Abstract
Background
Venous thromboembolism, including deep vein thrombosis and pulmonary embolism, results in a substantial healthcare system burden. This retrospective observational study compared hospital length of stay (LOS) and hospitalization costs for patients with venous thromboembolism treated with rivaroxaban versus those treated with warfarin.
Methods and Results
Hospitalizations for adult patients with a primary diagnosis of deep vein thrombosis or pulmonary embolism who were initiated on rivaroxaban or warfarin were selected from MarketScan's Hospital Drug Database between November 1, 2012, and December 31, 2013. Patients treated with warfarin were matched 1:1 to patients treated with rivaroxaban using exact and propensity score matching. Hospital LOS, time from first dose to discharge, and hospitalization costs were reported descriptively and with generalized linear models (GLMs). The final study cohorts each included 1223 patients (751 with pulmonary embolism and 472 with deep vein thrombosis). Cohorts were well matched for demographic and clinical characteristics. Mean (±SD) LOS was 3.7±3.1 days for patients taking rivaroxaban and 5.2±3.7 days for patients taking warfarin, confirmed by GLM‐adjusted results (rivaroxaban 3.7 days, warfarin 5.3 days, P<0.001). Patients with provoked venous thromboembolism admissions showed longer LOSs (rivaroxaban 5.1±4.5 days, warfarin 6.5±5.6 days, P<0.001) than those with unprovoked venous thromboembolism (rivaroxaban 3.3±2.4 days, warfarin 4.8±2.8 days, P<0.001). Days from first dose to discharge were 2.4±1.7 for patients treated with rivaroxaban and 3.9±3.7 for patients treated with warfarin when initiated with parenteral anticoagulants (P<0.001), and 2.7±1.7 and 3.7±2.1, respectively, when initiated without parenteral anticoagulants (P<0.001). Patients initiated on rivaroxaban incurred significantly lower mean total hospitalization costs ($8688±$9927 versus $9823±$9319, P=0.004), confirmed by modeling (rivaroxaban $8387 [95% confidence interval, $8035–$8739]; warfarin $10 275 [95% confidence interval, $9842–$10 708]).
Conclusions
Rivaroxaban was associated with significantly shorter hospital LOS and lower hospitalization costs compared with warfarin.
Keywords: anticoagulants, embolism, rivaroxaban, thrombosis, warfarin
Subject Categories: Thrombosis, Embolism, Quality and Outcomes
Introduction
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are manifestations of venous thromboembolic disease processes that result in serious and chronic comorbidities (eg, vascular insufficiencies, pulmonary hypertension) or death.1 In the United States, venous thromboembolism (VTE) is estimated to occur in about 100 persons per 100 000 annually, with about two thirds of cases being DVT.1, 2, 3 The economic and healthcare system burden of VTE is substantial, with estimated annual costs ranging from $13.5 to $27.2 billion (2011 US dollars).4
Standard therapy for VTE is early treatment with parenteral anticoagulation, with the main goals of preventing extension and recurrence of the thrombotic event.5 Acute DVT is more often treated at home than in the hospital, while acute PE is typically treated in the hospital since it is associated with much higher short‐term mortality.5 Anticoagulation has traditionally been initiated with subcutaneous or intravenous heparin, either low‐molecular‐weight heparin or unfractionated heparin, given in combination with a vitamin K antagonist (VKA), most commonly warfarin, started concurrently or shortly after initiating the heparin therapy.5 VKAs have several clinical limitations. Warfarin is slow‐acting, with a narrow therapeutic range, unpredictable anticoagulant effects due to food and drug interactions, and highly variable metabolism.6 Routine coagulation (laboratory) monitoring and dose adjustment are needed to maintain blood coagulation within the desired therapeutic window.
The newer oral anticoagulants currently available in the United States include the direct thrombin inhibitor dabigatran and Factor Xa inhibitors rivaroxaban, apixaban, and edoxaban. These offer promising alternatives to VKEs as standard oral therapy for VTE, exhibiting more rapid onset of action, fewer food and drug interactions, and predictable anticoagulant effects without the need for routine laboratory monitoring.6
Rivaroxaban was shown in the EINSTEIN‐DVT and ‐PE trials to be as effective as enoxaparin and warfarin (or acenocoumarol) in treating acute DVT and PE without the need for dose adjustment or routine laboratory monitoring.7, 8, 9 A post hoc analysis of the hospitalization records from the EINSTEIN clinical trials found that hospitalized patients treated with rivaroxaban had significantly shorter lengths of stay (LOSs) than those treated with enoxaparin/VKA.10 A separate analysis of the US and Canadian subset of EINSTEIN‐DVT and ‐PE patients found a 1.6‐day mean reduction in LOS for patients treated with rivaroxaban versus those treated with enoxaparin/VKA.11 A case‐control study found lower costs of medical care with rivaroxaban treatment, compared with warfarin treatment, owing primarily to shortened initial LOS and fewer readmissions over a 6‐month period.12
While clinical trial data showed the potential for rivaroxaban to reduce hospital LOS associated with VTE compared with standard treatment, real‐world data comparing the relative effectiveness of rivaroxaban and warfarin outside of the clinical trial population are limited. This study augments the information learned in the clinical trial setting by examining utilization and cost outcomes in a data set derived from a large and geographically diverse sample of US hospitals. The primary objective of this study was to compare hospital LOS and hospitalization costs among patients admitted with a primary VTE diagnosis who were initiated on oral anticoagulation therapy with rivaroxaban versus warfarin during their hospital stay.
Methods
Study Design and Data Source
This was a retrospective analysis of patients hospitalized with a primary diagnosis of either DVT or PE and who were initiated on oral anticoagulation pharmacotherapy with either rivaroxaban or warfarin during their stay. Data were drawn from the Truven Health MarketScan Hospital Drug Database (HDD) between November 1, 2012 and December 31, 2013. The HDD is derived from the ordering and billing systems of over 600 geographically and demographically diverse US hospitals, including detailed information on diagnoses, procedures, and drug administration in inpatient settings. All services recorded by the hospital are captured. Diagnoses, including the principal and all secondary patient diagnoses, use the International Classification of Disease, 9th Revision, Clinical Modifications (ICD‐9‐CM) classification system. Principal and secondary patient procedure records use the ICD‐9‐CM procedure code, and pharmacy records employ a proprietary identification system combining drug (generic) names and National Drug Codes. All study data are deidentified and compliant with the Health Insurance Portability and Accountability Act (HIPAA) of 1996. Because this study used only deidentified patient records and did not involve the collection, use, or transmittal of individually identifiable data, institutional review board approval to conduct this study was not required.
Patient Selection
Patients with a complete hospital admission record during the period from November 1, 2012, to December 31, 2013, with a primary VTE diagnosis (ICD‐9‐CM diagnosis codes 451.1x, 451.2x, 453.4x, 453.8x, 453.9x, or 415.1x recorded as the principal diagnosis) were identified. The requirement for the VTE diagnosis to be in the principal diagnosis position in the discharge record was intended to distinguish primary VTE from postoperative or other nosocomial‐attributed VTE. The first observed hospital admission record after November 1, 2012, was defined as the index hospitalization with the date of admission serving as the index date. Patients were required to have received a prescription for rivaroxaban or warfarin during the hospitalization. Patients may also have received low‐molecular‐weight heparin or unfractionated heparin therapy prior to receiving either rivaroxaban or warfarin.
To increase the likelihood that our study focused on a patient's initial (incident) VTE event, patients were excluded if they had a hospital admission record within 12 months prior to the index date with a VTE diagnosis in any position. Hospitalizations for patients receiving both rivaroxaban and warfarin and those receiving the drugs apixaban or dabigatran etexilate mesylate were also excluded (edoxaban was not yet available in the United States). Finally, patients younger than 18 on the index date or with a pregnancy diagnosis were excluded from analysis. The study period for any particular patient was the entire length of that hospitalization (admission through discharge).
Patients receiving warfarin were matched to patients receiving rivaroxaban on a 1:1 rivaroxaban‐to‐warfarin basis using a combination of exact matching on the primary diagnoses of DVT or PE followed by propensity score matching (Mahalanobis metric and nearest neighbor match without replacement). Propensity scores were calculated using a logistic regression model to predict the probability of initiating rivaroxaban treatment, with a vector of independent variables comprising hospital demographics (geographic location, teaching, urban, bed size), patient demographics (age, sex, payer, admission year, admission source), and patient clinical characteristics chosen to reflect baseline characteristics as closely as possible, with the limitation that patient characteristics prior to the hospital admission were not available. Clinical characteristics used in matching included a relative comorbidity index (count of unique 3‐digit ICD‐9‐CM codes during the hospitalization13) and presence of specific chronic comorbidities of anemia, arrhythmia, cancer, chronic obstructive pulmonary disease (COPD), congestive heart failure, diabetes mellitus, hypertension, ischemic heart disease, and renal disease. The balance achieved by the matching procedure was assessed by comparing prematch and postmatch distributions of the independent variables included in the propensity score model via standardized differences, which is not dependent on study sample sizes and therefore less susceptible than t tests and chi‐square tests to type I or type II error in either large or small samples, respectively.14, 15
Study Outcomes
The primary outcomes for this study were hospital LOS and hospitalization costs. LOS was evaluated for each index admission by a count of the days from admission to discharge. Time from admission to first treatment dose and from first treatment initiation to discharge were recorded. LOS results were further stratified for patients with evidence of provoked VTE versus unprovoked VTE, with provoked VTE defined as patients with a fracture diagnosis, a major surgical procedure, selected drugs (oral contraceptives, estrogens, progestins, selective estrogen receptor modulators, aromatase inhibitors, or erythropoiesis‐stimulating agents), a postpartum diagnosis, or a malignant neoplasm diagnosis.5
Hospitalization costs (those incurred by the hospital) were reported by service categories including room costs, inpatient medication costs, laboratory tests, procedures, and other services for the index admission. Costs for hospitals were calculated by applying the Centers for Medicare & Medicaid Services (CMS) hospital department‐level ratios of costs‐to‐charges (CCRs). Approximately 88% of patients were treated in facilities with CCRs available. The mean CCRs were used to impute costs in the other facilities. All dollar estimates were inflated to 2015 US dollars using the Medical Care Component of the Consumer Price Index.
Demographic and Clinical Characteristics
Patient demographics included age, sex, payer, and discharge status. Hospital characteristics included teaching status, urban‐rural hospital setting, licensed bed size, and US geographic region. Clinical characteristics included a relative comorbidity index (count of unique 3‐digit ICD‐9‐CM codes during the hospitalization), specific comorbidities based on ICD‐9‐CM diagnoses recorded during the hospitalization, and cardiovascular‐related concomitant medications administered during the hospitalization.
Statistical Analysis
Patient demographics, clinical characteristics, treatment patterns, and study outcomes were summarized descriptively. Counts and proportions were presented for categorical variables with chi‐square tests of differences between treatment groups. Means and SDs were reported for continuous variables. t tests and ANOVA were used for normally distributed continuous variables, Wilcoxon or Kruskal‐Wallis tests were applied to nonparametric data, and Mann‐Whitney tests were used for nonparametric median costs.
Multivariate regression models were fit to LOS and cost outcomes to provide additional robustness in cohort comparisons, adjusting for demographic factors (age, sex, payer, hospital type, hospital bed size, hospital location), patients' clinical characteristics, and other covariates including intensive care unit (ICU) stay days, treatment started with and without a parenteral anticoagulant, and treatment starting before or after 3 days into the admission. A generalized linear model (GLM) with a log link function and negative binomial error distribution was used for LOS, as well as a Cox proportional hazards model estimating effects of censoring on the time to discharge. GLMs involving a log link function and a gamma error distribution were estimated for cost outcomes. The level of statistical significance for all statistical tests was 0.05. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Sample Selection
The final matched rivaroxaban and warfarin treatment cohorts each included 1223 patient admissions, with 751 primary PE admissions and 472 primary DVT admissions. The sample counts resulting from each of the patient selection criteria are shown in Table 1.
Table 1.
Patient Selection
| Selection Criteria | Rivaroxaban | Warfarin | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Hospitalization with a primary diagnosis of DVT or PE during 11/1/2012 to 12/31/2013 | 22 781 | 100 | 22 781 | 100 |
| No hospitalization with any DVT or PE diagnosis 12 months preindex | 22 009 | 97 | 22 009 | 97 |
| Received rivaroxaban or warfarin during hospitalization | 1879 | 8 | 16 557 | 73 |
| Aged ≥18 y as of the index date | 1876 | 8 | 16 537 | 73 |
| No evidence of pregnancy at the time of hospitalization | 1876 | 8 | 16 534 | 73 |
| Did not receive both rivaroxaban and warfarin during admission | 1229 | 5 | 15 887 | 70 |
| No apixaban or dabigatran during hospitalization | 1223 | 5 | 15 873 | 70 |
| Study cohorts before matching | ||||
| Primary DVT or PE | 1223 | 100 | 15 873 | 100 |
| Primary PE | 751 | 61 | 9123 | 57 |
| Primary DVT | 472 | 39 | 6750 | 43 |
| Final study cohorts after matching | ||||
| Primary DVT or PE | 1223 | 100 | 1223 | 100 |
| Primary PE | 751 | 61 | 751 | 61 |
| Primary DVT | 472 | 39 | 472 | 39 |
DVT indicates deep vein thrombosis; PE, pulmonary embolism.
Demographic and Clinical Characteristics
The final rivaroxaban and warfarin treatment groups were demographically well matched with no statistically significant differences, as were the characteristics of the hospitals where patients were treated (Table 2). The mean ages were 64.3±16.6 years for rivaroxaban and 64.2±16.9 years for warfarin (P=0.944), with male patients ranging from 47% to 48% (P=0.571). The majority of admissions in both treatment cohorts were in urban‐based (87%) and nonteaching hospitals (84%). Distributions of hospital bed size were not statistically different between any comparison cohorts. Minor differences in the payer distribution were unremarkable, with 54% to 55% of patients covered by Medicare. Equal percentages of patients were discharged home (86%) or transferred (13%) to another facility (eg, skilled nursing).
Table 2.
Patient and Hospital Demographics
| Characteristics | Rivaroxaban | Warfarin | P Value |
|---|---|---|---|
| Total cohort, No. | 1223 | 1223 | |
| Age, mean±SD, y | 64.3±16.6 | 64.2±16.9 | 0.944 |
| Age group, %, y | 0.266 | ||
| 18 to 34 | 5.5 | 6.0 | |
| 35 to 44 | 8.0 | 8.2 | |
| 45 to 54 | 15.0 | 12.4 | |
| 55 to 64 | 17.3 | 19.8 | |
| 65 to 74 | 22.9 | 22.6 | |
| 75+ | 31.3 | 31.1 | |
| Sex, % | 0.571 | ||
| Male | 48.2 | 47.0 | |
| Female | 51.8 | 53.0 | |
| Principal payer, % | 0.450 | ||
| Private insurance | 34.2 | 36.6 | |
| Medicare | 55.0 | 54.1 | |
| Medicaid | 4.6 | 3.4 | |
| Other | 6.2 | 5.8 | |
| Hospital type, % | 0.620 | ||
| Teaching | 16.4 | 15.7 | |
| Nonteaching | 83.6 | 84.3 | |
| Hospital setting, % | 0.672 | ||
| Urban | 87.5 | 86.9 | |
| Rural | 12.5 | 13.1 | |
| Licensed bed size, % | 0.952 | ||
| 1–199 beds | 23.3 | 24.0 | |
| 200–299 beds | 18.9 | 19.1 | |
| 300–499 beds | 28.7 | 27.7 | |
| 500+ beds | 29.1 | 29.2 | |
| Discharge status, % | 0.364 | ||
| Home/home health | 85.9 | 86.2 | |
| Transferred | 13.2 | 13.3 | |
| Othera | 1.0 | 0.5 |
The “other” discharge status included inpatient death, which occurred in 0.8% of patients taking rivaroxaban and 0.5% of patients taking warfarin.
The rivaroxaban and warfarin treatment groups were also clinically well matched with no statistically significant differences between any of the comparator cohorts for either the relative comorbidity index, or any of the specific comorbidities of interest, or for use of any of the concomitant medication classes of interest with the exception of diuretics usage (rivaroxaban 30% versus warfarin 34%; P=0.030). Hypertension, dyslipidemia, ischemic heart disease, chronic obstructive pulmonary disease, and anemia were the most common chronic comorbid diagnoses (Table 3).
Table 3.
Clinical Characteristics
| Characteristics | Rivaroxaban | Warfarin | P Value |
|---|---|---|---|
| Total cohort, No. | 1223 | 1223 | |
| Comorbidity index, mean±SD | |||
| Number of diagnosesa | 9.5±4.6 | 9.7±4.7 | 0.415 |
| Comorbid conditions,b% | |||
| Hypertension | 61 | 61 | 0.967 |
| Dyslipidemia | 38 | 40 | 0.185 |
| Anemia | 21 | 23 | 0.352 |
| COPD | 21 | 20 | 0.689 |
| Cardiac ischemia/angina | 21 | 21 | 0.960 |
| Diabetes mellitus | 19 | 18 | 0.379 |
| Diseases of the esophagus | 19 | 19 | 0.606 |
| Cardiac arrhythmia | 18 | 19 | 0.876 |
| Overweight or obese | 18 | 21 | 0.081 |
| Renal disease | 15 | 15 | 0.909 |
| Nondependent drug abuse | 14 | 14 | 0.906 |
| Acquired hypothyroidism | 12 | 11 | 0.254 |
| Heart failure | 11 | 11 | 0.949 |
| Malignant cancer | 9 | 9 | 1.000 |
| Cardiometabolic medications, % | |||
| β‐Blockers | 37 | 40 | 0.213 |
| Antiplatelets | 36 | 36 | 0.736 |
| Statins | 34 | 34 | 0.966 |
| Diuretics | 30 | 34 | 0.030 |
| ACE inhibitors | 25 | 25 | 0.852 |
| Calcium channel blockers | 22 | 22 | 0.884 |
| Thrombolytics | 12 | 14 | 0.129 |
| ARBs | 11 | 10 | 0.843 |
ACE indicates angiotensin‐converting enzyme; ARBs, angiotensin II receptor blockers; COPD, chronic obstructive pulmonary disease.
Unique 3‐digit International Classification of Diseases, 9th Revision, Clinical Modification codes.
Comorbid conditions noted with diagnoses recorded in <5% of admissions included peripheral vascular disease, cerebrovascular disease, hepatic disease, major bleed, abdominal surgery, orthopedic surgery, and inflammatory bowel disease.
Hospital LOS
The rivaroxaban‐treated cohort had significantly shorter mean and median LOS than warfarin patients overall and across all stratified comparator groups (Table 4). Overall (all patients, all units), the mean LOS for patients taking rivaroxaban was 3.7±3.1 days compared with 5.2±3.7 days for those taking warfarin, P<0.001, an unadjusted mean difference of 1.5 fewer hospitalized days for the rivaroxaban cohort. Median LOS overall (all patients, all units) was 3 days for rivaroxaban and 5 days for warfarin, a difference of 2 fewer days for median LOS for the rivaroxaban cohort. Although provoked VTE hospitalizations overall were longer than unprovoked VTE, the rivaroxaban cohort's LOS was significantly shorter than the warfarin cohort whether their VTE was considered provoked (rivaroxaban 5.1±4.5 days, warfarin 6.5±5.6 days, P<0.001) or unprovoked (rivaroxaban 3.3±2.4 days, warfarin 4.8±2.8 days, P<0.001). Patients with ICU stays (≈40% for both cohorts) generally had 1 day longer LOS than patients without time in the ICU (≈60% for both cohorts). The Figure graphically shows the differences in time to discharge using a time‐to‐event analysis, with the median (probability of no discharge) for rivaroxaban and warfarin cohorts at discrete times of 3 days and 5 days, respectively. The GLM modeling of LOS, adjusting for the effects of demographic and clinical characteristics, confirmed the descriptive results, with adjusted overall LOS for VTE at 3.71 days (95% confidence interval [CI], 3.63–3.80 days) for rivaroxaban and 5.28 days (95% CI, 5.15–5.40 days) for warfarin, a significant difference of 1.57 days. Table 5 summarizes the predicted mean LOS estimated from the GLM model.
Table 4.
Observed Hospital Lengths of Stay
| Length of Stay Measure | All VTE Patients | Provoked VTE | Unprovoked VTE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rivaroxaban | Warfarin | P Value | Rivaroxaban | Warfarin | P Value | Rivaroxaban | Warfarin | P Value | |||||||
| All admissions (No., %) | 1223 | 100% | 1223 | 100% | 294 | 100% | 277 | 100% | 929 | 100% | 946 | 100% | |||
| Length of stay, d (mean, SD) | 3.7 | 3.1 | 5.2 | 3.7 | <0.001 | 5.1 | 4.5 | 6.5 | 5.6 | <0.001 | 3.3 | 2.4 | 4.8 | 2.8 | <0.001 |
| Median days | 3 | 5 | 4 | 5 | 3 | 4 | |||||||||
| Admissions with ICU stays (No., %) | 476 | 39% | 489 | 40% | 0.591 | 143 | 49% | 132 | 48% | 0.481 | 333 | 36% | 357 | 38% | 0.281 |
| Length of stay, d (mean, SD) | 4.3 | 3.5 | 6.0 | 4.3 | <0.001 | 5.5 | 4.9 | 7.4 | 6.7 | <0.001 | 3.7 | 2.5 | 5.5 | 2.8 | <0.001 |
| Median days | 4 | 5 | 4 | 6 | 3 | 5 | |||||||||
| Admissions without ICU stays (No., %) | 747 | 61% | 734 | 60% | 0.591 | 151 | 51% | 145 | 52% | 0.710 | 596 | 64% | 589 | 62% | 0.777 |
| Length of stay, d (mean, SD) | 3.4 | 2.8 | 4.7 | 3.1 | <0.001 | 4.6 | 4.0 | 5.7 | 4.2 | <0.001 | 3.1 | 2.3 | 4.5 | 2.7 | <0.001 |
| Median days | 3 | 4 | 4 | 5 | 3 | 4 | |||||||||
| Days from first dose to discharge | |||||||||||||||
| With parenteral anticoagulantsa (No., %) | 787 | 64.4% | 788 | 64.4% | 0.966 | 213 | 72.4% | 191 | 69.0% | 0.359 | 574 | 61.8% | 597 | 63.1% | 0.555 |
| Length of stay, d (mean, SD) | 2.41 | 1.74 | 3.91 | 2.73 | <0.001 | 2.50 | 1.95 | 4.17 | 3.79 | <0.001 | 2.38 | 1.66 | 3.83 | 2.29 | <0.001 |
| Median days | 2 | 3 | 2 | 3 | 2 | 4 | |||||||||
| Without parenteral anticoagulantsb (No., %) | 436 | 35.7% | 435 | 35.6% | 0.966 | 81 | 27.6% | 86 | 31% | 0.359 | 355 | 38.2% | 349 | 36.9% | 0.555 |
| Length of stay, d (mean, SD) | 2.73 | 1.72 | 3.72 | 2.14 | <0.001 | 2.72 | 2.19 | 3.71 | 2.46 | 0.007 | 2.74 | 1.60 | 3.73 | 2.06 | <0.001 |
| Median days | 2 | 3 | 2 | 3 | 2 | 3 | |||||||||
ICU indicates intensive care unit; VTE, venous thromboembolism.
Treatment with rivaroxaban or warfarin was initiated with or following parenteral anticoagulants.
Treatment with rivaroxaban or warfarin was initiated without prior or concomitant parenteral anticoagulants.
Figure 1.
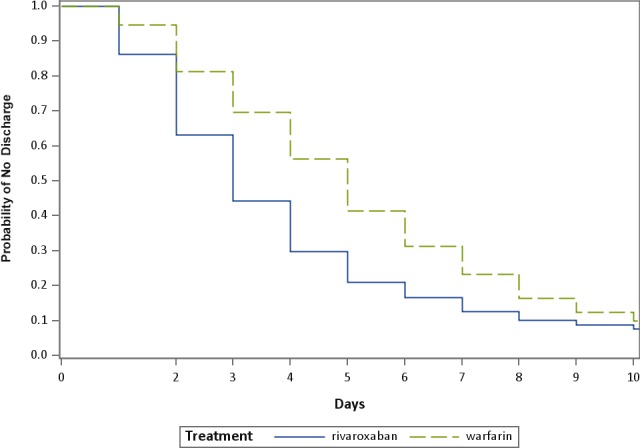
Kaplan‐Meier curve comparing times (in days) from index to discharge for rivaroxaban and warfarin cohorts (estimated using a Cox proportional hazards model for time to event where the censoring variable was discharge status).
Table 5.
Multivariate Regressiona Results for Hospital LOS, in Days
| Treatment Group | Predicted Mean LOS | Lower 95% CI | Upper 95% CI | Predicted Median LOS |
|---|---|---|---|---|
| Rivaroxaban, d | 3.71 | 3.63 | 3.80 | 3.38 |
| Warfarin, d | 5.28 | 5.15 | 5.40 | 4.82 |
CI indicates confidence interval; LOS, length of stay.
Generalized linear model with a log link function and negative binomial error distribution.
Treatment Patterns
The proportion of patients who initiated treatment with parenteral anticoagulants was 64% in both treatment groups (Table 4). The mean interval from the time of admission to first‐dose administration was higher in patients treated with rivaroxaban than those treated with warfarin (2.2±1.7 days versus 1.8±1.7 days; P<0.001), and likewise among patients who initiated treatment without anticoagulants; however, initiation was within the first day of admission (days from admission to first treatment dose: 0.9±1.8 days for rivaroxaban, 0.4±1.0 days for warfarin, P<0.001). As shown in Table 4, the mean and median number of days from initiation of treatment until discharge was significantly shorter for rivaroxaban‐treated patients than warfarin‐treated patients who had treatment initiated with parenteral anticoagulants (days from first dose through discharge: rivaroxaban 2.4±1.7 days, warfarin 3.9±2.7 days; P<0.001) and similarly for patients who initiated rivaroxaban or warfarin treatment without parenteral anticoagulants (rivaroxaban 2.7±1.7 days, warfarin 3.7±2.1 days, P<0.001).
Total Hospitalization Costs
The unadjusted mean total hospitalization costs were significantly lower for patients treated with rivaroxaban compared with patients treated with warfarin ($8688±$9927 versus $9823±$9319, P=0.004) (Table 6). Consistent with lower LOS, the mean room charges were significantly lower for patients treated with rivaroxaban than patients treated with warfarin ($2098±$2490 versus $3093±$3341, P<0.001). The inpatient pharmacy cost for rivaroxaban was higher compared with warfarin pharmacy cost; however, the overall mean pharmacy costs were not significantly different between the treatment cohorts ($647±$1357 versus $698±$1121, P=0.308) (Table 6).
Table 6.
Hospitalization Costsa (Unadjusted)
| Hospital Costs by Category | Rivaroxaban | Warfarin | P Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Inpatient pharmacy | $647 | $1357 | $698 | $1121 | 0.308 |
| Median | $279 | $377 | |||
| Rivaroxaban and warfarin | $21 | $29 | $5 | $10 | <0.001 |
| Median | $12 | $3 | |||
| Proceduresb | $416 | $1641 | $228 | $834 | <0.001 |
| Median | $0 | $0 | |||
| Laboratory tests | $421 | $555 | $491 | $467 | <0.001 |
| Median | $295 | $363 | |||
| Other services | $7225 | $7723 | $8372 | $7743 | <0.001 |
| Median | $4875 | $6392 | |||
| Room rate | $2098 | $2490 | $3093 | $3341 | <0.001 |
| Median | $1531 | $2343 | |||
| Total costs | $8688 | $9927 | $9823 | $9319 | 0.004 |
| Median | $5666 | $7386 | |||
Other services includes Room rate.
All costs were inflation adjusted to 2015 US dollars.
Procedures include operating room costs.
The GLM of the costs confirmed the statistically significant differences in mean costs between the matched rivaroxaban and warfarin cohorts (Table 7). With predicted mean total hospitalization costs at $8387 for the rivaroxaban cohort and $10 275 for the warfarin cohort, the rivaroxaban cohort's hospitalization costs were an average of $1888 less per admission. Although the median costs were generally lower, as is typical for non‐normally distributed healthcare costs, the difference between the median costs was similar at $1669 lower per admission for the rivaroxaban cohort.
Table 7.
| Treatment Group | Predicted Mean Cost | Lower 95% CI | Upper 95% CI | Predicted Median Cost |
|---|---|---|---|---|
| Rivaroxaban | $8387 | $8035 | $8739 | $6350 |
| Warfarin | $10 275 | $9842 | $10 708 | $8019 |
CI indicates confidence interval.
Generalized linear model with a log link function and gamma error distribution.
All costs were inflation adjusted to 2015 US dollars.
Discussion
This study found that patients admitted to the hospital for treatment of VTE had a significantly shorter mean LOS by 1.57 days, with fewer days from the first treatment dose to discharge, when initiating oral anticoagulation with rivaroxaban compared with warfarin, accompanied by significantly lower hospitalization costs. The reduction in LOS was consistent across both provoked and unprovoked patient strata. This finding generally agrees with recent study results examining LOS in the EINSTEIN clinical trial populations.10, 11 In North American EINSTEIN patients, rivaroxaban was associated with a mean LOS of 4.5 days in hospitalized patients compared with a mean LOS of 6.1 days in warfarin‐treated patients.11 The multinational post hoc analysis of the EINSTEIN trials saw variations in hospitalization rates and LOS across regions, but, in all cases, LOS was significantly shorter in the rivaroxaban‐treated group compared with standard treatment.10 The results of the current study (3.71 days for rivaroxaban and 5.28 days for warfarin) are important in that they corroborate the findings of the clinical trials with real‐world evidence across practice settings in a large sample of US hospitals.
A recent case‐control study by Kahler et al12 comparing rivaroxaban and warfarin treatment in low‐risk VTE patients over a 6‐month period similarly found significantly lower overall costs for the rivaroxaban‐treated cohort compared with the standard‐of‐care cohort. The Kahler study also found that the overall inpatient pharmacy costs were significantly higher for the warfarin‐treatment cohort, despite higher costs for rivaroxaban itself. Our study similarly found pharmacy costs trending higher (Table 6) for the warfarin cohort.
This study's results combined data from both primary DVT and primary PE patient hospitalizations, a priori, as it has been reported that a majority of patients diagnosed with DVT will also have PE (symptomatic or asymptomatic), and likewise for PE, and further that the anticoagulant clinical trials showed similar estimates of efficacy and safety with either indication alone and in patients with both DVT and PE.5 Our study reported a larger percentage of primary PE hospitalizations (61%) compared with primary DVT (39%), which is consistent with the 2012 evidence‐based guidelines for VTE antithrombotic therapy recommending home treatment (when possible) for acute DVT and hospitalization for PE due to higher associated short‐term PE mortality.5 This is also consistent with the EINSTEIN trials in which 52% of DVT patients and 90% of PE patients were hospitalized.10 DVT patients admitted to the hospital may therefore represent a potentially higher‐risk subpopulation than those treated as outpatients.
Demographically, the age distribution of the patient population in this study was in line with US national statistics for VTE from the US Centers for Disease Control and Prevention, as well as the slightly higher female (to male) percentage for VTE hospitalizations.16 Discharge status was in relative agreement with 2012 US Healthcare Cost and Utilization Project (HCUP) statistics showing 80.3% of patients hospitalized with primary VTE discharged to home or home health and 15.2% transferred to another facility.17
The mean LOS for VTE in the 2012 US HCUP data for primary DVT/PE hospitalizations was 4.9 days, and a study of 991 primary PE admissions at Brigham and Women's Hospital between 2003 and 2010 reported a mean LOS of 4.1±3.2 days (median 3 days).17, 18 The mean LOS in these (nontrial) populations of 4.1 to 4.9 days, in addition to the EINSTEIN trial results, are a range within which our combined results fall, albeit in somewhat different populations.
Differences in total hospitalization costs were largely affected by differences in the room rate, following the associated differences in LOS. The differences (rivaroxaban lower by $1135 unadjusted, $1888 adjusted) provide real‐world data corroborating a recent study by Mody et al,19 which evaluated the hospital costs of treating patients with rivaroxaban versus other anticoagulant agents using an economic model derived from the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET‐AF), EINSTEIN‐DVT and ‐PE, and the RECORD1‐3 randomized clinical trials, showing the potential cost‐savings at the upper bound of LOS difference as high as $2233 per patient (lower bound assumes no LOS difference). Further, the total hospital charges in our study are in reasonable agreement with other US data including the 2012 HCUP report, with mean costs for primary VTE at $9866, and the Brigham and Women's Hospital study reporting mean total costs at $8764 per patient (2003–2010 data).17, 18
Limitations
The HDD is derived primarily from hospital billing records, and, as such, the data were not collected for research purposes and may be missing elements found in medical records, such as the reasons for admission or clinical assessments. In addition, they may be subject to coding error, for which the extent of miscoding or undercoding that could result in bias is unknown, and can also result in measurement error in variables. Patient medical history was limited to that which was part of the patients' hospitalizations and thus incomplete, such that the patients' outpatient treatment prior to admission, particularly whether they had been receiving either rivaroxaban or warfarin prior to admission, was unknown. Hospital costs, including drug costs, were calculated on the basis of the CMS CCR for each hospital, and therefore actual hospital services costs may differ and may be subject to any inaccuracies of the CCRs. This study sought to reduce selection bias as a result of confounding through the use of propensity score matching to ensure cohorts had similar baseline demographic and clinical characteristics, and increasing the likelihood that the differences found in hospital LOSs were the result of the treatment received during hospitalization. Covariates were chosen that reflected baseline patient characteristics as closely as possible within the previously mentioned limitation of data available only during the index hospitalization. Multivariate analyses of LOS outcomes provided further adjustment for covariates used in the matching procedure and an additional degree of robustness in cohort comparisons. However, there is always the potential for unmeasured confounders, especially since information such as race, socioeconomic status, and other sociodemographic variables (eg, patient assessments such as height, weight, smoking status) were unavailable. The statistical analysis was not able to account for clustering of patients within hospitals because of very small sample sizes in the individual hospitals. Finally, the HDD is a convenience sample of contributing hospitals in the United States, and therefore findings in this study may not be representative of the entire US population.
Conclusions
In this real‐world study of US patients hospitalized for incident VTE, patients initiating oral anticoagulant treatment with rivaroxaban had significantly shorter mean hospital LOS by 1.57 days, attributable to fewer days from first dose to discharge, compared with those treated with warfarin. Patients treated with rivaroxaban incurred significantly lower mean hospitalization costs by $1888 per admission than warfarin‐treated patients. In view of rivaroxaban being found to be as effective as VKAs for treating VTE in clinical trials, this study provides evidence for clinicians to consider the potential for decreasing hospital stays and costs and simplifying treatment regimens using the newer oral anticoagulants to treat VTE when appropriate for their patients.
Sources of Funding
This research was sponsored by Janssen Scientific Affairs LLC, Raritan, NJ, USA.
Disclosures
Margolis, Tran, and Smith are employees of Truven Health Analytics, which was paid by Janssen Scientific Affairs LLC in connection with the conduct of the study and development of this manuscript. Crivera, Schein, and Bookhart are employees of Janssen Scientific Affairs LLC. Deitelzweig and Kline declare no competing financial interests.
Acknowledgments
The authors wish to acknowledge the key contributions of Donna O'Sullivan and Slim Benloucif, whose tireless work in defining, extracting, assembling, and analyzing the data made this research possible. Monika Raut, formerly with Janssen Scientific Affairs LLC, contributed to the concept, design, and analysis for the study. Editorial support for this manuscript was provided by Santosh Tiwari. O'Sullivan, Benloucif, and Tiwari are employees of Truven Health Analytics.
(J Am Heart Assoc. 2016;5:e003788 doi: 10.1161/JAHA.116.003788)
References
- 1. Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495–S501. [DOI] [PubMed] [Google Scholar]
- 2. Fields JM, Goyal M. Venothromboembolism. Emerg Med Clin North Am. 2008;26:649–683, viii. [DOI] [PubMed] [Google Scholar]
- 3. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ III. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158:585–593. [DOI] [PubMed] [Google Scholar]
- 4. Mahan CE, Borrego ME, Woersching AL, Federici R, Downey R, Tiongson J, Bieniarz MC, Cavanaugh BJ, Spyropoulos AC. Venous thromboembolism: annualised United States models for total, hospital‐acquired and preventable costs utilising long‐term attack rates. Thromb Haemost. 2012;108:291–302. [DOI] [PubMed] [Google Scholar]
- 5. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR; American College of Chest Physicians . Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e419S–e494S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eriksson BI, Quinlan DJ, Eikelboom JW. Novel oral factor Xa and thrombin inhibitors in the management of thromboembolism. Annu Rev Med. 2011;62:41–57. [DOI] [PubMed] [Google Scholar]
- 7. Einstein Investigators , Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S. Oral rivaroxaban for symptomatic venous thromboembolism. New Engl J Med. 2010;363:2499–2510. [DOI] [PubMed] [Google Scholar]
- 8. Einstein‐PE Investigators , Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J, Verhamme P, Wells P, Agnelli G, Cohen A, Berkowitz SD, Bounameaux H, Davidson BL, Misselwitz F, Gallus AS, Raskob GE, Schellong S, Segers A. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. New Engl J Med. 2012;366:1287–1297. [DOI] [PubMed] [Google Scholar]
- 9. Prins MH, Lensing AW, Bauersachs R, van Bellen B, Bounameaux H, Brighton TA, Cohen AT, Davidson BL, Decousus H, Raskob GE, Berkowitz SD, Wells PS. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN‐DVT and PE randomized studies. Thromb J. 2013;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Bellen B, Bamber L, Correa de Carvalho F, Prins M, Wang M, Lensing AW. Reduction in the length of stay with rivaroxaban as a single‐drug regimen for the treatment of deep vein thrombosis and pulmonary embolism. Curr Med Res Opin. 2014;30:829–837. [DOI] [PubMed] [Google Scholar]
- 11. Bookhart BK, Haskell L, Bamber L, Wang M, Schein J, Mody SH. Length of stay and economic consequences with rivaroxaban vs enoxaparin/vitamin K antagonist in patients with DVT and PE: findings from the North American EINSTEIN clinical trial program. J Med Econ. 2014;17:691–695. [DOI] [PubMed] [Google Scholar]
- 12. Kahler ZP, Beam DM, Kline JA. Cost of treating venous thromboembolism with heparin and warfarin versus home treatment with rivaroxaban. Acad Emerg Med. 2015;22:796–802. [DOI] [PubMed] [Google Scholar]
- 13. Farley JF, Harley CR, Devine JW. A comparison of comorbidity measurements to predict healthcare expenditures. Am J Manag Care. 2006;12:110–117. [PubMed] [Google Scholar]
- 14. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention . Venous thromboembolism in adult hospitalizations –United States, 2007–2009. MMWR. 2012;61:401–424. [PubMed] [Google Scholar]
- 17. National statistics—principal diagnosis only. Outcomes by ICD‐9‐CM diagnosis codes. Rockville, MD: Agency for Healthcare Research and Quality, 2012. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp. Accessed October 5, 2015.
- 18. Fanikos J, Rao A, Seger AC, Carter D, Piazza G, Goldhaber SZ. Hospital costs of acute pulmonary embolism. Am J Med. 2013;126:127–132. [DOI] [PubMed] [Google Scholar]
- 19. Mody SH, Huynh L, Zhuo DY, Tran KN, Lefebvre P, Bookhart B. A cost‐analysis model for anticoagulant treatment in the hospital setting. J Med Econ. 2014;17:492–498. [DOI] [PubMed] [Google Scholar]


