Abstract
Mucopolysaccharidosis type IVA or Morquio type-A disease is a hereditary lysosomal storage disorder caused by deficient activity of the lysosomal enzyme N-acetylgalactosamine-6-sulfate sulfatase (GALNS). The disease is caused by lysosomal accumulation of unprocessed glycosaminoglycans (GAGs) that manifests with severe to mild skeletal and cardiopulmonary abnormalities. We have developed a modified microtiter plate-based enzyme activity assay using dried blood spots and a fluorescent substrate for measuring specific GALNS activity to identify patients with MPS IVA.
Abbreviations: 4-MU, 4-methylumbelliferone; 4-MU-β-gal-S, 4-methylumbelliferyl-β-d-galactopyranoside sulfate sodium salt; β-Gal, β-galactosidase; BSA, bovine serum albumin; DBS, dried-blood spot; ERT, enzyme replacement therapy; GAG, glycosaminoglycan; GALNS, n-acetylgalactosamine-6-sulfate sulfatase; LSD, lysosomal storage disease; MPS IVA, mucopolysaccharidosis type IVA
Keywords: MPS, Mucopolysaccharidosis, Morquio type A, Fluorometric enzyme assay
1. Introduction
Mucopolysaccharidosis type IVA (MPS IVA) or Morquio syndrome type A is a hereditary lysosomal storage disorder (LSD) caused by deficient activity of the lysosomal enzyme N-acetylgalactosamine-6-sulfate sulfatase (GALNS). Autosomal recessive mutations in the GALNS gene that encodes the enzyme result in impaired breakdown of two glycosaminoglycans (GAGs) in particular; keratan sulfate (KS) and chondroitin-6-sulfate (C6S) [1], [2]. The excessive storage of GAGs in lysosomes causes disturbance in cellular metabolism resulting in tissue and organ dysfunction. Accumulated GAGs may also be excreted in urine, with elevated levels measured by chromatography or mass spectrometry techniques [3], [4], [5], [6]. Mild to severe symptoms include skeletal and joint deformities, auditory and visual impairment, cardiovascular abnormalities and significant compromise of the respiratory system.
Clinical diagnosis of this condition is challenging, particularly due to the variation in symptoms likely caused by the heterogeneity of mutations in the GALNS gene. Furthermore, many clinical manifestations are common to other MPS subtypes including MPS IVB which results from mutations in the GLB1 gene that encodes for the enzyme β-galactosidase. Thus, routine radiological and clinical findings need further laboratory testing, including GAG analysis and enzyme activity testing to confirm the diagnosis [2]. Enzyme activity testing is especially crucial to distinguish between MPS IVA and MPS IVB [7].
Until recently, treatment options for MPS IVA patients were limited to supportive care. In February 2014 the US Food and Drug Administration approved enzyme replacement therapy (ERT) with recombinant human GALNS (BioMarin Pharmaceutical Inc., San Rafael, CA) to treat the clinical manifestations of MPS IVA [8]. The availability of ERT necessitates accurate laboratory testing for early confirmatory diagnosis of patients suspected to have Morquio syndrome in order to prevent severe disease onset and long term damage. We have, therefore, developed a microtiter plate-based fluorescence assay for measuring GALNS activity in dried blood spots (DBSs). This assay is modified from the protocol previously described for testing GALNS activity in leukocytes [7] and further improves on the previously reported method for dried blood spots [9]. We have previously developed similar fluorometric microtiter plate-based enzyme assays for MPS I & II [10], [11]. These DBS assays are accurate, robust and have a fast turn-around time, thus reducing the time to diagnosis and treatment. Alternative methods based on tandem mass spectrometry have been published that are designed for mass screening of newborns and are less suitable in the clinical diagnostic setting [12], [13].
2. Materials and methods
2.1. Reagents and solutions
All reagents for preparation of buffers and sample extraction (sodium hydroxide, sodium chloride, sodium acetate (anhydrous), sodium carbonate, sodium bicarbonate, lead acetate trihydrate, glacial acetic acid, phosphoric acid, sodium azide, triton X-100, sodium phosphate dibasic, sodium phosphate monobasic, bovine serum albumin (BSA), β-galactosidase (Aspergillus oryzae), 4-methylumbelliferone sodium salt (4-MU)) were obtained from Sigma Aldrich Corp. (St. Louis, MO). Molecular biology grade water was obtained from VWR International (Radnor, PA). 4-Methylumbelliferyl-β-d-galactopyranoside-6-sulfate sodium salt (4-MUβgal-S) substrate was purchased from Toronto Research Chemicals (Toronto, Canada).
Sample extraction buffer was prepared by dissolving 30 mmol/L lead acetate trihydrate in 50 mmol/L sodium acetate (pH 5.0). A substrate dilution buffer with a final concentration of 0.1 mol/L sodium acetate, 0.1 mol/L sodium chloride, 0.02% sodium azide and 5 mmol/L lead acetate was prepared (pH 4.3) and lyophilized substrate was added to make a 20 mmol/L working stock solution that was stored at − 20 °C in 1 mL aliquots for up to 6 months. For the second incubation step of GALNS enzyme assay, 10 U/mL β-galactosidase solution was prepared in 0.2% BSA with 0.02% sodium azide; and sodium phosphate buffer (0.9 mol/L) with 0.02% sodium azide (pH 4.3). Enzyme reaction was terminated with a stop buffer containing 1 mol/L sodium carbonate–bicarbonate (pH 10.7) with 0.01% triton X-100.
2.2. DBS samples
The normal range for GALNS enzyme activity was determined using de-identified dried blood spots (n = 75) from the Duke Biochemical Genetics Laboratory (BGL). In order to set up the affected patient range, de-identified DBS from MPS IVA patients (n = 13) diagnosed using leukocyte enzyme assay and/or molecular sequencing was kindly provided by Greenwood Genetic Center (GGC), obtained under their IRB protocol.
2.3. Enzyme extraction and assay setup
To test enzyme activity, one 3.2 mm punch from a DBS card (~ 3 μL of blood) was added to 100 μL of extraction buffer in a microfuge tube and incubated for 5–6 h at ambient/room temperature on a rocker. GALNS enzyme activity in the DBS extract was tested in a two-step incubation procedure.
2.3.1. First incubation
For the first incubation step, 20 μL aliquots of the DBS extract were transferred to four duplicate wells of the 96-well microtiter plate. Ten μL of 4-MUβgal-S substrate solution was added to two duplicate reaction wells in the first two rows, while the remaining two wells served as duplicate blanks (no substrate). Microtiter plates were sealed with adhesive aluminum foil and incubated for 20 h at 37 °C along with the remaining 4-MUβgal-S solution in microfuge tubes.
2.3.2. Second incubation
The next day, five μL of sodium phosphate buffer (pH 4.3) and 10 μL of exogenous β-galactosidase solution (10 U/mL) were added to each reaction well only. The microtiter plate was resealed and incubated for an additional 4 h at 37 °C.
2.3.3. Assay termination and GALNS measurement
The enzyme reaction was finally terminated by adding 105 μL of stop buffer to all wells (including blank wells). Five μL of sodium phosphate buffer, 10 μL of β-galactosidase (10 U/mL) and 10 μL of the remaining 4-MUβgal-S substrate were added to the duplicate blank wells to measure background fluorescence from the undigested substrate. After a brief centrifugation of the microplate, the fluorescence in individual wells was read using a bench-top fluorometer (Tecan Group Ltd., Männedorf, Switzerland). The mean fluorescence of the duplicate blank wells for each individual sample was subtracted from that of the reaction wells to calculate the relative fluorescence units (RFU). The RFU was then plotted against a freshly prepared 4MU standard curve and converted to enzyme activity units, expressed in pmol/punch/h, based on the standard curve.
2.4. Assay validation
Assay variability, sensitivity, precision and stability were tested to validate the protocol.
2.4.1. Variability
The intra-day assay variability was determined by testing a set of eight normal control DBS five times within one plate and calculating the % CV. Ten normal control spots were assayed individually on six different days to establish the inter-day assay variability. Two different analysts tested the same set of ten DBS to determine operator variability as part of the validation process.
2.4.2. Sensitivity
To establish the minimum detectable enzyme activity measurement, DBS extracts were serially diluted (1:5, 1:10, 1:20, 1:50 and 1:100) and assayed by the described method.
2.4.3. Stability
Stability of GALNS enzyme activity in DBS stored under different temperatures and time durations was determined by exposing ten individual DBS samples at − 20 °C, 4 °C and 22 °C (RT) for 7 days. DBS was also stored at higher temperatures (30 °C & 37 °C) for various lengths of time to mimic exposure during transportation from distant places in a delivery truck or when samples are held transiently during shipping and receiving in warm climates. Higher temperature exposure times were for 6 h, 16 h & 30 h before measuring GALNS enzyme activity in DBS.
2.4.4. Precision and specificity
Specificity of the assay was tested by assaying for GALNS activity in DBS samples from MPS IVA patients as well as those with other known LSDs (MPS I, MPS II, MPS VI and Fabry).
3. Results and discussion
Using appropriate substrates is critical for accurate measurement of enzyme activity levels. We tested substrates for GALNS activity measurement from four different commercial sources for optimal separation between enzyme activity levels using quality control (QC) DBS with low, intermediate and high activity levels. We selected the substrate from Toronto Research Chemicals (TRC) since it showed the best separation between the DBS activities tested under our assay conditions (data not shown).
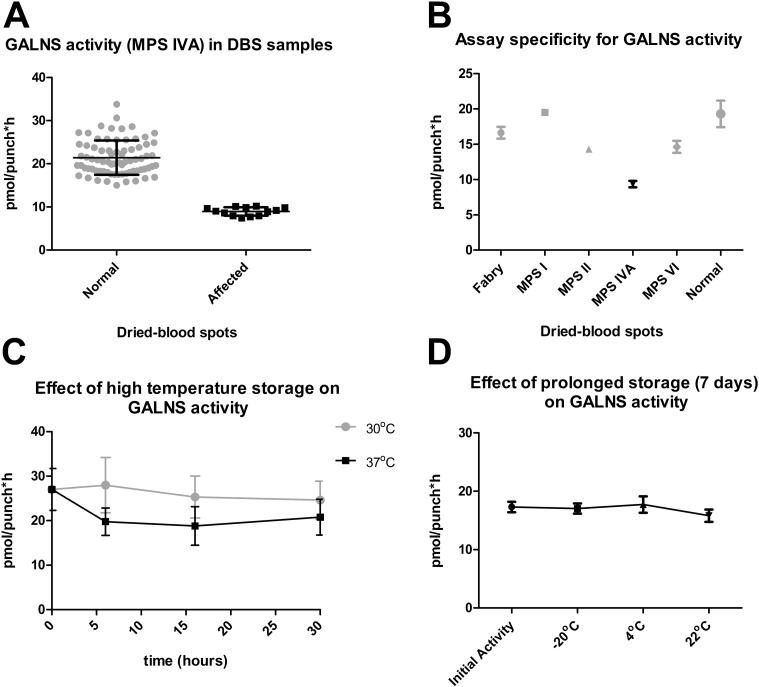
We then determined relative GALNS enzyme activity levels in the DBS from known MPS IVA patients (n = 13) and normal control DBS (n = 75). The DBS from normal individuals (gray circles) from the Duke repository had enzyme activities in the range of 15.0–33.8 pmol/punch/h, with calculated mean ± standard deviation values of 21.4 ± 3.97 pmol/punch/h. The MPS IVA-affected DBS (black squares) had GALNS activity ranging from 7.4 to 10.2 pmol/punch/h, with a mean value of 8.94 ± 0.97 (mean ± std. dev.). GALNS-deficient, MPS-IVA DBS values were clearly distinguishable from the normal control spots as shown in Fig. 1A (p < 0.0001, Tukey's two-tailed t-test).
Fig. 1.
A) Comparison of GALNS enzyme activity in normal control (circles) and MPS IVA (squares) dried blood spots. B) Specificity of assay for GALNS activity in DBS from affected MPS IVA compared to other known LSDs and normal controls. C) Stability of GALNS activity in dried-blood spots was tested by incubating DBS (n = 10) at 30 °C (circles) & 37 °C (squares) for 6 h, 16 h and 30 h. D) Enzyme activity was measured in ten DBS stored long-term (7 days) at − 20 °C, 4 °C & 22 °C.
The intra-day (n = 8) and inter-day (n = 10) variations (% CV) of this fluorometric assay were within acceptable limits (< 8.7% and < 14.6%, respectively). Results of the assay performed by two different operators were also consistent, with observed variation at < 8.9% CV (n = 10). These results demonstrate that this microplate assay methodology is highly reproducible and accurate. The assay was sensitive enough to detect enzymatic activity in normal control DBS extracts at 1:20 dilution. DBS from known patients with MPS IVA showed low activity as compared to DBS from other LSDs (MPS I, MPS II, MPS VI and Fabry) that showed GALNS activities in the normal ranges (14.0–25.3 pmol/punch ∗ h), thus demonstrating specificity for the target enzyme (Fig. 1B).
We also tested the stability of GALNS activity in DBS samples (n = 10) over a range of temperatures and time periods. DBS was stored at 30 °C or 37 °C for 6 h, 16 h and 30 h to test for exposure to high temperatures expected during transportation and handling. GALNS enzyme activity decreased gradually in DBS stored at 30 °C but did not change significantly for up to 30 h (p > 0.05; Tukey's multiple comparison test). The enzyme activity was less stable at 37 °C, showing significant reduction (p < 0.0001) as early as 6 h (Fig. 1C). However, GALNS activity was stable for up to 7 days in DBS stored at − 20 °C, 4 °C and 22 °C (Fig. 1D) with mean enzyme activity values between 15.8 and 17.7 pmol/punch ∗ h.
Our studies demonstrate that GALNS enzyme activity can be readily measured in DBS to identify enzyme deficient patients. This method improves the time to diagnosis by reducing the total incubation time by up to 24 h compared to the previously described methodology [9]. The time required to perform this assay by our method is comparable to that of other enzyme assays for similar hereditary metabolic conditions (e.g., MPS I, MPS II, Gaucher, Fabry, Pompe) including those performed using leukocyte preparations [7], [14]. In addition, the ability to test enzyme activities in DBS offers the advantage of assaying very small sample volumes from a relatively stable source (i.e., DBS) that can be conveniently shipped and processed. It is recommended that the DBS enzyme activity results be confirmed using a second method if possible, like enzyme activity testing in leukocytes or fibroblasts or by molecular analysis of the GALNS gene.
4. Conclusions
We have developed and validated an improved dried blood spot-based microplate assay for measuring GALNS enzyme activity using a fluorescent substrate to diagnose patients with MPS IVA. This method combines an initial enzyme extraction step with a shortened assay time for completing the enzymatic reaction compared to existing enzyme assays. This assay is robust, reproducible and can be completed within two days as compared with existing protocols that require three days for activity measurement.
Acknowledgments
This study was supported by a sponsored research agreement with BioMarin Pharmaceutical Inc. (3938453-190276). We wish to thank Dr. Tim Wood, Greenwood Genetic Center, for providing invaluable MPS IVA patient DBS specimens as well as substrate reagents. We thank Dr. Maira Burin, Dr. Gabriel Civallero, Dr. Roberto Guigliani and Dr. Zoltan Lukacs for their invaluable comments and technical feedback.
References
- 1.Rivera-Colon Y., Schutsky E.K., Kita A.Z., Garman S.C. The structure of human GALNS reveals the molecular basis for mucopolysaccharidosis IV A. J. Mol. Biol. 2012;423:736–751. doi: 10.1016/j.jmb.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood T.C., Harvey K., Beck M., Burin M.G., Chien Y.H., Church H.J., D'Almeida V., van Diggelen O.P., Fietz M., Giugliani R., Harmatz P., Hawley S.M., Hwu W.L., Ketteridge D., Lukacs Z., Miller N., Pasquali M., Schenone A., Thompson J.N., Tylee K., Yu C., Hendriksz C.J. Diagnosing mucopolysaccharidosis IVA. J. Inherit. Metab. Dis. 2013;36:293–307. doi: 10.1007/s10545-013-9587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martell L.A., Cunico R.L., Ohh J., Fulkerson W., Furneaux R., Foehr E.D. Validation of an LC–MS/MS assay for detecting relevant disaccharides from keratan sulfate as a biomarker for Morquio A syndrome. Bioanalysis. 2011;3:1855–1866. doi: 10.4155/bio.11.172. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H., Young S.P., Millington D.S. Quantification of glycosaminoglycans in urine by isotope-dilution liquid chromatography-electrospray ionization tandem mass spectrometry. Curr. Protoc. Hum. Genet. 2013 doi: 10.1002/0471142905.hg1712s76. (Chapter 17:Unit 17.12) [DOI] [PubMed] [Google Scholar]
- 5.de Jong J.G., Wevers R.A., Laarakkers C., Poorthuis B.J. Dimethylmethylene blue-based spectrophotometry of glycosaminoglycans in untreated urine: a rapid screening procedure for mucopolysaccharidoses. Clin. Chem. 1989;35:1472–1477. [PubMed] [Google Scholar]
- 6.Byers S., Rozaklis T., Brumfield L.K., Ranieri E., Hopwood J.J. Glycosaminoglycan accumulation and excretion in the mucopolysaccharidoses: characterization and basis of a diagnostic test for MPS. Mol. Genet. Metab. 1998;65:282–290. doi: 10.1006/mgme.1998.2761. [DOI] [PubMed] [Google Scholar]
- 7.van Diggelen O.P., Zhao H., Kleijer W.J., Janse H.C., Poorthuis B.J., van Pelt J., Kamerling J.P., Galjaard H. A fluorimetric enzyme assay for the diagnosis of Morquio disease type A (MPS IV A) Clin. Chim. Acta. 1990;187:131–139. doi: 10.1016/0009-8981(90)90339-t. [DOI] [PubMed] [Google Scholar]
- 8.Hendriksz C.J., Burton B., Fleming T.R., Harmatz P., Hughes D., Jones S.A., Lin S.P., Mengel E., Scarpa M., Valayannopoulos V., Giugliani R., Investigators S., Slasor P., Lounsbury D., Dummer W. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): a phase 3 randomised placebo-controlled study. J. Inherit. Metab. Dis. 2014 doi: 10.1007/s10545-014-9715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camelier M.V., Burin M.G., De Mari J., Vieira T.A., Marasca G., Giugliani R. Practical and reliable enzyme test for the detection of mucopolysaccharidosis IVA (Morquio syndrome type A) in dried blood samples. Clin. Chim. Acta. 2011;412:1805–1808. doi: 10.1016/j.cca.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Sista R.S., Wang T., Wu N., Graham C., Eckhardt A., Bali D., Millington D.S., Pamula V.K. Rapid assays for Gaucher and Hurler diseases in dried blood spots using digital microfluidics. Mol. Genet. Metab. 2013;109:218–220. doi: 10.1016/j.ymgme.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tolun A.A., Graham C., Shi Q., Sista R.S., Wang T., Eckhardt A.E., Pamula V.K., Millington D.S., Bali D.S. A novel fluorometric enzyme analysis method for Hunter syndrome using dried blood spots. Mol. Genet. Metab. 2012;105:519–521. doi: 10.1016/j.ymgme.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Gelb M.H., Turecek F., Scott C.R., Chamoles N.A. Direct multiplex assay of enzymes in dried blood spots by tandem mass spectrometry for the newborn screening of lysosomal storage disorders. J. Inherit. Metab. Dis. 2006;29:397–404. doi: 10.1007/s10545-006-0265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khaliq T., Sadilek M., Scott C.R., Turecek F., Gelb M.H. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis IVA. Clin. Chem. 2011;57:128–131. doi: 10.1373/clinchem.2010.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Civallero G., Michelin K., de Mari J., Viapiana M., Burin M., Coelho J.C., Giugliani R. Twelve different enzyme assays on dried-blood filter paper samples for detection of patients with selected inherited lysosomal storage diseases. Clin. Chim. Acta. 2006;372:98–102. doi: 10.1016/j.cca.2006.03.029. [DOI] [PubMed] [Google Scholar]