Abstract
Automated flow cytometry of urine remains an incompletely validated method to rule out urinary tract infection (UTI) in children. This cross-sectional analytical study was performed to compare the predictive values of flow cytometry and a dipstick test as initial diagnostic tests for UTI in febrile children and prospectively included 1,106 children (1,247 episodes). Urine culture was used as the gold standard test for diagnosing UTI. The performance of screening tests to diagnose UTI were established using receiver operating characteristic (ROC) analysis. Among these 1,247 febrile episodes, 221 UTIs were diagnosed (17.7% [95% confidence interval {CI}, 15.6 to 19.8%]). The area under the ROC curve for flow cytometry white blood cell (WBC) counts (0.99 [95% CI, 0.98 to 0.99]) was significantly superior to that for red blood cell (0.74 [95% CI, 0.70 to 0.78]) and bacterial counts (0.89 [95% CI, 0.87 to 0.92]) (P < 0.001). Urinary WBC counts also had a significantly higher area under the ROC curve than that of the leukocyte esterase (LE) dipstick (0.92 [95% CI, 0.90 to 0.94]), nitrite dipstick (0.83 [95% CI, 0.80 to 0.87]), or the combination of positive LE and/or nitrite dipstick (0.91 [95% CI, 0.89 to 0.93]) test (P < 0.001). The presence of ≥35 WBC/μl of urine was the best cutoff point, yielding both a high sensitivity (99.5% [95% CI, 99 to 100%]) and an acceptable specificity (80.6% [95% CI, 78 to 83%]). Using this cutoff point would have reduced the number of samples sent to the laboratory for culture by 67%. In conclusion, the determination of urinary WBC counts by flow cytometry provides optimal performance as an initial diagnostic test for UTI in febrile children.
INTRODUCTION
A diagnosis of urinary tract infection (UTI) requires at least 18 h until confirmation of a positive urine culture (1, 2). The growing need to enhance the performance of urine culture combined with the need to free up resources by rejecting negative samples quickly and economically has drawn attention to tests that can be used as initial diagnostic tests to rapidly rule out UTI in a two-tiered diagnostic process (3). For that purpose, several rapid urinary tests are commonly used, including dipstick biochemical analysis of urine for nitrites or leukocyte esterase (LE), and microscopic examination of urine for formed elements, including white blood cells (WBCs) and bacteria (4). In a recent meta-analysis, a positive dipstick for either LE or nitrite had 88% sensitivity and 79% specificity, whereas a nitrite-only positive dipstick had 49% sensitivity and 98% specificity for diagnosing UTI (4). Microscopic analysis of WBCs from centrifuged urine had a lower sensitivity and specificity than dipstick analysis and offered no advantage. However, microscopic examination for the detection of bacteria after Gram stain seems to be the most accurate test, with estimates of 91% sensitivity and 96% specificity (4). However, manual microscopy is technically demanding, and maintaining a 24-h service is restricted by the need for qualified laboratory staff, consumption of resources, and a considerable workload (5).
Urinalysis with automated flow cytometers performed by personnel with minimal training provides instant results for bacterial, red blood cell (RBC), and WBC counts (6). Furthermore, the small amount of urine needed (±1 ml) makes this method suitable for pediatric primary care facilities (5). Several adult studies reported good diagnostic performance of this technique, suggesting that automated flow cytometry might become the reference technique for urinalysis (3, 4, 7–9). However, only a few relatively small studies have investigated the diagnostic performance of this technique in children (5, 10).
The present study was designed to compare the diagnostic performance of urinary automated flow cytometry analysis and urinary dipstick analysis of nitrites and LE as screening tests for UTI in a pediatric population. For this purpose, screening with both tests was performed in febrile children in whom UTI was considered a possibility on clinical grounds and for whom a urine sample was sent for culture. Further objectives of this study were to determine the optimal leukocyturia cutoff value to detect a UTI in febrile children, and to model how automated flow cytometry WBC counts perform as a diagnostic test in populations with different disease probabilities of UTI (11).
MATERIALS AND METHODS
This prospective cross-sectional analytical study took place over 2 years in children (up to 16 years of age) screened for UTI at the emergency department of the Hôpital Universitaire des Enfants Reine Fabiola in Brussels, Belgium. Children were included in the study when the pediatrician in charge requested urinalysis to rule out a suspicion of UTI. Eligibility was limited to children with body temperature of ≥38°C recorded in the emergency department or a history of fever of ≥38°C recorded within the previous 24 h. All consecutive eligible infectious episodes between July 2006 and July 2008 were included.
For every child screened for UTI, a fresh urine sample was sent to the local laboratory for dipstick and automated cytometer analyses as well as quantitative urine culture. For children younger than 24 months, urine samples were obtained by suprapubic aspiration or bladder catheterization. For older children, samples were obtained by clean catch or bladder catheterization.
Dipstick urinalysis (Medi-Test Combi 11; Macherey-Nagel, Düren, Germany) was considered positive if there was a trace or more of LE and/or nitrite. Bacterial, RBC, and WBC counts were assessed on uncentrifuged urine using the automated flow cytometer Sysmex UF-100 (Merck-Eurolab, Leuven, Belgium). The threshold for abnormal leukocyturia was set at ≥35 leukocytes/μl of urine by the manufacturer. Urine osmolality was not taken into account in order to process or not process samples for culture. The laboratory staff was blinded to clinical information.
UTI was confirmed in children having a urine culture growing ≥100,000 CFU/ml of a single pathogen (12). In urine samples obtained by suprapubic aspiration, any growth of pathogen was considered significant (12). Urine culture was realized in the routine of the hospital bacteriology laboratory by technicians who were unaware that the specimens were part of a clinical study.
The study protocol was reviewed and approved by the ethics committee of our institution. The study was designed as a quality control of a diagnostic workup used in routine clinical practice in all patients admitted with a suspicion of UTI in our institution. Written informed consent was therefore not required from the parents of children who contributed data to the present study. To guarantee data confidentiality, data were anonymized, birthdates were deleted after the age had been calculated, and the data file was conserved on a password-protected laptop computer.
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were expressed as proportions with 95% confidence intervals (CIs) using the exact binominal distribution. Receiver operating characteristic (ROC) curves were drawn with UTI as outcome variable and the different diagnostic procedures as the classifier variable. The overall diagnostic performance of screening tests was expressed by the ROC area under the curve (AUC) with estimation of the 95% CI. The null hypothesis of equal test performance was tested by comparing ROC AUCs of the different types of diagnostic tests (reject null hypothesis at P < 0.05).
To further investigate the diagnostic performance of urinary WBC determinations by automated flow cytometry, we modeled PPV, NPV, value-added PPV, and value-added NPV, as well as proportional reduction in uncertainty (PRU) for populations with different levels of disease prevalence according to the methodology developed by Coulthard (11). Value-added PPV and value-added NPV express the absolute increase in diagnostic certainty provided by the diagnostic test: the value-added PPV was computed as PPV − expected prevalence, and the value-added NPV was calculated as NPV − (1− expected prevalence).
PRU measures the proportionate reduction in the outstanding diagnostic uncertainty and was calculated as (value-added PPV)/(1 − prevalence) and (value-added NPV)/prevalence for positive and negative test results, respectively (11).
The manuscript has been prepared according to STARD guidelines using the 2015 STARD checklist (http://www.equator-network.org/reporting-guidelines/stard/).
All statistical analyses were done using STATA 12.0 for Windows (Stata Corporation, College Station, TX).
RESULTS
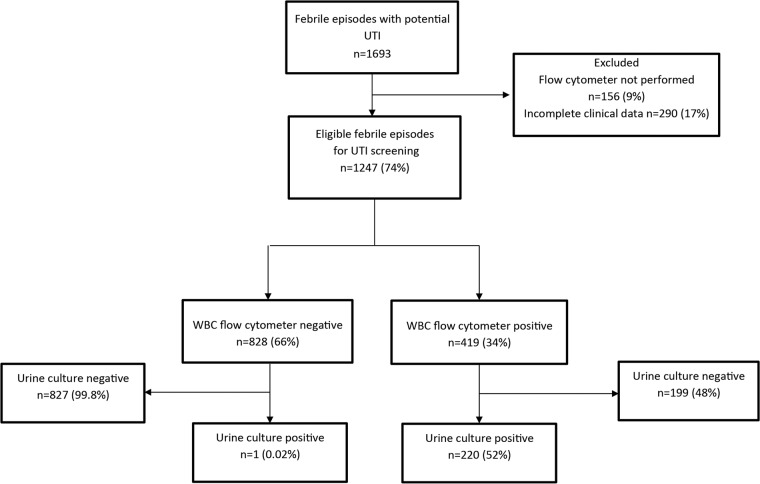
Over a prospective 2-year inclusion period, 1,693 consecutive episodes of fever (1,521 children) were screened for UTI. The diagnosis was confirmed by urine culture in 272 episodes (249 children). Fifty-one episodes (51 children) with positive urine cultures were excluded from the study because of incomplete urinalysis and/or clinical data. The remaining 221 episodes (198 children) were included in the study. Among the 1,421 episodes of fever (1,272 children) suspected for UTI but having negative urine cultures, 1,026 episodes (908 children) had complete blood and urinary tests and were therefore included in this study (Fig. 1).
FIG 1.
Flow diagram of febrile episodes screened for inclusion in the study.
The prevalence of UTI was of 17.7% (95% CI, 15.6 to 19.8%). Children with UTI were significantly younger, more frequently were girls, and had higher fever and C-reactive protein (CRP) levels than those without (Table 1).
TABLE 1.
Demographics and clinical data of episodes with fever screened for UTI
| Dataa | Total cohort (n = 1,247) | Cases with UTI (n = 221) | Cases without UTI (n = 1,026) | Pb |
|---|---|---|---|---|
| Gender | ||||
| Male | 508 (41) | 75 (34) | 433 (42) | |
| Female | 739 (59) | 146 (66) | 593 (58) | 0.023 |
| Age distribution (mo)c | ||||
| 0–3 | 133 (11) | 34 (15) | 99 (10) | |
| 4–12 | 167 (13) | 68 (31) | 99 (10) | |
| 13–24 | 112 (9) | 40 (18) | 72 (7) | |
| 25–36 | 115 (9) | 15 (7) | 100 (10) | |
| >36 | 716 (58) | 64 (29) | 652 (63) | |
| Age (median [IQR]) | 44 (13–93) | 14 (6–44) | 50 (22–107) | <0.0001 |
| Fever at entry (°C) | ||||
| 38–38.5 | 559 (45) | 37 (17) | 522 (51) | |
| 38.6–39 | 309 (25) | 85 (38) | 224 (22) | |
| 39.1–40 | 332 (27) | 92 (42) | 240 (23) | |
| >40 | 40 (4) | 7 (3) | 40 (4) | |
| Temp (median [IQR]) | 38.7 (38.0–39.4) | 39 (38.9–39.8) | 38.5 (38.0–39.2) | <0.0001 |
| Level of CRP (mg/dl) | ||||
| <4 | 828 (66) | 54 (24) | 774 (75) | |
| 4–10 | 234 (19) | 80 (36) | 154 (15) | |
| >10 | 185 (15) | 87 (39) | 98 (10) | |
| CRP level (median [IQR]) | 1.4 (0.5–6.4) | 8.5 (4.0–13.3) | 0.7 (0.5–3.9) | <0.0001 |
Data are presented as number (%), unless otherwise stated.
P value for testing of null hypothesis of no difference between children with and without UTI.
Information on age missing in 4 children.
The most common pathogen isolated during UTI episodes was Escherichia coli, which grew in 189 (86%) of the positive cultures. Febrile conditions other than UTI were distributed as follows: viral upper respiratory tract infections, n = 434 (42%); gastroenteritis, n = 103 (10%); appendicitis, n = 47 (5%); pneumonia, n = 44 (4%); bronchitis, n = 28 (3%); unknown origin, n = 122 (12%); and other, n = 248 (24%).
Automated flow cytometry results.
ROC curves for each urinalysis test (WBC, RBC, and bacterial counts) are presented in Fig. 2. The AUC for WBC counts was 0.99 (95% CI, 0.98 to 0.99), compared with 0.74 (95% CI, 0.70 to 0.78) for RBC counts and 0.89 (95% CI, 0.87 to 0.92) for bacterial counts. The overall diagnostic performance of urinary WBC counts was by far superior to that of RBC and bacterial counts (P < 0.001 for both comparisons; Fig. 2).
FIG 2.
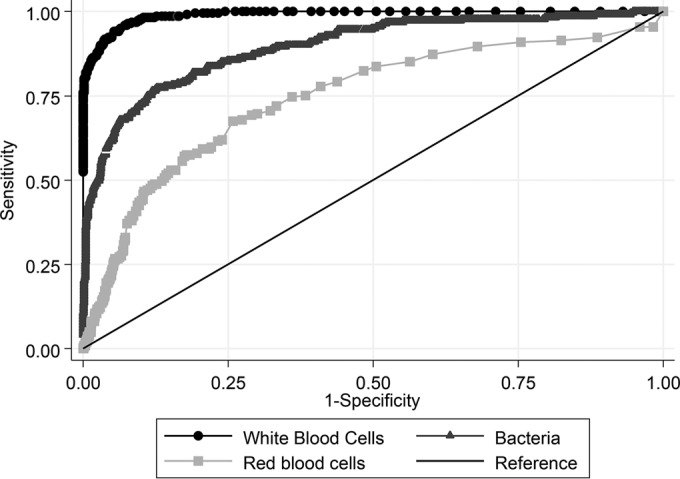
The ROC curves for each urinalysis test are presented. The AUC for red blood cells was 0.74 (95% CI, 0.70 to 0.78), compared with 0.89 (95% CI, 0.87 to 0.92) for bacterial counts and 0.99 (95% CI, 0.98 to 0.99) for cytometry leukocyte counts (P < 0.001 compared to red blood cell and bacterial counts).
We therefore restricted the analysis of appropriate cutoff values as screening tests for UTI to urinary WBC counts. The median number of urinary WBC was 764 WBC/μl (interquartile range [IQR], 176 to 2,378 WBC/μl) in cases of UTI and 15 WBC/μl (IQR, 7 to 29 WBC/μl) in other infectious episodes (P < 0.001). The best cutoff point was 35 WBC/μl, resulting in only 1 false-negative result and a sensitivity of 99.5% (95% CI, 99 to 100%), a specificity of 81% (95% CI, 78 to 83%), and an NPV of 99.9% (95% CI, 99.3 to 100%) (Table 2). Increasing the cutoff point to 100 WBC/μl of urine resulted in a marked improvement of specificity from 81 to 97%, corresponding to 3% of false-positive results and an overall increase in patients that were correctly classified by the test. However, sensitivity dropped from 99.5% to 89%, which would result in missing more than 10% of UTI episodes due to false-negative results of the screening test (Table 2).
TABLE 2.
Distribution of urinary cytometry WBC counts in febrile children with and without UTI and diagnostic test performance at different cutoff levels
| WBC count/μl of urine cutoff | No. with: |
Performance (% [95% CI])a |
|||||
|---|---|---|---|---|---|---|---|
| UTI | Other infection | Total infections | Sensitivity | Specificity | PPV | NPV | |
| 0–34 | 1 | 827 | 828 | NA | NA | NA | NA |
| 35–99 | 23 | 169 | 192 | 99.5 (97.5–100) | 80.6 (78.0–83.0) | 52.5 (47.6–57.4) | 99.9 (99.3–100) |
| ≥100 | 197 | 30 | 227 | 89.1 (84.3–92.9) | 97.1 (95.9–98.0) | 86.8 (81.7–90.9) | 97.6 (96.5–98.5) |
| Total | 221 | 1,026 | 1,247 | ||||
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) are calculated for leukocyte counts equal to or above the lower limit of the indicated range. NA, not applicable.
The use of the test with a cutoff point of 35 WBC/μl of urine to rule out UTI would have reduced the number of samples sent to the laboratory for culture by 830 (67% [95% CI, 64 to 70%]).
Urine dipstick results.
The LE dipstick test had a sensitivity of 89% (95% CI, 84 to 93%) and an NPV of 97% (95% CI, 96 to 98%) (Table 3). The nitrite dipstick test had a sensitivity of 67% (95% CI, 60 to 73%) and an NPV of 93% (95% CI, 92 to 95%). When positivity of the LE and/or nitrite dipstick was used as a screening test, it had a sensitivity of 93% (95% CI, 89 to 96%) and an NPV of 98% (95% CI, 97 to 99%) (Table 3).
TABLE 3.
Sensitivity, specificity, and predictive values of dipstick in predicting positive urine culture
| Result by test | No. with: |
Performance (% [95% CI]) |
||||
|---|---|---|---|---|---|---|
| UTI | No UTI | Sensitivity | Specificity | PPV | NPV | |
| LE | ||||||
| Positive | 197 | 102 | 89 (84–93) | 90 (88–92) | 66 (61–71) | 97 (96–98) |
| Negative | 24 | 924 | ||||
| Nitrites | ||||||
| Positive | 148 | 1 | 67 (60–73) | 99.9 (99–100) | 99 (97–100) | 93 (92–95) |
| Negative | 73 | 1025 | ||||
| LE and/or nitrites | ||||||
| Positive | 205 | 103 | 93 (89–96) | 90 (88–92) | 67 (64–69) | 98 (97–99) |
| Negative | 16 | 923 | ||||
| Total | 221 | 1,026 | ||||
Comparison of overall diagnostic performance between automated flow cytometry urinary WBC counts and urinary dipstick.
Urinary WBC counts had an ROC AUC of 0.99 that was significantly higher than the ROC AUC for the LE dipstick (0.92), nitrite dipstick (0.83), or the combination of positive results for LE and/or nitrite (0.91) (Table 4; see also Fig. S1 in the supplemental material; P < 0.001 for all comparisons).
TABLE 4.
Comparison of overall diagnostic performance between urinary dipstick and cytometry urinary WBC counts
| Test | ROC AUC (95% CI)a | Pb |
|---|---|---|
| Flow cytometry | ||
| WBC counts | 0.99 (0.98–0.99) | Reference |
| Dipstick test | ||
| LE | 0.92 (0.90–0.94) | <0.0001 |
| Nitrites | 0.83 (0.80–0.87) | <0.0001 |
| LE and/or nitrites | 0.91 (0.89–0.93) | <0.0001 |
ROC AUC, receiver operating characteristic area under the curve.
P values of null hypothesis of equal ROC AUC compared to cytometry urinary WBC counts.
Stratification of results by age.
The diagnostic performance of urinary dipstick and cytometry WBC count tests with a cutoff of 35 WBC/μl of urine was investigated after stratifying patients into five age categories (Table 5). There were no differences in test performance as expressed by the AUC of ROC curves between the age categories for any of the tests. The analysis also demonstrated excellent diagnostic performance of cytometry WBC counts, which remained superior to dipstick analysis in all age categories (Table 5).
TABLE 5.
AUC with 95% CI of urinary dipstick and cytometry WBC count ROC curves stratified for age
| Age (mo) | n | AUC (95% CI) |
|||
|---|---|---|---|---|---|
| Cytometry WBC count | Dipstick |
||||
| LE | Nitrite | LE and/or nitrites | |||
| Total | 1,247 | 0.99 (0.98–0.99) | 0.92 (0.90–0.94) | 0.83 (0.80–0.87) | 0.91 (0.89–0.93) |
| 0–3 | 133 | 0.99 (0.99–1.00) | 0.93 (0.91–0.95) | 0.81 (0.80–0.84) | 0.93 (0.90–0.95) |
| 4–12 | 167 | 0.98 (0.96–0.99) | 0.91 (0.89–0.93) | 0.85 (0.82–0.87) | 0.94 (0.92–0.95) |
| 13–24 | 112 | 0.99 (0.98–1.00) | 0.91 (0.90–0.93) | 0.84 (0.80–0.86) | 0.90 (0.89–0.93) |
| 25–36 | 115 | 0.98 (0.96–1.00) | 0.90 (0.88–0.92) | 0.80 (0.78–0.83) | 0.91 (0.90–0.93) |
| >36 | 716 | 0.99 (0.98–0.99) | 0.89 (0.87–0.91) | 0.84 (0.81–0.86) | 0.90 (0.89–0.92) |
Modeling the diagnostic performance of urinary automated flow cytometry WBC counts for populations with different prevalence of UTI.
Positive and negative predictive values of diagnostic tests depend on the prevalence of disease. The proportion of false-positive test results decreases in populations with higher disease prevalence, which results in higher PPV, whereas the proportion of false-negative test results increases, causing a reduction in NPV.
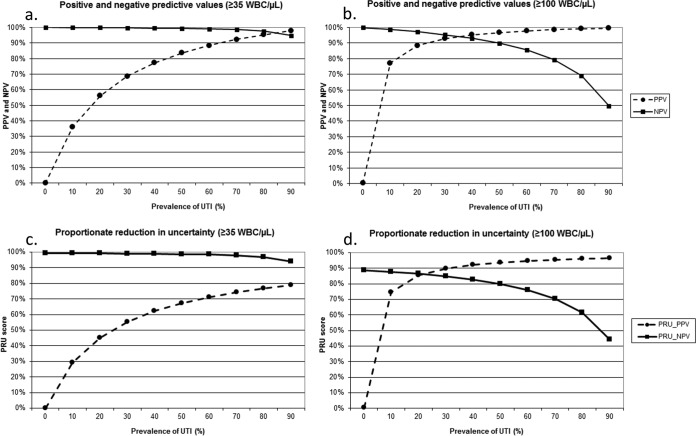
The PPV and NPV of urinary automated flow cytometry WBC counts with positivity cutoffs at 35 and 100 per μl were therefore modeled for populations with UTI prevalence varying between 0 and 90%, using a previously published methodology (11) (Fig. 3a and b). PPV was lower at the 35-WBC/μl cutoff than at the 100-WBC/μl cutoff because of the higher number of false-positive test results (Fig. 3a and b). This was observed in particular at low prevalence of UTI, when the impact of the false-positive tests on PPV is stronger (Fig. 3a and b). The drawback of the higher 100-WBC/μl positivity cutoff was a larger number of false-negative tests and a lower NPV, particularly in populations with a high prevalence of UTI (Fig. 3b). On the contrary, at the 35-WBC/μl cutoff, false-negative results are exceptional, and NPV remains excellent even at a very high prevalence of disease (Fig. 3a).
FIG 3.
(a and b) Modeling of the positive and negative predictive values (PPV and NPV, respectively) of cytometry leukocyte counts with positivity cutoff at ≥35 WBC/μl of urine (a) and ≥100 WBC/μl (b) at different population prevalences of UTI. (c and d) Modeling of the positive and negative proportionate reduction in uncertainty (PRU_PPV and PRU_NPV, respectively) of cytometry leukocyte counts with positivity cutoff at ≥35 WBC/μl of urine (c) and ≥100 WBC/μl of urine (d) at different population prevalences of UTI.
The proportionate reduction of diagnostic uncertainty (PRU) expresses the percent reduction in false diagnosis by the test in relation to disease classification based on prevalence alone. These plots confirm that the capacity of a positive test to reduce diagnostic uncertainty is improved at the higher WBC cutoff, particularly in populations with low disease prevalence (Fig. 3c and d). However, for the 100-WBC/μl cutoff, this happened at the price of a major loss in the NPV PRU at higher disease prevalence (Fig. 3d). In contrast, at a cutoff of 35 WBC/μl, negative urinary automated flow cytometry reduced diagnostic uncertainty by about 99% in populations with UTI prevalence up to 50%, reflecting the very strong capacity of a negative test to rule out UTI even in populations with high disease prevalence (Fig. 3c).
DISCUSSION
Negative urine cultures constitute a large percentage of the clinical microbiology workload (13, 14). A sensitive diagnostic test to rule out UTI in children might avoid both unnecessary urine cultures and antibiotic treatments for suspected UTI.
Our prospective cross-sectional study in a large cohort of febrile children shows that automated flow cytometry WBC counts has an excellent overall performance as a diagnostic test. With an ROC AUC of 0.99, it performs significantly better than RBC and bacterial counts with ROC AUCs of 0.74 and 0.89, respectively. We therefore focused on automated flow cytometry WBC counts and made a detailed analysis in order to determine the optimal positivity cutoff as a screening test for UTI. This analysis showed that the 35-WBC/μl cutoff resulted in only one false-negative result, with sensitivity and NPV close to 100%. Using this cutoff point would make the procedure an ideal first test in a two-tiered diagnostic strategy because it would have reduced the number of samples sent to the laboratory for culture by 67%. Under these circumstances, urine culture for a definitive bacteriological diagnosis and antibiotic sensitivity testing could be restricted to patients with a high probability of UTI with a minimal risk of missing the diagnosis due to false-negative flow cytometry results.
Several adult studies published on the accuracy of the automated flow cytometry WBC counts by urinalysis state 15 to 17 WBC/μl as the abnormal cutoff points (9, 15). A study by Lunn et al. on 186 urine samples reported that the presence of ≥40 WBC/μl of urine had a sensitivity of 89% and a specificity of 85% for predicting positive culture (5). These authors recommend that only samples which are positive on automated microscopy criteria should be sent for culture. Our results are completely in line with those of Lunn et al. (5).
At the time of the study design in 2006, our protocol was in accordance with the diagnostic criteria of the American Academy of Pediatrics (APP), which recommended ≥100,000 CFU/ml in the urine culture in order to confirm UTI (12). At present, the diagnostic criteria for UTI in children are under debate, and the APP recommends a threshold of ≥50,000 CFU/ml in their latest guidelines (2). The sensitivity and NPV of cytometer leukocyte counts should therefore be confirmed in a clinical setting which uses the present cutoff of ≥50,000 CFU/ml for the diagnosis of UTI.
Another limitation may be that urinary tests were done on fresh urine samples immediately analyzed after procurement at the local laboratory. This of course differs from the other clinical situations where samples were likely to be tested after a certain delay, during which WBCs may disappear from urine (16). Therefore, our results should only be extrapolated to clinical settings where rapid processing and analysis of fresh urine are feasible.
Urinary dipsticks are an alternative method to rapidly screen febrile children for UTI. Our data show that the automated flow cytometry WBC count test performed significantly better than urine dipstick analysis as a screening test for UTI. This was due mainly to the higher sensitivity of the automated flow cytometry-based test. Positivity for LE or nitrites had a sensitivity of 93%, which would result in missing 7% of the children with UTIs compared to 0.5% with automated flow cytometry. This test performance of the urinary dipstick is insufficient for use as a first test in a two-tiered diagnostic strategy, where the first test is mainly used to rule out the condition of interest with an extremely low risk of false-negative results in order to limit the second confirmatory test to a restricted number of patients at high probability of disease. Of interest, when urinalysis is negative for both dipstick and automated flow cytometry examinations, urine cultures were negative in 100% of cases.
NPV and PPV are two essential measures of test performance to guide clinical decision making. For a given test, both vary with disease prevalence, which has repercussions on the proportions of patients with false-positive and false-negative tests in the overall population (13). We therefore modeled NPV, PPV, and the PRU score of the urinary automated flow cytometry WBC count test for populations with different prevalence of UTIs using a methodology previously described by Coulthard (11). False-negative results remained exceptional, with an NPV above 95% for a prevalence of UTI up to 80% when urinary automated flow cytometry WBC count was used with the 35-WBC per μl cutoff. This confirms that urinary cytometry WBC count remains an excellent screening test to exclude UTI in febrile children even in populations with a high prevalence of the disease.
In this study, automated flow cytometry WBC count analysis was not compared with microscopic analysis of bacteria after Gram stain in the urine samples, which has been previously reported as the best predictive test for UTI (4). Microscopic urinalysis is no longer available as a routine procedure in our center because of the need for a 24-h service with trained personnel. Furthermore, our data are only applicable to febrile patients seen at a pediatric emergency department with a diagnosis of possible UTI. The applicability to mildly febrile or nonfebrile children as well as immunocompromised patients unable to mount a substantial inflammatory response remains to be investigated. However, we modeled the diagnostic performance of automated flow cytometry WBC count by plotting the PPV and NPV for all levels of prevalence, so that clinicians operating in high- or low-risk clinics, or with children known to have a high or low chance of UTIs, can extrapolate rough estimates of test performance for the patient populations that are relevant to their practice. However, these are estimates, and the diagnostic performance of the test should ideally be tested again in a second pediatric cohort with a different prevalence of UTI.
We conclude that automated flow cytometry urinary WBC count is an accurate diagnostic test to rule out UTI and has superior diagnostic performance compared to dipstick urinalysis for the prediction of UTI in febrile children. A cutoff value of 35 WBC/μl provides an extremely low false-negative rate and is therefore appropriate to select febrile children for whom urine culture is warranted, while ensuring that clinically important cases of UTI would not be missed.
Supplementary Material
ACKNOWLEDGMENTS
We declare no conflicts of interest.
This study was funded by the Department of Pediatric Nephrology of the Hôpital Universitaire des Enfants Reine Fabiola in Brussels, Belgium.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01382-16.
REFERENCES
- 1.Hellström A, Hanson E, Hansson S, Hjalmas K, Jodal U. 1991. Association between urinary symptoms at 7 years old and previous urinary tract infections. Arch Dis Child 66:232–234. doi: 10.1136/adc.66.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management. 2011. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 128:595–610. doi: 10.1542/peds.2011-1330. [DOI] [PubMed] [Google Scholar]
- 3.Pieretti B, Brunati P, Pini B, Colzani C, Congedo P, Rocchi M, Terramocci R. 2010. Diagnosis of bacteriuria and leukocyturia by automated flow cytometry compared with urine culture. J Clin Microbiol 48:3990–3996. doi: 10.1128/JCM.00975-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams GJ, Macaskill P, Turner RM, Hodson E, Craig JC. 2010. Absolute and relative accuracy of rapid urine tests for urinary tract infection in children: a meta-analysis. Lancet Infect Dis 10:240–250. doi: 10.1016/S1473-3099(10)70031-1. [DOI] [PubMed] [Google Scholar]
- 5.Lunn A, Holden S, Boswell T, Watson AR. 2010. Automated microscopy, dipsticks and the diagnosis of urinary tract infection. Arch Dis Child 95:193–197. doi: 10.1136/adc.2009.166835. [DOI] [PubMed] [Google Scholar]
- 6.Hanneman-Pohl K, Kampf SC. 1999. Automation of urine sediment examination: a comparison of the Sysmex UF-100 automated flow cytometer with routine manual diagnosis (microscopy, test strips and bacterial culture). Clin Chem Lab Med 37:753–764. [DOI] [PubMed] [Google Scholar]
- 7.Evans R, Davinson MM, Sim LRW, Hay AJ. 2006. Testing by Sysmex UF-100 flow cytometer and with bacterial culture in a diagnostic laboratory: a comparison. J Clin Pathol 59:661–662. doi: 10.1136/jcp.2005.032847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SY, Kim YJ, Lee SM, Hwang SH, Kim HH, Lee HY. 2007. Evaluation of Sysmex UF-100 urine cell analyzer as a screening test to reduce the need for urine cultures for community-acquired urinary tract infections. Am J Clin Pathol 128:922–925. doi: 10.1309/4606EC29U50DVAFY. [DOI] [PubMed] [Google Scholar]
- 9.Brilha S, Proença H, Cristino JM, Hänscheid T. 2010. Use of flow cytometry (Sysmex UF-100) to screen for positive urine cultures: in search for the ideal cut-off. Clin Chem Lab Med 48:289–292. [DOI] [PubMed] [Google Scholar]
- 10.Hoberman A, Chao HP, Keller DM, Hickey R, Davis HW, Ellis D. 1993. Prevalence of urinary tract infection in febrile infants. J Pediatr 123:17–23. doi: 10.1016/S0022-3476(05)81531-8. [DOI] [PubMed] [Google Scholar]
- 11.Coulthard MG. 2007. Quantifying how tests reduce diagnostic uncertainty. Arch Dis Child 92:404–408. doi: 10.1136/adc.2006.111633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Academy of Pediatrics Committee on Quality Improvement, Subcommittee on Urinary Tract Infection. 1999. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics 103:843–852. [DOI] [PubMed] [Google Scholar]
- 13.Kellogg JA, Manzella JP, Shaffer SN, Schwartz BB. 1987. Clinical relevance of culture versus screens for the detection of microbial pathogens in urine specimens. Am J Med 83:739–745. doi: 10.1016/0002-9343(87)90907-7. [DOI] [PubMed] [Google Scholar]
- 14.Zaman Z, Roggeman S, Verhaegen J. 2001. Unsatisfactory performance of flow cytometer UF-100 and urine strips in predicting outcome of urine cultures. J Clin Microbiol 39:4169–4171. doi: 10.1128/JCM.39.11.4169-4171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jolkonnen S, Paattiniemi EL, Karpanoja P, Sarkkinen H. 2010. Screening urine samples by flow cytometry reduces the need for culture. J Clin Microbiol 48:3117–3121. doi: 10.1128/JCM.00617-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coulthard MG, Nelson A, Smith T, Perry JD. 2010. Point-of-care diagnostic tests for childhood urinary tract infection: phase-contrast microscopy for bacteria, stick-testing, and counting white blood cells. J Clin Pathol 63:823–829. doi: 10.1136/jcp.2010.077990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.