Abstract
Blood cultures (BCs) are the standard method for diagnosis of bloodstream infections (BSIs). However, the average BC contamination rate (CR) in U.S. hospitals is 2.9%, potentially resulting in unnecessary antibiotic use and excessive therapy costs. Several studies have compared various skin antisepsis agents without a clear consensus as to which agent is most effective in reducing contamination. A prospective, randomized crossover study directly comparing blood culture contamination rates using chlorhexidine versus iodine tincture for skin antisepsis was performed at Robert Wood Johnson University Hospital (RWJUH). Eight nursing units at RWJUH were provided with blood culture kits containing either chlorhexidine (CH) or iodine tincture (IT) for skin antisepsis prior to all blood culture venipunctures, which were obtained by nurses or clinical care technicians. At quarterly intervals, the antiseptic agent used on each nursing unit was switched. Analyses of positive BCs were performed to distinguish true BSIs from contaminants. Of the 6,095 total BC sets obtained from the participating nursing units, 667 (10.94%) were positive and 238 (3.90%) were judged by the investigators to be contaminated. Of the 3,130 BCs obtained using IT, 340 (10.86%) were positive and 123 (3.93%) were contaminated. Of 2,965 BCs obtained using CH, 327 (11.03%) were positive and 115 (3.88%) were contaminated. The rates of contaminated BCs were not statistically significant between the two antiseptic agents (P = 1.0). We conclude that CH and IT are equivalent agents for blood culture skin antisepsis.
INTRODUCTION
Blood cultures (BCs) have long been the standard method of diagnosis of bacteremia during hospitalization. However, the average BC contamination rate (CR) in U.S. hospitals is 2.9%, resulting in unnecessary antibiotic use and potential excessive therapy costs of >$8,000 per contamination event (1, 2). Studies have shown that the use of prepackaged antisepsis kits aid in reduction of contamination from 8.4% to 4.8% (3). Additionally, dedicated phlebotomy teams with proper training in aseptic technique have been shown to further reduce CRs from 4.8% to 1.2% (3). The most likely source for BC contamination is the patient's skin at the venipuncture site, signifying that adequate skin antisepsis is critical in reducing CRs.
Chlorhexidine (CH) and iodine tincture (IT) have been shown to be more effective than povidone iodine (PI) for reduction of contamination (4, 5, 6); however, no statistically significant difference has thus far been found to exist between CH and IT (6, 7). A recent prospective randomized crossover trial reported by Washer et al. (6) directly compared CRs between CH, IT, and PI as antisepsis methods. They found no statistically significant difference between them; however, the overall CR for the study period was only 0.76%, which is considerably lower than the average CR at most medical centers across the United States (6). This low baseline CR may make significant differences between antisepsis methods difficult to distinguish.
Both CH and IT are currently used at Robert Wood Johnson University Hospital (RWJUH) for skin antisepsis prior to BC venipuncture. We have conducted a prospective, randomized crossover trial directly comparing CH and IT to distinguish BC CRs in a medical center with an overall CR of >3%. The higher CR at our institution also increases the likelihood that our study might detect a measurable difference.
MATERIALS AND METHODS
This study was conducted in eight nursing units within RWJUH over a 1-year time period from July 2014 through June 2015. The nursing units were designated as follows: MICU, medical intensive care unit; RCU, respiratory care unit; BMTU, bone marrow transplant unit, MO1, medical oncology unit 1, MO2, medical oncology unit 2, SO, surgical oncology unit; MS1, medical-surgical unit 1; and MS2, medical-surgical unit 2. Five nursing units (MICU, BMTU, MO1, MO2, MS1) were initially assigned to the CH arm, while the other three (RCU, SO, MS2) were assigned to the IT arm. Nurses and clinical care technicians (CCTs) in each unit underwent in-service training in aseptic technique, and the objectives of the study were outlined to the participating staff. BC kits, each containing two labeled culture bottles (one aerobic and one anaerobic) with either CH or IT were provided to each unit by the microbiology lab for use over 3-month time periods.
At the end of each 3-month block, the units switched to culture kits containing the other antiseptic, thus alternating skin preparation methods on a quarterly basis over the course of 1 year. The study investigators confirmed the switch with the microbiology lab at the end of each 3-month block.
Aseptic protocols were as follows. The venipuncture site was scrubbed with an isopropyl alcohol pad for 30 s, IT (2% iodine tincture solution; CareFusion, San Diego, CA) was then applied in concentric circles moving away from the venipuncture site to an approximately 5-cm diameter and allowed to dry for 30 s. CH (a ChloraPrep 1-step applicator, containing 2% chlorhexidine gluconate; CareFusion) was applied in a back-and-forth motion over the venipuncture site to an approximately 5-cm diameter and allowed to dry for 30 s.
BC bottles were labeled with colored dots to specify which preparation method was used (i.e., white for CH and orange for IT), and they were processed according to the RWJUH clinical microbiology laboratory standard operating procedure, which entails incubation at 35°C using the Bactec FX system (BD, Franklin Lakes, NJ) for 5 days. All positive cultures were then subcultured and processed further using standard laboratory techniques (8). The microbiology technologists recorded the culture results with color codes and patient accession numbers for use in data analysis.
BCs included in the study were those collected by unit nurses or CCTs via peripheral venipuncture from any patient admitted to each of the participating nursing units over the 1-year course of the study. BCs from blood samples that were drawn using an antiseptic not assigned to a particular nursing unit at the time of collection were excluded from the study.
BCs were considered positive if one or more microorganisms grew in at least one culture set. Positive cultures were considered contaminated if only one culture set grew common skin organisms, including coagulase-negative staphylococci, viridans group streptococci, Bacillus species, Neisseria species (other than Neisseria meningitidis or Neisseria gonorrhoeae), Micrococcus species, or aerobic Gram-positive rods. If two culture sets were positive with the same skin microorganism, they were considered true positives. A chart review of all contaminants was performed by one of the investigators (E.S.-R.) to confirm that they were, in fact, true contaminants in the context of the patient's clinical picture. This was accomplished by review of progress notes within the electronic medical record (EMR) to determine whether or not the patient was treated for the potential contaminant. If the clinical care team or infectious disease consultant determined treatment was necessary, the culture was considered a true positive.
This study was evaluated by the RWJUH institutional review board (IRB) and found to be exempt from IRB approval, as it was considered a quality assurance assessment and did not meet the regulatory definition of human subject research.
Statistical analysis.
CRs were calculated by dividing the number of contaminated cultures by the total number of BCs drawn via peripheral venipuncture using each antiseptic agent. A Pearson chi-square test was used to analyze categorical data. The number of total culture sets planned for the study was 6,000, approximately 3,000 each from the CH and IT arms. If power is calculated at 80% with this sample size, we would be able to detect a 1.3% difference in CRs, with alpha = 0.05.
RESULTS
Of the 6,095 total blood culture sets obtained from the participating nursing units, 667 (10.94%) were positive and 238 (3.90%) were judged by the investigators to be contaminated. As shown in Table 1, of the 3,130 BCs obtained using IT, 340 (10.86%) were positive and 123 (3.93%) were contaminated. Of the 2,965 BCs obtained using CH, 327 (11.03%) were positive, and 115 (3.88%) were contaminated. The rates of contaminated BCs between the two antiseptic agents were not different statistically (P = 1.0) (9).
TABLE 1.
Number of cultures obtained and contamination rates using chlorhexidine and iodine tincture
| Parameter | Chlorhexidine | Iodine tincture | P value |
|---|---|---|---|
| Total no. of cultures drawn | 2,965 | 3,130 | |
| Total no. of positive cultures | 327 | 340 | |
| Total no. of contaminated cultures | 115 | 123 | |
| Positive cultures (%) | 11.03 | 10.86 | 0.84 |
| Contaminated cultures (%) | 3.88 | 3.93 | 1.0 |
The most commonly isolated contaminant organisms were coagulase-negative staphylococcus (77.7%), viridans group streptococci (7.6%), and Corynebacterium species (3.4%). Other organisms included Propionibacterium species, Bacillus species, Micrococcus species, and Lactobacillus species (Table 2). There was no difference in contaminant organisms when analyzed by antiseptic agent.
TABLE 2.
Microorganisms judged to be contaminants from blood cultures obtained using chlorhexidine and iodine tincture
| Microorganism | No. (%) of contaminated blood cultures |
||
|---|---|---|---|
| Chlorhexidine (n = 115) | Iodine tincture (n = 123) | Total (n = 238) | |
| Coagulase-negative Staphylococcus | 90 (78.3) | 95 (77.2) | 185 (77.7) |
| Viridans group Streptococci | 8 (7.0) | 10 (8.1) | 18 (7.6) |
| Corynebacterium species | 5 (4.3) | 3 (2.4) | 8 (3.4) |
| Bacillus species | 1 (0.9) | 5 (4.1) | 6 (2.5) |
| Propionibacterium species | 2 (1.7) | 2 (1.6) | 4 (1.7) |
| Micrococcus species | 1 (0.9) | 4 (3.3) | 5 (2.1) |
| Lactobacillus species | 2 (1.7) | 1 (0.8) | 3 (1.3) |
| Polymicrobial | 6 (5.2) | 3 (2.4) | 9 (3.8) |
DISCUSSION
This study corroborates and supports the recent findings of Washer et al. (6). Similar to those investigators, we found virtually no difference in CRs between CH and IT. This remained true even in the setting of our institution's relatively high overall contamination rate of nearly 4%. The CRs observed in this study were similar to the average institution-wide CR at RWJUH as reported by the microbiology laboratory. This allows us to extrapolate our quality improvement data as representative of the hospital as a whole.
Given that in-service training of nurses and CCTs was only performed at the study initiation and there was no periodic monitoring of aseptic technique, we compared the overall CRs for each month of the study (Fig. 1). There was a moderate degree of month-to-month variability, and a slight upward trend was noted during the first 3 months of the study, but this trend did not persist during the following months.
FIG 1.
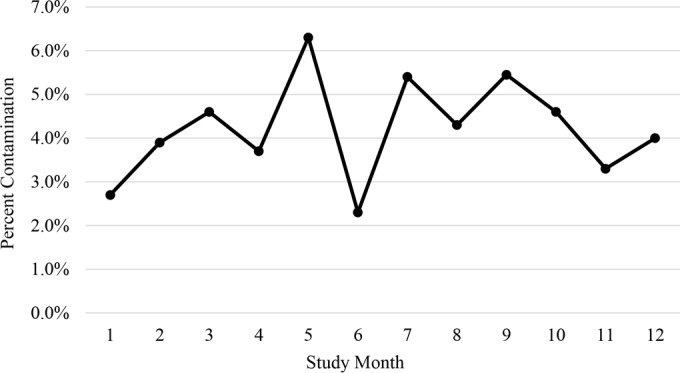
Comparison of overall contamination rates by study month.
When CR data were evaluated by nursing unit, our study hypothesis held true in that there was little difference between IT and CH. However, CRs tended to vary significantly between nursing units. The general medical/surgical units and medical intensive care unit exhibited higher average CRs of 5.3% (and as high as 6.6% for one unit) compared to 3.9% for the study as a whole. In contrast, the four oncology units (including surgical oncology, medical oncology, and bone marrow transplant units) all had much lower average CRs of 2.3%, with the BMTU being the lowest at 1% (Table 3). This is an intriguing finding from a quality improvement standpoint, in that it likely reflects the diligence of nurses and CCTs in maintaining aseptic conditions because of the immunocompromised state of the majority of patients admitted to these units. Ideally, this marked difference in CRs between units should not exist, as proper aseptic technique should be reinforced and practiced in all nursing units, regardless of the patient population. These findings will be used to promote quality improvement measures at our institution.
TABLE 3.
Percent contamination by nursing unit
| Nursing unita | % contamination using: |
||
|---|---|---|---|
| Chlorhexidine | Iodine tincture | Total | |
| BMTU | 1.9 | 0 | 1.0 |
| MOU1 | 2.5 | 3.1 | 2.8 |
| MOU2 | 2.0 | 2.7 | 2.3 |
| SOU | 2.7 | 3.5 | 3.1 |
| SU | 3.0 | 2.7 | 2.9 |
| MU | 6.9 | 6.2 | 6.5 |
| MICU | 4.6 | 6.0 | 5.2 |
| RCU | 9.1 | 4.1 | 6.6 |
BMTU, bone marrow transplant unit; MOU1, medical oncology unit 1; MOU2, medical oncology unit 2; SOU, surgical oncology unit; SU, surgical inpatient unit; MU, medical inpatient unit; MICU, medical intensive care unit; RCU, respiratory care unit.
There were several limitations to this study. We did not perform periodic observations of phlebotomies performed by nurses and CCTs in participating nursing units to ensure proper aseptic technique. In-service training took place at the start of the study, but it is likely that new nurses and technicians were hired or transferred to the participating units throughout the 1-year course of the study. These individuals would not have received the training by the investigators and may have been unaware of the study taking place. Our study included a wide range of patient populations, including ICU, general medical, general surgical, oncology, leukemia/lymphoma, and bone marrow transplant recipients; however, it did not include pediatric or emergency department patients.
The results of this study, as well as those of Washer et al. (6), suggest that iodine tincture and chlorhexidine tincture are equivalent antiseptic agents for skin antisepsis in patients who require blood cultures. Therefore, other factors may be considered in the decision of which product to choose in a given institution. These factors might include cost, ease of use and esthetics, shelf life, allergic reactions and other toxicities, and availability. Both products are easy to use, and both require only 30 s of drying time. Allergic reactions, although rare, are more common with iodine, and iodine can stain sheets and clothing. Chlorhexidine is generally more expensive. In recent years, both products have at times been unavailable from manufacturers. Based on cost and availability, iodine tincture is the only agent currently used for BC antisepsis at RWJUH.
ACKNOWLEDGMENTS
We thank the microbiology laboratory staff, in particular Kim Joho and Shandline Estime, at Robert Wood Johnson University Hospital.
We declare no competing financial interests.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Bates DW, Goldman L, Lee TH. 1991. Contaminant blood cultures and resource utilization: the true consequences of false-positive results. JAMA 265:365–369. [PubMed] [Google Scholar]
- 2.Gander RM, Byrd L, DeCrescenzo M, Hirany S, Bowen M, Baughman J. 2009. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol 47:1021–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinbaum FI, Lavie S, Danek M, Sixsmith D, Heinrich GF, Mills SS. 1997. Doing it right the first time: quality improvement and the contaminant blood culture. J Clin Microbiol 35:563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little JR, Murray PR, Traynor PS, Spitznagel E. 1999. A randomized trial of povidone iodine compared with iodine tincture for venipuncture site disinfection: effects on rates of blood culture contamination. Am J Med 107:119–125. [DOI] [PubMed] [Google Scholar]
- 5.Mimoz O, Karim A, Mercat A, Cosseron M, Falissard B, Parker F, Richard C, Samii K, Nordmann P. 1999. Chlorhexidine compared with povidone iodine as skin preparation before blood culture: a randomized, controlled trial. Ann Intern Med 131:834–837. [DOI] [PubMed] [Google Scholar]
- 6.Washer LL, Chenoweth C, Kim HW, Rogers MA, Malani AN, Riddell J 4th, Kuhn L, Noeyack B Jr, Neusius H, Newton DW, Saint S, Flanders SA. 2013. Blood culture contamination: a randomized trial evaluating the comparative effectiveness of 3 skin antiseptic interventions. Infect Control Hosp Epidemiol 34:15–21. [DOI] [PubMed] [Google Scholar]
- 7.Barenfanger J, Drake C, Lawhorn J, Verhulst SJ. 2004. Comparison of chlorhexidine and tincture of iodine for skin antisepsis in preparation for blood sample collection. J Clin Microbiol 42:2216–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2007. Procedures for the collection of diagnostic blood specimens by venipuncture; approved standard—6th ed CLSI document H3-A6. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.US Department of Health, Education and Welfare Center for Disease Control. 1977. Analytical statistics: statistical methods—testing for significance. Center for Disease Control, Atlanta, GA. [Google Scholar]


