scale 1–10), and if they had missed any information.
We used parts of existing standardized questionnaires to prevent the questionnaire being too long and to improve the response. We selected 10 questions of the TAPQOL [12], [13] concerning the child's health, emotions and behavioral problems in the last three months (never/sometimes/often). The selected health items were based on possible CF-related physical complaints (stomach pain, cramps, airway problems, dyspnoea, bad appetite), behavioral problems (sleeping disturbances, crying) and emotions (happiness). In addition, parents were asked how often their child had been ill compared to other children (more often/equally/less frequently), their concern about the child's health (scale 1–5; 1 = very concerned 5 = not concerned), and if they had visited their GP.
Parents were also asked to complete four items of the HADS questionnaire, three anxiety items and one item on depression (scale 1–4; 0 = negative to 4 = positive) [11].
2.6. Interviews
Twenty-five additional semi-structured in-depth interviews were done by a research nurse (SR) at the parents' home to investigate the experiences of parents and the impact of the false-positive result in more detail. Parents were selected from the known false-positive infants, about five parents per 20 false-positive results.
The semi-structured questions contained six areas; 1) knowledge and education about CF screening, 2) performance of the heel prick, 3) information about the positive screening result, 4) experiences with the follow-up care, 5) feelings after the diagnosis was ruled out and 6) opinions about NBS for CF in general. All interviews were recorded on tape and transcribed. Two researchers (AV and SR) analyzed the results by independently assigning quotes to themes and comparing these themes.
2.7. Statistical analysis of the questionnaires
The two groups were compared using the Student's t-test for quantitative variables, the Chi-square test for dichotomous variables, and the Mann–Whitney-U or Kruskal–Wallis test for variables on an ordinal scale. In paired series, quantitative variables were compared by using the paired t-test (HADS score after NBS result and six months later). Cronbach's alpha was used to determine whether questions belonging to the same scale could be summed up in a single scale score (Cronbach's alpha of 0.7 or higher). For all tests, a p-value < 0.05 was considered to be significant.
The correlation between being well-informed (high total test score (8–11 out of 11 points)) and the level of stress and anxiety (HADS-sum scale) and the relationship between knowledge and negative feelings after being informed was explored using the Spearman correlation.
3. Results
3.1. Study population
There were 109 infants with a false positive screening test for CF in the IRT-PAP program. Parents of three infants with a false-positive result were excluded; two infants died soon after birth and parents of one child declined further participation. Therefore, we sent questionnaires to 106 cases and 318 controls. A total of 62 (59%) questionnaires from the false-positive (FP) group and 146 (46%) from the control (C) group were returned. Both groups had comparable demographic backgrounds, except for the number of mothers that completed the questionnaire in the control group (Table 1). Twenty-four mothers and three fathers participated in the 25 semi-structured interviews. The demographic data of this group were not significantly different from the false-positive group as a whole.
Table 1.
Demographic data of the population.
| False-positive n(%) |
Controls n(%) |
p value | |
|---|---|---|---|
| Total | 62 (58.8) | 146 (45.9) | 0.002b |
| -Mother | 48 (77.4) | 136 (93.8) | |
| -Father | 10 (16.1) | 8 (5.5) | |
| -Both | 4 (6.5) | 1 (0.7) | |
| Mean age (years) (SD; range) | 32 (4.7; 19–43) | 32 (4.1; 24–41) | 0.806c |
| Married status | |||
| -Married | 41 | 94 | 0.967b |
| -Living together | 20 | 48 | |
| -Single parent | 1 | 3 | |
| Educational level | |||
| -No education | 2 (3.2) | 3 (2.1) | 0.133a |
| -Primary school | 1 (1.6) | 0 | |
| -Secondary school | 33 (53.3) | 64 (44.1) | |
| -Postgraduate | 26 (41.9) | 78 (53.8) | |
| Number of children | |||
| -1 | 33 (53.2) | 63 (43.4) | 0.155b |
| -2 | 19 (30.6) | 66 (45.6) | |
| -3 | 5 (8.1) | 11 (7.6) | |
| -4 | 4 (6.5) | 5 (3.4) | |
| -More than 4 | 1 (1.6) | 0 | |
| Land of origin | |||
| -The Netherlands | 57 (91.9) | 137 (94.4) | 0.536b |
| -Other European | 1 (1.6) | 4 (2.8) | |
| -Other non-European | 4 (6.5) | 4 (2.8) | |
| First language | |||
| -Dutch | 53 (85.5) | 128 (88.3) | 0.297b |
| -Dutch and other language | 8 (12.9) | 17 (11.7) | |
| -Other | 1 (1.6) | 0 | |
| Do you understand Dutch? | |||
| Yes | 60 (98.4) | 143 (98.6) | 0.887a |
| No | 0 | 2 (1.4) |
Mann–Whitney-U.
Chi-square test.
Student's t-test.
3.2. Knowledge and education
Parents in the FP group had significantly higher knowledge test scores compared to the C group, with a mean score of 8.6 (SD 1.86) versus 7.5 (SD 2.27) (p = 0.014;Table 2). Although parents in the FP group who searched for extra information had higher scores in the knowledge test, this difference was not significant (p = 0.051). About a third of the parents were informed about CF screening by their midwife/gynecologist (Table 3) and a third received the leaflet. The number of parents in the FP group wholooked for additional information on the internet was higher than in the control group (Table 3). The additional interviews showed that although a third (9/25 interviewed parents) of the GPs discouraged parents to search on the internet, almost all (21/25) did this anyway.
Table 2.
Correctly answered knowledge items in the false-positive group and control group.
| FP (n = 62) n(%) |
C (n = 146) n(%) |
p value⁎ | ||
|---|---|---|---|---|
| 1 | Early identification and treatment of CF will help to decrease health problems belonging to CF | 53 (86) | 130 (89) | 0.471 |
| 2 | The risk for a child to have CF is very small (< 1%) | 28 (45) | 64 (43) | 0.860 |
| 3 | Participation to the CF study is obligated for every newborn baby | 49 (79) | 107 (73) | 0.381 |
| 4 | More blood is needed to perform CF screening | 41 (66) | 77 (52) | 0.075 |
| 5 | All newborns with a positive test result are affected with CF | 60 (97) | 89 (60) | < 0.001 |
| 6 | Parents always receive the test result also in the case that the test is negative | 48 (77) | 127 (87) | 0.084 |
| 7 | Data from this study may only be used for this study on NBSCF | 43 (69) | 115 (79) | 0.381 |
| 8 | A healthy person can be a carrier of CF | 56 (90) | 91 (62) | < 0.001 |
| 9 | If the screening result is positive you will be informed by your general practitioner | 59 (95) | 104 (71) | < 0.001 |
| 10 | During this study, you will also be informed about the carrier status of your child | 35 (56) | 46 (32) | 0.001 |
| 11 | My child is affected with CF | 59 (95) | 135 (93) | 0.478 |
FP = false-positive group, C = control group (screen-negative), NBSCF = newborn screening for cystic fibrosis.
Chi-square test.
Table 3.
Information sources and the number of parents that received information by the different sources and their evaluation (scores 1–10) of the information they received.
| False-positive (n = 62) |
Control (n = 146) |
p value | |
|---|---|---|---|
| Midwife/gynecologist (%(n)) Score (SD; range) |
29 (18) 6.3 (1.19; 3–8) |
40 (59) 7.1 (1.40; 1–10) |
0.155⁎ 0.045# |
| Leaflet (%(n)) Score (SD; range) |
60 (37) 6.7 (1.55; 1–9) |
53 (78) 7.4 (0.90; 4–10) |
0.273⁎ 0.001# |
| Website (%(n)) Score (SD; range) |
32 (20) 7.1 (1.06; 5–9) |
8 (11) 7.0 (1.14; 5–8) |
0.001⁎ 0.831# |
| Screener (%(n)) Score (SD; range) |
45 (19) 6.7 (1.59; 2–10) |
62 (91) 7.1 (1.37; 1–10) |
0.599⁎ 0.236# |
| Satisfied with information (%(n)) | 36 (22) | 47 (146) | 0.117⁎ |
| Knew that child would be tested for CF (%(n)) | 38 (62.2) | Not asked |
Chi-square test.
Student's t-test.
Overall, the FP group was less positive about the given information compared to the C group, although this difference was only significant for the leaflet (p < 0.001) and the midwife/gynecologist (p = 0.045). The FP group gave the highest value for the information on the website, but parents in the FP group (39%) missed information about what would happen after a positive screening result. Parents' opinions about being informed face-to-face or by telephone varied; 7/25 interviewed parents were informed by telephone, 18 face-to-face of which five were invited to the doctor's practice and in 13 cases the doctor visited the parents at home. Some parents preferred being informed personally, whereas others got the idea that the problem must be very serious if the doctor would pay them a visit.
3.3. Estimated risk of being affected with CF, reliability of screening test and regret
The majority of parents in both groups estimated the risk of their child being affected with CF low, 95% (FP; 59/62) and 92% (C; 134/146) scored one or two on a five-point scale (1 = low 5 = high). Parents in the FP group considered the CF screening test less reliable (p < 0.001). None of the parents in the C group regretted their participation in the NBSCF study, versus 11% (7/62) in the FP group (p < 0.001). Most parents in the C group would participate again in a next pregnancy (142/146), while 6% (4/62) of the FP group would not participate again and 16% (10/62) was not certain (p < 0.001).
3.4. Anxiety and depression
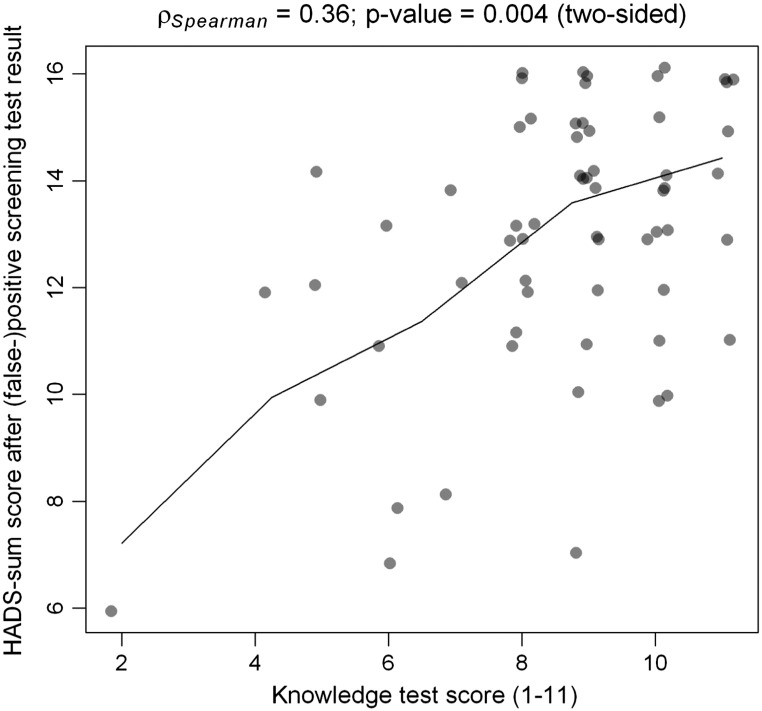
Parents were asked about their feelings of anxiety and depression in the last week (HADS score, Table 4). The Cronbach's alpha was 0.771, therefore we made a sum-HADS score (range 4–16; high HADS scores mean low levels of anxiety and depression). The mean HADS score was 12.95 (SD 2.47, range 6–16) in the FP group and 13.39 (SD 2.16, range 5–16) in the C group, which was not significantly different (p = 0.206). Well-informed (knowledge test score 8–11) parents in the FP group showed significantly higher sum-HADS scores (Fig. 1), even when corrected for the parental educational level, which means that they experienced less feelings of anxiety and depression (p < 0.018).
Table 4.
Parental stress and concern (HADS score) six months after NBSCF.
| FP %(n) n = 62 |
C %(n) n = 146 |
p value | |
|---|---|---|---|
| I feel tense | 0.688 | ||
| Mostly | 3 (2) | 2 (3) | |
| Often | 15 (9) | 8 (11) | |
| Sometimes | 53 (33) | 56 (81) | |
| Never | 29 (18) | 35 (50) | |
| I can sit down quietly and feel relaxed | 0.195 | ||
| Not at all | 5 (3) | 3 (5) | |
| Not often | 16 (10) | 23 (33) | |
| Mostly | 50 (31) | 37 (53) | |
| Always | 29 (18) | 37 (54) | |
| I am worried | 0.520 | ||
| Very often | 7 (4) | 3 (4) | |
| Often | 10 (6) | 8 (11) | |
| Sometimes, but not too often | 40 (25) | 44 (64) | |
| Sometimes | 44 (27) | 46 (66) | |
| I feel cheerful | 0.117 | ||
| Not at all | 0 | 1 (2) | |
| Not often | 8 (5) | 3 (4) | |
| Sometimes | 21 (13) | 15 (22) | |
| Mostly | 71 (44) | 81 (117) | |
| Mean score (4–16) | 12.95 | 13.39 | 0.289 |
Numbers do not always add up to the numbers at the top of the columns due to incomplete filled in questionnaires. P values calculated with Mann–Whitney-U test.
NBSCF = newborn screening for cystic fibrosis, HADS = Hospital Anxiety and Depression Score. FP = false positive group C = control group (screen negative).
Fig. 1.
The relation between parental knowledge about NBSCF measured by the knowledge test score (range 0–11) and the level of parental anxiety and depression measured by the HADS-questionnaire. The questionnaire included four items of the Hospital Anxiety And depression Scale (HADS), namely three anxiety items and one item on depression (scale 1 to 4; 0 = negative and 4 = positive). We made a sum scale of the four HADS items (Cronbach'salpha was 0771) with a range of 4 to 16 points. High sum scores mean low levels of anxiety and depression. The knowledge test has 11 questions about cystic fibrosis and the screening test subjects that were listed in the leaflet that parents received (1 point for each good answer, score 1 to 11 points).
3.5. Emotions after a positive screening test result
We asked the FP group how they felt after being informed about the positive screening result. The Cronbach's alpha was 0.837, therefore we established a sum-scale ranging from 6 to 30 points (negative to positive). Parents showed strong negative feelings after being informed about the positive screening result, with a mean score of 9.1 (SD 4.93, range 6–30). Six months later, the diagnosis of CF excluded, the mean score increased to 26.5 (SD 5.16, range 8–30). Fig. 2a and b shows the difference between emotional feelings immediately after the positive test result and six months thereafter (p < 0.001). There was no significant correlation between being well-informed and negative emotional feelings (p = 0.589).
Fig. 2.
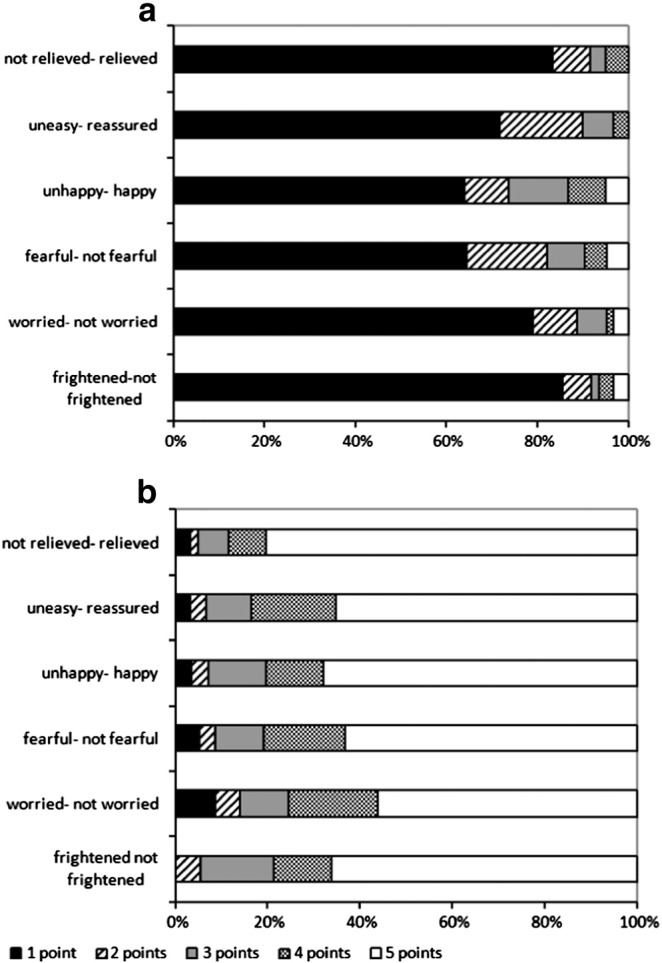
Emotional feelings of parents expressed (a) after they were informed about the positive screening test result for CF and (b) six months after exclusion of the diagnosis cystic fibrosis. Parents were asked to report their feelings and emotions on a five point scale (1 = positive, 5 = negative). They were asked how they felt after being informed about the positive screening test for cystic fibrosis and six months after exclusion of the CF diagnosis. The figure represents the percentage of parents with a certain score (1–5).
3.6. Parents' opinion of their child's health (TAPQOL)
Parents in the FP group expressed similar physical complaints compared to the C group (Table 5). Parental concern about their baby's health or the number of visits to their GP did not differ significantly between both groups (p = 0.223 and p = 0.198 respectively). About half of the interviewed parents (48%) observed symptoms that match with CF, mostly during airway infections. Although almost all (22/25) were sure that their child was not affected with CF, five stated that they had had some doubts about the diagnosis in the past and three of them still felt unsure.
Table 5.
Parents opinion about their child's health in the last three months (TAPQOL).
| FP %(n) n = 62 |
C %(n) n = 146 |
p value | |
|---|---|---|---|
| Health problems? | 0.761 | ||
| Yes | 22.5 (14) | 20.7 (30) | |
| No | 77.5 (48) | 79.3 (115) | |
| How often did your child suffer from stomach ages? | 0.939 | ||
| Never | 72.5 (45) | 71.1 (101) | |
| Sometimes | 22.5 (14) | 24.7 (35) | |
| Often | 5.0 (3) | 4.2 (6) | |
| How often did your child suffer from abdominal cramps? | 0.545 | ||
| Never | 36.1 (22) | 30.3 (43) | |
| Sometimes | 50.8 (31) | 59.2 (84) | |
| Often | 13.1 (8) | 10.5 (15) | |
| How often did you notice airway problems? | 0.838 | ||
| Never | 80.3 (49) | 80.9 (115) | |
| Sometimes | 16.4 (10) | 14.1 (20) | |
| Often | 3.3 (2) | 5.0 (7) | |
| How often was your child short of breath? | 0.836 | ||
| Never | 83.6 (51) | 80.4 (115) | |
| Sometimes | 13.1 (8) | 15.4 (22) | |
| Often | 3.3 (2) | 4.2 (6) | |
| Sleeping disturbances? | 0.521 | ||
| Never | 58.1 (36) | 50.6 (73) | |
| Sometimes | 33.8 (21) | 42.4 (61) | |
| Often | 8.1 (5) | 7.0 (10) | |
| How often did your child cry at night? | 0.058 | ||
| Never | 66.2 (41) | 48.6 (70) | |
| Sometimes | 29.0 (18) | 46.5 (67) | |
| Often | 4.8 (3) | 4.9 (7) | |
| Were there any feeding difficulties? | 0.256 | ||
| Never | 82.2 (51) | 77.2 (112) | |
| Sometimes | 13.0 (8) | 20.7 (30) | |
| Often | 4.8 (3) | 2.1 (3) | |
| Number of health visits to the general practitioner? | |||
| Not visited | 0.198 | ||
| Once | 40 (25) | 56 (79) | |
| 2–3 times | 34 (21) | 30 (44) | |
| ≥ 4 times | 18 (11) | 11 (16) | |
| 8 (5) | 4 (6) |
Numbers do not always add up to the numbers at the top of the columns due to incomplete filled in questionnaires. P values calculated with Chi-square test.
FP = false positive group, C = control group (screen-negative), TAPQOL = TNO-AZL Preschool children Quality Of Life questionnaire.
3.7. Follow-up care after a positive screening result
About 88% of the parents were informed by their GP and 12% by a pediatrician. Most parents were satisfied about how they were informed. Satisfaction with time of referral was negatively associated with the number of days between being informed and the appointment at the hospital (Spearman correlation coefficient 0.402; p = 0.001). A third of the infants was referred to the hospital the next day, 20% on the second day, 20% on the third day, and about a third of the parents after three days.
Most parents appreciated the way in which they were counseled in the hospital. We saw a trend between negative parental feelings and the number of days between the positive test and the diagnosis, although this was not significant (Spearman p = 0.059). The time between performing the sweat test and the result varied; about half of the parents got the result on the same day, 16% the next day, and 34% had to wait two days or longer. In 17% of the infants the sweat test failed and had to be repeated.
4. Discussion
This study showed that most parents experience strong feelings of concern and anxiety when they receive a positive screening result for NBSCF. However, six months later, most parents were reassured and sure that their child did not have CF. Parents showed no significant differences in concern about their child's health compared to a screen negative control group. Stress-levels were similar in both groups.
Our results confirm the results of a recent non-controlled study showing that the early anxiety in parents induced by a positive screening result for CF was mostly decreased within three months thereafter, or that parents were reassured after genetic counseling and sweat test results [14]. The results after one and two years did not differ significantly [5].
Well-informed parents showed less feelings of anxiety and depression. This finding underscores the importance of high quality health education for parents who receive a (false-)positive result. A previous controlled study in NBS for biochemical genetic disorders also showed that parental stress is linked to poor understanding of the test and the disease that was investigated [3]. Some studies show that face-to-face communication leads to better parental understanding, and might prevent misunderstanding of results, especially in low-educated parents and parents with difficulty with the English language [7].
Despite the specially developed website which was noted in the leaflet and GP-information, part of the GPs discouraged parents to look on the internet, something that parents did anyway. Very few studies report on internet as an information source for parents about NBS [14], [15], [16]. Two studies showed that half respectively 82% of the parents use internet after a positive screening result to look for additional information, which is comparable to our study (69%) [16]. We think that parents should be guided to reliable websites with information that focuses on the screening itself and some additional information about CF.
The number of health visits during the six months after a false-positive result did not differ from the C group, similar to the findings of another controlled study showing no difference in primary care utilization, emergency room use and hospitalization by the age of six months [17]. Two other studies show a higher hospitalization rate in the FP group [3], [8]. However, in these studies the FP group differed from the control group in age and socio-economic status. Both studies came from the same group as the one that showed no difference, maybe as a consequence of improved information to parents about screening over time reducing parental concern.
Despite their experience with a false-positive test result, many parents were in favor of newborn screening (89%), similar to other studies [18]. Parents were most unsatisfied about not being informed about the possibility and implications of a false-positive result.
This study differs from most other studies evaluating the effect of a false-positive NBSCF result, as no carriers were identified. For some parents, the knowledge of being a carrier leads to misunderstanding, anxiety, and stress [19], [20], [21].
4.1. Strengths and limitations of the study
The design of this study, prospective, controlled, and of ample size, ensures that the results of our study can be considered as reliable. Also, additional interviews with a small sample of the parents allowed us to receive in depth information about parent's argumentation.
Most responders were born in the Netherlands and spoke Dutch as maternal language. Based on the population data we expected about 8% to be immigrants, of which 56% were of non-western origin, which was the case. Therefore multi-ethnicity cannot have influenced the results of our study.
However, when interpreting the results we must also consider some limitations. First, the response rate of 59% and 46% was acceptable but not very high. This response rate is common in questionnaire studies, higher response rates are seen in telephone interviews [3], [8], [15], [21]. We decided to use questionnaires so parents could decide whether they wanted to participate in private, and because we could reach a larger group. Second, the educational level of the responders was high and may not be representative for the entire population. Third, the relatively higher number of fathers in the false-positive group might have influenced the results, as mothers are often more anxious about the health of their child [4]. Recall bias may have influenced the differences we found about the information parents remembered to have obtained, as the questionnaires were sent about six months after the positive screening test result. However, during the interviews parents described this stressful period very well and detailed. We choose to send one questionnaire and not two at different time points because we wanted a high response rate and complete data.
5. Conclusions
False-positive results in NBSCF lead to strong negative feelings immediately after the positive test, but do not seem to cause long-term parental anxiety. Never-the-less, about one fifth of the parents continued to feel worried about their child's health when asked face-to-face. Parents do not assess their child's health differently when compared with a screen-negative control group. Referral within 24–28 h is important, satisfaction about time of referral was negatively associated with the number of days between being informed and the appointment at the hospital. Well-informed parents showed less anxiety and depression, which shows the importance of providing parents and professionals with adequate educational material. When the parents receive the result of a positive screening test, easy accessible, specially designed websites offer probably the best opportunities for providing the necessary information at the right time.
Footnotes
Data were previously presented on the ECFS in Hamburg 2011 as a poster and oral presentation. Abstract: “Do false positive test results for newborn screening for cystic fibrosis lead to long term parental anxiety”. A.M. Vernooij-van Langen, S.J. Reijntjens, S.M. van der Pal, J.G. Loeber et al. Journal of Cystic Fibrosis Vol. 10 Suppl. 1, page S11.
Contributor Information
A.M.M. Vernooij-van Langen, Email: amm.vernooij@gmail.com.
S.M. van der Pal, Email: Sylvia.vanderpal@tno.nl.
A.J.T. Reijntjens, Email: srs07@atriummc.nl.
J.G. Loeber, Email: Gerard.loeber@gmail.com.
E. Dompeling, Email: Edward.dompeling@mumc.nl.
J.E. Dankert-Roelse, Email: Jeannettedankert@gmail.com.
References
- 1.Southern K.W., on behalf of the ECFS CF Neonatal Screening Working Group A survey of newborn screening for cystic fibrosis in Europe. J. Cyst. Fibros. 2007;6:57–65. doi: 10.1016/j.jcf.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Castellani C., Southern K.W., Brownlee K., Dankert Roelse J., Duff A., Farrell M. European best practice guidelines for cystic fibrosis neonatal screening. J. Cyst. Fibros. 2009;8:153–173. doi: 10.1016/j.jcf.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Gurian E.A., Kinnamon D.D., Henry J.J., Waisbren S.E. Expanded newborn screening for biochemical disorders: the effect of a false-positive result. Pediatrics. 2006;117:1915–1921. doi: 10.1542/peds.2005-2294. [DOI] [PubMed] [Google Scholar]
- 4.Hewlett J., Waisbren S.E. A review of the psychosocial effects of false-positive results on parents and current communication practices in newborn screening. J. Inherit. Metab. Dis. 2006;29:677–682. doi: 10.1007/s10545-006-0381-1. [DOI] [PubMed] [Google Scholar]
- 5.Beucher J., Leray E., Deneuville E., Roblin M., Pin I., Bremont F. Psychological effects of false-positive results in cystic fibrosis newborn screening: a two-year follow-up. J. Pediatr. 2010;156:771–776. doi: 10.1016/j.jpeds.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 6.van der Ploeg C.P., Lanting C.I., Kauffman-de Boer M.A., Uilenburg N.N., de Ridder-Sluiter J.G., Verkerk P.H. Examination of long-lasting parental concern after false-positive results of neonatal hearing screening. Arch. Dis. Child. 2008;93:508–511. doi: 10.1136/adc.2007.129320. [DOI] [PubMed] [Google Scholar]
- 7.Tluczek A., Mischler E.H., Bowers B., Peterson N.M., Morris M.E., Farrell P.M. Psychological impact of false-positive results when screening for cystic fibrosis. Pediatr. Pulmonol. Suppl. 1991;7:29–37. doi: 10.1002/ppul.1950110707. [DOI] [PubMed] [Google Scholar]
- 8.Waisbren S.E., Albers S., Amato S., Ampola M., Brewster T.G., Demmer L. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. JAMA. 2003;290:2564–2572. doi: 10.1001/jama.290.19.2564. [DOI] [PubMed] [Google Scholar]
- 9.Vernooij-van Langen A.M., Loeber J.G., Elvers B., Triepels R.H., Gille J.J., Van der Ploeg C.P. Novel strategies in newborn screening for cystic fibrosis: a prospective controlled study. Thorax. 2012;67:289–295. doi: 10.1136/thoraxjnl-2011-200730. [DOI] [PubMed] [Google Scholar]
- 10.Schrik Pennings J. maar ook begrip. Reacties van ouders op fout-positieve uitslag na neonatale screening. Med. Contact [Dutch] 2007;62:58–60. [Google Scholar]
- 11.Zigmond A.S., Snaith R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 12.Fekkes M., Bruil J., Vogels T. TNO Report PG/JGD2003221. 2004. TAPQOL Manual. Developed by Leiden Center for Child health and Pediatrics LUMC TNO. [Google Scholar]
- 13.Bunge E.M., Essink-Bot M.L., Kobussen M.P., van Suijlekom-Smit L.W., Moll H.A., Raat H. Reliability and validity of health status measurement by the TAPQOL. Arch. Dis. Child. 2005;90:351–358. doi: 10.1136/adc.2003.048645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang C.W., McColley S.A., Lester L.A., Ross L.F. Parental understanding of newborn screening for cystic fibrosis after a negative sweat-test. Pediatrics. 2011;127:276–283. doi: 10.1542/peds.2010-2284. [DOI] [PubMed] [Google Scholar]
- 15.Moran J., Quirk K., Duff A.J., Brownlee K.G. Newborn screening for CF in a regional paediatric centre: the psychosocial effects of false-positive IRT results on parents. J. Cyst. Fibros. 2007;6:250–254. doi: 10.1016/j.jcf.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Feldman M.D. Munchausen by internet: detecting factitious illness and crisis on the Internet. South. Med. J. 2000;93:669–672. [PubMed] [Google Scholar]
- 17.Lipstein E.A., Perrin J.M., Waisbren S.E., Prosser L.A. Impact of false-positive newborn metabolic screening results on early health care utilization. Genet. Med. 2009;11:716–721. doi: 10.1097/GIM.0b013e3181b3a61e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tluczek A., Koscik R.L., Farrell P.M., Rock M.J. Psychosocial risk associated with newborn screening for cystic fibrosis: parents' experience while awaiting the sweat-test appointment. Pediatrics. 2005;115:1692–1703. doi: 10.1542/peds.2004-0275. [DOI] [PubMed] [Google Scholar]
- 19.Ciske D.J., Haavisto A., Laxova A., Rock L.Z., Farrell P.M. Genetic counseling and neonatal screening for cystic fibrosis: an assessment of the communication process. Pediatrics. 2001;107:699–705. doi: 10.1542/peds.107.4.699. [DOI] [PubMed] [Google Scholar]
- 20.Hayeems R.Z., Bytautas J.P., Miller F.A. A systematic review of the effects of disclosing carrier results generated through newborn screening. J. Genet. Couns. 2008;17:538–549. doi: 10.1007/s10897-008-9180-1. [DOI] [PubMed] [Google Scholar]
- 21.Tijmstra T. False positive results in screening tests: experiences of parents of children screened for congenital hypothyroidism. Fam. Pract. 1986;3:92–96. doi: 10.1093/fampra/3.2.92. [DOI] [PubMed] [Google Scholar]