Abstract
Our objective was to establish reference MIC quality control (QC) ranges for drug susceptibility testing of antimycobacterials, including first-line agents, second-line injectables, fluoroquinolones, and World Health Organization category 5 drugs for multidrug-resistant tuberculosis using a 7H9 broth microdilution MIC method. A tier-2 reproducibility study was conducted in eight participating laboratories using Clinical Laboratory and Standards Institute (CLSI) guidelines. Three lots of custom-made frozen 96-well polystyrene microtiter plates were used and prepared with 2× prediluted drugs in 7H9 broth-oleic acid albumin dextrose catalase. The QC reference strain was Mycobacterium tuberculosis H37Rv. MIC frequency, mode, and geometric mean were calculated for each drug. QC ranges were derived based on predefined, strict CLSI criteria. Any data lying outside CLSI criteria resulted in exclusion of the entire laboratory data set. Data from one laboratory were excluded due to higher MIC values than other laboratories. QC ranges were established for 11 drugs: isoniazid (0.03 to 0.12 μg/ml), rifampin (0.03 to 0.25 μg/ml), ethambutol (0.25 to 2 μg/ml), levofloxacin (0.12 to 1 μg/ml), moxifloxacin (0.06 to 0.5 μg/ml), ofloxacin (0.25 to 2 μg/ml), amikacin (0.25 to 2 μg/ml), kanamycin (0.25 to 2 μg/ml), capreomycin (0.5 to 4 μg/ml), linezolid (0.25 to 2 μg/ml), and clofazimine (0.03 to 0.25 μg/ml). QC ranges could not be established for nicotinamide (pyrazinamide surrogate), prothionamide, or ethionamide, which were assay nonperformers. Using strict CLSI criteria, QC ranges against the M. tuberculosis H37Rv reference strain were established for the majority of commonly used antituberculosis drugs, with a convenient 7H9 broth microdilution MIC method suitable for use in resource-limited settings.
INTRODUCTION
Managing an estimated global annual incidence of 9.6 million Mycobacterium tuberculosis cases requires an arsenal of effective antituberculosis (anti-TB) drugs that are active against both susceptible and multidrug-resistant strains (1). Furthermore, maximizing the effectiveness of treatment and minimizing the risk of development of further resistance requires accurate drug susceptibility testing (DST) for M. tuberculosis clinical isolates (2, 3). To improve reliability of DST for M. tuberculosis, regulators have introduced a requirement to establish quality control (QC) ranges for new and repurposed anti-TB drugs (4).
Two approaches to M. tuberculosis DST are currently employed: critical concentration (CC)-based agar proportion assay (APA) and MIC-based methodology. CC is well established and used widely for classifying the sensitivity of M. tuberculosis to anti-TB drugs. The CC, also known as the antimicrobial susceptibility testing breakpoint, is defined by international convention as the lowest concentration of a drug that inhibits growth of 95% (90% for pyrazinamide) of wild-type M. tuberculosis strains, while at the same time it does not inhibit strains that are considered to be clinically resistant (i.e., isolated from patients unresponsive to therapy) (3). However, several disadvantages are associated with using CC values in DST. First, up to 5% of wild-type M. tuberculosis strains (10% for pyrazinamide) are effectively classified as drug resistant, and second, clinical outcome data for individual drugs often are not available because combination therapy is the norm for TB treatment (5). In addition, the CCs for many older anti-TB drugs, including some examined in this study, were established many years ago and have not since been revalidated; hence, any genetic variation over time, as well as the development of resistance, will not have been accounted for (3). For example, one analysis has reported that regional variability of M. tuberculosis susceptibility to ofloxacin would result in different CC values from isolates in different settings (6, 7). As a result of these factors, together with the common occurrence that the CC is close to the MIC, there is a high probability of misclassification of M. tuberculosis strains as susceptible or resistant when using a CC-based APA method (5).
The second approach used in M. tuberculosis DST is based on the MIC, defined as the lowest concentration of a drug (in micrograms per milliliter) that results in complete inhibition of visual growth in vitro (8). In DST, a susceptible reference isolate is tested alongside the clinical isolates to act as a quality control. H37Rv is an established M. tuberculosis reference strain, being well documented as a pansusceptible wild-type isolate, and is used in laboratories worldwide for routine monitoring of assay performance (3). Ongoing quality control and quality assurance of DST requires QC ranges to be established by MIC testing of such a reference isolate.
Therefore, our objective was to establish, by means of a tier-2 study, DST QC MIC ranges against the H37Rv reference strain for a broad panel of anti-TB drugs using a 7H9 broth microdilution MIC method recognized by the Clinical Laboratory and Standards Institute (CLSI). Our choice of a broth microdilution MIC method that could be commercially available in the future was influenced by the advantage it offers over agar dilution MIC methods, in that DST results are produced in half the time (incubation period of 10 days or less compared with 21 days or more for agar media) as that required with current methodologies (9). Drugs were selected for the study on the basis of having an important role in M. tuberculosis treatment, as well as having no established QC range for M. tuberculosis H37Rv using a broth microdilution MIC method. The 14 anti-TB drugs tested included first-line agents, second-line injectables, fluoroquinolones, and World Health Organization (WHO) category 5 drugs (linezolid and clofazimine).
MATERIALS AND METHODS
Study design.
This tier-2 reproducibility study (TMC207-PMR-1988-004) was designed according to CLSI criteria (10), with the appropriate modifications required for M. tuberculosis as described in the companion paper on bedaquiline (9). As such, the results were generated in eight different laboratories based in eight separate and distinct institutions (tier-2 criteria specify a minimum of seven laboratories). Furthermore, each laboratory was instructed to perform 10 replicate tests on separate days using the internationally recognized M. tuberculosis reference strain H37Rv and separate lots of custom-made frozen 96-well polystyrene microtiter plates (Thermo Fisher Sensititre for research use only; Thermo Fisher Scientific Inc., Waltham, MA). For the purpose of the analysis, each lot was assigned a number from 1 to 3. The study protocol was developed through a consensus approach, based on feedback from the participating investigators. The study was performed in a blinded fashion, as none of the laboratories had access to any results from other laboratories.
The broth microdilution MIC method employed was adapted from that described in CLSI document M07-A9, section 10.4 (8). Modifications included the preparation of the drug stock concentration, the QC reference strain, and the preparation of the inoculum. The reproducibility of the DST methods was evaluated within each participating laboratory, between different laboratories, and between reagent lots.
Participating laboratories.
The eight participating laboratories were spread across the world, located on three different continents. The investigators and their laboratories included CLSI members or advisors, the WHO Supranational Reference Laboratory Network (SRLN), the U.S. Centers for Disease Control and Prevention (CDC), and other internationally recognized TB laboratories.
Materials.
The reference strain used was M. tuberculosis H37Rv, originating from the American Type Culture Collection (ATCC 27294). Each laboratory used its own H37Rv strain, rather than it being provided centrally, as well as its own standard medium for its culture.
Other materials provided centrally included 12.5-ml sterile deionized water tubes and saline Tween broth to reconstitute the mycobacterial inoculum. Middlebrook 7H9 broth base and oleic acid albumin dextrose catalase (OADC) (7H9-S) were obtained from Becton Dickinson (Franklin Lakes, NJ).
A total of 14 anti-TB drugs were evaluated, comprising first-line drugs (isoniazid [INH], rifampin, and ethambutol), fluoroquinolones (levofloxacin, moxifloxacin, and ofloxacin), second-line injectable agents (amikacin, kanamycin, and capreomycin), oral bacteriostatic drugs (prothionamide and ethionamide), and WHO category 5 drugs (linezolid and clofazimine). Given that pyrazinamide DST is problematic due to a low-pH requirement, nicotinamide was evaluated as a potential surrogate (11). The frozen 96-well polystyrene microtiter plates contained prediluted drugs at 2× the final concentration made in 2× 7H9-S (Thermo Fisher Sensititre for research use only). The concentration ranges in the MIC frozen panels for the drugs tested are summarized in Table 1.
TABLE 1.
Summary of 7H9 broth microdilution MIC QC ranges against M. tuberculosis H37Rv strain for anti-TB drugs
| Drug | Dilution range used in the study (μg/ml) | MIC QC range (μg/ml) |
|---|---|---|
| First-line drugs | ||
| Isoniazida | 0.03–1 | 0.03–0.12 |
| Rifampin | 0.06–1 | 0.03–0.25 |
| Ethambutol | 0.25–8 | 0.25–2 |
| Nicotinamideb | 16–512 | NAe |
| Fluoroquinolones | ||
| Levofloxacin | 0.12–4 | 0.12–1 |
| Moxifloxacin | 0.06–2 | 0.06–0.5 |
| Ofloxacin | 0.12–4 | 0.25–2 |
| Second-line injectables | ||
| Amikacin | 0.12–8 | 0.25–2 |
| Kanamycinc | 0.12–8 | 0.25–2 |
| Capreomycin | 0.12–4 | 0.5–4 |
| Oral bacteriostatic drugs | ||
| Prothionamide | 0.06–2 | NA |
| Ethionamide | 0.12–4 | NA |
| WHO category 5 drugs | ||
| Linezolid | 0.12–4 | 0.25–2 |
| Clofazimined | 0.06–1 | 0.03–0.25 |
QC range derived from microtiter plate Lot-1 and Lot-2 only.
Nicotinamide was included as a potential surrogate for pyrazinamide.
QC range derived from Lot-2 and Lot-3 only.
The modal concentration of clofazimine, 0.06 μg/ml, was the lowest concentration tested.
NA, not applicable as QC ranges that included at least 95% of the observed values could not be set.
Broth microdilution MIC method.
The 7H9 broth microdilution MIC method was conducted as described in the companion paper (9). In brief, M. tuberculosis H37Rv was grown in 7H11 or Lowenstein-Jensen medium and resuspended in saline Tween with glass beads. The turbidity of the resulting suspension was adjusted with sterile deionized water to match that of a McFarland standard 1 suspension, corresponding to ∼5 × 107 CFU/ml. There is good concordance between the McFarland scale and CFU per milliliter for M. tuberculosis (12). A 2× inoculum was to be prepared by inoculating 12.5-ml sterile deionized water tubes with 255 μl of the 1 McFarland suspension to give a 50-fold dilution from 1 McFarland (∼1 × 106 CFU/ml). The 2× M. tuberculosis H37Rv suspension was then poured into a disposable inoculum reservoir, and then 100 μl was transferred to the microtiter plate wells using an 8- or 12-channel micropipette and sterile tips with filters. The final inoculum size in each well was ∼5 × 105 CFU/ml. The growth control well, but not the well containing the negative control, was also inoculated with M. tuberculosis H37Rv. After inoculation, the plates were sealed in plastic bags (10 plates per bag) and incubated at 35 to 37°C. Each drug was tested over a 5- to 7-fold range, taking into consideration the CLSI guidance that whenever possible, the low end of the QC range for dilution testing should only include concentrations that can be accurately prepared and that dilutions should extend to no more than five dilutions below a drug's susceptibility breakpoint (10). The final drug concentration ranges in the wells following inoculation are given in Table 1; e.g., for isoniazid the concentrations were 1, 0.5, 0.25, 0.12, 0.06, and 0.03 μg/ml.
Data collection and statistical methods.
Microtiter plates were read by visual inspection at days 7, 10, and 14 after inoculation to establish MICs for each drug. MIC was defined as the lowest concentration of the drug (in micrograms per milliliter) that prevented visual growth. Growth and negative-control wells were checked to ensure adequate growth and no contamination, respectively. Any negative-control well with a turbid appearance was suspected of contamination; the results were discounted as invalid and the procedures and reagents checked.
Standardized forms for data capture and reporting were provided to all principal investigators ahead of study start to ensure consistency (9). Quality control was conducted on data sets from each laboratory, and data quality was monitored, with any data inconsistencies queried to the providing investigator.
The MIC distribution, mode, and geometric mean were calculated for each drug. Initial QC ranges were determined by centering on the MIC mode ±1 dilution and doubling dilution over a 3-dilution QC range (10). However, if the mode occurred at the end of the proposed dilution range, the geometric mean was used instead. The proposed QC range was required to encompass at least 95% of the observed MIC values. In the event that a QC range over 3 dilutions could not be established, a 4-dilution QC range was analyzed. In such cases, the initial proposed range was adjusted (i) to include at least 95% of the observed MIC values to accommodate variability expected in routine testing, (ii) if the frequency distribution showed a shoulder off the modal value that included ≥60% of the data points, and/or (iii) if a bimodal distribution was observed. If these criteria were not met, it was concluded that the QC range could not be set.
Upon observation of outlying ranges for any given drug tested, the entire data set (all three microtiter plate lots) from the laboratory providing the outlying data for that drug was excluded. The analyses for that drug were then repeated using the data from the remaining laboratories. Also in accordance with CLSI recommendations, any nonperforming lot was excluded from the analyses (10). Therefore, as explained in the companion paper (9), the number of planned observations (240; 8 laboratories times 30 tests) was not always achieved.
RESULTS
Overall findings.
Using the CLSI M23-A3 guidelines, QC ranges were established for 11 anti-TB drugs using the 7H9 broth microdilution MIC (Table 1). The three remaining drugs (nicotinamide, prothionamide, and ethionamide) were deemed to be nonperformers in the assay, as QC ranges that included at least 95% of the observed MIC values could not be established. Details are given below of how MIC QC ranges were established for four of the most commonly used drugs (rifampin, isoniazid, levofloxacin, and amikacin). A summary of observations, including geometric mean and mode, for all of the drugs tested is provided in the online supplement (see Table S1 in the supplemental material). For most of the drugs tested, no significant differences were reported between results for the three different microtiter plate lots. Data from one laboratory (Lab-1) were excluded due to potential contamination during shipment, as some plates were thawed upon arrival at the laboratory. The raw data from all laboratories are shown in the supplemental material (see Table S2). Day 10 MICs were used in the analyses, except for Lab-1 and Lab-8, which recorded data at day 7 and day 14, respectively.
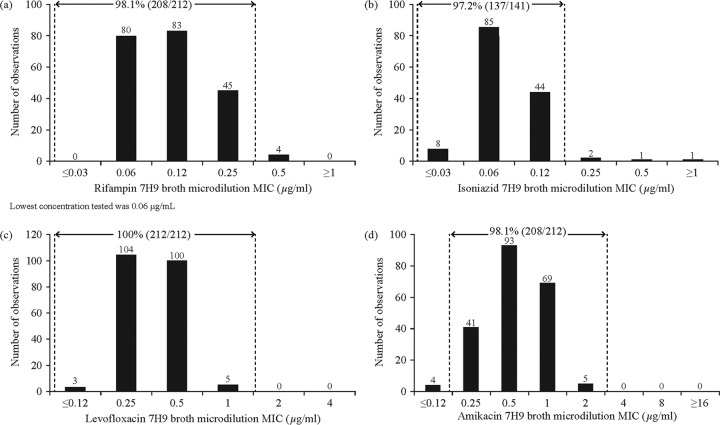
Setting the QC range for rifampin.
A 3-dilution QC range which was centered on the mode value of 0.12 μg/ml for the rifampin MIC distribution was found to include 208/212 (98.1%) of the observations (Fig. 1a). However, a shoulder was observed at 0.06 μg/ml, with observations at this concentration representing 96.4% (80/83) of the mode value. Since this was well above the 60% defined threshold, the QC range was extended to a 4-dilution range in accordance with the recommendation of CLSI document M23-A3. As a result, the MIC QC range for rifampin broth microdilution MIC was set at 0.03 to 0.25 μg/ml.
FIG 1.
7H9 broth MIC distribution against M. tuberculosis H37Rv and quality control range for rifampin (a), isoniazid (b), levofloxacin (c), and amikacin (d).
Setting the QC range for isoniazid.
Preliminary testing was performed at a TB laboratory not participating in the current study on the frozen plates prior to initiating the study. The results indicated that INH MICs for Lot-3 were an order of magnitude higher than those for Lot-1 and Lot-2. Therefore, Lot-3 data for INH only were excluded from the analyses (see Fig. S1 in the supplemental material), and the total number of included observations was 141. The mode was 0.06 μg/ml and geometric mean was 0.076 μg/ml (Fig. 1b). A 3-dilution QC range of 0.03 to 0.12 μg/ml, centered on the mode, was found to include 97.2% (137/141) of the observations.
Setting the QC range for levofloxacin.
The 3-dilution QC range for the levofloxacin MIC distribution centered on the mode included 207/212 (97.6%) of observations, with a mode value of 0.25 μg/ml and a geometric mean of 0.355 μg/ml (Fig. 1c). However, since the shoulder at 0.5 μg/ml was 96.1% of the mode value, the QC range was extended to a 4-dilution range. Therefore, the MIC QC range for levofloxacin was set at 0.12 to 1 μg/ml.
Setting the QC range for amikacin.
A 3-dilution QC range which was centered on the mode value of 0.5 μg/ml for the amikacin MIC distribution was found to include 203/212 (95.8%) of the observations (Fig. 1d). However, since the shoulder at 1 μg/ml was 74.2% of the mode value, the QC range was extended to a 4-dilution range. Therefore, the MIC QC range for amikacin was set at 0.25 to 2 μg/ml.
DISCUSSION
This multicenter reproducibility study has defined 7H9 broth microdilution MIC QC ranges for commonly used anti-TB drugs, using the M. tuberculosis reference strain H37Rv. The study employed a consensus methodology devised by experienced M. tuberculosis investigators from various geographically separate laboratories, in accordance with CLSI guidance (8, 10).
To our knowledge, MIC ranges for TB drugs have not previously been established for the M. tuberculosis reference strain H37Rv in a tier-2 study. One study assessed 7H9 broth microdilution for DST of common anti-TB drugs but did not report the actual MIC values (13). However, similar MIC distributions have been reported for the same reference strain between different liquid culture systems, with MIC variability of a 2-fold dilution or less (5). Studies using 7H10 agar or Bactec MGIT 960 and Bactec 460 determined MICs against H37Rv of 0.5 μg/ml for ofloxacin, 0.125 μg/ml for moxifloxacin, 0.25 μg/ml for levofloxacin, 2 μg/ml for ethambutol, 0.5 μg/ml for amikacin, and 1 to 2 μg/ml for both kanamycin and capreomycin (14–16); all of these values lie within the MIC QC ranges we report for these drugs. For isoniazid, a microdilution method using 7H10 agar or Bactec 460 yielded a MIC range of 0.064 to 0.125 μg/ml for H37Rv, which is essentially within the MIC QC range of 0.03 to 0.12 μg/ml determined in this study (16). Thus, while MIC distributions will vary according to the particular DST method used, our results are in line with existing data generated using other liquid culture methods.
The microbiological method described here is standardized, reliable, and convenient. Such methods represent the most practical DST approach for existing anti-TB drugs. While it would be ideal to establish clinical breakpoints for each TB drug, such an approach would be prohibitively expensive, as it would require new clinical trials and would still be complicated by the fact that a combination of drugs is used to treat M. tuberculosis. Although MIC data alone will not always be sufficient to make informed clinical decisions in the field, such data can often be supplemented by knowledge of pharmacokinetics and pharmacodynamics (PK/PD) data for the drugs in question when available. Thus, PK/PD cutoffs can be applied to the MIC distributions to set breakpoints that can be used to select the appropriate drugs for the patient, optimizing therapy (16, 17). One important consideration in this regard is that PK/PD data determined in serum cannot always be used to predict susceptibility of an intracellular mycobacterium. For example, reduced moxifloxacin and ethambutol efficacy within macrophages has been shown against Staphylococcus aureus and Mycobacterium avium (18–21).
Strengths of the study include a design that followed strict CLSI criteria (10). Furthermore, the methodology was developed by consensus by participating laboratories that were highly experienced in M. tuberculosis DST. Given that the eight participating laboratories were located in geographically diverse countries, the study findings are likely to be representative of a tier-2 study. While the study did not include any laboratories in resource-limited settings, future data generated in those settings using the H37Rv strain will help further refine the tier-3 quality control ranges.
The study experienced a logistical challenge in that it was not practical for all participating laboratories to use the same batch of M. tuberculosis H37Rv strain; hence, each used its own stock. A second study limitation was that some data had to be excluded from the analysis; however, this was conducted according to CLSI guidance (10). Excluded data were from Lab-1, which reported that some plates were thawed upon arrival. These factors may have contributed to inconsistencies of MICs from Lab-1. Therefore, it was not surprising that the MICs for all drugs tested in Lab-1 were somewhat affected. Also, Lot-3 data for INH were excluded from the analysis. Indeed, preliminary DST performed on all lots indicated that INH MICs for Lot-3 were an order of magnitude higher than those for Lot-1 and Lot-2. Therefore, Lot-3 data for INH were excluded without significant effect on the remaining data, since two other lots were tested by eight laboratories 10 times each. Finally, we were unable to establish QC ranges for nicotinamide, prothionamide, or ethionamide, as no range of tested concentrations encompassed at least 95% of the observed MIC values. The inclusion of nicotinamide was experimental in nature and yielded an alternative and simple substitute for pyrazinamide (which requires acidic conditions for DST) (11). Since ethionamide and prothionamide are bacteriostatic rather than bactericidal against M. tuberculosis, the rules for antifungal DST had to be applied, with the lowest concentration that resulted in 80% inhibition of growth determined. Such endpoints are expected to lead to inconsistent readings that will vary between personnel in the same laboratory and, consequently, among laboratories.
Using the convenient 7H9 broth microdilution method, which is suitable for use in resource-limited settings, we were able to establish QC ranges against the M. tuberculosis H37Rv reference strain for the majority of commonly used anti-TB drugs, including both first- and second-line agents, as well as WHO category 5 drugs.
Supplementary Material
ACKNOWLEDGMENTS
We thank Janssen team members, in particular Koen Andries, Brian Dannemann, Myriam Theeuwes, and Chrispin Kambili, for their input into this paper and Ben Van Baelen (independent contractor) for statistical analyses. We acknowledge Emanuele Borroni (TB Supranational Reference Laboratory, San Raffaele Scientific Institute, Milan, Italy), Maria Wijkander and Juan Carlos Toro (TB Supranational Reference Laboratory, The Public Health Agency of Sweden, Solna, Sweden), Shaheed Vally Omar (National TB Reference Laboratory, Center for Tuberculosis, National Institute of Communicable Diseases, Johannesburg and Department of Medical Microbiology, University of Pretoria, Pretoria, South Africa), Luc Vranckx (Janssen Pharmaceutica NV, Beerse, Belgium), David Sikes (Reference Laboratory, Division of TB Elimination, U.S. Centers for Disease Control and Prevention, Atlanta, GA, USA), Franziska Wittwer (Department of Medical Microbiology, Center of Laboratory Medicine, Luzerner Kantonsspital LUKS, Luzern, Switzerland), Alex Sloutsky (Department of Public Health Massachusetts State Laboratory Institute, Jamaica Plain, MA, USA), and Joni Mostert (TASK Applied Science, SA MRC Centre for TB Research, DST/NRF Center of Excellence for Biomedical TB Research, Division of Molecular Biology and Human Genetics, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg, South Africa) for their important contributions to these studies. Medical writing support was provided by Ian Woolveridge of Zoetic Science, Macclesfield, UK, an Ashfield Company.
K.K. and N.L. are full-time employees of Janssen. We have no additional financial conflicts of interest to declare.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the CDC.
This study was done in part to satisfy FDA postmarketing requirements.
All authors substantially contributed to the study's design and protocol and execution of the work described. All authors were involved in the development of the primary manuscript and interpretation of the data, have read and approved the final version, and have met the criteria for authorship as established by the ICMJE.
Funding Statement
All participating laboratories received funds for this study from Janssen Pharmaceuticals except the Reference Laboratory, Division of TB Elimination, U.S. Centers for Disease Control and Prevention, Atlanta, GA, USA. Support for medical writing assistance was provided by Janssen Pharmaceuticals.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01138-16.
For a companion article on this topic, see doi:10.1128/JCM.01123-16.
REFERENCES
- 1.WHO. 2015. Global tuberculosis report 2015. WHO, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1. [Google Scholar]
- 2.WHO. 2008. Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. WHO, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/70500/1/WHO_HTM_TB_2008.392_eng.pdf?ua=1&ua=1. [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2011. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard, 2nd ed CLSI document M24-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services, Food and Drug Administration. 2009. Guidance for industry: microbiological data for systematic antibacterial drug products–development, analysis, and presentation. U.S. FDA, Washington, DC: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm182288.pdf. [Google Scholar]
- 5.Ängeby K, Juréen P, Kahlmeter G, Hoffner SE, Schön T. 2012. Challenging a dogma: antimicrobial susceptibility testing breakpoints for Mycobacterium tuberculosis. Bull World Health Organ 90:693–698. doi: 10.2471/BLT.11.096644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasipanodya J, Srivastava S, Gumbo T. 2012. New susceptibility breakpoints and the regional variability of MIC distribution in Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 56:5428. doi: 10.1128/AAC.00976-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chigutsa E, Meredith S, Wiesner L, Padayatchi N, Harding J, Moodley P, Mac Kenzie WR, Weiner M, McIlleron H, Kirkpatrick CM. 2012. Population pharmacokinetics and pharmacodynamics of ofloxacin in South African patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother 56:3857–3863. doi: 10.1128/AAC.00048-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed. CLSI document M07-A9 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.Kaniga K, Cirillo DM, Hoffner S, Ismail NA, Kaur D, Lounis N, Metchock B, Pfyffer GE, Venter A. 2016. A multilaboratory, multicountry study to determine bedaquiline MIC quality control ranges for phenotypic drug-susceptibility testing. J Clin Microbiol 54:2956–2962. doi: 10.1128/JCM.01123-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2008. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline, 3rd ed CLSI document M23-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Martin A, Takiff H, Vandamme P, Swings J, Palomino JC, Portaels F. 2006. A new rapid and simple colorimetric method to detect pyrazinamide resistance in Mycobacterium tuberculosis using nicotinamide. J Antimicrob Chemother 58:327–331. doi: 10.1093/jac/dkl231. [DOI] [PubMed] [Google Scholar]
- 12.Peñuelas-Urquides K, Villarreal-Treviño L, Silva-Ramírez B, Rivadeneyra-Espinoza L, Said-Fernández S, de León MB. 2013. Measuring of Mycobacterium tuberculosis growth. A correlation of the optical measurements with colony forming units. Braz J Microbiol 44:287–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coban AY, Birinci A, Ekinci B, Durupinar B. 2004. Drug susceptibility testing of Mycobacterium tuberculosis by the broth microdilution method with 7H9 broth. Mem Inst Oswaldo Cruz 99:111–113. doi: 10.1590/S0074-02762004000100020. [DOI] [PubMed] [Google Scholar]
- 14.Angeby KA, Jureen P, Giske CG, Chryssanthou E, Sturegård E, Nordvall M, Johansson AG, Werngren J, Kahlmeter G, Hoffner SE, Schön T. 2010. Wild-type MIC distributions of four fluoroquinolones active against Mycobacterium tuberculosis in relation to current critical concentrations and available pharmacokinetic and pharmacodynamic data. J Antimicrob Chemother 65:946–952. doi: 10.1093/jac/dkq091. [DOI] [PubMed] [Google Scholar]
- 15.Juréen P, Angeby K, Sturegård E, Chryssanthou E, Giske CG, Werngren J, Nordvall M, Johansson A, Kahlmeter G, Hoffner S, Schön T. 2010. Wild-type MIC distributions for aminoglycoside and cyclic polypeptide antibiotics used for treatment of Mycobacterium tuberculosis infections. J Clin Microbiol 48:1853–1858. doi: 10.1128/JCM.00240-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schön T, Juréen P, Giske CG, Chryssanthou E, Sturegård E, Werngren J, Kahlmeter G, Hoffner SE, Angeby KA. 2009. Evaluation of wild-type MIC distributions as a tool for determination of clinical breakpoints for Mycobacterium tuberculosis. J Antimicrob Chemother 64:786–793. doi: 10.1093/jac/dkp262. [DOI] [PubMed] [Google Scholar]
- 17.Turnidge J, Paterson DL. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev 20:391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pascual A, Garcia I, Ballesta S, Perea EJ. 1999. Uptake and intracellular activity of moxifloxacin in human neutrophils and tissue-cultured epithelial cells. Antimicrob Agents Chemother 43:12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke BF. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother 50:841–851. doi: 10.1128/AAC.50.3.841-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshpande D, Srivastava S, Meek C, Leff R, Hall GS, Gumbo T. 2010. Moxifloxacin pharmacokinetics/pharmacodynamics and optimal dose and susceptibility breakpoint identification for treatment of disseminated Mycobacterium avium infection. Antimicrob Agents Chemother 54:2534–2539. doi: 10.1128/AAC.01761-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deshpande D, Srivastava S, Meek C, Leff R, Gumbo T. 2010. Ethambutol optimal clinical dose and susceptibility breakpoint identification by use of a novel pharmacokinetic-pharmacodynamic model of disseminated intracellular Mycobacterium avium. Antimicrob Agents Chemother 54:1728–1733. doi: 10.1128/AAC.01355-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.