Abstract
Background
Incomplete endothelialization is the primary substrate of late stent thrombosis; however, recent reports have revealed that abnormal vascular responses are also responsible for the occurrence of late stent failure. The aim of the current study was to assess vascular response following deployment of biodegradable polymer‐based Synergy (Boston Scientific) and Nobori (Terumo) drug‐eluting stents and the durable polymer‐based Resolute Integrity stent (Medtronic) in an atherosclerotic rabbit iliac artery model.
Methods and Results
A total of 24 rabbits were fed an atherogenic diet, and then a balloon injury was used to induce atheroma formation. Synergy, Nobori, and Resolute Integrity stents were randomly implanted in iliac arteries. Animals were euthanized at 28 days for scanning electron microscopic evaluation and at 90 days for histological analysis. The percentage of uncovered strut area at 28 days was lowest with Synergy, followed by Resolute Integrity, and was significantly higher with Nobori stents (Synergy 1.1±2.2%, Resolute Integrity 2.0±3.9%, Nobori 4.6±3.0%; P<0.001). At 90 days, inflammation score was lowest for Synergy (0.27±0.45), followed by Nobori (0.62±0.59), and was highest for Resolute Integrity (0.89±0.46, P<0.001). Foamy macrophage infiltration within neointima (ie, neoatherosclerosis) was significantly less with Synergy (0.62±0.82) compared with Nobori (0.85±0.74) and Resolute Integrity (1.39±1.32; P=0.034).
Conclusions
The biodegradable polymer‐coated thin‐strut Synergy drug‐eluting stent showed the fastest stent strut neointimal coverage and the lowest incidence of neoatherosclerosis in the current animal model.
Keywords: atherosclerosis, endothelialization, polymer, stent
Subject Categories: Animal Models of Human Disease, Stent
Introduction
Drug‐eluting stents (DESs) have reduced restenosis by inhibiting neointimal growth,1, 2 but there are still certain indications of late stent failure such as late stent thrombosis and restenosis. Although incomplete endothelialization was reported as the primary substrate of late stent thrombosis in an initial pathological study,3, 4 recent reports revealed that abnormal vascular responses are also responsible for the occurrence of late stent failure.5, 6, 7 Such abnormal responses include hypersensitivity reactions, extensive fibrin deposition along with malapposition, and neoatherosclerosis. Consequently, both early reendothelialization and high biocompatibility of the implant device are important for better long‐term clinical outcomes.
Newer generation DESs incorporate thinner stent struts, biocompatible polymers, and optimal drug dose and release kinetics, all of which accelerate greater arterial healing with faster neointimal coverage.8 Such optimizations have resulted in better clinical outcomes with new‐generation DESs compared with first‐generation DESs9, 10 and, in turn, have led to extended application of DES usage in various clinical settings including patients presenting with acute myocardial infarction.11, 12
A recent pathological study demonstrated that cobalt–chromium everolimus‐eluting stents showed greater arterial healing, as demonstrated by faster reendothelialization and lower incidence of inflammatory reactions compared with first‐generation DESs.13 Surprisingly, however, the incidence of neoatherosclerosis was similar in cobalt–chromium everolimus‐eluting stents and first‐generation DESs in this study. This result suggested that long‐term biocompatibility remained uncertain, even with the safest and most effective DESs in current use. Most clinical studies revealed that patients treated with current‐generation DESs uniformly experienced late target revascularization in ≈2% of cases.9, 14, 15 It could be concluded that the existence of polymer may have been responsible for the development of neoatherosclerosis, and thus biodegradable polymer‐based DESs would be expected to reduce the incidence of neoatherosclerosis. No clinical study, however, has proven this concept because the detection of neoatherosclerosis may be challenging in the clinical setting, and underlying plaque and patient backgrounds vary. We aimed to evaluate the vascular response following implantation of biodegradable polymer‐based Synergy (everolimus‐eluting; Boston Scientific) and Nobori (biolimus‐eluting; Terumo) stents and the durable polymer‐based Resolute Integrity (zotarolimus‐eluting; Medtronic) stent in an atherosclerotic rabbit iliac artery model. In addition, the extent of early reendothelialization was evaluated in the same model.
Methods
Atherosclerotic Rabbit Model
The study protocol was reviewed and approved by the Education and Research Support Center in the Department of Animal Care at Tokai University.
The atherosclerotic rabbit model was used, as described previously.16 Briefly, rabbits (Tokyo Laboratory Animals Science Co., Ltd., Tokyo, Japan) were fed an atherogenic diet (1% cholesterol and 6% peanut oil; Oriental Yeast Co, Ltd) to induce atheroma formation. At 1 week after initiation of the atherogenic diet, balloon injury was performed using a 3.0×15‐mm balloon catheter by withdrawing the inflated balloon at a nominal pressure from the distal iliac artery to the aorta. After balloon injury, animals were maintained on an atherogenic diet for another 6 weeks (total of 7 weeks). The diet was then switched to regular rabbit chow until euthanasia (Figure 1). Stents were implanted 2 weeks after the regular rabbit chow started.
Figure 1.
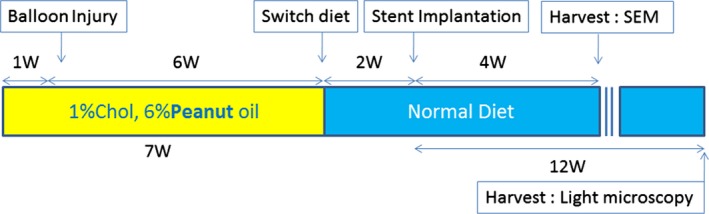
Atherosclerotic rabbit model. Balloon injury was performed after initiation of high cholesterol diet followed by switching of diet to normal chow. Animals euthanized at 28 days were used for scanning electron microscopy (SEM), and those euthanized at 90 days were used for light microscopic analysis. W indicates weeks.
Stent Implantation
All animals were premedicated with oral aspirin (40 mg/day) 2 days before the procedure.4, 16, 17 Rabbits were anesthetized with 2.5% isoflurane via facemask. Surgical access was obtained via the carotid artery using a general sterile technique, and a 5F vascular sheath was inserted through the left carotid artery. Heparin (100 IU/kg) was injected before catheterization. Angiography of the aorta and bilateral iliac artery was performed. Biodegradable polymer‐based everolimus‐eluting stents (Synergy), biodegradable polymer‐based biolimus‐eluting stents (Nobori), and durable polymer‐based zotarolimus‐eluting stents (Resolute Integrity) were randomized and implanted in each iliac artery, resulting in each animal receiving 2 different stents (eg, right iliac: Synergy; left iliac: Nobori). The characteristics of the 3 stents are shown in Table 1. Stents were deployed by 30 seconds of balloon inflation to nominal pressure, resulting in a target stent:artery ratio of ≈1.1 to 1.2:1. Following stent deployment, angiography was performed to document patency. Thereafter, all catheters were removed, the proximal portion of the carotid artery was ligated, the muscle and fascia were sutured with a 3.0 dexon absorbable suture, and the neck incision was closed with a 1.0 silk nonabsorbable suture. All animals received 40 mg aspirin daily postoperatively.
Table 1.
Characteristics of Tested Drug‐Eluting Stents
| Synergy | Resolute Integrity | Nobori | |
|---|---|---|---|
| Stent | Platinum–chromium | Cobalt–nickel | Stainless steel 316L |
| Strut thickness | 74 μm | 91 μm | 125 μm |
| Coating | Abluminal | Conformal | Abluminal |
| Polymer | PLGA (biodegradable) | BiolLynxa (durable) | PLA (biodegradable) |
| Drug | Everolimus | Zotarolimus | Biolimus A9 |
| Drug dose | 1.0 μg/mm2 | 1.6 μg/mm2 | 15.6 μg/mm |
PLA indicates polylactic acid; PLGA, poly(lactic‐co‐glycolic) acid.
Copolymer of hydrophobic C10 polymer, hydrophilic C19 polymer, and polyvinylpyrrolidinone.
Euthanasia and Fixation
Rabbits were anesthetized with isoflurane via facemask. Two 4F sheaths were positioned in the right carotid artery and jugular vein. Rabbits were administered intravenous heparin (100 IU/kg). Angiography was performed to document patency (Figure 2). Euthanasia was accomplished with an overdose of pentobarbital intravenously. The lower extremities were perfused at 80 mm Hg (dripping from a height of ≈2 m) with Ringer's lactate until the perfusate from the jugular vein was clear of blood. The solution was then switched to 10% neutral buffered formalin for 15 minutes (or 1500 mL). The segment from the distal aorta to the proximal femoral arteries was dissected free and cleaned of periadventitial tissue.
Figure 2.
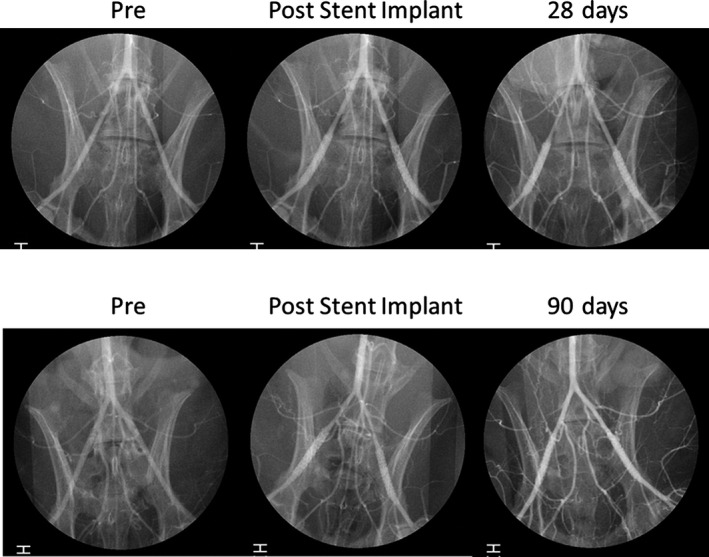
Representative angiographic images of 12 rabbits for 28 days and 10 rabbits for 90 days. Angiography was performed before (pre) and after (post) stent implantation and during follow‐up.
Scanning Electron Microscopy
Intact stented arterial segments were bisected longitudinally to expose the luminal surface and photographed. Specimens were visualized using a scanning electron microscope. Regions of interest were photographed at incremental magnifications of ×50, ×200, and ×600. From low‐power photographs (×15), exposed stent struts were traced using offline digital software (WinROOF image processing software, version 6; Mitani Corp), and percentage of exposed stent strut per stented area was calculated (Figure 3).
Figure 3.
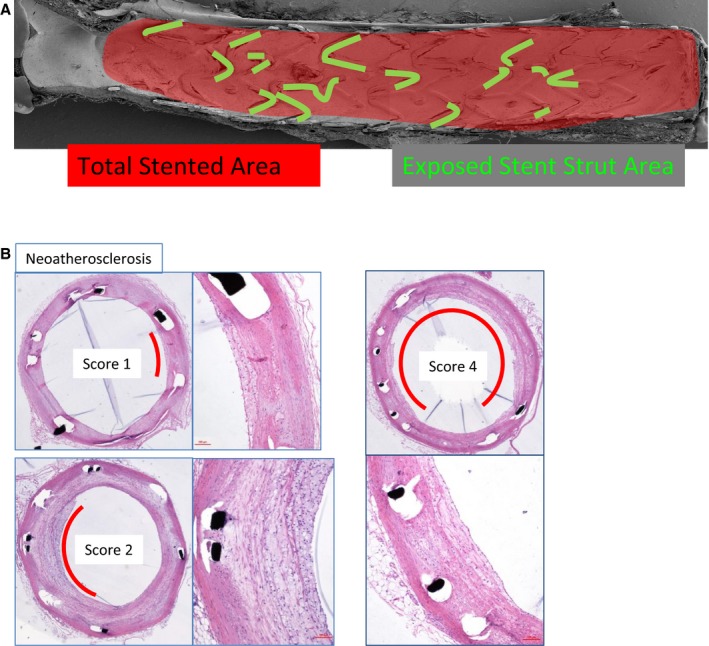
A method to quantify uncovered struts. Areas of strut without endothelial cells were traced and divided by total stented area. Score is based on the number of quadrants in which each vascular reaction is involved. A, Inflammation. B, Neoatherosclerosis.
Histological Evaluation
Stented sections were stained with hematoxylin and eosin. Cross‐sectional areas (stent area and lumen) of each section were measured with digital morphometry. Neointimal volume and percentage of stenosis were calculated using the following formulas; (neointimal volume)=(stent area)−(lumen) and (percentage stenosis)=(neointimal volume)/(stent area)×100. Inflammation and foamy macrophage infiltration within the neointima (ie, neoatherosclerosis) were examined on serial hematoxylin and eosin–stained sections of each stent part (proximal, middle, distal) and graded based on the number of quadrants that were involved in accordance with the above pathological findings (Figure 3).
Statistical Analysis
All values were expressed as mean±SD. Because of the clustered nature of 2 stents measured from 1 animal resulting in unknown correlations among measurements within these lesion clusters, statistical comparisons of all parameters between stent groups were performed by linear generalized estimating equation modeling with an assumed Gaussian distribution or ordinal variables, an identity link function, and an assumed exchangeable structure for the within‐cluster correlation matrix. Comparison of each pair of stents was based on the estimated marginal means with sequential Bonferroni correction. P<0.05 was considered statistically significant. All statistics were calculated with SPSS version 23 (IBM Corp) software.
Results
Animal and Stent Conditions During the Study Period
A total of 2 animals died from severe weight loss caused by anorexia. The final numbers of animals used were 12 atherosclerotic rabbits for 28 days and 10 atherosclerotic rabbits for 90 days that were euthanized at the scheduled time points.
Measurements of Percentage of Uncovered Strut Area (Animals for 28 Days)
All stents (n=8 each) were confirmed to be patent at euthanasia. Scanning electron microscopy analysis revealed no major thrombus formation on the luminal surface, and most of the surface area was covered by endothelial cells with partially uncovered stent struts. Platelet adhesion was generally observed at the site of uncovered struts. Percentage of uncovered strut area in Synergy was the lowest, followed by Resolute Integrity, and was significantly higher with Nobori stents (Synergy 1.1±2.2%, Resolute Integrity 2.0±3.9%, Nobori 4.6±3.0%; P<0.001). The difference was significant between Synergy and Nobori (Figure 4).
Figure 4.
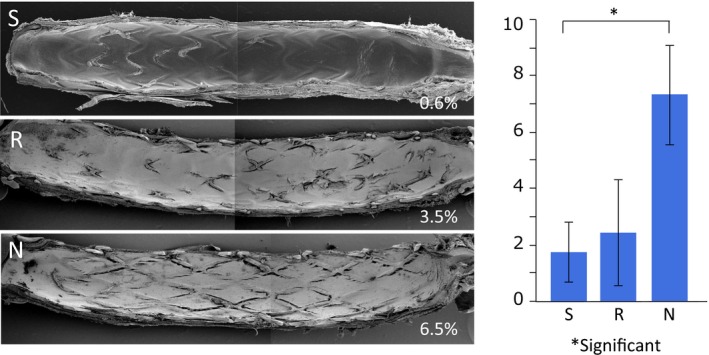
Representative scanning electron microscopy images of 8 stents each harvested from animals euthanized at 28 days. The percentage of uncovered strut area was significantly higher with Nobori compared with Synergy. N indicates Nobori; R, Resolute Integrity; S, Synergy.
Histological Evaluation (Animals for 90 Days)
Morphometric analysis revealed that the outer stent area was significantly smaller for Synergy compared with Resolute Integrity and Nobori (stent area: Synergy 5.8±0.3 mm2, Resolute Integrity 6.6±0.6 mm2, Nobori 6.4±0.5 mm2; P<0.001) (Table 2). In contrast, neointimal area was significantly greater with Resolute Integrity compared with Synergy (Synergy 1.6±0.5 mm2, Resolute Integrity 2.1±0.5 mm2, Nobori 1.7±0.4 mm2; P<0.001; difference was significant between Synergy and Resolute Integrity), whereas percentage of stenosis did not reach statistical significance (Synergy 26.7±8.0%, Resolute Integrity 30.9±6.2%, Nobori 26.8±5.8%; P=0.11) (Figure 5).
Table 2.
Histological Analysis at 90 Days
| Variable | Synergy (n=7) | Resolute Integrity (n=6) | Nobori (n=7) | P Value |
|---|---|---|---|---|
| Stent area, mm2 | 5.8±0.3 | 6.6±0.6 | 6.4±0.5 | <0.001a |
| Neointima, mm2 | 1.6±0.5 | 2.1±0.5 | 1.7±0.4 | <0.001b |
| Stenosis, % | 26.7±8.0 | 30.9±6.2 | 26.8±5.8 | 0.11 |
| Inflammation score | 0.27±0.45 | 0.89±0.46 | 0.62±0.59 | <0.001c |
| Neoatherosclerosis score | 0.62±0.82 | 1.39±1.32 | 0.85±0.74 | 0.034b |
Significantly different between Synergy vs Resolute Integrity and Nobori.
Significantly different between Synergy vs Resolute Integrity.
Significantly different between each stent.
Figure 5.
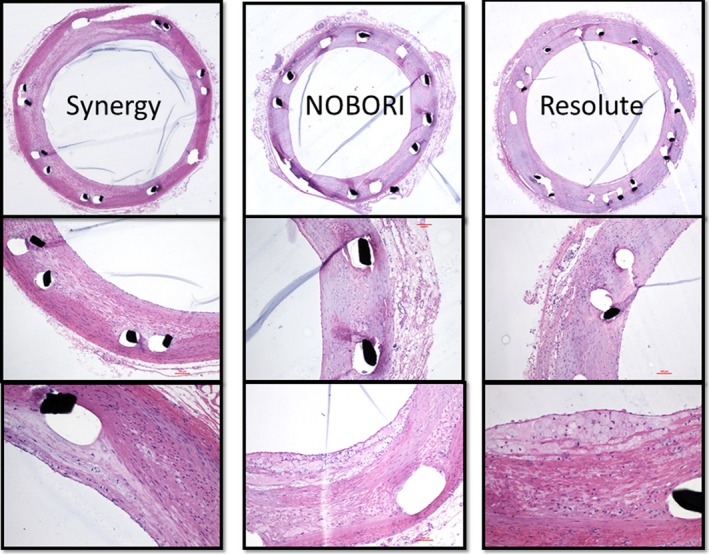
Representative histological images of 7 Synergy, 7 Nobori, and 6 Resolute Integrity stents harvested from animals euthanized at 90 days. All stent types were patent at euthanasia and showed various extents of inflammation and neoatherosclerosis.
Inflammation score was lowest for Synergy (0.27±0.45), followed by Nobori (0.62±0.59), and highest for Resolute Integrity (0.89±0.46; P<0.001). Differences were significant for Synergy versus Nobori and Resolute Integrity and for Nobori versus Resolute Integrity.
Foamy macrophage infiltration within neointima (ie, neoatherosclerosis) was significantly less with Synergy (0.62±0.82) compared with Nobori (0.85±0.74) and Resolute Integrity (1.39±1.32; P=0.034; difference was significant for Synergy versus Resolute Integrity) (Table 2).
Discussion
The current study demonstrated (1) that Synergy showed the lowest number of uncovered stent struts compared with Resolute Integrity and Nobori stents (although the difference between Synergy and Resolute Integrity was not statistically significant) and (2) that inflammation and neoatherosclerosis were significantly less evident with Synergy compared with Resolute Integrity and Nobori.
Neointimal Coverage
As the previous studies reported, reendothelialization following stent implantation is very important to prevent stent thrombosis3, 4 because the exposure of stent strut surfaces to the bloodstream may result in thrombus formation.16 Synergy showed the fastest neointimal coverage in atherosclerotic rabbits among the DESs investigated. This atherosclerotic rabbit model is well known to reflect the human vascular response following DES implantation.16 We previously reported that second‐generation DESs demonstrate greater endothelial coverage with higher expression of functional markers such as platelet and endothelial cell adhesion molecule 1 and thrombomodulin compared with first‐generation DESs.8, 16 Second‐generation DESs incorporate thin stent strut thickness, ideal drug type (ie, “‐limus”), dose, and optimal release kinetics, suggesting that the combination of these components positively influences vascular repair. Although there are differences among sirolimus, everolimus, and biolimus A9 in “position 40,” all of these ‐limus drugs show antiproliferative and immunosuppressive effects that are not able to differentiate endothelial cells from smooth muscle cells; therefore, it is unlikely that the difference in drug affects the extent of reendothelialization.18 The current study reinforces the contention that strut thickness is one of the most important factors in strut coverage; Resolute Integrity also showed better coverage compared with Nobori stents, although it should be noted that stent platform, polymer, and release kinetics are different between Resolute Integrity and Nobori.
Although an abnormal vascular response is responsible for the occurrence of late stent failure,5 incomplete reendothelialization is still a primary cause of early and late stent thrombosis. In fact, most recent clinical studies revealed that the majority of stent thrombosis cases are clustered in the early phase. In contrast, second‐generation DESs recently received the European CE mark for the use of dual antiplatelet therapy for at least 1 to 3 months after stent implantation19, 20; therefore, early reendothelialization of the stent strut is an essential feature of current‐generation DESs. This study suggests that it is safe to minimize the duration of dual antiplatelet therapy in patients who received new‐generation biodegradable polymer‐based everolimus‐eluting stent (ie, Synergy) implantation.
Vascular Response
Although there is no clearly established animal model for evaluation of the incidence of “neoatherosclerosis” following stent implantation, the current study showed varied degrees of foamy macrophage infiltration within neointima (ie, neoatherosclerosis); therefore, we semiquantified the extent of this phenomenon. Neoatherosclerosis was significantly less frequent with Synergy compared with Resolute Integrity and Nobori. Furthermore, the extent of the inflammatory reaction with Synergy was lowest among the stents studied, suggesting a relationship between inflammation and neoatherosclerosis. These findings reinforce the contention that biocompatibility is one of the most important factors in the occurrence of neoatherosclerosis. Although Nobori also incorporates the use of a biodegradable polymer, drug elution and the polymer‐degradation process take ≈6 to 9 months; therefore, the vascular wall reaction would still have been active over the period of the current study. In contrast, Synergy has faster drug‐release kinetics and a shorter degradation period; therefore, the inflammatory reaction may be concluded at an early time point, which would explain the findings of the current study. In fact, fibrous neointimal tissue was more frequently observed with Synergy stents compared with other DESs. Nakano et al reported that neointimal tissue in DESs consisted predominantly of extracellular matrix including proteoglycan, whereas bare metal stents usually showed fibrous neointima21; this may explain the fact that vulnerable neointima in DESs is prone to induction of neoatherosclerosis. Because the lower incidence of neoatherosclerosis may lead to lower incidence of very late stent thrombosis, it may be expected that the biodegradable polymer‐based DESs show better clinical outcomes compared with durable stents in long‐term follow‐up. Another factor related to the occurrence of neoatherosclerosis is the patient's background, especially lipid metabolism. Lee et al found that higher low‐density lipoprotein cholesterol is an independent predictor of neoatherosclerosis by using optical coherence tomography follow‐up data.22 A study recently reported that inhibition of some microRNAs associated with cholesterol metabolism and atherosclerosis may be a potential target of progression of atherosclerosis.23 Consequently, both local approach and systemic treatment are key to preventing the occurrence of neoatherosclerosis.
Although biodegradable polymer‐based DESs effectively become bare metal stents after polymer absorption, it should be emphasized that completion of the inflammatory reaction requires a certain time period, and early degradation may be optimal. Most previous effective DESs release the drug within 3 months, suggesting that the neointimal proliferation process occurs within the same time period, and thus an ideal duration of drug release should be ≈3 months.
Limitations
As with all other preclinical studies using animals, the rabbit iliac artery model may not adequately represent biological responses of human atherosclerotic arteries. The extent of reendothelialization was assessed only by scanning electron microscopy. Additional data including immune‐staining of an endothelial marker8 or biomarker reflecting coagulability such as d‐dimer plasma level24 may further corroborate our findings.
Although the extent of foamy macrophage infiltration (ie, neoatherosclerosis) was semiquantified by grading based on the number of quadrants, neither the depth of infiltration nor the actual area of neoatherosclerosis could be taken into account. No previous report described the incidence of neoatherosclerosis in the animal model, so we believe the current study gave an important insight into the mechanism of neoatherosclerosis.
Finally, we used 3 different DESs applying different stent platforms, drugs, and polymers; therefore, we cannot assert that degradation of the polymer was related solely to lower incidence of neoatherosclerosis in the stents with biodegradable polymer.
Conclusion
The biodegradable polymer‐coated thin‐strut Synergy DES showed the greatest stent strut neointimal coverage compared with Resolute Integrity and Nobori stents. Incidence of neoatherosclerosis was significantly lower with Synergy.
Disclosures
Nakazawa is a consultant for Abbott Vascular Japan, Terumo Corporation, Japan Medical Device Engineering, and St. Jude Medical and is in receipt of research grants from Abbott Vascular, Terumo Corporation, Boston Scientific, Daiichi Sankyo, and Japan Medical Device Engineering. The remaining authors have no conflicts of interest.
Acknowledgments
The authors thank Masayoshi Tokunaga and Yoshiko Ito (the Education and Research Support Center, Department of Cell Biology and Histology, Tokai University), Sachie Tanaka and Katsuko Naito (the Education and Research Support Center, Department of Animal Care, Tokai University) for their valuable technical assistance.
(J Am Heart Assoc. 2016;5:e003803 doi: 10.1161/JAHA.116.003803)
References
- 1. Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R. A randomized comparison of a sirolimus‐eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. [DOI] [PubMed] [Google Scholar]
- 2. Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME. A polymer‐based, paclitaxel‐eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. [DOI] [PubMed] [Google Scholar]
- 3. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug‐eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. [DOI] [PubMed] [Google Scholar]
- 4. Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug‐eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. [DOI] [PubMed] [Google Scholar]
- 5. Nakazawa G. Stent thrombosis of drug eluting stent: pathological perspective. J Cardiol. 2011;58:84–91. [DOI] [PubMed] [Google Scholar]
- 6. Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first‐generation sirolimus‐ and paclitaxel‐eluting stents. J Am Coll Cardiol. 2011;57:390–398. [DOI] [PubMed] [Google Scholar]
- 7. Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R. The pathology of neoatherosclerosis in human coronary implants bare‐metal and drug‐eluting stents. J Am Coll Cardiol. 2011;57:1314–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, Wilson PS, Skorija K, Cheng Q, Xu X, Gold HK, Kolodgie FD, Virmani R. Endothelial cell recovery between comparator polymer‐based drug‐eluting stents. J Am Coll Cardiol. 2008;52:333–342. [DOI] [PubMed] [Google Scholar]
- 9. Gada H, Kirtane AJ, Newman W, Sanz M, Hermiller JB, Mahaffey KW, Cutlip DE, Sudhir K, Hou L, Koo K, Stone GW. 5‐year results of a randomized comparison of XIENCE V everolimus‐eluting and TAXUS paclitaxel‐eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6:1263–1266. [DOI] [PubMed] [Google Scholar]
- 10. Palmerini T, Biondi‐Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D'Ascenzo F, Kimura T, Briguori C, Sabate M, Kim HS, De Waha A, Kedhi E, Smits PC, Kaiser C, Sardella G, Marullo A, Kirtane AJ, Leon MB, Stone GW. Stent thrombosis with drug‐eluting and bare‐metal stents: evidence from a comprehensive network meta‐analysis. Lancet. 2012;379:1393–1402. [DOI] [PubMed] [Google Scholar]
- 11. Raber L, Kelbaek H, Ostojic M, Baumbach A, Heg D, Tuller D, von Birgelen C, Roffi M, Moschovitis A, Khattab AA, Wenaweser P, Bonvini R, Pedrazzini G, Kornowski R, Weber K, Trelle S, Luscher TF, Taniwaki M, Matter CM, Meier B, Juni P, Windecker S; Investigators CAT . Effect of biolimus‐eluting stents with biodegradable polymer vs bare‐metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308:777–787. [DOI] [PubMed] [Google Scholar]
- 12. Sabate M, Cequier A, Iniguez A, Serra A, Hernandez‐Antolin R, Mainar V, Valgimigli M, Tespili M, den Heijer P, Bethencourt A, Vazquez N, Gomez‐Hospital JA, Baz JA, Martin‐Yuste V, van Geuns RJ, Alfonso F, Bordes P, Tebaldi M, Masotti M, Silvestro A, Backx B, Brugaletta S, van Es GA, Serruys PW. Everolimus‐eluting stent versus bare‐metal stent in ST‐segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380:1482–1490. [DOI] [PubMed] [Google Scholar]
- 13. Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, Kutys R, Ladich E, Finn AV, Kolodgie FD, Virmani R. Pathology of second‐generation everolimus‐eluting stents versus first‐generation sirolimus‐ and paclitaxel‐eluting stents in humans. Circulation. 2014;129:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taniwaki M, Raber L, Magro M, Kalesan B, Onuma Y, Stefanini GG, van Domburg RT, Moschovitis A, Meier B, Juni P, Serruys PW, Windecker S. Long‐term comparison of everolimus‐eluting stents with sirolimus‐ and paclitaxel‐eluting stents for percutaneous coronary intervention of saphenous vein grafts. EuroIntervention. 2014;9:1432–1440. [DOI] [PubMed] [Google Scholar]
- 15. Sabate M, Brugaletta S, Cequier A, Iniguez A, Serra A, Jimenez‐Quevedo P, Mainar V, Campo G, Tespili M, den Heijer P, Bethencourt A, Vazquez N, van Es GA, Backx B, Valgimigli M, Serruys PW. Clinical outcomes in patients with ST‐segment elevation myocardial infarction treated with everolimus‐eluting stents versus bare‐metal stents (EXAMINATION): 5‐year results of a randomised trial. Lancet. 2016;387:357–366. [DOI] [PubMed] [Google Scholar]
- 16. Nakazawa G, Nakano M, Otsuka F, Wilcox JN, Melder R, Pruitt S, Kolodgie FD, Virmani R. Evaluation of polymer‐based comparator drug‐eluting stents using a rabbit model of iliac artery atherosclerosis. Circ Cardiovasc Interv. 2011;4:38–46. [DOI] [PubMed] [Google Scholar]
- 17. Joner M, Farb A, Cheng Q, Finn AV, Acampado E, Burke AP, Skorija K, Creighton W, Kolodgie FD, Gold HK, Virmani R. Pioglitazone inhibits in‐stent restenosis in atherosclerotic rabbits by targeting transforming growth factor‐beta and MCP‐1. Arterioscler Thromb Vasc Biol. 2007;27:182–189. [DOI] [PubMed] [Google Scholar]
- 18. Santulli G. microRNAs distinctively regulate vascular smooth muscle and endothelial cells: functional implications in angiogenesis, atherosclerosis, and in‐stent restenosis. Adv Exp Med Biol. 2015;887:53–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silber S, Kirtane AJ, Belardi JA, Liu M, Brar S, Rothman M, Windecker S. Lack of association between dual antiplatelet therapy use and stent thrombosis between 1 and 12 months following resolute zotarolimus‐eluting stent implantation. Eur Heart J. 2014;35:1949–1956. [DOI] [PubMed] [Google Scholar]
- 20. Kedhi E, Stone GW, Kereiakes DJ, Serruys PW, Parise H, Fahy M, Simonton CA, Sudhir K, Sood P, Smits PC. Stent thrombosis: insights on outcomes, predictors and impact of dual antiplatelet therapy interruption from the SPIRIT II, SPIRIT III, SPIRIT IV and COMPARE trials. EuroIntervention. 2012;8:599–606. [DOI] [PubMed] [Google Scholar]
- 21. Nakano M, Otsuka F, Yahagi K, Sakakura K, Kutys R, Ladich ER, Finn AV, Kolodgie FD, Virmani R. Human autopsy study of drug‐eluting stents restenosis: histomorphological predictors and neointimal characteristics. Eur Heart J. 2013;34:3304–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee SY, Hur SH, Lee SG, Kim SW, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Hong MK. Optical coherence tomographic observation of in‐stent neoatherosclerosis in lesions with more than 50% neointimal area stenosis after second‐generation drug‐eluting stent implantation. Circ Cardiovasc Interv. 2015;8:e001878. [DOI] [PubMed] [Google Scholar]
- 23. Novak J, Olejnickova V, Tkacova N, Santulli G. Mechanistic role of microRNAs in coupling lipid metabolism and atherosclerosis. Adv Exp Med Biol. 2015;887:79–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santulli G, Wronska A, Uryu K, Diacovo TG, Gao M, Marx SO, Kitajewski J, Chilton JM, Akat KM, Tuschl T, Marks AR, Totary‐Jain H. A selective microRNA‐based strategy inhibits restenosis while preserving endothelial function. J Clin Invest. 2014;124:4102–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]


