Abstract
Background
Higher concentrations of the apolipoprotein B (apoB) lipoproteins increase the risk of cardiovascular disease. However, whether the risk associated with apoB lipoproteins varies with age has not been well examined.
Methods and Results
We determined the associations for total cholesterol, low‐density lipoprotein (LDL)‐cholesterol (LDL‐C), non‐high‐density lipoprotein‐cholesterol (non‐HDL‐C), apoB, apolipoprotein A‐I (apoA‐I), and HDL‐cholesterol (HDL‐C) with myocardial infarction at different ages in 11 760 controls and 8998 myocardial infarction cases of the INTERHEART Study. Logistic regression was used to compute the odds ratio of myocardial infarction for 1 SD change in each lipid marker by decade from <40 to >70 years of age. Except for those >70, plasma levels of total cholesterol, LDL‐C, and non‐HDL‐C and apoB were greater in cases than controls. However, the average levels of these markers decreased significantly as age increased. By contrast, levels of apoA‐I and HDL‐C were significantly greater in controls than cases but increased significantly as age increased. The cardiovascular risk associated with the atherogenic lipid markers differed at different ages. Most notably, there was a significant decline in the odds ratio for total cholesterol, LDL‐C, and non‐HDL‐C, and apoB with increases in age whereas the odds ratios associated with apoA‐I and HDL‐C were consistent across the age groups.
Conclusions
These data indicate that the risk of cardiovascular events associated with apoB particles is greater in younger compared to older individuals. This finding is consistent with greater relative benefit from LDL‐lowering therapy in younger compared to older individuals and so argues for therapy in younger individuals with elevated lipids.
Keywords: apolipoprotein B, cardiovascular events, low‐density lipoprotein cholesterol, non‐high‐density lipoprotein cholesterol, primary prevention, risk assessment, risk factor, risk prediction
Subject Categories: Lipids and Cholesterol, Primary Prevention, Mortality/Survival
Introduction
Low‐density lipoprotein cholesterol (LDL‐C), non‐high‐density lipoprotein cholesterol (non‐HDL‐C), and apolipoprotein B (apoB) have all been proposed as markers to quantitate the atherogenic damage attributable to apoB particles. LDL‐C is a measure of the mass of cholesterol within LDL particles, whereas non‐HDL‐C is a measure of the mass of cholesterol within very‐low‐density lipoprotein, LDL, and lipoprotein Lp(a) particles. Each of these lipoprotein particles contains 1 molecule of apoB.1 However, except for unusual circumstances such as remnant lipoprotein disorder, typically 90% of apoB particles are LDL particles. Thus, apoB is determined by LDL particle number even though apoB also includes very‐low‐density lipoprotein and Lp(a) particles.1
Given that apoB lipoprotein particles are a major determinant of cardiovascular risk and given the rapid increase in cardiovascular risk with age, the cardiovascular risk associated with apoB particles might be assumed to increase with age. However, multiple prospective epidemiological studies have shown that LDL‐C, total cholesterol (TC), and apoB all appear to be more potent risk factors for a coronary event for those who are <70 years of age compared to those who are >70.2, 3, 4, 5, 6 Nevertheless, because age is such a strong determinant of risk,7, 8, 9 the algorithms adopted by Guidelines such as American College of Cardiology/American Heart Association strongly favor lowering of LDL‐C for primary prevention of those over 60 compared those who are under 60.10
However, this practice is hard to reconcile with the fact that even moderately lower levels of LDL‐C over a lifetime are associated with markedly lower cardiovascular risk over a lifetime.11, 12 Moreover, moderate elevation of LDL‐C and non‐HDL‐C, particularly if persistent, identify a group of individuals at age 55 who are at substantial cardiovascular risk over the next 15 years, most of whom would not have been recommended for preventive therapy based on calculations of global risk.13
Until now, attention has focused on whether markers of the atherogenic lipoproteins remain predictive in older individuals. The alternate question—whether these particles create greater risk in younger individuals and, if so, how much—has not been specifically addressed. Were this to be the case, the relative benefit from lowering of apoB particles might be even greater in those who are younger compared to those who are older. Accordingly, the objective of the present study was to examine the major lipid and apolipoprotein markers as predictors of coronary risk over a broad range of age. The INTERHEART study includes more than 25 000 participants from all the major regions of the world and also allows comparison at different ages of the association of LDL‐C, non‐HDL‐C, and apoB with coronary events.14
Methods
Details of the design and methods of the INTERHEART study have been published previously.14 Institutional Review Board ethics approval was obtained from all participating sites and all subjects gave informed consent. INTERHEART consisted of 12 461 cases with a first acute myocardial infarction and 14 637 age and sex‐matched controls without known cardiovascular disease. Subjects were recruited from 262 centers in 52 countries. The use of lipid‐lowering medications was documented. Nonfasting blood samples were obtained from 9345 cases and 12 120 controls. Concentrations of TC, HDL‐C, apoA1, and apoB were measured with the Roche Hitachi 917 analyzer and concentrations of non‐HDL‐C were calculated as TC minus HDL‐C. Apolipoprotein concentrations were measured using the Tina‐ quant apoB and apoA1 kits (version 2, with the International Federation of Clinical Chemistry SP3‐07 reference standard and International Federation of Clinical Chemistry SP1‐01 reference preparations), which are standardized methods for measurement of apoB and apoA1. Cholesterol concentrations were measured with an enzymatic colorimetric method (CHOD‐PAP) with cholesterol esterase, cholesterol oxidase, and 4 aminoantipyrine. Concentrations of HDL‐C were measured with a homogeneous enzymatic colorimetric assay (HDL‐C plus, 2nd generation) that uses cholesterol esterase and cholesterol oxidase coupled with polyethylene glycol to the amino groups.
Statistical Analysis
Of the 27 098 subjects recruited in the INTERHEART study, data for this analysis were available in 20 758 individuals. Subjects were categorized by age in decades. Then the mean and SD of selected lipid parameters across different age groups overall and by case or control status of the participants were computed. These included apoB, apoA‐1, LDL‐C, HDL‐C, TC, and non‐ HDL‐C, which was computed by subtracting HDL‐C from TC. Standardized variables were computed by subtracting the sample mean and dividing it by sample SD of each lipid parameter under consideration. Simple logistic regression was used to compute the odds ratio of myocardial infarction for 1 SD change in each lipid marker. In addition to sex and ethnicity, we have also adjusted the odds ratio estimates for diabetes, smoking, and systolic and diastolic blood pressure and lipid‐lowering medications. Tests of linear trend were also performed using orthogonal contrast across age categories and using corresponding coefficients from the logistic regression model. All analyses were performed using SAS version 9.2 and figures were prepared using S‐Plus version 8.2.
Results
The number of cases and controls and the average lipid and apolipoprotein levels by age are described in Table 1. As would be expected, the smallest numbers of cases were <40 years of age. Nevertheless, even in this age category, data on 555 cases and 903 controls are included. For the total group, that is, for cases and controls taken together, the average apoB, non‐HDL‐C, and TC is higher in younger than older individuals but the differences are small and inconsistent. By contrast, apoA‐I and HDL‐C are clearly greater in the older subjects than the younger ones (P<0.0001).
Table 1.
Lipid and Apolipoprotein Concentrations by Age
| Age, y | Age <40 | 40≤ Age <50 | 50≤ Age <60 | 60≤ Age <70 | Age ≥70 | P‐Trend |
|---|---|---|---|---|---|---|
| Total number: 20 758 | 1458 | 4327 | 5702 | 5566 | 3705 | |
| ApoB, mg/100 mL | 92 (27) | 96 (27) | 95 (26) | 93 (26) | 91 (24) | 0.0003 |
| Total cholesterol, mg/100 mL | 197 (48) | 204 (47) | 203 (47) | 201 (47) | 199 (46) | 0.726 |
| LDL‐C, mg/100 mL | 123 (42) | 128 (41) | 128 (40) | 128 (40) | 125 (38) | 0.069 |
| Non‐HDL‐C, mg/100 mL | 159 (49) | 165 (47) | 162 (46) | 159 (45) | 154 (44) | <0.0001 |
| Apo‐A1, mg/100 mL | 111 (25) | 114 (25) | 117 (26) | 120 (27) | 120 (28) | <0.0001 |
| HDL‐C | 38 (13) | 39 (13) | 40 (14) | 43 (14) | 45 (15) | <0.0001 |
Numbers in parentheses indicate 95% CI. ApoA‐1 indicates apolipoprotein A1; ApoB, apolipoprotein B; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; non‐HDL‐C, non‐high‐density lipoprotein cholesterol; SD, standard deviation.
Table 2 describes the average levels of the lipid markers by age categories separately for controls and Table 3 for cases. Comparing cases to controls, except for the oldest subjects, apoB (P<0.001), non‐HDL‐C (P<0.001 age <40, >40<50; P<0.015>60<70) and LDL‐C (P<0.001) are significantly higher in cases than controls. However, apoA‐I is significantly higher in controls than in cases at all ages (P<0.001 at all ages) and HDL‐C is significantly higher (P<0.001) at all ages in controls compared to case except for those >70. The concentrations of apoB, TC, and LDL‐C are slightly, but significantly, higher in the older controls whereas the converse is observed for apoA‐I and HDL‐C. By contrast, apoB, LDL‐C, and non‐HDL‐C levels are significantly lower in older compared to younger cases whereas apoA‐I and HDL‐C are significantly higher in younger cases compared to older cases.
Table 2.
Cholesterol and Apolipoprotein Concentrations by Age for Controls
| Age, y | Age <40 (903) | Age ≤40 to <50 (2535) | Age ≤50 to <60 (3241) | Age ≤60 to <70 (3135) | Age ≥70 (1946) | P‐Trend |
|---|---|---|---|---|---|---|
| ApoB, mg/100 mL | 88 (25) a | 92 (24)a | 92 (25)a | 92 (25)a | 91 (24)b | 0.003 |
| Total cholesterol, mg/100 mL | 192 (45)a | 198 (44)a | 200 (46)a | 201 (45)a | 200 (46)b | <0.0001 |
| LDL‐C, mg/100 mL | 117 (38)a | 122 (38)a | 124 (39)a | 126 (38)a | 124 (38)b | <0.0001 |
| Non‐HDL‐C, mg/100 mL | 153 (46)a | 159 (44)a | 159 (46)a | 157 (43)c | 155 (43)b | NS |
| ApoA‐1, mg/100 mL | 115 (25)a | 118 (25)a | 121 (27)a | 124 (29)a | 124 (30)a | <0.0001 |
| HDL‐C, mg/100 mL | 39 (14)a | 39 (14)a | 41 (14)a | 44 (15)a | 45 (15)b | <0.0001 |
Numbers in parentheses indicate 95% CI. ApoA‐1 indicates apolipoprotein A1; ApoB, apolipoprotein B; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; non‐HDL‐C, non‐high‐density lipoprotein cholesterol; SD, standard deviation.
P<0.001 controls vs cases.
NS P not significant controls vs cases.
P<0.015 controls vs cases.
Table 3.
Cholesterol and Apolipoprotein Concentrations by Age for Cases
| Age, y | Age <40 (555) | Age ≤40 to <50 (1792) | Age ≤50 to <60 (2461) | Age ≤60 to <70 (2431) | Age ≥70 (1759) | P‐Trend |
|---|---|---|---|---|---|---|
| ApoB, mg/100 mL | 100 (30) | 103 (28) | 99 (26) | 95 (27) | 91 (25) | <0.0001 |
| Total cholesterol, mg/100 mL | 205 (52) | 211 (50) | 206 (47) | 202 (49) | 198 (46) | <0.0001 |
| LDL‐C, mg/100 mL | 133 (47) | 137 (43) | 133 (40) | 131 (41) | 126 (39) | <0.0001 |
| Non‐HDL‐C, mg/100 mL | 169 (52) | 174 (49) | 167 (46) | 160 (47) | 154 (44) | <0.0001 |
| ApoA‐1, mg/100 mL | 105 (23) | 109 (23) | 111 (24) | 114 (24) | 116 (25) | <0.0001 |
| HDL‐C, mg/100 mL | 36 (13) | 37 (12) | 39 (12) | 41 (12) | 44 (14) | <0.0001 |
Numbers in parentheses indicate 95% CI. ApoA‐1 indicates apolipoprotein A1; ApoB, apolipoprotein B; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; non‐HDL‐C, non‐high‐density lipoprotein cholesterol.
Table 4 displays the odds ratios (OR) by age group, adjusted for sex, ethnicity, systolic and diastolic blood pressure, smoking, and lipid‐lowering medications for the pro‐ and anti‐atherogenic lipoprotein markers. The ORs for apoB, LDL‐C, and non‐HDL‐C are highest at <40 and 40 to 50 and decrease steadily thereafter. The trends with increased age are all statistically significant (P<0.001). Except for the extremes, the age‐related ORs for apoB are significantly higher than for non‐HDL‐C (P<0.001), whereas the differences between apoB and LDL‐C were not. With regard to the anti‐atherogenic markers, the OR for apoA‐I is substantially lower than for HDL‐C at all ages (P<0.001). Moreover, and in contrast to the proatherogenic markers, except for those >70, there is little absolute change in values at the different ages for both apoA‐I and HDL‐C.
Table 4.
Odds Ratio for 1 SD Change LDL‐C, Non‐HDL‐C, and apoB Adjusted for Age, Sex, Ethnicity, Smoking, SBP and DBP, and Lipid‐Lowering Medication
| Age, y | ApoBa | LDL‐Ca | Non‐HDL‐Ca | TCa | ApoA1a | HDL‐Cb |
|---|---|---|---|---|---|---|
| 0: Overall | 1.36 (1.32–1.41) | 1.33 (1.29–1.37) | 1.25 (1.22–1.29) | 1.20 (1.17–1.24) | 0.70 (0.68–0.73) | 0.87 (0.84–0.90) |
| 1: Age <40 | 1.51 (1.34–1.70) | 1.38 (1.23–1.55) | 1.32 (1.18–1.48) | 1.29 (1.14–1.45) | 0.66 (0.57–0.76) | 0.87 (0.76–0.99) |
| 2: Age ≤40 to <50 | 1.62 (1.51–1.74) | 1.55 (1.44–1.67) | 1.47 (1.37–1.58) | 1.43 (1.33–1.53) | 0.70 (0.64–0.75) | 0.87 (0.80–0.94) |
| 3: Age ≤50 to <60 | 1.41 (1.33–1.49) | 1.35 (1.27–1.44) | 1.27 (1.20–1.34) | 1.22 (1.15–1.29) | 0.70 (0.66–0.75) | 0.85 (0.80–0.90) |
| 4: Age ≤60 to <70 | 1.25 (1.18–1.33) | 1.25 (1.18–1.33) | 1.18 (1.11–1.25) | 1.12 (1.05–1.19) | 0.69 (0.65–0.74) | 0.84 (0.79–0.89) |
| 5: Age ≥70 | 1.12 (1.04–1.21) | 1.18 (1.09–1.27) | 1.08 (1.00–1.16) | 1.05 (0.97–1.13) | 0.75 (0.70–0.81) | 0.94 (0.88–1.01) |
Numbers in parentheses indicate 95% CI. ApoA‐1 indicates apolipoprotein A1; ApoB, apolipoprotein B; DBP, indicates diastolic blood pressure; SBP, systolic blood pressure; LDL‐C, low‐density lipoprotein cholesterol; non‐HDL‐C, non‐high‐density lipoprotein cholesterol; TC, total cholesterol.
P<0.001 significant decrease as age increased.
P not significant across range of ages.
These trends are presented in Figures 1 and 2, which display the ORs for the markers at different ages when adjusted for age, sex, ethnicity, diabetes, smoking, and systolic and diastolic blood pressures. The similarity of the downward trends as age increased for all the proatherogenic markers are all significant (P<0.001). By contrast, no clear trend across the age spectrum is apparent for the anti‐atherogenic markers.
Figure 1.
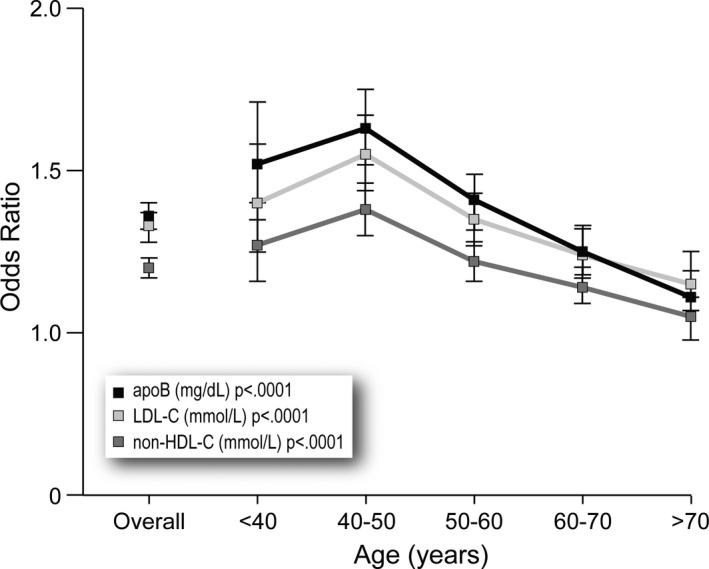
Odds ratios for low‐density lipoprotein cholesterol (LDL‐C), non‐high‐density lipoprotein cholesterol (non‐HDL‐C), and apolipoprotein B (apoB) overall and for each decade.
Figure 2.

Odds ratios for high‐density lipoprotein cholesterol (HDL‐C) and apolipoprotein A‐1 (apoA‐I) overall and for each decade.
Discussion
This is the first study to systematically evaluate the effects of LDL‐C, non‐HDL‐C, apoB, HDL‐C, and apoA‐I on the risk of a coronary event at different ages. Overall, the ORs were higher for apoB, non‐HDL‐C, and LDL‐C in myocardial infarction cases compared to controls. However, contrary to what might have been expected from the fact that the risk of cardiovascular events dramatically increases with age, these ORs all significantly decreased with age. We also found significant decreases in the concentrations of the 3 LDL‐related markers of cardiovascular risk as age increased. By contrast, while there were significant increases in the concentrations of apoA‐I and HDL‐C at the older ages, there was no significant trend to change in the ORs for apoA‐I and HDL‐C.
At the present time, risk is the primary criterion that has been adopted by guidelines to initiate lipid‐lowering therapy to reduce the subsequent risk of cardiovascular disease. Because age is the principal determinant of risk as calculated by guideline algorithms,8, 9 except for those with markedly elevated levels of LDL‐C or diabetes, lipid‐lowering interventions become common only after the age of 60.10 The evidence that even moderately lower lifelong levels of LDL‐C are associated with substantially lower levels of cardiovascular events11, 12 and that even moderate elevations of LDL‐C and non‐HDL‐C, if sustained, can produce considerable 15‐year cardiovascular risk suggest that important treatment opportunities are being lost.13 There is no doubt, therefore, that elevated levels of the apoB lipoproteins, if present during the third to sixth decades of life, are a potent cause of cardiovascular events, an observation that argues for earlier intervention in such individuals.
The use of risk models by guidelines has substantially advanced cardiovascular prevention because it integrates the adverse effects of the accepted cardiovascular risk factors. However, while risk algorithms generate reasonably precise estimates of the incidence of disease in a group, such estimates are often unacceptably imprecise for the individuals who make up the group.15 Moreover, basing the decision as to whether preventive therapy is justified solely upon calculated risk assumes that the benefit of statin therapy in an individual is determined only by the level of risk in that individual, whereas the Cholesterol Treatment Trialists (CTT) study demonstrated that benefit will also relate to the baseline level of LDL‐C: the higher the baseline level of LDL‐C, the greater the absolute lowering that is possible and therefore the greater the absolute benefit that is possible.16 Based on these principles, we have shown that the potential benefit from statin therapy can be calculated for each individual—the estimated individual benefit—and doing so would extend preventive therapy to a substantial number of younger individuals with moderately high levels of LDL‐C without increasing the maximum number to prevent 1 cardiovascular event.17
The present study demonstrates that the atherogenic risk attributable to the apoB lipoproteins varies at different ages. The absolute risk due to LDL would be greatest at younger ages due to the higher levels and to the higher OR. Since the level of LDL is a major determinant of the benefit of LDL‐lowering treatment,18 then the benefit of LDL lowering would be expected to be relatively greater in younger compared to older subjects.
Because cardiovascular risk, and therefore the incidence of cardiovascular events, increases so sharply after age 60, cardiovascular disease is thought to be a disease of older age groups.10 But half of all cardiovascular events in men and one third in women occur before age 65.19 Forty‐ and 50‐year‐olds account, quantitatively, for an important fraction of the total number of events. Given that the population at risk in these age groups is so large and that the 10‐year risk for the great majority of individuals is low, more emphasis will have to be placed on identifying those subjects in whom the causes of cardiovascular disease such as high apoB lipoproteins are present. Similarly, more emphasis must be placed on those who are younger regarding the causes of vascular disease. The present findings add to that argument by demonstrating that the risk associated with the apoB lipoproteins is greater in younger compared to older individuals.
A strength of the INTERHEART design is that it includes sufficiently large numbers of younger as well as older individuals, with and without clinical events, to allow comparison of the atherogenic markers for their relation to the chance of a clinical event at the different ages. Nevertheless, INTERHEART is not a prospective observational study.14 The Prospective Collaboration Study, a meta‐analysis, which included 51 000 cardiovascular deaths (nonfatal myocardial infarctions were not collected) demonstrated that the risk of cardiovascular deaths associated with higher total cholesterol levels is greater in younger compared to older individuals.7 More recently, investigators from the Malmo Preventive Project performed a matched case–control analysis to examine the relation of risk factors and age of incident myocardial infarction.20 They reported an inverse relation between the OR for total cholesterol and quartiles of age for incident myocardial infarction but not for the other conventional risk factors. A positive family history was also associated with premature disease. Triglycerides were measured but not apoB or the other lipoprotein lipids. Our results are consistent with and extend these observations.
What might explain the inverse relation between cardiovascular risk and the apoB lipoproteins that we have observed? We hypothesize that the inverse relation of LDL to the risk of a cardiovascular event is based on the anatomic stage and extent of atherosclerotic disease present within the arterial wall. Detailed pathological studies demonstrated that lesions with a substantial mass of extracellular cholesterol—hence lesions that can precipitate a clinical event due to plaque rupture—as a rule appear only in the fourth decade but progress rapidly up to the sixth decade with more gradual advancement after that.21, 22 Cholesterol‐rich lesions dominate early disease but are less prominent later as extensive scarring and calcification progressively develop within the atherosclerotic arteries. The evolution of these structural features suggests that plaque rupture, which is the lesion most closely related to LDL, may predominate in the earlier life history of the disease but may be less prominent as a mechanism of an acute ischemic event later when intramural hematoma and endothelial erosion may become more common. To be sure, depending on the level of LDL, as well as other factors, progression in lesion pathology will occur at different rates in different individuals. Nevertheless, such a general anatomical sequence would be consistent with the temporal pattern we have observed. These observations suggest that earlier LDL lowering will be associated with greater clinical benefit, a relation that has been documented in the CTT meta‐analysis.
Except for those >70, the OR for apoB was greater at each age than LDL‐C or non‐HDL‐C. These differences were statistically significant for non‐HDL‐C but not for LDL‐C. More stringent testing of their relative predictive powers by discordance analysis of the INTERHEART study demonstrates that cardiovascular risk is more powerfully predicted by apoB than by LDL‐C and non‐HDL‐C,23 results that are consistent with a series of other results of discordance analysis.24, 25, 26, 27, 28 Moreover, apoB has been shown to correlate more closely with the clinical benefit of statin therapy than LDL‐C or non‐HDL‐C29 and more closely than LDL‐C or non‐HDL‐C with a decrease in angiographic progression of coronary lesions.30 Finally, our results are consistent with the findings of Wiesbauer and colleagues31 and Zambon et al32 that an apoB that is disproportionately elevated compared to LDL‐C is more common in individuals with premature coronary artery disease.
In summary, the present data demonstrate an inverse relation between the cardiovascular risk posed by apoB, LDL‐C, and non‐HDL‐C and age, a relationship that is consistent with potentially greater relative benefit of preventive LDL lowering in younger age groups.
Sources of Funding
This research was supported by an unrestricted grant from the Doggone Foundation.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003665 doi: 10.1161/JAHA.116.003665)
References
- 1. De Graaf J, Couture P, Sniderman A. ApoB in Clinical Care. Houten: Springer; 2015. [Google Scholar]
- 2. Batty GD, Shipley M, Smith GD, Kivimaki M. Long term risk factors for coronary heart disease and stroke: influence of duration of follow‐up over four decades of mortality surveillance. Eur J Prev Cardiol. 2015;22:1139–1145. [DOI] [PubMed] [Google Scholar]
- 3. Simons LA, Simons J, Friedlander Y, McCallum J. Cholesterol and other lipids predict coronary heart disease and ischaemic stroke in the elderly, but only in those below 70 years. Atherosclerosis. 2001;159:201–208. [DOI] [PubMed] [Google Scholar]
- 4. Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A‐I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. [DOI] [PubMed] [Google Scholar]
- 5. Krumholz HM, Seeman TE, Merrill SS, Mendes de Leon CF, Vaccarino V, Silverman DI, Tsukahara R, Ostfeld AM, Berkman LF. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all‐cause mortality in persons older than 70 years. JAMA. 1994;272:1335–1340. [PubMed] [Google Scholar]
- 6. Clarke R, Lewington S, Youngman L, Sherliker P, Peto R, Collins R. Underestimation of the importance of blood pressure and cholesterol for coronary heart disease mortality in old age. Eur Heart J. 2002;23:286–293. [DOI] [PubMed] [Google Scholar]
- 7. Prospective Studies Collaboration , Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta‐analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. [DOI] [PubMed] [Google Scholar]
- 8. Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton‐Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. [DOI] [PubMed] [Google Scholar]
- 9. Sniderman AD, Furberg CD. Age as a modifiable risk factor for cardiovascular disease. Lancet. 2008;371:1547–1549. [DOI] [PubMed] [Google Scholar]
- 10. Pencina MJ, Navar‐Boggan AM, D'Agostino RB, Williams K, Neely B, Sniderman AD, Peterson ED. Application of new cholesterol guidelines to a population‐based sample. N Engl J Med. 2014;370:1422–1431. [DOI] [PubMed] [Google Scholar]
- 11. Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. [DOI] [PubMed] [Google Scholar]
- 12. Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, Kahn J, Afonso L, Williams KA, Flack JM. Effect of long‐term exposure to lower low‐density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60:2631–2639. [DOI] [PubMed] [Google Scholar]
- 13. Navar‐Boggan AM, Peterson ED, D'Agostino RB, Neely B, Sniderman AD, Pencina MJ. Hyperlipidemia in early adulthood increases long‐term risk of coronary heart disease. Circulation. 2015;131:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, Kazmi K, Yusuf S; INTERHEART study investigators . Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case‐control study. Lancet. 2008;372:224–233. [DOI] [PubMed] [Google Scholar]
- 15. Sniderman AD, D'Agostino RB, Pencina MJ. The role of physicians in the era of predictive analytics. JAMA. 2015;314:25–26. [DOI] [PubMed] [Google Scholar]
- 16. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R; Cholesterol Treatment Trialists' (CTT) Collaborators . Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 17. Thanassoulis G, Williams K, Altobelli KK, Pencina MJ, Cannon CP, Sniderman AD. Individualized statin benefit for determining statin eligibility in the primary prevention of cardiovascular disease. Circulation. 2016;133:1574–1581. [DOI] [PubMed] [Google Scholar]
- 18. Toth PP, Thanassoulis G, Williams K, Furberg CD, Sniderman A. The risk‐benefit paradigm vs the causal exposure paradigm: LDL as a primary cause of vascular disease. J Clin Lipidol. 2014;8:594–605. [DOI] [PubMed] [Google Scholar]
- 19. Sniderman AD, Thanassoulis G, Williams K, Pencina M. Risk of premature cardiovascular disease vs the number of premature cardiovascular events. JAMA Cardiol. 2016;1:492–494. [DOI] [PubMed] [Google Scholar]
- 20. Gränsbo K, Almgren P, Nilsson PM, Hedblad B, Engström G, Melander O. Risk factor exposure in individuals free from cardiovascular disease differs according to age at first myocardial infarction. Eur Heart J. 2016;37:1977–1981. [DOI] [PubMed] [Google Scholar]
- 21. Stary HC. The sequence of cell and matrix changes in atherosclerotic lesions of coronary arteries in the first forty years of life. Eur Heart J. 1990;11(suppl E):3–19. [DOI] [PubMed] [Google Scholar]
- 22. Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. [DOI] [PubMed] [Google Scholar]
- 23. Sniderman AD, Islam S, Yusuf S, McQueen MJ. Discordance analysis of apolipoprotein B and non‐high density lipoprotein cholesterol as markers of cardiovascular risk in the INTERHEART study. Atherosclerosis. 2012;225:444–449. [DOI] [PubMed] [Google Scholar]
- 24. Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, Vasan RS, Wilson PWF, D'Agostino RB. LDL particle number and risk of future cardiovascular disease in the Framingham Offspring Study—implications for LDL management. J Clin Lipidol. 2007;1:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC. Clinical implications of discordance between low‐density lipoprotein cholesterol and particle number. J Clin Lipidol. 2011;5:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mora S, Buring JE, Ridker PM. Discordance of low‐density lipoprotein (LDL) cholesterol with alternative LDL‐related measures and future coronary events. Circulation. 2014;129:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pencina MJ, D'Agostino RB, Zdrojewski T, Williams K, Thanassoulis G, Furberg CD, Peterson ED, Vasan RS, Sniderman AD. Apolipoprotein B improves risk assessment of future coronary heart disease in the Framingham Heart Study beyond LDL‐C and non‐HDL‐C. Eur J Prev Cardiol. 2015;22:1321–1327. [DOI] [PubMed] [Google Scholar]
- 28. Wilkins JT, Li RC, Sniderman A, Chan C, Lloyd‐Jones DM. Discordance between apolipoprotein B and LDL‐cholesterol in young adults predicts coronary artery calcification: the CARDIA study. J Am Coll Cardiol. 2016;67:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thanassoulis G, Williams K, Ye K, Brook R, Couture P, Lawler PR, De Graaf J, Furberg CD, Sniderman A. Relations of change in plasma levels of LDL‐C, non‐HDL‐C and apoB with risk reduction from statin therapy: a meta‐analysis of randomized trials. J Am Heart Assoc. 2014;3:e000759 doi: 10.1161/JAHA.113.000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Masson W, Siniawski D, Lobo M, Molinero G, Giorgi M, Huerín M. Association between LDL‐C, non HDL‐C, and apolipoprotein B levels with coronary plaque regression. Arq Bras Cardiol. 2015;105:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wiesbauer F, Blessberger H, Azar D, Goliasch G, Wagner O, Gerhold L, Huber K, Widhalm K, Abdolvahab F, Sodeck G, Maurer G, Schillinger M. Familial‐combined hyperlipidaemia in very young myocardial infarction survivors (< or =40 years of age). Eur Heart J. 2009;30:1073–1079. [DOI] [PubMed] [Google Scholar]
- 32. Zambon A, Brown BG, Deeb SS, Brunzell JD. Genetics of apolipoprotein B and apolipoprotein AI and premature coronary artery disease. J Intern Med. 2006;259:473–480. [DOI] [PubMed] [Google Scholar]


