Abstract
Disk diffusion testing is widely used to detect methicillin resistance in staphylococci, and cefoxitin is currently considered the best marker for mecA-mediated methicillin resistance. In low-inoculum diffusion testing (colony suspension at 106 CFU/ml), the addition of moxalactam in combination with cefoxitin has been reported to improve on cefoxitin alone for the detection of methicillin-heteroresistant staphylococci. However, moxalactam is absent from EUCAST and CLSI guidelines, which use high-inoculum diffusion testing (colony suspension at 108 CFU/ml), calling into question the potential interest of including moxalactam in their recommendations. The inhibition zone diameters of cefoxitin and moxalactam, alone and in combination, were evaluated for concordance with mecA and mecC positivity in a large collection of clinical Staphylococcus isolates (611 Staphylococcus aureus, Staphylococcus lugdunensis, and Staphylococcus saprophyticus isolates and 307 coagulase-negative staphylococci other than S. lugdunensis and S. saprophyticus isolates, of which 22% and 53% were mecA-positive, respectively) and in 25 mecC-positive S. aureus isolates using high-inoculum diffusion testing. Receiver operating characteristic, sensitivity, and specificity analyses indicated that the detection of mecA- and mecC-positive and negative isolates did not improve with moxalactam, either alone or in combination with cefoxitin, compared to cefoxitin alone. These findings were similar in both the S. aureus/S. lugdunensis/S. saprophyticus group and in the coagulase-negative staphylococci group. Our results do not support the use of moxalactam as an additional marker of methicillin resistance when testing with high-inoculum disk diffusion.
INTRODUCTION
Staphylococcus aureus is an important human pathogen that causes a wide variety of community- and health care-associated infections (1). This pathogen has a proven ability to adapt to the selective pressure of antibiotics. S. aureus was initially methicillin-susceptible, but isolates resistant to this antibiotic were identified soon after its introduction (2), first in hospital settings and currently in the community and in livestock (3, 4). Methicillin-resistant S. aureus (MRSA) is currently a major cause of morbidity and mortality worldwide (5).
Coagulase-negative staphylococci (CoNS) have been increasingly recognized to cause clinically significant infections, and CoNS were reported to be one of the most common pathogens responsible for nosocomial infections (6). As observed with S. aureus, CoNS were initially susceptible to methicillin, but the emergence of methicillin-resistant CoNS rapidly followed that of MRSA (7), and CoNS eventually became predominant in hospital settings.
The main mechanism of staphylococci resistance to methicillin is the production of an auxiliary penicillin-binding protein (PBP), PBP2a, encoded by the mecA gene, or its variant mecALGA251, which has been renamed mecC and its product designated PBP2c (8, 9). PBP2a and PBP2c expression renders the isolate resistant to all β-lactams, except for the new subclass of cephalosporins with anti-MRSA activity consisting of ceftaroline and ceftobiprole, which have sufficiently high affinity for PBP2a (8, 10). The mecA and mecC genes are foreign to the core genome of staphylococci, and they are not present in methicillin-susceptible strains (9, 11).
Methicillin resistance can be detected phenotypically by disk diffusion testing (12) with β-lactam molecules, such as oxacillin or cefoxitin. However, heterogeneous expression of PBP2a resistance particularly affects the results obtained with oxacillin (13). In contrast, cefoxitin is a very sensitive and specific marker of mecA-mediated methicillin resistance, and it is considered the agent of choice for disk diffusion tests (14). Until 2013, the Comité de l'Antibiogramme de la Société Française de Microbiologie (CA-SFM) recommended additional testing with moxalactam, an oxacephem, together with cefoxitin for the detection of mecA-mediated methicillin resistance (http://www.sfm-microbiologie.org/). This recommendation was based on the observation that disk diffusion with both cefoxitin and moxalactam was more accurate than cefoxitin alone in differentiating heteroresistant S. aureus and CoNS isolates from methicillin-susceptible isolates at low inoculum levels (colony suspension at 106 CFU/ml) (15–17). However, scarce discrepant data, available only at higher inoculum levels (colony suspension at 108 CFU/ml), have led to questions regarding a potential interest in including moxalactam in the EUCAST and CLSI recommendations (17, 18).
Here, we reexamined the performance of cefoxitin and moxalactam alone and in combination to discriminate mecA-positive and mecC-positive from mecA-negative and mecC-negative staphylococci with the disk diffusion method at a high inoculum level (colony suspension at 108 CFU/ml).
MATERIALS AND METHODS
Bacterial collection.
During a 6-month period (1 May 2014 to 31 September 2014), we collected all clinically significant Staphylococcus isolates that were either cultivated from diverse human clinical specimens sent to the clinical laboratory of the Centre Hospitalier, Lyon Sud, or transmitted to the French National Reference Center for Staphylococci (FNRCS), for expert consultation. Of note, staphylococcal isolates are typically referred to the reference center by French clinical laboratories for clinically severe infections or a particular antimicrobial susceptibility pattern. Because mecC-positive human isolates are uncommon in clinical practice, 25 were randomly selected from within an international collection of representative strains belonging to the various European clones that harbor this gene (clonal complex 130 [CC130], n = 12; CC1943, n = 8; CC425, n = 5) from the FNRCS strains collection. Expression of PBP2c by these strains was confirmed using the PBP2a culture colony test (Alere, Scarborough, MA) after induction (i.e., using colonies around the cefoxitin disk), thanks to the cross-reaction of this immunoassay with PBP2c.
Because all of these isolates were collected anonymously and no clinical data were recorded, this study did not require specific ethics approval and patient consent was not sought in accordance with French regulations. Species were identified with the Vitek MS (bioMérieux) matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) system according to manufacturer recommendations. S. aureus CIP 65-8T (MRSA) and ATCC 25923 (mecA-negative) were used as the quality controls for all of the experiments.
mecA and mecC detection.
Genomic DNA was extracted from the cultures of all staphylococcal isolates with a standard procedure. These DNA extracts served as the templates for PCR amplification with primers previously shown to be specific for mecA or mecC, strictly following the protocol described by the authors (19, 20). mecA and mecC expression was detected using the PBP2a culture colony test (Alere, Scarborough, MA) after induction (i.e., using colonies around the cefoxitin disk) as previously described (20).
Susceptibility testing.
Disk diffusion tests were performed according to EUCAST instructions (http://www.eucast.org) with Mueller-Hinton agar plates (Mueller-Hinton 2; bioMérieux, Marcy l'Etoile, France), a bacterial suspension in 0.9% NaCl solution at a density equivalent to a 0.5 McFarland barium sulfate standard (1 to 2 108 CFU/ml), and disks loaded with moxalactam (30 μg) and cefoxitin (30 μg) (I2A, Toulouse, France). After incubation, inhibition zone diameters (IZDs) were measured with an automated plate reader (SirScan; I2A) and reviewed manually by the operator when required. When heterogeneous growth occurred, the innermost limit of the inhibition zone was read. S. aureus CIP 65-8T (MRSA) and ATCC 25923 (mecA-negative) served as the quality controls in each series of plates. The cefoxitin IZD was interpreted based on the EUCAST breakpoint. Results for these control strains were concordant in all series.
As advised by EUCAST, staphylococcal species were divided into two groups for IZD interpretation. The first group comprised S. aureus, Staphylococcus lugdunensis, and Staphylococcus saprophyticus with a cefoxitin IZD breakpoint for resistance (R) of <22 or 25 mm according to EUCAST/CLSI or CA-SFM guidelines, respectively. The second group included other CoNS with R breakpoints of <25 or 26 mm, respectively (http://www.eucast.org; http://www.sfm-microbiologie.org/) (21).
The MIC of oxacillin was determined by the broth microdilution method and interpreted following EUCAST recommendations (21).
Statistics.
We applied receiver operating characteristic (ROC) curve analyses to evaluate the performance of each molecule in detecting mecA positivity, independent of the chosen breakpoint. Pointwise estimates of the area under the ROC curve are reported with 95% confidence intervals (CIs). Comparisons between estimates were made using Delong's method (22). ROC computations were performed with the pROC software package (23). The optimal breakpoints for mecA and mecC detection in our collection, as well as for discrimination of mecA- and mecC-positive S. aureus, were computed by maximizing the sum of sensitivity and specificity (Youden's index). The sensitivity and specificity were compared among different cefoxitin breakpoints and among different combinations of molecules with McNemar's test. The significance threshold was set to 0.05 for all tests. No P value correction for multiple testing was applied, which is consistent with the exploratory nature of the study. All computations were performed with R software version 3.0.1 “Good Sport” (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS AND DISCUSSION
A total of 918 staphylococcal isolates were collected during the 6-month period of this study. These isolates included 597 S. aureus isolates, 9 S. saprophyticus isolates, 5 S. lugdunensis isolates, and 307 CoNS. The distribution of mecA positivity among the remaining 918 different isolates is shown in Table S1 in the supplemental material. We found mecA in 130/597 S. aureus isolates, 1/5 S. lugdunensis isolate, 0/9 S. saprophyticus isolates, and 164/307 CoNS.
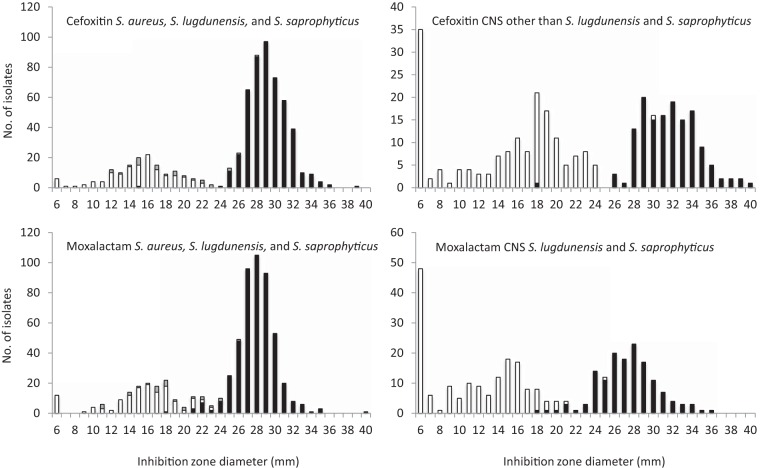
The distribution of cefoxitin and moxalactam IZDs for S. aureus, S. lugdunensis, S. saprophyticus, and CoNS is shown in Fig. 1. In mecA-positive and mecC-positive S. aureus, S. lugdunensis, and S. saprophyticus isolates, cefoxitin IZDs ranged from 6 to 28 mm (median, 16 mm) and moxalactam IZDs ranged from 6 to 23 mm (median, 16 mm). One mecA-positive S. lugdunensis isolate and five mecC-positive S. aureus isolates repeatedly had cefoxitin IZDs that were greater than the EUCAST breakpoint (Table 1). The six isolates with cefoxitin IZDs that were greater than the EUCAST breakpoint expressed additional PBP2a or PBP2c after induction; however, 2/6 isolates had oxacillin MICs that were below 2 mg/liter, suggesting a low expression of PBP2c by these isolates.
FIG 1.
Distribution of inhibition zone diameters for 30-μg cefoxitin disks and 30-μg moxalactam disks. The cultures tested included 597 S. aureus isolates, 5 S. lugdunensis isolates, and 307 CoNS. White, mecA-positive; gray, mecC-positive; black, mecA-and mecC-negative.
TABLE 1.
Results observed with isolates with aberrant cefoxitin inhibition zone diameters
| Isolate | Species | Gene | Inhibition zone diameter (mm)a |
Oxacillin MIC (mg/liter)a | PBP2a/c productionb | |
|---|---|---|---|---|---|---|
| Cefoxitin | Moxalactam | |||||
| ST20110751 | S. aureus | mecC | 25 | 24 | 4 | Positive |
| ST20110820 | S. aureus | mecC | 26 | 24 | 0.5 | Positive |
| ST20112295 | S. aureus | mecC | 23 | 22 | 16 | Positive |
| ST20112313 | S. aureus | mecC | 25 | 26 | 8 | Positive |
| ST20112317 | S. aureus | mecC | 23 | 22 | 0.5 | Positive |
| ST20131725 | S. aureus | 15 | 28 | 0.25 | Negative | |
| 14016737201 | S. lugdunensis | mecA | 28 | 23 | 4 | Positive |
| 15031834601 | S. epidermidis | mecA | 30 | 25 | 4 | Positive |
Inhibition zone diameters and oxacillin MICs were determined following EUCAST recommendations (disks loaded with 30 μg of cefoxitin or moxalactam). The presence of mecA and mecC genes was determined by specific PCR as described in the Material and Methods.
PBP2a and PBP2c production was detected by an immunochromatographic method after induction using colonies around cefoxitin.
In mecA-negative S. aureus, S. lugdunensis, and S. saprophyticus isolates, cefoxitin IZDs ranged from 15 to 39 mm (median, 29 mm) and moxalactam IZDs ranged from 18 to 40 mm (median, 28 mm). One mecA-negative S. aureus isolate repeatedly had a 15-mm cefoxitin IZD, which was below the breakpoint, with an 18-mm moxalactam IZD. This isolate tested negative for mecC in PCR experiments and did not produce high levels of β-lactamase (data not shown). Its oxacillin MIC was 0.25 mg/liter (Table 1). We did not detect the production of additional PBP with the PBP2a culture colony test assay after induction with cefoxitin. These findings led us to suspect that this isolate expresses PBP variants with low affinities for β-lactams (24).
In mecA-positive CoNS, cefoxitin and moxalactam IZDs ranged from 6 to 30 mm (median, 17 mm) and from 6 to 25 mm (median, 12 mm), respectively. Only one mecA-positive Staphylococcus epidermidis isolate repeatedly had cefoxitin IZDs that were greater than the 25-mm EUCAST breakpoint with an oxacillin MIC at 4 mg/liter and a positive PBP2a culture colony test after induction with cefoxitin (Table 1). Finally, all mecA-negative CoNS isolates had cefoxitin IZDs that were above the breakpoint, ranging from 26 to 40 mm (median, 32 mm), and the moxalactam IZDs ranged from 18 to 36 mm (median, 27 mm).
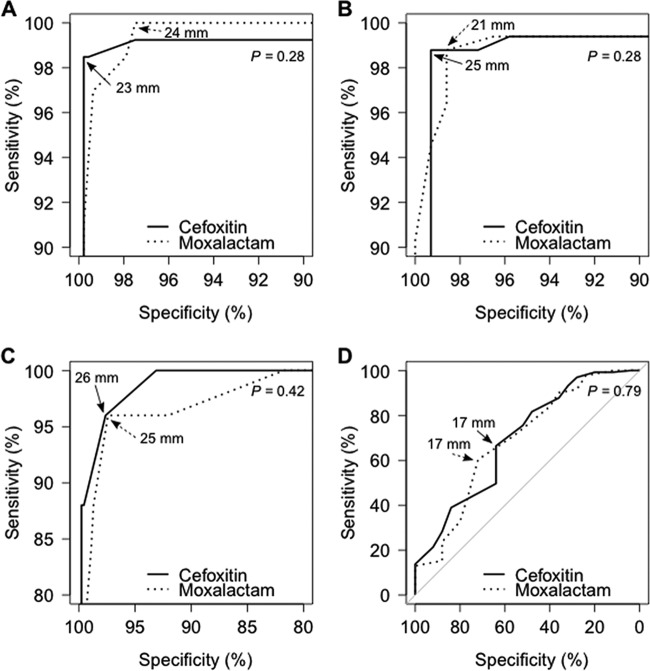
In the ROC analysis, cefoxitin and moxalactam as markers of mecA positivity had similar discriminatory power, which was indicated by their similar areas under the ROC curve (Fig. 2; see also Table S2 in the supplemental material). The calculated optimal breakpoints for the S. aureus/S. lugdunensis/S. saprophyticus group and for the group of other staphylococcal species were, respectively, an R of <23 mm and an R of <25 mm for cefoxitin and an R of <24 mm and an R of <21 mm for moxalactam. Compared to the cefoxitin breakpoints recommended by EUCAST and CLSI for both groups, our calculated breakpoints differed by only 1 mm for the S. aureus/S. lugdunensis/S. saprophyticus group and matched perfectly for other CoNS. This finding highlights the remarkable stability of cefoxitin IZD distributions across different strain collections.
FIG 2.
ROC analysis of the inhibition zone diameters of cefoxitin and moxalactam as markers for mecA and mecC gene positivity in Staphylococcus spp. Cefoxitin and moxalactam ROC curves were compared in the following contexts: mecA detection in S. aureus, S. lugdunensis, and S. saprophyticus (A); mecA detection in other staphylococci (B); mecC detection in S. aureus (C); and mecA versus mecC discrimination in S. aureus (D). P values comparing ROC curves were computed using Delong's method. Optimal diameter breakpoints for cefoxitin and moxalactam are indicated by plain and dotted arrows, respectively. Axes of A, B, and C were clipped to 90%, 90%, and 80%, respectively.
We used McNemar's test to compare the sensitivity and specificity of mecA detection with different cefoxitin breakpoints (our calculated breakpoints, those from the EUCAST and CLSI guidelines, and those from CA-SFM EUCAST guidelines) and for the combination of cefoxitin and moxalactam (see Table S3 in the supplemental material). For all of the clinical breakpoints used, discrimination between mecA-positive and mecA-negative strains in the S. aureus/S. lugdunensis/S. saprophyticus group and the CoNS groups was not superior using moxalactam, alone or in combination with cefoxitin, compared to that using cefoxitin alone. Thus, additional testing with moxalactam did not improve the performance of disk diffusion testing in our collection. In contrast, 6/8 discordant isolates with cefoxitin IZDs were correctly reclassified using oxacillin MICs. Values reached 8/8 based on the PBP2a culture colony test assay after induction. The results suggested that the addition of the oxacillin MIC or expression of additional PBP2 in the cefoxitin disk diffusion test is more efficient in improving detection of MRSA than the addition of the moxalactam disk diffusion test.
In contrast to observations by Join-Lambert et al. (17), our results did not indicate that any performance benefit can be expected from systematic addition of moxalactam testing at high inoculum levels for discriminating between mecA-positive and mecA-negative staphylococci. The argument of Join-Lambert et al. for moxalactam use was that this molecule may be better than cefoxitin for detecting heteroresistant isolates. Their study and ours were performed with strain collection from different time periods, and the prevalence of staphylococcal methicillin heteroresistance may have changed in France between 2006 and 2014 (Join-Lambert et al. study [17] and our collection, respectively). We hypothesize that the use of a higher inoculum may have attenuated the difference in performance between cefoxitin and moxalactam for detecting heteroresistant isolates as previously noted by Roisin et al. (18). Overall, the results of this study suggest that cefoxitin should remain the preferred marker for detecting methicillin resistance in current disk diffusion testing protocols.
Supplementary Material
ACKNOWLEDGMENTS
We thank Isabelle Frédenucci, Michèle Demontclos, François Vandenesch, CA-SFM members, and EUCAST members for their fruitful comments. We also thank Annie Martra and Franck Bes for technical assistance.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01195-16.
REFERENCES
- 1.Lowy F. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Jevons M. 1961. Celbenin-resistant staphylococci. Br Med J 1:124–125. doi: 10.1136/bmj.1.5219.124-a. [DOI] [Google Scholar]
- 3.Vandenesch F, Naimi T, Enright M, Lina G, Nimmo G, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy M, Etienne J. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wulf M, Voss A. 2008. MRSA in livestock animals-an epidemic waiting to happen? Clin Microbiol Infect 14:519–521. doi: 10.1111/j.1469-0691.2008.01970.x. [DOI] [PubMed] [Google Scholar]
- 5.de Kraker M, Wolkewitz M, Davey P, Koller W, Berger J, Nagler J, Icket C, Kalenic S, Horvatic J, Seifert H, Kaasch A, Paniara O, Argyropoulou A, Bompola M, Smyth E, Skally M, Raglio A, Dumpis U, Kelmere A, Borg M, Xuereb D, Ghita M, Noble M, Kolman J, Grabljevec S, Turner D, Lansbury L, Grundmann H, Group BS. 2011. Clinical impact of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 55:1598–1605. doi: 10.1128/AAC.01157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Eiff C, Peters G, Heilmann C. 2002. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis 2:677–685. doi: 10.1016/S1473-3099(02)00438-3. [DOI] [PubMed] [Google Scholar]
- 7.Barber M. 1964. Naturally occuring methicillin-resistant staphylococci. J Gen Microbiol 35:183–190. doi: 10.1099/00221287-35-2-183. [DOI] [PubMed] [Google Scholar]
- 8.Ubukata K, Yamashita N, Konno M. 1985. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother 25:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Álvarez L, Holden MT, Lindsay H, Webb CR, Brown DF, Curran MD, Walpole E, Brooks K, Pickard DJ, Teale C, Parkhill J, Bentley SD, Edwards GF, Girvan EK, Kearns AM, Pichon B, Hill RL, Larsen AR, Skov RL, Peacock SJ, Maskell DJ, Holmes MA. 2011. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect Dis 11:595–603. doi: 10.1016/S1473-3099(11)70126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ubukata K, Nonoguchi R, Matsuhashi M, Konno M. 1989. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J Bacteriol 17:2882–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katayama Y, Ito T, Hiramatsu K. 2000. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 44:1549–1555. doi: 10.1128/AAC.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EUCAST. 2013. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_v1.0_20131211.pdf.
- 13.Cauwelier B, Gordts B, Descheemaecker P, Van Landuyt H. 2004. Evaluation of a disk diffusion method with cefoxitin (30 microg) for detection of methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis 23:389–392. doi: 10.1007/s10096-004-1130-8. [DOI] [PubMed] [Google Scholar]
- 14.Skov R, Smyth R, Larsen A, Frimodt-Møller N, Kahlmeter G. 2005. Evaluation of cefoxitin 5 and 10 microg discs for the detection of methicillin resistance in staphylococci. J Antimicrob Chemother 55:157–161. doi: 10.1093/jac/dkh514. [DOI] [PubMed] [Google Scholar]
- 15.Felten A, Grandry B, Lagrange P, Casin I. 2002. Evaluation of three techniques for detection of low-level methicillin-resistant Staphylococcus aureus (MRSA): a disk diffusion method with cefoxitin and moxalactam, the Vitek 2 system, and the MRSA-screen latex agglutination test. J Clin Microbiol 40:2766–2771. doi: 10.1128/JCM.40.8.2766-2771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felten A, Casin I. 2003. Détection simple des staphylocoques résistants à la méticilline grâce à un disque de céfoxitine ou de latamoxef. Rev Fr Lab 352:27–30. [Google Scholar]
- 17.Join-Lambert O, Clauser S, Guillet C, Jais J, Abachin E, Quesnes G, Carbonnelle E, Le Monnier A, Zahar J, Kayal S, Berche P, Ferroni A. 2007. Comparison of cefoxitin and moxalactam 30 microg disc diffusion methods for detection of methicillin resistance in coagulase-negative staphylococci. J Antimicrob Chemother 9:763–766. [DOI] [PubMed] [Google Scholar]
- 18.Roisin S, Nonhoff C, Denis O, Struelens M. 2008. Evaluation of new Vitek 2 card and disk diffusion method for determining susceptibility of Staphylococcus aureus to oxacillin. J Clin Microbiol 46:2525–2528. doi: 10.1128/JCM.00291-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, Watanabe S. 1991. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol 29:2240–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pichon B, Hill R, Laurent F, Larsen A, Skov R, Holmes M, Edwards G, Teale C, Kearns A. 2012. Development of a real-time quadruplex PCR assay for simultaneous detection of nuc, Panton-Valentine leucocidin (PVL), mecA and homologue mecALGA251. J Antimicrob Chemother 67:2338–2341. [DOI] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing; 23rd information supplement. CLSI M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.DeLong E, DeLong D, Clarke-Pearson D. 1988. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 23.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J, Müller M. 2011. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorgensen J. 1991. Mechanisms of methicillin resistance in Staphylococcus aureus and methods for laboratory detection. Infect Control Hosp Epidemiol 12:14–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.