Abstract
Diagnosis of Propionibacterium acnes bone and joint infection is challenging due to the long cultivation time of up to 14 days. We retrospectively studied whether reducing the cultivation time to 7 days allows accurate diagnosis without losing sensitivity. We identified patients with at least one positive P. acnes sample between 2005 and 2015 and grouped them into “infection” and “no infection.” An infection was defined when at least two samples from the same case were positive. Clinical and microbiological data, including time to positivity for different cultivation methods, were recorded. We found 70 cases of proven P. acnes infection with a significant faster median time to positivity of 6 days (range, 2 to 11 days) compared to 9 days in 47 cases with P. acnes identified as a contamination (P < 0.0001). In 15 of 70 (21.4%) patients with an infection, tissue samples were positive after day 7 and in 6 patients (8.6%) after day 10 when a blind subculture of the thioglycolate broth was performed. The highest sensitivity was detected for thioglycolate broth (66.3%) and the best positive predictive values for anaerobic agar plates (96.5%). A prolonged transportation time from the operating theater to the microbiological laboratory did not influence time to positivity of P. acnes growth. By reducing the cultivation time to 7 days, false-negative diagnoses would increase by 21.4%; thus, we recommend that biopsy specimens from bone and joint infections be cultivated to detect P. acnes for 10 days with a blind subculture at the end.
INTRODUCTION
Propionibacterium acnes is a facultative anaerobic Gram-positive rod, abundant on the human skin, and mainly associated with the sebaceous glands of the shoulder and axilla (1). It is most commonly associated with the chronic skin disease acne vulgaris. However, it may also cause bone and joint infections, including implant-associated infections. P. acnes has been recognized as an emerging cause of shoulder infections (2, 3) and is among the most common pathogens isolated in shoulder periprosthetic joint infections (PJI) (4, 5). P. acnes has also been implicated in other biofilm-related infections (6–8), such as cardiovascular implant-associated infections (9), spinal osteomyelitis (10, 11), and endophthalmitis (12, 13).
Diagnosis of P. acnes bone and joint infections is challenging since pain is often the only symptom (14, 15). For a long time, P. acnes was underdiagnosed in bone and joint infections due to the short cultivation time routinely used in diagnostic laboratories. In general, biofilm-forming bacteria are known to replicate at a slow rate due to low metabolism (16). Since recent studies recommended a prolonged cultivation time of up to 14 days for bone and joint infections (17, 18), the diagnosis of P. acnes infections has become more frequently documented (19). In view of the high costs of a long incubation period, a recent study suggested that 7 days of incubation should be sufficient for accurately diagnosing orthopedic implant-associated infections (20). In this study, 96.6% of the infections were detected within 7 days. However, P. acnes caused only one out of the 58 infections. The majority of cases were caused by Staphylococcus aureus and coagulase-negative staphylococci. Other studies attempted to improve diagnostic quality using better cultivation methods or sonication of implants to improve sensitivity and expedite diagnosis (21–24). However, none of these studies included a sufficient number of P. acnes infections to perform statistical analysis, which would allow drawing conclusions about the ideal cultivation time for slow-growing microorganisms such as P. acnes.
This study reports the results from a large cohort of patients with P. acnes-positive samples, with analysis and comparison of the times to detection in bone and tissue biopsy specimens and of sonicated implants with detailed information about transportation times, media types, and handling of specimens. We hypothesized that a higher inoculum as found in bone and joint infections would show growth of P. acnes earlier than a lower inoculum due to contamination. Therefore, we compared times to positivity in patients with an infection caused by P. acnes and isolation of P. acnes interpreted as a contaminant. The main aim of the study was to answer the question of whether reducing the cultivation time to 7 days allows diagnosis of P. acnes-related bone and joint infections without loss of sensitivity.
MATERIALS AND METHODS
Study population.
The orthopedic University Hospital Balgrist in Zurich, Switzerland, is a 120-bed orthopedic center specialized for bone and joint infections. Approximately 5,000 surgical procedures are performed annually there; in 2015, there were 326 primary hip, 186 knee, and 143 shoulder arthroplasties. In this single-center study, we retrospectively searched for patients with an orthopedic surgery (bone- or joint related) with at least one positive sample for P. acnes isolated between January 2005 and December 2015. All positive and negative samples within the same patient, same hospitalization period, and same infection site were grouped as a diagnostic set in a patient case.
Microbiological data were searched using the database of the Institute of Medical Microbiology, University of Zurich, Zurich, Switzerland. Clinical and demographic parameters of the patients' medical history at the time of diagnostic work-up, including diagnosis, were investigated using the patient clinical database of the orthopedic department and the prospective database of the Infectious Diseases Consultation service. We grouped patients into the following 2 groups: “infection” with growth of P. acnes in 2 or more samples within the same case and “no infection” with only 1 sample positive for P. acnes, suggesting contamination of the sample. For optimal and accurate allocation in one of the two groups, infection and no infection, only cases with 3 or more samples were included for our analysis (25, 26). Although the diagnosis of infection was strictly microbiologically defined, we also reviewed clinical presentations of all patients (presence of sinus tract) and investigated the intraoperative presentation. Infections were further grouped as (i) associated with a joint arthroplasty (PJI), (ii) associated with an orthopedic implant other than arthroplasty (implant-associated infections), (iii) septic arthritis (often arthroscopy associated), or (iv) osteomyelitis. Implants other than arthroplasties were labeled as small if screws or anchors were in place and as large if plates or intramedullary nails were used. Intraoperative samples for microbiological diagnostics might be tissue or bone biopsy specimens (peri- or intraarticular, periimplant) for conventional culture and the implant itself for culture of sonication fluid if it was removed (27). In cases with septic arthritis or osteomyelitis, only tissue or bone biopsy specimens were investigated.
Synovial fluid samples were excluded because we assumed faster time to positivity from recovered planktonic bacteria than from biofilm bacteria isolated from periimplant tissue or bone samples. Samples for analysis should have a minimal acceptable cultivation time so that statistical analysis is not distorted. Consequentially, we excluded negative samples with a too-short cultivation time according to Table 1. Since antibiotic treatment might influence the time to positivity of P. acnes growth, we excluded samples from patients who had taken antibiotics for ≥24 h within 14 days prior to sample acquisition. Furthermore, we excluded patients with polymicrobial infections since other bacteria in the same sample, in particular virulent bacteria, might decrease detection of P. acnes. All excluded samples and cases are enumerated in Table S1 in the supplemental material. The study was performed in line with the current ethical guidelines and approved by the institutional review board in Zurich, Switzerland.
TABLE 1.
Standard cultivation times for periimplant tissue, implant (sonication fluid), and bone and accepted minimum cultivation time to be included in our study
| Culture type | Cultivation method | Standard cultivation time (days) | Accepted cultivation time for inclusion (days) |
|---|---|---|---|
| Tissue | Direct aerobic | 7 | ≥6 |
| Direct anaerobic | 7 | ≥6 | |
| Thioglycolate aerobic | 10 (−14)a | ≥9 | |
| Implant | Direct aerobic | 2–3 | ≥2 |
| Direct anaerobic | 2–3 | ≥2 | |
| Aerobic BCBb | 7 | ≥6 | |
| Anaerobic BCB | 7 | ≥6 | |
| Bone | Thioglycolate aerobic | 10 (−14)a | ≥9 |
Prolonged culture with blind subculture at the end.
BCB, blood culture bottles.
Analysis and statistical methods.
For each sample of a patient diagnostic set, we recorded culture details such as type (e.g., tissue, bone, sonication fluid, synovial fluid, or wound swab), culture method, and Gram staining. We calculated time to positivity of each positive sample and culture type in the group of cases with an infection and cases with no infection.
Transportation time was calculated as the difference in hours between time of acquisition of samples during surgery and arrival at the microbiological laboratory. Since the exact time of biopsy specimen acquisition had not been recorded in the medical chart, we defined time of acquisition as the mean in time between the start and end times of surgery.
We analyzed time to positivity, sensitivity, specificity, and positive and negative predictive values for each culture method (direct aerobic, direct anaerobic, and enrichment) for tissue and bone biopsy specimens and sonication fluid from explanted hardware. To calculate diagnostic sensitivity, we compared test performance in patients with an infection (number of positive samples divided by all samples taken within a case) and specificity in patients with no infection (number of negative samples divided by all samples taken within a case).
Statistical analysis was conducted using Stata 14.1 SE (StataCorp, College Station, TX). We used nonparametric test statistics: Wilcoxon rank sum tests to compare continuous variables and Fisher's exact tests to compare categorical variables. To assess if transportation time from the operation theater to the microbiological laboratory was correlated with culture time to positivity, we used Spearman's rank correlation coefficient. Kaplan-Meier curves were used to illustrate the number of days from sampling to culture positivity. For patients belonging to the group of P. acnes infections, time was measured until culture positivity of the second sample to confirm infection. Differences between groups were analyzed with log-rank tests.
Microbiological processing. (i) Prediagnostic cultures.
Periimplant tissue, explanted hardware (implant), and bone samples were transported from the operating room at the orthopedic hospital (Balgrist) to the microbiology laboratory, which is a 30- to 60 min drive by car. The microbiological samples are transported twice daily. Tissue or bone samples were transported in culture tubes without addition of transporting media; implants for sonication were transported in Ringer-lactate solution.
(ii) Diagnostic cultures.
To extract bacteria from the tissue, samples were vortexed using 4-mm glass beads (Sarstedt, Nürmbrecht, Germany). After homogenization, samples were directly incubated under aerobic and anaerobic conditions on agar plates and in aerobic thioglycolate broth (BD, Allschwil, Switzerland) for enrichment. The bone samples were inoculated in aerobic thioglycolate broth only. See Table 1 for the cultivation times used in this study. For aerobic cultivation, Columbia sheep blood agar without antibiotics (bioMérieux, Mary-l'Etoile, France), colistin-nalidixic acid (CNA) blood agar (bioMérieux), MacConkey agar (bioMérieux), and Crowe agar (chocolate agar supplemented with bacitracin and IsoVitaleX [Difco GC medium; Becton Dickinson]) were used. Brucella agar (in-house sheep blood agar plates with hemin and vitamin K1; BD), kanamycin-vancomycin agar (laked sheep blood Brucella agar plates with kanamycin and vancomycin; BD), and phenylethyl alcohol agar plates (BD) were used for anaerobic cultivation (Whitley anaerobic workstation MG1000; Don Whitley Scientific, West Yorkshire, England). Thioglycolate broth medium was inspected daily for cloudiness and then subsequently plated onto chocolate (aerobic) and Brucella (anaerobic) agar plates for further identification. If thioglycolate broth cultures were negative after 10 days of cultivation, blind subcultures plated on chocolate and Brucella agar plates were performed and cultivated for another 2 to 3 days.
Explanted implants were directly placed either in a plastic bag (double bagged) or in a sterile Tupperware container in the operating room and transported to the microbiological laboratory for sonication using BactoSonic (Berlin, Germany) as previously described (28, 29). Under a laminar flow, the future opening sites of the bags were thoroughly disinfected using 70% alcohol, and the bags were sliced open using a sterile scalpel. All of the fluid inside the inner bag or the Tupperware container was pipetted into a falcon tube (BD) and inoculated onto the corresponding culture media. Of the sonication fluid, 0.5 ml was plated onto the distinct agar plates for aerobic (chocolate and sheep blood agar) and anaerobic (Brucella) cultivation as described for tissue previously. Ten milliliters of the liquid was inoculated into blood culture flasks (BacT/Alert FA and FN bottles; Bio-Mérieux) for aerobic and anaerobic cultivation. The bottles were vertically incubated and slightly shaken at 60 Hz. Flasks with a positive growth signal were subcultured using the set of agar-based media described above. Cultivation times for the different methods are summarized in Table 1. A threshold of ≥50 CFU/ml bacteria on direct agar plates was considered positive.
All microorganisms have been identified by standard identification methods, including matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF) since 2012. Before 2012, P. acnes was identified by conventional reactions, such as positive reactions for CAMP, indole, nitrate reduction, catalase, and detection of propionic acid as metabolic fatty acids formed by glucose fermentation.
(iii) Microscopy.
A Gram stain was performed routinely for tissue samples and sonication fluid from implants parallel with all methods mentioned on day zero in the clinical microbiology laboratory.
(iv) Time to positivity of P. acnes growth.
We defined time to positivity as the time at which either (i) P. acnes typical colonies grew on agar plates, (ii) turbidity appeared in the thioglycolate broth, or (iii) a positive signal was detected in a blood culture bottle for which P. acnes was finally identified on agar plates.
RESULTS
Clinical data.
We identified 70 cases with a P. acnes infection, in which 262 out of 379 samples were positive (69.1%). Forty-seven cases did not fulfill our criteria for a proven infection with only one positive sample (47 out of 215 samples, 21.9%). The most common sample site was shoulder (total n = 77), followed by hip (n = 26). In the infection group, we diagnosed PJI in 35 (50%) cases, and implant-associated infections with large (plates, intramedullary nail) or small implants (screws, anchors) in 10 (14.3%) and 17 (24.3%) cases, respectively. Five cases presented with septic arthritis (arthroscopy-associated), and 3 cases with osteomyelitis (2 in the shoulder after infiltration or trauma and 1 in the clavicula associated with B-cell lymphoma). In the no infection group, there were pain due to mechanical reasons in 22 (46.8%), aseptic loosening of an implant in 9 (19.2%), and other reasons in 16 (34%) cases diagnosed at revision surgery.
There was no significant difference in the number of samples taken between the infection and no infection group (means, 5.4 and 4.6 samples, respectively; P = 0.06). The characteristics of cases are shown in Table 2. We found no statistically significant difference with regard to sex, age, or presence of foreign bodies between the two groups. Intraoperative findings described by the surgeon did not help to distinguish between infection and no infection. Isolation of P. acnes in the shoulder was more prevalent in the infection group (51 and 26 cases, respectively; P = 0.073), but isolation in the knee was more frequent in the no infection group (1 and 5 cases, respectively; P = 0.038).
TABLE 2.
Characteristics of 70 cases with a bone and joint infection (≥2 positive P. acnes samples) and 47 cases with no infection (1 positive P. acnes sample)
| Parameter | Infection (n = 70) | No infection (n = 47) | P |
|---|---|---|---|
| Patient characteristic | |||
| Female sex (no. [%]) | 21 (30) | 15 (32) | 0.84 |
| Age (median [range]) (yr) | 58 (21–81) | 58 (16–86) | 0.49 |
| Sample site (no. [%]) | |||
| Shoulder | 51 (72.9) | 26 (55.3) | 0.07 |
| Hip | 13 (18.6) | 13 (27.7) | 0.27 |
| Spine | 4 (5.7) | 1 (2.1) | 0.65 |
| Knee | 1 (1.4) | 5 (10.6) | 0.04 |
| Other | 1 (1.4) | 2 (4.3) | 0.56 |
| Presence of (no. [%]) | |||
| Any foreign body | 62 (88.6) | 39 (83) | 0.42 |
| Prosthesis | 35 (50) | 27 (57.4) | 0.46 |
| Intraoperative presentation | |||
| Normal | 7 (10) | 6 (12.8) | 0.77 |
| Pus | 9 (12.9) | 2 (4.3) | 0.20 |
| Inflammation | 41 (58.6) | 26 (55.3) | 0.85 |
| Wear of the implant | 1 (1.4) | 2 (4.3) | 0.56 |
| Adhesion | 10 (14.3) | 7 (14.9) | 1 |
| No data available | 2 (2.8) | 4 (8.5) | 0.22 |
Time to positivity.
The proportion of sample positivity was 55.4% after 7 days and 65.7% after 10 days in the infection group, which was significantly higher than in the no infection group (8.4% and 19.1%, respectively; P < 0.0001) (Fig. 1).
FIG 1.
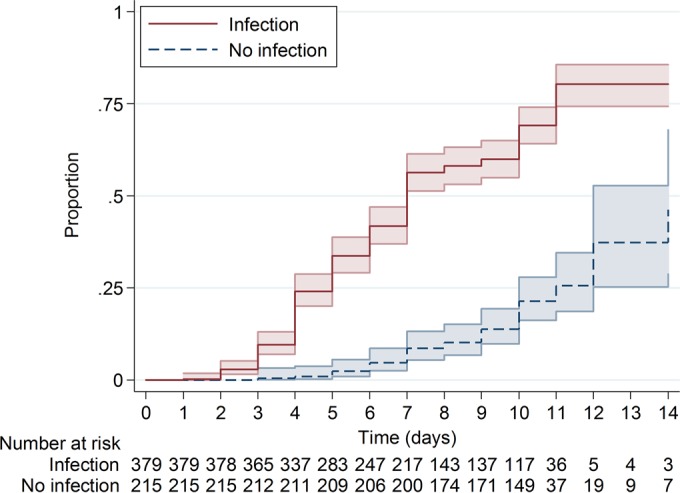
Proportion of sample positivity with P. acnes in the infection and no infection groups. The colored areas represent the 95% confidence intervals. The proportion of positivity at day 7 was 55.4% and that at day 10 was 65.7% in the infection group. In the no infection group only 8.4% were positive at day 7 and 19.1% at day 10 (P < 0.0001), and discontinuation of sample cultivation would have resulted in missing 52 of 262 (19.9%) positive samples or 15 of 70 (21.4%) cases.
Overall, the median time to the first positive sample was different in samples from the infection group (5 days, 95% confidence interval [CI], 4 to 5 days) from that in the no infection group (9 days, 95% CI, 7 to 10 days, P < 0.0001) (Fig. 2A). Time to confirmation of infection, according to our definition equal to time to the second positive sample, was also significantly different (6 days, 95% CI, 5 to 7 days) to the single positive sample in the no infection group (9 days, 95% CI, 7 to 10 days; P < 0.0001) (Fig. 2B).
FIG 2.
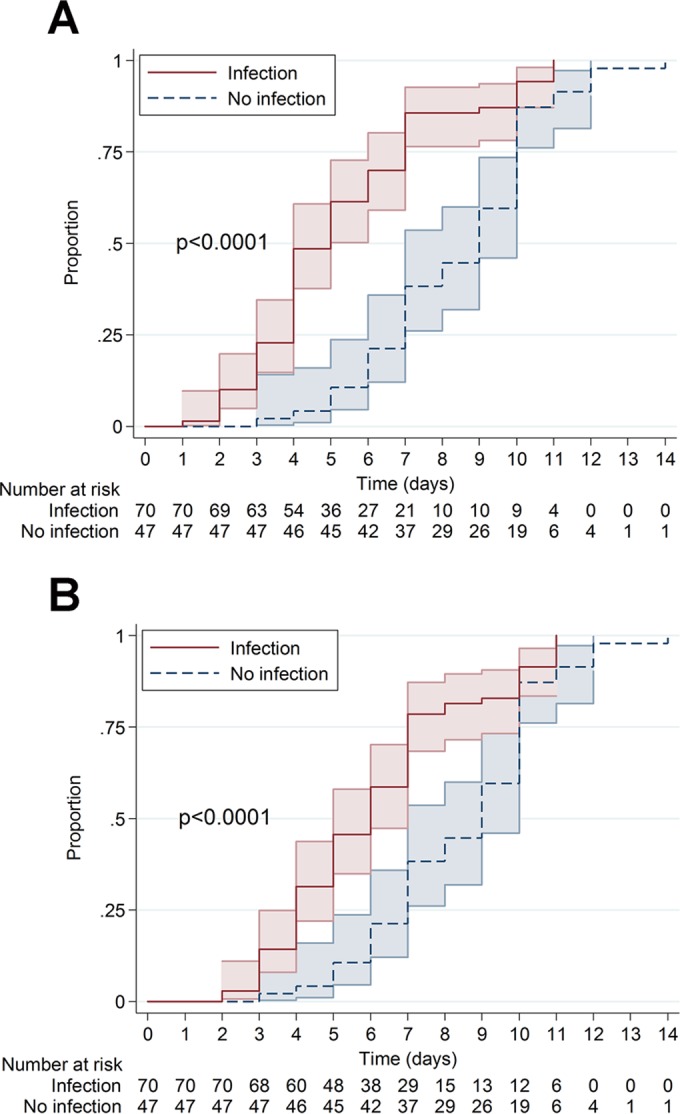
Proportions of first (A) or second (confirmation) (B) positive sample in the infection group compared to the single positive sample in the no infection group. The colored areas represent the 95% confidence intervals. Median time to positivity was 5 days (95% CI, 4 to 5 days) for the first sample, 6 days (95% CI, 5 to 7 days) for the second positive sample in the infection group to confirm infection, and 9 days (95% CI, 7 to 10 days) in the no infection group.
Within the infection group, discontinuation of cultures at 7 days would have led to missing diagnosis of infection in 15 out of 70 (21.4%) cases (Fig. 2B). On day 10, a P. acnes infection was still not diagnosed in 6 cases (8.6%). These cultures finally turned positive on day 11, all from blind subcultures at the end of regular cultivation of thioglycolate broth. In the no infection group, the majority of cases with a single positive P. acnes sample (29 of 47 cases, 61.7%), suggesting a contamination of the sample, were recorded after day 7 (Fig. 2B).
Comparing different cultivation methods (Table 3) in the infection and no infection group, we found that thioglycolate broth of tissue biopsy specimens showed a significant difference in median time to positivity (6 days, 95% CI, 5 to 6 days; 9 days, 95% CI, 8 to 10 days; P < 0.0001) as opposed to other methods, which did not significantly differ.
TABLE 3.
Median time to positivity for different cultivation methods in the infection and no infection groups
| Culture type | Culture method | Results for infection group |
Results for no infection group |
||
|---|---|---|---|---|---|
| No. of positive cultures | TtPa (median [95% CI]) (days) | No. of positive cultures | TtP (median [95% CI]) (days) | ||
| Tissue | Direct aerobic | 15 | 7 (5–7) | 2 | 7 |
| Direct anaerobic | 136 | 6 (6–6) | 5 | 6 | |
| Thioglycolate aerobic | 205 | 6 (5–6) | 38 | 9 (8–10) | |
| Implant | Direct aerobic | 0 | 0 | ||
| Direct anaerobic | 13 | 4 | 2 | 3 | |
| Aerobic BCB | 0 | 0 | |||
| Anaerobic BCB | 4 | 4 | 1 | 5 | |
| Bone | Thioglycolate aerobic | 6 | 7 | 2 | 8 |
TtP, time to positivity.
b BCB, blood culture bottles.
Sensitivity, specificity, and positive and negative predictive values.
Thioglycolate broth as the enrichment method used for tissue and bone biopsy specimens was most effective in correctly identifying P. acnes (sensitivity, 66.3% in tissue samples and 75% in bone samples) and significantly different from those for aerobic and anaerobic agar plates (sensitivity, 5.1% and 42.1%, respectively; P = 0.0001). Low sensitivity was observed in all aerobic cultures (direct aerobic agar or aerobic blood culture bottles) (Table 4). Thioglycolate broth as the method with the highest sensitivity, however, showed low specificity in tissue (79.1%) and bone (66.7%) samples.
TABLE 4.
Sensitivity, specificity, positive predictive value, and negative predictive value of different culture methods
| Culture type | Culture method | Sensitivity (%) | Specificity (%) | PPVa (%) | NPVb (%) |
|---|---|---|---|---|---|
| Tissue | Direct aerobic | 5.1 | 98.9 | 88.2 | 38.8 |
| Direct anaerobic | 42.1 | 97.1 | 96.5 | 47.3 | |
| Thioglycolate aerobic | 66.3 | 79.1 | 84.4 | 58.1 | |
| Implant | Direct aerobic | 0 | 100 | 0 | 36.4 |
| Direct anaerobic | 37.1 | 91.3 | 86.7 | 48.8 | |
| Aerobic BCBc | 0 | 100 | 0 | 38.3 | |
| Anaerobic BCB | 13.3 | 95.2 | 80.0 | 43.5 | |
| Bone | Thioglycolate aerobic | 75.0 | 66.7 | 75.0 | 66.7 |
PPV, positive predictive value.
NPV, negative predictive value.
BCB, blood culture bottles.
The best specificity with low false-positive results was observed when aerobic agar or aerobic blood culture bottles were used as the enrichment method. Among tissue and sonication fluid cultures, both direct anaerobic cultivation methods showed the best positive predictive value with 96.5% and 86.7%, respectively (Table 4).
Gram staining.
Only 10 out of 311 (3.2%) samples in the group of patients with a bone and joint infection with an available Gram stain showed a positive result with Gram-positive rods. No positive Gram staining was observed in any of the samples from noninfected individuals, resulting in a 100% positive predictive value and 36.9% negative predictive value.
Influence of transportation time on diagnostic output.
Transportation time was available in 528 of 594 samples (88.9%) out of which 336 samples were allocated to the infection group and 192 to the no infection group. Transportation time of less than 24 h was seen in 94% of the positive samples in the infection group and in 92% in the no infection group. Transportation time did not correlate with time to positivity (Spearman's rho = −0.0195; P = 0.66).
Subanalysis of 35 cases with a PJI caused by P. acnes.
In a subanalysis of 35 cases with a PJI caused by P. acnes, we compared diagnostic sensitivity of sonication fluid with tissue biopsy specimen cultures. Only 26 cases could be included for this subanalysis with both sonication fluid and tissue biopsy specimen cultures available. Eight cases could not be included due to only having tissue samples and 1 case due to only having tissue and bone samples analyzed.
For tissue biopsy specimens, the sensitivity was 96.2% (25/26 cases) with at least 1 positive culture as opposed to sonication fluid with 46.2% (12/26). Twenty-three cases had ≤2 positive tissue samples, 2 had only 1, and 1 had no positive tissue at all. All 3 cases (11.5%) with <2 positive samples had positive sonication of >100 CFU/ml to be classified as an infection and would have been misclassified as a contaminant if only tissue biopsy specimens had been cultivated.
DISCUSSION
In this large cohort study of 70 P. acnes infections, we showed that median time to positivity to confirm an infection was 6 days with a range up to 11 days. However, reducing the cultivation time to 7 days as proposed by previous studies (20, 23) would have resulted in missing diagnosis in 15 patients (21.4%), suggesting that in patients with a high prevalence of P. acnes infections, a prolonged cultivation is crucial for detecting infections. This supports the results of a study, including fewer P. acnes infections (37 cases), which showed that 14% of positive samples were detected after day 7 (3). Furthermore, the study showed that a prolonged cultivation time of >10 days did not improve sensitivity. Therefore, Frangiamore et al. (3) recommended that a routine cultivation time of 10 days is optimal to ensure accurate diagnosis of P. acnes infections. Our data support this recommendation. However, we recommend a 10-day cultivation with a blind subculture in thioglycolate broth at the end in certain cases with high suspicion of P. acnes infection. We would have missed 6 cases (8.6%) if we had stopped cultures at day 10.
We reported a median time to the first positive sample of 5 days. A study by Minassian et al. (23) using blood culture bottles as an enrichment method for tissue biopsy specimens showed similar results (median time to positivity of 5 days; range, 3 to 13 days), but it was of smaller size with 30 Propionibacterium species isolates from 16 patients. Butler-Wu et al. described mean time to positivity of 7.3 ± 2.6 days with a range of up to 13 days for 19 infected cases undergoing revision arthroplasty (18). P. acnes grew significantly faster in samples isolated from patients with a P. acnes infection compared to patients with no infection. This difference was most pronounced in enrichment thioglycolate broth, supporting previous studies (3), and our hypothesis that a higher inoculum is to be found in infected samples than in contaminated samples.
Thioglycolate broth enrichment during 10 days is a sensitive method to detect P. acnes. However, a prolonged incubation time also increases the risk of cultivating a bacterial contamination (30, 31) as shown by our result that 61.7% of samples belonging to our no infection cases were recorded after day 7. Our results are consistent with those of a previous study showing that 21.7% of the cases with only 1 positive P. acnes sample, labeled as no infection, became positive after day 13 (18). A previous study published in 2013 showed a faster time to positivity when thioglycolate broth was cultivated anaerobically (30), which might be investigated in a prospective study in our orthopedic center. Avoiding the problem of low specificity, we found that the best positive predictive value was for tissue biopsy specimens grown directly on anaerobic agar. All aerobic cultivation methods showed low sensitivity, which is not surprising since P. acnes is facultative anaerobic. We did not find an increased sensitivity using either aerobic or anaerobic cultures as Butler-Wu et al. reported (18).
Gram staining is not a useful tool for excluding bone and joint infections. All of our 10 positive Gram stains were from infected cases. This supports published data showing that positive results prove infection, while a negative result does not exclude an infection (32, 33).
Cultivation of sonication fluid of implants is known to show sensitivity better than conventional tissue cultures (27). This is also shown in a recent study by Portillo et al., who investigated the sensitivity of sonication in 39 orthopedic implant-associated infections, including 5 cases with a P. acnes infection. They detected all 5 P. acnes infections by sonication but only 2 by conventional tissue cultures (22). A retrospective cohort study of 20 PJI caused by P. acnes showed an 89% sensitivity for sonication fluid cultures and 60% for conventional tissue cultures (15). Our results showing lower sensitivity for sonication than for tissue cultures refute this observation: The most obvious explanation for our findings is our short cultivation time of only 3 days on agar plates and 7 days in blood culture bottles compared to a longer cultivation time in tissue or bone biopsy specimen cultures. Other studies using cultivation times of up to 14 days in blood culture bottles reported a high sensitivity for sonication (21, 22, 24). An alternative explanation for our low sensitivity with sonication is the possibility that P. acnes might be inhibited or killed using ultrasound baths as has been shown for other pathogens (34). We had initially used plastic bags instead of Tupperware containers for sonication for many years (using a higher ultrasound frequency of 45 kHz), which might contribute to the difference and further emphasizes the necessity to adhere to a standard protocol. Our results showing better sensitivity of direct agar plating for 2 to 3 days compared to cultivation using blood culture bottles for 7 days is intriguing particularly in light of the increasing number of studies that point to improved performance of blood culture media for the recovery of pathogens causing PJI (21, 22, 24). An advantage of using blood culture bottles instead of thioglycolate broth medium with the presence of antibiotic-absorbing agents could not be assessed at this point since we excluded patients taking antibiotics.
We showed that a longer transportation time from the operating theater to the microbiology laboratory did not influence time to positivity of P. acnes growth. This is in contrast to the recent Infectious Diseases Society of America (IDSA) recommendation (35) emphasizing that anaerobic tissue cultures should be transported at room temperature in anaerobic containers as soon as possible with an optimal time of 2 h. However, our result supports the hypothesis that microorganisms in the biofilm state, in particular P. acnes as a facultative anaerobic bacterium, as found in bone and joint infections are robust to environmental changes. We did not find another study investigating the influence of time of transportation or use of anaerobic transport container for recovery of P. acnes in bone and joint infections.
The strength of our study is the large cohort of 70 cases with a P. acnes infection. To our knowledge, this is the largest study to date with analysis of the applied cultivation methods and time to positivity in bone and joint infections focusing on P. acnes as a slow growing microorganism. Most study results in this field are influenced by various variables. To strengthen the conclusions of our analysis, we excluded samples obtained from individuals already receiving antibiotics as well as cases with a polymicrobial infection. The strength of our study is the time period of more than 10 years in which the same microbiological protocols were used for all bone and joint infection samples. A limitation of our study is that different culture media were not incubated for the same period of time, which makes comparison difficult but due to the retrospective study design also difficult to correct.
We conclude that a prolonged cultivation time of 10 days is necessary for P. acnes identification. We do not recommend reducing the cultivation time to 7 days in patient cohorts with a high incidence of P. acnes infections (e.g., those with shoulder PJI or vertebral osteomyelitis). We would have missed 21.4% of the P. acnes infections if the cultivation time had been reduced to 7 days. Thioglycolate broth as an enrichment method in tissue biopsy specimens showed high sensitivity. The best positive predictive value was seen with direct incubation on anaerobic agar plates. Time to positivity of P. acnes growth does not seem to be affected by a prolonged transportation time, which shows that P. acnes in the biofilm of musculoskeletal infections can survive for hours even in a fastidious environment.
Supplementary Material
ACKNOWLEDGMENTS
We thank Thomas Klein and the technicians of the Institute of Medical Microbiology of the University of Zurich for expert help and assistance.
Funding Statement
Yvonne Achermann is supported by the academic career program “Filling the Gap” of the Medical Faculty of the University of Zurich. The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01435-16.
REFERENCES
- 1.Patel A, Calfee RP, Plante M, Fischer SA, Green A. 2009. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elbow Surg 18:897–902. doi: 10.1016/j.jse.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 2.Levy PY, Fenollar F, Stein A, Borrione F, Cohen E, Lebail B, Raoult D. 2008. Propionibacterium acnes postoperative shoulder arthritis: an emerging clinical entity. Clin Infect Dis 46:1884–1886. doi: 10.1086/588477. [DOI] [PubMed] [Google Scholar]
- 3.Frangiamore SJ, Saleh A, Grosso MJ, Alolabi B, Bauer TW, Iannotti JP, Ricchetti ET. 2015. Early versus late culture growth of Propionibacterium acnes in revision shoulder arthroplasty. J Bone Joint Surg Am 97:1149–1158. doi: 10.2106/JBJS.N.00881. [DOI] [PubMed] [Google Scholar]
- 4.Achermann Y, Sahin F, Schwyzer HK, Kolling C, Wust J, Vogt M. 2013. Characteristics and outcome of 16 periprosthetic shoulder joint infections. Infection 41:613–620. doi: 10.1007/s15010-012-0360-4. [DOI] [PubMed] [Google Scholar]
- 5.Levy O, Iyer S, Atoun E, Peter N, Hous N, Cash D, Musa F, Narvani AA. 2013. Propionibacterium acnes: an underestimated etiology in the pathogenesis of osteoarthritis? J Shoulder Elbow Surg 22:505–511. doi: 10.1016/j.jse.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Bayston R, Ashraf W, Barker-Davies R, Tucker E, Clement R, Clayton J, Freeman BJ, Nuradeen B. 2007. Biofilm formation by Propionibacterium acnes on biomaterials in vitro and in vivo: impact on diagnosis and treatment. J Biomed Mater Res A 81:705–709. [DOI] [PubMed] [Google Scholar]
- 7.Ramage G, Tunney MM, Patrick S, Gorman SP, Nixon JR. 2003. Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials 24:3221–3227. doi: 10.1016/S0142-9612(03)00173-X. [DOI] [PubMed] [Google Scholar]
- 8.Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. 2012. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother 56:1885–1891. doi: 10.1128/AAC.05552-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohacek M, Weisser M, Kobza R, Schoenenberger AW, Pfyffer GE, Frei R, Erne P, Trampuz A. 2010. Bacterial colonization and infection of electrophysiological cardiac devices detected with sonication and swab culture. Circulation 121:1691–1697. doi: 10.1161/CIRCULATIONAHA.109.906461. [DOI] [PubMed] [Google Scholar]
- 10.Hahn F, Zbinden R, Min K. 2005. Late implant infections caused by Propionibacterium acnes in scoliosis surgery. Eur Spine J 14:783–788. doi: 10.1007/s00586-004-0854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uçkay I, Dinh A, Vauthey L, Asseray N, Passuti N, Rottman M, Biziragusenyuka J, Riche A, Rohner P, Wendling D, Mammou S, Stern R, Hoffmeyer P, Bernard L. 2010. Spondylodiscitis due to Propionibacterium acnes: report of twenty-nine cases and a review of the literature. Clin Microbiol Infect 16:353–358. doi: 10.1111/j.1469-0691.2009.02801.x. [DOI] [PubMed] [Google Scholar]
- 12.Jakab E, Zbinden R, Gubler J, Ruef C, von Graevenitz A, Krause M. 1996. Severe infections caused by Propionibacterium acnes: an underestimated pathogen in late postoperative infections. Yale J Biol Med 69:477–482. [PMC free article] [PubMed] [Google Scholar]
- 13.Lee BJ, Smith SD, Jeng BH. 2009. Suture-related corneal infections after clear corneal cataract surgery. J Cataract Refract Surg 35:939–942. doi: 10.1016/j.jcrs.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 14.Del Pozo JL, Patel R. 2009. Clinical practice. Infection associated with prosthetic joints. N Engl J Med 361:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renz N, Rienmüller A, Borens O, Scheibel M, Trampuz A. 2016. Shoulder periprosthetic joint infection caused by Propionibacterium acnes. Obere Extremität 11:96–100. [Google Scholar]
- 16.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. 1987. Bacterial biofilms in nature and disease. Annu Rev Microbiol 41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 17.Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. 2008. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis 47:1403–1409. doi: 10.1086/592973. [DOI] [PubMed] [Google Scholar]
- 18.Butler-Wu SM, Burns EM, Pottinger PS, Magaret AS, Rakeman JL, Matsen FA III, Cookson BT. 2011. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol 49:2490–2495. doi: 10.1128/JCM.00450-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Portillo ME, Corvec S, Borens O, Trampuz A. 2013. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. Biomed Res Int 2013:804391. doi: 10.1155/2013/804391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwotzer N, Wahl P, Fracheboud D, Gautier E, Chuard C. 2014. Optimal culture incubation time in orthopedic device-associated infections: a retrospective analysis of prolonged 14-day incubation. J Clin Microbiol 52:61–66. doi: 10.1128/JCM.01766-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen H, Tang J, Wang Q, Jiang Y, Zhang X. 2015. Sonication of explanted prosthesis combined with incubation in BD Bactec bottles for pathogen-based diagnosis of prosthetic joint infection. J Clin Microbiol 53:777–781. doi: 10.1128/JCM.02863-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portillo ME, Salvado M, Trampuz A, Siverio A, Alier A, Sorli L, Martinez S, Perez-Prieto D, Horcajada JP, Puig-Verdie L. 2015. Improved diagnosis of orthopedic implant-associated infection by inoculation of sonication fluid into blood culture bottles. J Clin Microbiol 53:1622–1627. doi: 10.1128/JCM.03683-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minassian AM, Newnham R, Kalimeris E, Bejon P, Atkins BL, Bowler IC. 2014. Use of an automated blood culture system (BD Bactec) for diagnosis of prosthetic joint infections: easy and fast. BMC Infect Dis 14:233. doi: 10.1186/1471-2334-14-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peel TN, Dylla BL, Hughes JG, Lynch DT, Greenwood-Quaintance KE, Cheng AC, Mandrekar JN, Patel R. 2016. Improved diagnosis of prosthetic joint infection by culturing periprosthetic tissue specimens in blood culture bottles. mBio 7:e01776–15. doi: 10.1128/mBio.01776-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. 2013. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:1–10. doi: 10.1093/cid/cis966. [DOI] [PubMed] [Google Scholar]
- 26.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR. 1998. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol 36:2932–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 28.Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A. 2010. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol 48:1208–1214. doi: 10.1128/JCM.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Achermann Y, Goldstein EJ, Coenye T, Shirtliff ME. 2014. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev 27:419–440. doi: 10.1128/CMR.00092-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shannon SK, Mandrekar J, Gustafson DR, Rucinski SL, Dailey AL, Segner RE, Burman MK, Boelman KJ, Lynch DT, Rosenblatt JE, Patel R. 2013. Anaerobic thioglycolate broth culture for recovery of Propionibacterium acnes from shoulder tissue and fluid specimens. J Clin Microbiol 51:731–732. doi: 10.1128/JCM.02695-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dora C, Altwegg M, Gerber C, Bottger EC, Zbinden R. 2008. Evaluation of conventional microbiological procedures and molecular genetic techniques for diagnosis of infections in patients with implanted orthopedic devices. J Clin Microbiol 46:824–825. doi: 10.1128/JCM.01227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson AJ, Zywiel MG, Stroh DA, Marker DR, Mont MA. 2010. Should gram stains have a role in diagnosing hip arthroplasty infections? Clin Orthop Relat Res 468:2387–2391. doi: 10.1007/s11999-009-1216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spangehl MJ, Masterson E, Masri BA, O'Connell JX, Duncan CP. 1999. The role of intraoperative gram stain in the diagnosis of infection during revision total hip arthroplasty. J Arthroplasty 14:952–956. doi: 10.1016/S0883-5403(99)90009-8. [DOI] [PubMed] [Google Scholar]
- 34.Monsen T, Lovgren E, Widerstrom M, Wallinder L. 2009. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections. J Clin Microbiol 47:2496–2501. doi: 10.1128/JCM.02316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baron EJ, Miller JM, Weinstein MP, Richter SS, Gilligan PH, Thomson RB Jr, Bourbeau P, Carroll KC, Kehl SC, Dunne WM, Robinson-Dunn B, Schwartzman JD, Chapin KC, Snyder JW, Forbes BA, Patel R, Rosenblatt JE, Pritt BS. 2013. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM) (a). Clin Infect Dis 57:e22–e121. doi: 10.1093/cid/cit278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


