Abstract
Background
Studies of kidney disease associated with cardiac catheterization typically rely on billing records rather than laboratory data. We examined the associations between percutaneous coronary interventions, acute kidney injury, and chronic kidney disease progression using comprehensive Veterans Affairs clinical and laboratory databases.
Methods and Results
Patients undergoing percutaneous coronary interventions between 2005 and 2010 (N=24 405) were identified in the Veterans Affairs Clinical Assessment, Reporting, and Tracking registry and examined for associated acute kidney injury and chronic kidney disease development or progression relative to 24 405 matched population controls. Secondary outcomes analyzed included dialysis, acute myocardial infarction, and mortality. The incidence of chronic kidney disease progression following percutaneous coronary interventions complicated by acute kidney injury, following uncomplicated coronary interventions, and in matched controls were 28.66, 11.15, and 6.81 per 100 person‐years, respectively. Percutaneous coronary intervention also increased the likelihood of chronic kidney disease progression in both the presence and absence of acute injury relative to controls in adjusted analyses (hazard ratio [HR], 5.02 [95% CI, 4.68–5.39]; and HR, 1.76 [95% CI, 1.70–1.86]). Among patients with estimated glomerular filtration rate <60 mL/min per 1.73 m2, acute kidney injury increased the likelihood of disease progression by 8‐fold. Similar results were observed for all secondary outcomes.
Conclusions
Acute kidney injury following percutaneous coronary intervention was associated with increased chronic kidney disease development and progression and mortality.
Keywords: angioplasty, contrast media, kidney, morbidity, survival
Subject Categories: Mortality/Survival, Percutaneous Coronary Intervention, Quality and Outcomes, Epidemiology, Nephrology and Kidney
Introduction
The relationships between cardiovascular procedure outcomes, including acute kidney injury (AKI), chronic kidney disease (CKD) development and progression,1, 2, 3, 4, 5 and mortality6, 7, 8 are incompletely delineated.9, 10, 11, 12 We sought to improve the methodological capture of periprocedural renal outcomes using integrated Veterans Affairs (VA) databases and objective laboratory information to both confirm and extend prior observations of CKD development and progression among percutaneous coronary intervention (PCI) patients and matched population controls. Recognizing that not all catheterization‐associated AKI results from contrast exposure, we also propose minor nosological reform to include common language describing AKI following cardiac catheterization and PCI as cardiac catheterization–associated AKI (CCA‐AKI), representing a broad category of AKI that includes contrast‐induced nephropathy, as well as AKI attributable to atheroembolization, hypotension, and other causes associated with these procedures.
The National Quality Forum has made CCA‐AKI prevention a major patient safety objective, albeit with limited success.13 Our understanding of the long‐term patient safety consequences of PCI and CCA‐AKI is incomplete, in part, because of reliance on administrative data to establish renal outcomes, inconsistent use of rigorous clinical definitions of CKD progression, and a general lack of proper controls.3, 14, 15 However, other studies have used serum creatinine to define baseline estimated glomerular filtration rate (eGFR) and CCA‐AKI.16, 17, 18 Prior studies have examined the relationship between AKI, CKD, and death in VA populations; however, none have incorporated rigorous laboratory definitions of CKD progression or incorporated the use of matched population controls.19, 20
Given the low sensitivity of administrative capture of CKD progression using International Classification of Diseases, 9th Revision (ICD‐9) codes,21 we sought to add to advance the field of AKI and CKD progression by evaluating renal outcomes following PCI using laboratory data and rigorous clinical definitions of CKD development and progression in a large national cohort of consecutive PCI patients and matched population controls using integrated VA databases containing longitudinal clinical and laboratory data.22 The addition of disease‐matched controls who did not undergo cardiac catheterization provides a reference group for comparison with cohorts characterized by both PCI complicated by CCA‐AKI and uncomplicated PCI (ie, without CCA‐AKI development). We hypothesized that CCA‐AKI would be associated with CKD development and progression, increased dialysis‐dependent renal failure, acute myocardial infarction (AMI), and increased all‐cause mortality relative to PCI patients without associated AKI, as well as population controls.
Methods
Cohort
All consecutive patients undergoing PCI between 2005 and 2010 in the VA Health Care System were reported to the VA Clinical Assessment, Reporting, and Tracking System for Cardiac Catheterization Laboratories (CART‐CL) program (N=35 627) with follow‐up through 2011. Only the first PCI procedure was included in the analysis, resulting in 35 410 index procedures. Covariates were defined at presentation for PCI. Patients receiving dialysis or with a history of renal replacement therapy or a prior hospitalization for renal failure (578) were excluded. Patients with missing serum creatinine data were also excluded (10 427), leaving a final PCI cohort of 24 405 patients (Figure 1). VA and Dartmouth institutional review boards approved the study and waived all patient consent requirements.
Figure 1.
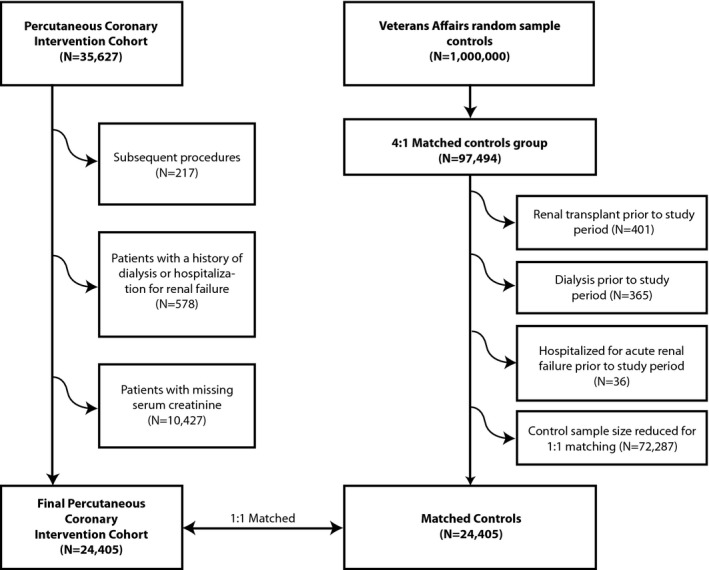
Study diagram. Generation of the percutaneous coronary intervention (PCI) and control cohorts. VA indicates Veterans Affairs.
PCI data were linked to the Corporate Data Warehouse (CDW) to determine preexisting renal disease and dialysis treatment, as well as postdischarge clinical end points (dialysis‐dependent renal failure, hospitalization with a principal diagnosis of renal failure, and AMI). The Austin Information Technology Center database was also linked to provide corresponding serum creatinine laboratory results from the period extending from 1 year prior to the procedure through the end of the study period. The vital status record was similarly linked to determine associated all‐cause mortality.
Population controls were abstracted from the CDW using the methods reported for the PCI cohort above. One million controls were randomly selected from CDW from 2005 through 2011. Covariates were defined at the start of the cohort period. A 4:1 matched control group consisting of VA patients not exposed to cardiac catheterization were matched to the PCI cohort using age category, diabetes mellitus, congestive heart failure, hypertension, and CKD stage. Control patients were excluded if they received a renal transplant (401), underwent dialysis (365), or were hospitalized for renal failure (36) prior to the study period, leaving 96 660 matched controls. Since not all matched covariates were balanced, we conducted a second round of matching using Coarsened Exact Matching using a 1:1 ratio resulting in perfect matches for age category, diabetes mellitus, congestive heart failure, hypertension, and CKD stage, leaving 24 405 matched controls. The date of the renal function measure in the population control patient was not matched to the PCI patient's procedure date.
CCA‐Acute Kidney Injury
CCA‐AKI was defined as an absolute ≥0.5 mg/dL or relative ≥25% increase from the last serum creatinine prior to PCI to the highest serum creatinine or new onset of dialysis up to 7 days post‐PCI. Due to the latency period of serum creatinine elevation of 3 to 5 days post‐insult, the window for ascertainment of CCA‐AKI was extended to 7 days.23, 24, 25, 26 This definition was requested by CART‐CL at the time of the data use agreement. Results were repeated using the Kidney Disease Outcomes Quality Initiative (KDOQI) definitions for AKI staging.
Kidney Outcomes
CKD progression was defined as incremental progression to a higher National Kidney Foundation KDOQI CKD stage (in mL/min per 1.73 m2: eGFR ≥60; stage 3: 30–59; stage 4: 15–29; stage 5: <15)22 as determined by 2 independent eGFR measures at least 90 days apart, or by progression to dialysis initiation or hospitalization for renal failure.22 However, albumin creatinine ratio (ACR) was not used as it was not routinely available in patients during the study period. Since we could not measure ACR we could not define CKD stages 1 to 2 and we could not further stratify CKD stages 3 to 5 by ACR criteria.27 The second eGFR date was uniformly employed as the event date for CKD progression. Progression of CKD in the first 90 days following PCI could only be defined by dialysis or hospitalization for renal failure. For example, patients with a baseline eGFR >60 mL/min per 1.73 m2 progressing with 2 independent eGFR measures at least 90 days apart <60 mL/min per 1.73 m2 or dialysis or hospitalization for renal failure would qualify for CKD progression, or patients with a baseline eGFR 30 to 59 mL/min per 1.73 m2 progressing to 2 independent eGFR measures <30 mL/min per 1.73 m2 or dialysis or hospitalization for renal failure would qualify for CKD progression, and so on.
Incident (or new) CKD was defined as progression from normal kidney function (eGFR ≥60 mL/min per 1.73 m2) to stage 3 or greater (eGFR <60 mL/min per 1.73 m2), or dialysis initiation or hospitalization for renal failure. For example, patients with a baseline eGFR ≥60 mL/min per 1.73 m2 progressing with 2 independent eGFR measures at least 90 days apart to <60 mL/min per 1.73 m2 or dialysis or hospitalization for renal failure would qualify for incident CKD progression.
Disease progression in patients with prevalent CKD was defined as an incremental increase from CKD stage 3 or 4 to a higher CKD stage. For example, patients with a baseline eGFR 30 to 59 mL/min per 1.73 m2 progressing to 2 independent eGFR measures <30 mL/min per 1.73 m2 or dialysis or hospitalization for renal failure would qualify for CKD progression, and patients with a baseline eGFR 15 to 29 mL/min per 1.73 m2 progressing to 2 independent eGFR measures <15 mL/min per 1.73 m2 or dialysis or hospitalization for renal failure would qualify for CKD progression.
Dialysis initiation was defined by documented receipt of either inpatient or outpatient renal replacement therapy.
We repeated our measure of CKD progression by conducting sensitivity analyses by defining progression only using eGFR laboratory values and thereby not allowing dialysis or hospitalization for renal failure to qualify CKD progression alone.
Cardiovascular Events and Mortality
Time to dialysis was defined from the date of PCI to the date of the first use of any dialysis. AMI was defined by hospitalization discharge diagnoses. Time to death was defined from the date of PCI to the date of death recorded in the vital status record. Censoring was determined through 2011 for PCI patients and controls.
Covariates
All covariates were recorded from the CART‐CL registry for PCI patients and from ICD‐9 codes from population controls. Tables 1 and 2 list the covariates captured for both PCI patients and population controls. Table 2 lists the perioperative procedural covariates collected on PCI patients alone.
Table 1.
Baseline Patient and Disease Characteristics
| Variables | Control | PCI | P Valuea |
|---|---|---|---|
| No. of patients (N=48 810) | 24 405 | 24 405 | |
| Age, y | |||
| <50 | 4.41 | 4.41 | 1.000 |
| 50 to 59 | 25.51 | 25.51 | |
| 60 to 69 | 42.35 | 42.35 | |
| 70 to 79 | 19.21 | 19.21 | |
| 80+ | 8.52 | 8.52 | |
| Female | 3.44 | 1.66 | <0.001 |
| White | 32.99 | 80.96 | <0.001 |
| Black | 8.70 | 12.40 | |
| American Indian | 0.12 | 1.02 | |
| Asian | 0.22 | 0.42 | |
| Unknown | 57.97 | 5.20 | |
| Current smoker | 16.65 | 19.15 | <0.001 |
| Comorbidities | |||
| Diabetes mellitus | 28.96 | 28.96 | 1.000 |
| Congestive heart failure | 17.95 | 17.95 | 1.000 |
| Hypertension | 79.83 | 79.83 | 1.000 |
| Posttraumatic stress disorder | 7.33 | 11.42 | <0.001 |
| Depression | 15.28 | 23.54 | <0.001 |
| Sleep apnea | 5.54 | 5.27 | 0.186 |
| Dyslipidemia | 59.17 | 77.91 | <0.001 |
| Cerebral vascular disease | 5.46 | 14.74 | <0.001 |
| Chronic obstructive pulmonary disease | 14.58 | 13.63 | 0.002 |
| Deep vein thrombosis | 1.97 | 1.31 | <0.001 |
| Myocardial infarction | 3.66 | 25.79 | <0.001 |
| Stroke or transient ischemic accident | 3.22 | 6.25 | <0.001 |
| Baseline renal function | |||
| CKD stage | |||
| eGFR ≥60 mL/min per 1.73 m2 | 75.93 | 75.93 | 1.000 |
| eGFR 30 to 59 mL/min per 1.73 m2 | 21.79 | 21.79 | |
| eGFR 15 to 29 mL/min per 1.73 m2 | 1.36 | 1.36 | |
| eGFR <15 mL/min per 1.73 m2 | 0.92 | 0.92 | |
| eGFR mL/min per 1.73 m2, median | 71.21 | 75.39 | <0.001 |
| Prior procedures | |||
| Stress test | 4.77 | 50.20 | <0.001 |
| Coronary artery bypass graft surgery | 4.74 | 22.57 | <0.001 |
| Valve surgery | 0.98 | 0.98 | 0.927 |
| Cardiac catheterization | 0.00 | 44.7 | <0.001 |
| Percutaneous coronary intervention | 0.00 | 23.24 | <0.001 |
Data are presented as percentages unless otherwise indicated. CKD indicates chronic kidney disease; eGFR, estimated glomerular filtration rate.
P value, compares population controls to all percutaneous coronary intervention (PCI) patients.
Table 2.
PCI Procedural Characteristics and Complications
| Variables | Uncomplicated PCI | PCI With CCA‐AKI | P Valuea |
|---|---|---|---|
| Indications for PCI procedure | |||
| STEMI | 7.01 | 11.84 | <0.001 |
| Restenosis | 1.29 | 0.91 | |
| Preoperative revascularization | 0.97 | 0.65 | |
| Asymptomatic ischemia | 1.42 | 0.91 | |
| Stable angina | 22.99 | 12.06 | |
| Unstable angina | 19.79 | 18.85 | |
| NSTEMI | 14.55 | 23.57 | |
| Cardiogenic shock | 0.18 | 1.31 | |
| Other | 6.86 | 6.43 | |
| Missing | 24.94 | 23.47 | |
| Status of PCI procedure | |||
| Elective | 58.62 | 38.45 | <0.001 |
| Urgent | 31.72 | 42.08 | |
| Emergent | 9.51 | 19.12 | |
| Salvage | 0.15 | 0.36 | |
| Procedure | |||
| Days from cardiac catheterization | 413.7 (374.5) | 380.7 (400.5) | 0.054 |
| Prior cardiac catheterization | 45.21 | 41.85 | <0.001 |
| Inpatient cardiac catheterization | 58.56 | 38.54 | <0.001 |
| Inpatient PCI | 55.55 | 75.99 | <0.001 |
| Cath/PCI same visit | 78.20 | 77.04 | <0.001 |
| Native lesion | 1.23 (0.82) | 1.24 (0.89) | 0.368 |
| Graft lesion | 0.10 (0.37) | 0.13 (0.42) | 0.0001 |
| Total lesion length (total lesion length, add together all lesions) | 2.5 (1.6) | 2.6 (1.8) | 0.021 |
| Lesion count (total number of lesions intervened upon) | 1.33 (0.76) | 1.38 (0.82) | 0.004 |
| Lesion length, mm (median) | 16.41 (9.72) | 16.73 (9.38) | 0.107 |
| Previously treated lesion | 0.08 (0.31) | 0.07 (0.29) | 0.045 |
| In‐stent restenosis | 0.06 (0.27) | 0.05 (0.23) | 0.001 |
| Chronic total occlusion | 0.06 (0.25) | 0.05 (0.24) | 0.079 |
| Thrombus | 0.08 (0.29) | 0.13 (0.38) | <0.001 |
| Acute closure | 0.005 (0.076) | 0.008 (0.091) | 0.028 |
The Table reports procedural complications among the percutaneous coronary intervention (PCI) cohort alone (not for population controls). Data are presented as percentage or mean (SD). CCA‐AKI indicates cardiac catheterization–associated acute kidney injury; NSTEMI, non–ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction.
P value, compares no CCA‐AKI to CCA‐AKI patients.
Statistical Analysis
Standard statistical methods were used to compare baseline univariate associations across all 3 groups (PCI complicated by CCA‐AKI, uncomplicated PCI, and controls) using chi‐square tests and ANOVA. We conducted Kaplan–Meier time‐to‐event analysis with a log‐rank test and Cox's proportional hazards modeling for any CKD progression, incident CKD development, progression in patients with prevalent CKD, dialysis initiation, AMI, and all‐cause mortality. To examine the association between CCA‐AKI–complicated and –uncomplicated PCI and major outcomes relative to controls, indicator variables for uncomplicated PCI and CCA‐AKI with the population control group as the referent category were included in the regression models including the listed covariates for adjustment. To account for potential confounding, all variables from Table 1 were evaluated as risk factors for adjusting the relationships between controls, uncomplicated PCI, and PCI with CCA‐AKI development, with CKD progression and other clinical end points. Covariates for adjustment were identified through the development of forward and backward stepwise logistic regression models for CCA‐AKI. These covariates were then used in all multivariate Cox's proportional hazard models used to estimate hazard ratios (HR) after adjusting for age, race, current smoker, diabetes mellitus, congestive heart failure, dyslipidemia on presentation, cerebrovascular disease, posttraumatic stress disorder, depression, cerebrovascular disease, history of stroke, chronic obstructive pulmonary disease, prior myocardial infarction, and baseline eGFR (mL/min per 1.73 m2). For comparison between PCI patients alone (uncomplicated PCI and PCI with CCA‐AKI) all risk factors in Table 2 were evaluated as risk factors for adjusting the PCI only analyses including adjusting for age, race, current smoker, diabetes mellitus, congestive heart failure, dyslipidemia on presentation, cerebrovascular disease, posttraumatic stress disorder, depression, cerebrovascular disease, history of stroke, chronic obstructive pulmonary disease, prior myocardial infarction, and baseline eGFR (mL/min per 1.73 m2), New York Heart Association class, indication of PCI procedure, status of PCI procedure (elective, urgent, emergent, salvage), and total number of diseased lesions. An alpha error of 0.05 was used to determine statistical significance. We repeated the CKD progression analysis using the Kidney Disease: Improving Global Outcomes (KDIGO) AKI staging criteria. Stata 11 (StataCorp, College Station, TX) statistical software was used for all analyses.
Results
Cohorts
A total of 48 810 patients (24 405 PCI and 24 405 matched controls) were evaluated (Figure 1). Controls were well matched to PCI patients for age, diabetes mellitus, hypertension, heart failure, and baseline CKD stage. Controls were less likely to have other chronic comorbidities and differed by several comorbidities not included in the a priori matching algorithm (Table 1).
CCA‐Acute Kidney Injury
PCI patients were stratified by those developing CCA‐AKI and those with uncomplicated PCI. Notably, CCA‐AKI developed in 13% of the PCI cohort (N=2763). Consistent with other reports, PCI patients developing CCA‐AKI were more likely to have urgent or emergent indications for intervention, exhibited greater temporal proximity to the diagnostic cardiac catheterization, and had angiographic evidence of more severe coronary disease, including the total number and length of disease lesions (Table 2). The median length of follow‐up for CCA‐AKI patients, uncomplicated PCI, and controls was 1.69 (person‐years), 1.86, and 6.0, respectively.
CKD Development or Progression
Overall incidence rates of CKD development or progression for CCA‐AKI, uncomplicated PCI (ie, without CCA‐AKI development), and matched controls were 28.66 (per 100 person‐years), 11.15, and 6.81, respectively (Figure 2, Table 3). PCI patients—both with and without CCA‐AKI—exhibited a 3‐ to 8‐fold higher likelihood of CKD progression over 5 years in adjusted analyses (HRCCA‐AKI, 5.02; 95% CI, 4.68–5.39 [P<0.001]; HRPCI, 1.76; 95% CI, 1.70–1.86 [P<0.001]; Table 3). CCA‐AKI increased CKD progression risk by ≈2‐ to 3‐fold in PCI patients over 5 years in adjusted analyses (HRCCA‐AKI, 2.51; 95% CI, 2.34–2.69 [P<0.001]). CCA‐AKI similarly increased the risk of CKD development in PCI patients with prior normal kidney function (Table 3, Figures 3 and 4).
Figure 2.
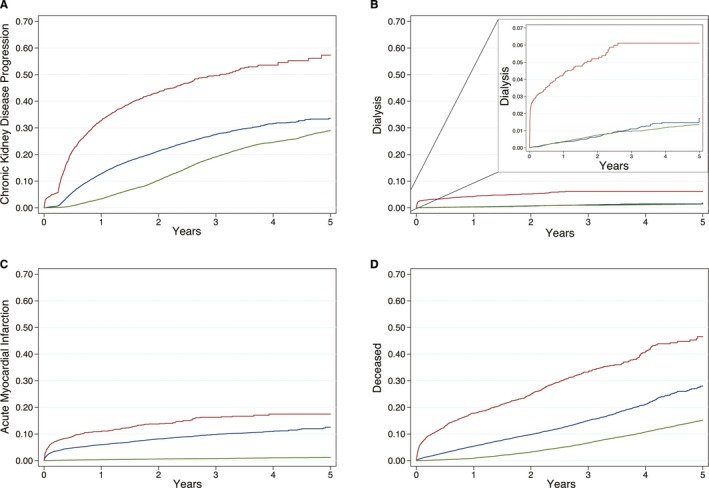
Kaplan–Meier time to event plots for study end points. Kaplan–Meier curves are plotted from the time of percutaneous coronary intervention (or the beginning of the study period for controls) to each study end point event and stratified by 3 groups: percutaneous coronary intervention complicated by cardiac catheterization–associated acute kidney injury (red), uncomplicated percutaneous coronary intervention (ie, without cardiac catheterization–associated acute kidney injury; blue), and matched controls (green). Plot A depicts the proportion of patients progressing to either new chronic kidney disease or a higher chronic kidney disease stage. Plot B depicts the proportion of patients undergoing a dialysis procedure with the internal plot y‐axis rescaled to 0 to 0.07. Plot C depicts the proportion of patients developing a recurrent postprocedural acute myocardial infarction. Plot D depicts the proportion of all‐cause patient deaths.
Table 3.
Multivariable HRs and Incidence Rates of CKD, Dialysis, Readmission, AMI, and Death
| Control | Uncomplicated PCI | PCI With CCA‐AKI | |
|---|---|---|---|
| Time to any CKD progression | |||
| No. of patients | 24 405 | 21 652 | 2753 |
| No. of CKD progression cases, No. (%) | 6761 (27.70) | 4130 (19.07) | 1025 (37.23) |
| Person‐years of follow‐up | 100 002.42 | 37 031.22 | 3520.93 |
| Incidence rate of CKD progression/100 person‐years of follow‐up | 6.81 | 11.15 | 28.66 |
| Adjusted HRa (control as referent) | 1.76 (1.70–1.86) | 5.02 (4.68–5.39) | |
| Adjusted HR (uncomplicated PCI as referent) | 2.51 (2.34–2.69) | ||
| Time to incident CKD (among patients with no CKD at time of catheterization/PCI eGFR ≥60) | |||
| No. of patients | 18 530 | 16 649 | 1880 |
| No. of new CKD cases, No. (%) | 5854 (31.59) | 3148 (18.91) | 643 (34.18) |
| Person‐years of follow‐up | 75 121.97 | 28 742.67 | 2669.10 |
| Incidence rate of new CKD/100 person‐years of follow‐up | 7.79 | 10.95 | 24.05 |
| Adjusted HR (control as referent) | 1.78 (1.69–1.88) | 4.99 (4.56–5.47) | |
| Adjusted HR (uncomplicated PCI as referent) | 3.38 (3.09–3.69) | ||
| Time to progressive CKD 4+ (among patients with eGFR <60 at time of catheterization/PCI) | |||
| No. of patients with CKD stage 3 and 4 | 5319 | 4682 | 637 |
| No. of new CKD cases, No. (%) | 812 (15.27) | 897 (19.16) | 266 (41.76) |
| Person‐years of follow‐up | 22 730.11 | 7814.69 | 681.94 |
| Incidence rate of new CKD/100 person‐years of follow‐up | 3.57 | 11.48 | 38.42 |
| Adjusted HR (control as referent) | 2.50 (2.23–2.80) | 8.03 (6.88–9.38) | |
| Adjusted HR (uncomplicated PCI as referent) | 2.96 (2.56–3.41) | ||
| Time to progressive CKD 5 (among patients with eGFR <30 at time of catheterization/PCI) | |||
| No. of patients with CKD stage 4 | 332 | 204 | 128 |
| No. of new CKD cases, No. (%) | 90 (27.11) | 71 (34.80) | 73 (57.03) |
| Person‐years of follow‐up | 1319.53 | 254.64 | 79.38 |
| Incidence rate of new CKD/100 person‐years of follow‐up | 6.82 | 27.88 | 84.40 |
| Adjusted HR (control as referent) | 3.13 (1.99–4.95) | 8.85 (5.41–14.45) | |
| Adjusted HR (uncomplicated PCI as referent) | 2.60 (1.79–3.79) | ||
| Time to first dialysis | |||
| No. of patients | 24 405 | 21 652 | 2753 |
| No. of new dialysis cases, No. (%) | 322 (1.32) | 155 (0.72) | 146 (5.30) |
| Person‐years of follow‐up | 114 341.44 | 43 444.24 | 4990.11 |
| Incidence rate of new dialysis/100 person‐years of follow‐up | 0.28 | 0.36 | 2.65 |
| Adjusted HR (control as referent) | 2.15 (1.72–2.68) | 7.83 (6.24–9.83) | |
| Adjusted HR (uncomplicated PCI as referent) | 4.24 (3.29–5.47) | ||
| Time to first AMI | |||
| No. of patients | 24 405 | 21 652 | 2753 |
| No. of new AMI cases, No. (%) | 267 (1.09) | 3526 (16.28) | 703 (25.54) |
| Person‐years of follow‐up | 114 379.69 | 37 049.65 | 3912.15 |
| Incidence rate of new AMI/100 person‐years of follow‐up | 0.23 | 4.12 | 7.57 |
| Adjusted HR (control as referent) | 12.43 (10.76–14.35) | 21.39 (17.92–25.53) | |
| Adjusted HR (uncomplicated PCI as referent) | 1.41 (1.24–1.60) | ||
| Time to death | |||
| No. of patients | 24 405 | 21 652 | 2753 |
| No. of deaths, No. (%) | 3710 (15.20) | 2444 (11.29) | 771 (28.01) |
| Person‐years of follow‐up | 114 975.79 | 43 594.02 | 5162.94 |
| Incidence rate of death/100 person‐years of follow‐up | 3.23 | 5.61 | 14.93 |
| Adjusted HR (control as referent) | 2.69 (2.52–2.86) | 6.67 (6.12–7.27) | |
| Adjusted HR (uncomplicated PCI as referent) | 2.07 (1.90–2.25) | ||
The Table reports on the incidence rates and adjusted hazard ratios (HRs) for chronic kidney disease (CKD) progression and cardiovascular disease longitudinal end points using time‐to‐event statistics and multivariate Cox's proportional hazard modeling. AMI indicates acute myocardial infarction; CCA‐AKI, cardiac catheterization–associated acute kidney injury; eGFR, estimated glomerular filtration rate.
HR: adjusted HR from Cox's proportional hazards model adjusting for age, race, current smoker, diabetes mellitus, congestive heart failure, dyslipidemia on presentation, cerebrovascular disease, posttraumatic stress disorder, depression, cerebrovascular disease, history of stroke, chronic obstructive pulmonary disease, prior myocardial infarction, and baseline estimated glomerular filtration rate (eGFR; mL/min per 1.73 m2), New York Heart Association class, indication of percutaneous coronary intervention (PCI) procedure, status of PCI procedure (elective, urgent, emergent, salvage), and total number of diseased lesions.
Figure 3.
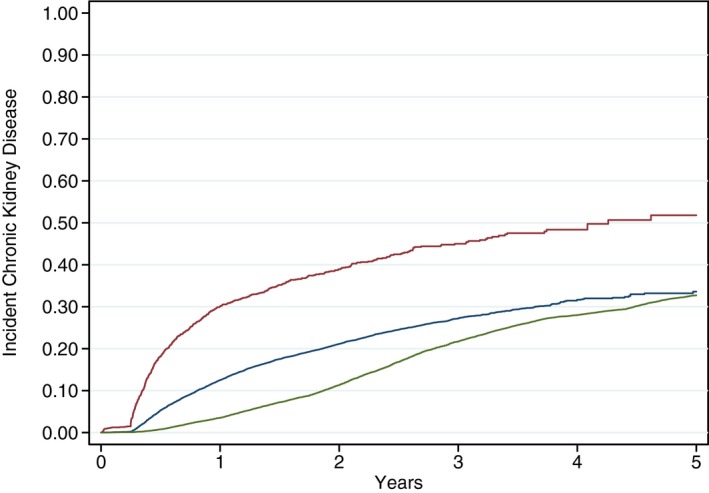
Kaplan–Meier time to event plots for incident chronic kidney disease (CKD) (laboratory values and dialysis). Kaplan–Meier curves are plotted from the time of percutaneous coronary intervention (or the beginning of the study period for controls) to incident CKD events defined by laboratory values and dialysis and stratified by 3 groups: percutaneous coronary intervention complicated by cardiac catheterization–associated acute kidney injury (red), uncomplicated percutaneous coronary intervention (ie, without cardiac catheterization–associated acute kidney injury; blue), and matched controls (green).
Figure 4.
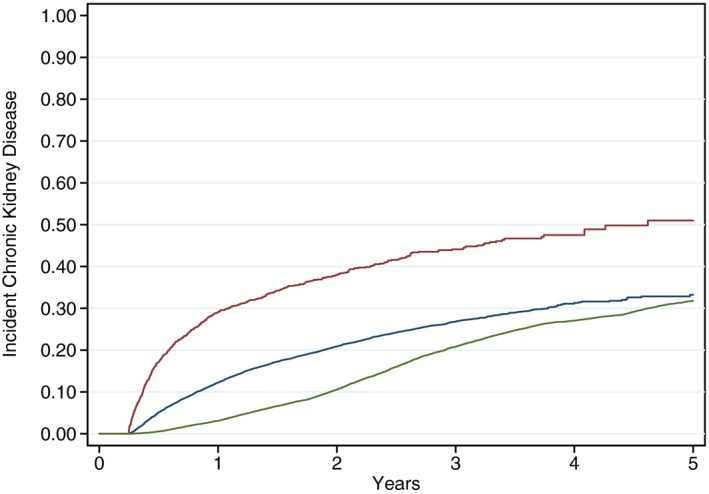
Kaplan–Meier time to event plots for incident chronic kidney disease (CKD) (laboratory values only). Kaplan–Meier curves are plotted from the time of percutaneous coronary intervention (or the beginning of the study period for controls) to incident CKD events defined by laboratory values only and stratified by 3 groups: percutaneous coronary intervention complicated by cardiac catheterization–associated acute kidney injury (red), uncomplicated percutaneous coronary intervention (ie, without cardiac catheterization–associated acute kidney injury; blue), and matched controls (green).
Dialysis
Incidence rates for dialysis initiation following PCI complicated by CCA‐AKI development, following uncomplicated PCI (ie, without CCA‐AKI development), and in matched controls were 2.65 (per 100 person‐years), 0.36, and 0.28, respectively (Figure 2B, Table 3). Relative to controls, PCI complicated by CCA‐AKI increased the likelihood of dialysis over 5 years nearly 8‐fold (HRCCA‐AKI, 7.83; 95% CI, 6.24, 9.83; P<0.001), whereas PCI uncomplicated by overt CCA‐AKI increased this risk over 2‐fold (HRPCI, 2.15; 95% CI, 1.72–2.68; P<0.001). Correspondingly, CCA‐AKI development independently increased the 5‐year risk of dialysis initiation over 4‐fold (HRCCA‐AKI, 4.24; 95% CI, 3.29–5.47; P<0.001).
Acute Myocardial Infarction
AMI incidence rates following PCI complicated by CCA‐AKI development, following uncomplicated PCI, and in matched controls were 7.57 (per 100 person‐years), 4.12, and 0.23, respectively (Figure 2C, Table 3). PCI patients with or without CCA‐AKI were 4 to 7 times more likely than controls to develop AMI over 5 years (HRCCA‐AKI, 21.39; 95% CI, 17.92–25.53 [P<0.001]; HRPCI, 12.43; 95% CI, 10.76–14.35 [P<0.001]). Among PCI patients, CCA‐AKI development also independently increased the 5‐year AMI risk by over 41% (HRCCA‐AKI, 1.41; 95% CI, 1.24–1.60; P<0.001). Risk increased dramatically immediately following PCI, after which it asymptotically plateaued (Figure 2C).
Mortality
The rates of all‐cause death associated with CCA‐AKI development, with uncomplicated PCI, and in controls were 14.93 (per 100 person‐years), 5.61, and 3.23, respectively (Figure 2D, Table 3). PCI patients with or without CCA‐AKI were 3 to 7 times more likely to die over 5 years relative to controls (HRCCA‐AKI, 6.67; 95% CI, 6.12–7.27 [P<0.001]; HRPCI, 2.69; 95% CI, 2.52–2.86 [P<0.001]). CCA‐AKI more than doubled this risk in PCI patients (HRCCA‐AKI, 2.07; 95% CI, 1.90–2.25; P<0.001).
KDIGO AKI Staging
Similar results were observed with KDIGO AKI definition and staging of AKI, whereby adjusted KDIGO AKI stage 1 had an HR of 2.08 (95% CI, 1.91–2.26), stage 2 had an HR of 3.24 (95% CI, 2.50–4.20), and stage 3 had an HR of 2.88 (95% CI, 2.42–3.41) compared with population controls (Figure 5).
Figure 5.
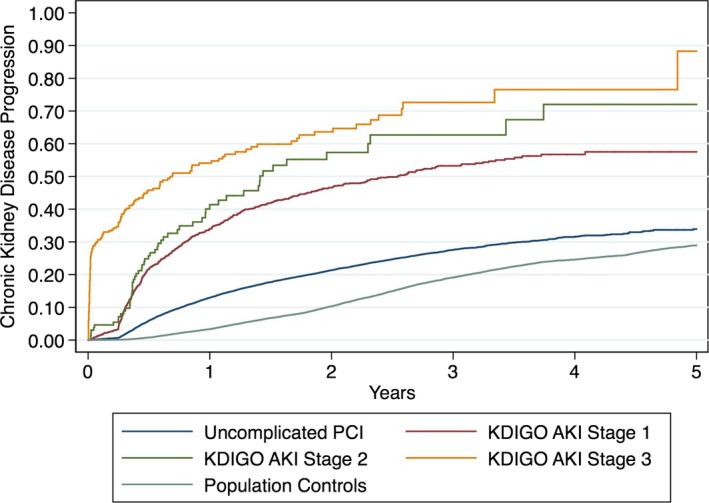
Kaplan–Meier time to event plots for chronic kidney disease (CKD) progression by Kidney Disease: Improving Global Outcomes (KDIGO) acute kidney injury (AKI) stage. Kaplan–Meier curves are plotted from the time of percutaneous coronary intervention (PCI; or the beginning of the study period for controls) to either new chronic kidney disease or a higher chronic kidney disease stage. The plot is stratified by 5 groups: PCI complicated by KDIGO AKI stage 1 (red), KDIGO AKI stage 2 (orange), KDIGO AKI stage 3 (yellow), uncomplicated PCI (ie, without cardiac catheterization–associated acute kidney injury; blue), and matched population controls (green).
Discussion
Summary
We provide new evidence of CKD development and progression following PCI in a national cohort of patients with matched population controls. Overall, we demonstrate that patients developing CCA‐AKI after PCI are 5 times more likely to develop CKD progression than matched controls. In established CKD, CCA‐AKI development is associated with an even greater (8‐fold) likelihood of progression. PCI associated with CCA‐AKI also increased the risk of CKD progression by 2‐ to 3‐fold relative to uncomplicated PCI. CCA‐AKI also markedly increased the risk of dialysis initiation.
CKD Progression in the Literature
Recent studies have examined the relationship between AKI and CKD in VA populations. Chawla et al constructed a prediction model for stage 4 CKD; however, this study only included patients hospitalized for AKI defined by ICD‐9 codes.19 Similarly, Thakar and colleagues examined the relationship between AKI and stage 4 CKD, measuring an HR of 3.56 (95% CI, 2.76–4.61).20 However, the population used for the Thakar study included only diabetic patients in the VA and did not evaluate the relationship between AKI and CKD progression among patients undergoing PCI exposed to contrast dye.20 Neither study used matched population controls.
Unlike previous retrospective investigations, we used laboratory eGFR‐based rules for identifying CKD development and progression instead of discharge billing diagnoses for hospitalized patients or for dialysis initiation. We utilized an objective and clinically rigorous metric of CKD progression—namely 2 independent eGFR values obtained at least 90 days apart—allowing for identification of CKD development or progression based primarily on laboratory, rather than administrative, data. Inclusion of a control group matched for major demographic variables and comorbidities also facilitates direct comparisons and strengthens our conclusions. Previous studies have reported an association between CCA‐AKI and subsequent declines in kidney function. A retrospective study of 14 782 Canadian patients undergoing cardiac angiography found that associated AKI increased ESRD risk by 4‐ to 12‐fold and increased risk of hospitalization for renal failure by 2‐ to 5‐fold.3 In a similar cohort of 3986 Italian patients 19% of patients developing AKI had persistent renal dysfunction at 3 months, compared with only 0.9% of patients without associated AKI.4 Hospitalized veterans discharged with AKI (identified using hospital billing data) have also been shown to be at increased risk of adverse events including death, dialysis, and worsening renal function (defined as a 25% decrease in eGFR).1 Our research significantly advances our understanding of CKD progression in several ways. First, we report on CCA‐AKI development and CKD progression in a large population cohort in the United States capturing 100% of all PCI procedures performed across 70 VA medical centers nationwide between 2005 and 2010. Second, we included matched population controls from the underlying population. Finally, we used laboratory values to define CKD progression instead of administrative billing codes.21
Our study shows that CCA‐AKI development following PCI increases the risk for CKD progression, dialysis initiation, AMI, and death compared with patients without CCA‐AKI. We also show that these risks are increased in PCI patients, even in the absence of overt CCA‐AKI. We recently reported that CCA‐AKI can be prevented in 1 of 5 PCI patients through improvement efforts,28, 29 yet the current evidence base has not yet established whether CCA‐AKI prevention can, in fact, prevent CKD development or progression. As such, the precise roles of aggressive prophylactic efforts in CCA‐AKI prevention and mitigation of CKD development and progression remain to be established.
Pathophysiology of CCA‐AKI and CKD Progression
There are a number of potential explanations for CKD development and progression following CCA‐AKI. Renal damage can result from either direct toxicity of the contrast medium or from renal hemodynamic changes associated with its use.30, 31, 32 Increased complement activation and a combination of both unopposed secondary vasoconstriction and tubular obstructive changes that serve to increase epithelial contrast exposure have also been implicated.33, 34, 35 Although initially considered fully reversible, recent evidence has suggested that these events may promote progression in the longer term.1, 6, 36 Interestingly, in the Coronary Artery Bypass Surgery Off‐ or On‐Pump Revascularization Study (CORONARY),2 reduced AKI risk within 30 days of coronary artery bypass surgery performed off‐pump was not associated with better preservation of kidney function at 1 year.2 As such, in addition to the causal explanations above, it is possible that CCA‐AKI identifies patients at high risk for progression. We attempt to address this in our analyses through matching and multivariable adjustment, but residual and unmeasured confounding remain possible.
PCI was associated with accelerated CKD progression relative to matched population controls, even in the absence of overt CCA‐AKI. This does not, however, exclude a causal role for contrast administration. In principle, subclinical contrast‐associated injury below conventional detection thresholds could directly contribute to both CKD progression and other adverse outcomes. Although speculative, associated hemodynamic changes and atheroembolization have also been implicated as causal contributors to CCA‐AKI.37, 38, 39 It is also possible that cardiac catheterization selects for vasculopathic patients prone to CKD progression independent of intervention.40, 41 Despite matching and multivariable analyses, residual or unmeasured confounding also remains possible.
Limitations
Several potential limitations warrant consideration. This was a retrospective study and the associations we found between AKI and progression of CKD and other longitudinal events may have been biased as a result of missing data in relevant confounders.42 However, we captured patient comorbidities, laboratory values, cardiac anatomy and disease severity, and posthospitalization events related to organ injury and organ failure from comprehensive databases within the VA to minimize measurement bias. We also did not prospectively or directly measure GFR nor did we determine whether all patients used VA services for all laboratory test or dialysis services. Nonetheless, we believe the use of 2 independent creatinine‐based eGFR calculations obtained at least 90 days apart represents a robust and rigorous method of monitoring CKD development and progression. By definition, CKD progression determined by this method cannot be assessed prior to 3 months. As such, the first phase of the multiphasic curve in Figure 2A can be fully accounted for by acute hospitalizations for kidney failure or initiation of dialysis. Neither of these end points fully excludes the inclusion of cases of severe reversible AKI, although these numbers are expected to be low and the exclusion of nonlaboratory CKD end point data did not fundamentally change either the shapes or relationships between curves (Figures 3 and 4). We also recognize that death may function as a competing risk for CKD progression; however, Figures 3 and 4 suggest that the progression of CKD appears to be driven by the change in renal function and not primarily by clinical end points. In addition, our principal aim was to identify incremental changes in CKD stage, and we did not focus on eGFR as a continuous variable either within or across individual CKD stages. As such, our analysis may underestimate the full impact on CKD progression. We also did not match individual patients in the population cohort to individual PCI patients to a renal function measure at the same time of the PCI patient's procedure. Progression of CKD in the controls was between 2005 and 2010, whereas progression of CKD in the PCI procedure started at the time of the PCI procedure. Second, we did not take albuminuria into account in classifying CKD,27 as this information was not routinely available for analysis. The associated inability to distinguish normal kidney function from CKD stages 1 and 2 for patients with eGFR ≥60 mL/min per 1.73 m2 thus limited analysis of CKD development and progression to creatinine‐based eGFR groupings alone. Third, we randomly selected over 1 million patients from the general veteran population. From this sample, we used a matching algorithm to match for age, diabetes mellitus, hypertension, heart failure, and baseline CKD stage. Patients were well matched for each of these comorbidities or conditions, although additional comorbidities not included in the a priori cardiovascular matching algorithm were found to differ in the PCI cohort compared with controls (Table 1). These unanticipated differences notwithstanding, both PCI patients and controls did not differ according to the 5 most important factors selected for a priori matching. Multivariable analyses were conducted to account for remaining differences in matched population controls and in patients both with and without CCA‐AKI. We did not link our VA cohorts to Medicare or State‐all payer claims as our data use agreement did not allow for these linkages to determine whether all veterans in our PCI and population cohort were routine users of the VA health system. Therefore, we may underestimate the true incidence of our longitudinal end points including hospitalizations, procedures such as dialysis, and laboratory measures. Future investigations should track healthcare services by participants in the VA and outside the VA. We lost a portion of our PCI cohort because of missing serum creatinine laboratory measures from CART‐CL and Austin; the selection bias in missing renal function through these data sources is not fully understood. Lastly, the generalizability of our findings are limited for several reasons: (1) the study population consisted of VA patients only, which can underrepresent women and minorities; (2) the definition of AKI that we used does not match the more contemporary KDIGO definition; and (3) our AKI definition includes more than just contrast nephropathy. In AKI definition sensitivity analyses, we obtained similar results with the KDIGO AKI definition in the association between AKI and CKD progression and other end points.
Conclusions
Our results suggest that CCA‐AKI developing after PCI is associated with significantly increased risk of CKD progression, AMI, and all‐cause death when compared with uncomplicated PCI and matched population controls. However, it is likely that this increased risk of AMI is related to underlying differences in risk between the PCI group and the controls. Consistent with previous reports, these risks were most marked in the subset of patients developing clinically evident CCA‐AKI. CCA‐AKI was also associated with a markedly increased risk of dialysis initiation following AKI development. Our findings suggest a need to identify and better understand these factors and their contributions, as well as the need to review the risk calculus associated with these procedures. We recommend further investigations to identify both system improvements and novel therapeutic targets to prevent CCA‐AKI and to reduce CKD progression and associated morbidity and mortality following PCI.
Sources of Funding
Dr Brown received funding from the Agency for Healthcare Research and Quality (K01 HS018443) and Veterans Affairs Health Services Research and Development Investigator Initiated Research (11‐292) for this research. Additional funding was provided for Dr Sarnak from the National Institute of Diabetes and Digestive and Kidney Diseases (DK078204). Dr Maddox was supported by a Veterans Affairs Health Services Research and Development Career Development Award. Dr Robey has received support from the Veterans Affairs Office of Rural Health Specialty Care Access Network‐Extension for Community Healthcare Outcomes Program and the Veterans Affairs Cooperative Studies Program Million Veteran Program. Dr Matheny was supported by a Veterans Affairs Health Services Research and Development Career Development Award (CDA‐08‐020) and Veterans Affairs Health Services Research and Development Investigator Initiated Research 11‐292.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003812 doi: 10.1161/JAHA.116.003812)
References
- 1. Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between AKI and long‐term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014;9:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garg AX, Devereaux PJ, Yusuf S, Cuerden MS, Parikh CR, Coca SG, Walsh M, Novick R, Cook RJ, Jain AR, Pan X, Noiseux N, Vik K, Stolf NA, Ritchie A, Favaloro RR, Parvathaneni S, Whitlock RP, Ou Y, Lawrence M, Lamy A; Investigators C . Kidney function after off‐pump or on‐pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA. 2014;311:2191–2198. [DOI] [PubMed] [Google Scholar]
- 3. James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, Pannu N, Manns BJ, Klarenbach SW, Hemmelgarn BR. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123:409–416. [DOI] [PubMed] [Google Scholar]
- 4. Maioli M, Toso A, Leoncini M, Gallopin M, Musilli N, Bellandi F. Persistent renal damage after contrast‐induced acute kidney injury: incidence, evolution, risk factors, and prognosis. Circulation. 2012;125:3099–3107. [DOI] [PubMed] [Google Scholar]
- 5. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta‐analysis. Kidney Int. 2012;81:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown JR, Malenka DJ, DeVries JT, Robb JF, Jayne JE, Friedman BJ, Hettleman BD, Niles NW, Kaplan AV, Schoolwerth AC, Thompson CA. Transient and persistent renal dysfunction are predictors of survival after percutaneous coronary intervention: insights from the Dartmouth Dynamic Registry. Catheter Cardiovasc Interv. 2008;72:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCullough P. Outcomes of contrast‐induced nephropathy: experience in patients undergoing cardiovascular intervention. Catheter Cardiovasc Interv. 2006;67:335–343. [DOI] [PubMed] [Google Scholar]
- 8. Brown JR, Cochran RP, Dacey LJ, Ross CS, Kunzelman KS, Dunton RF, Braxton JH, Charlesworth DC, Clough RA, Helm RE, Leavitt BJ, Mackenzie TA, O'Connor GT; Northern New England Cardiovascular Disease Study G . Perioperative increases in serum creatinine are predictive of increased 90‐day mortality after coronary artery bypass graft surgery. Circulation. 2006;114:I409–I413. [DOI] [PubMed] [Google Scholar]
- 9. Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coca SG, Cho KC, Hsu CY. Acute kidney injury in the elderly: predisposition to chronic kidney disease and vice versa. Nephron Clin Pract. 2011;119(suppl 1):c19–c24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsu CY. Yes, AKI truly leads to CKD. J Am Soc Nephrol. 2012;23:967–969. [DOI] [PubMed] [Google Scholar]
- 12. Ishani A, Nelson D, Clothier B, Schult T, Nugent S, Greer N, Slinin Y, Ensrud KE. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171:226–233. [DOI] [PubMed] [Google Scholar]
- 13. National Quality Forum . Safe Practices for Better Healthcare 2006 Update: A Consensus Report. Washington, DC: National Quality Forum; 2007. [Google Scholar]
- 14. Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vuurmans T, Byrne J, Fretz E, Janssen C, Hilton JD, Klinke WP, Djurdjev O, Levin A. Chronic kidney injury in patients after cardiac catheterisation or percutaneous coronary intervention: a comparison of radial and femoral approaches (from the British Columbia Cardiac and Renal Registries). Heart. 2010;96:1538–1542. [DOI] [PubMed] [Google Scholar]
- 16. Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009;76:1089–1097. [DOI] [PubMed] [Google Scholar]
- 17. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Hsu CY. Dialysis‐requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6:2567–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kern EF, Maney M, Miller DR, Tseng CL, Tiwari A, Rajan M, Aron D, Pogach L. Failure of ICD‐9‐CM codes to identify patients with comorbid chronic kidney disease in diabetes. Health Serv Res. 2006;41:564–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Kidney F . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 23. Maioli M, Toso A, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, Bellandi F. Sodium bicarbonate versus saline for the prevention of contrast‐induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol. 2008;52:599–604. [DOI] [PubMed] [Google Scholar]
- 24. Maioli M, Toso A, Leoncini M, Micheletti C, Bellandi F. Effects of hydration in contrast‐induced acute kidney injury after primary angioplasty: a randomized, controlled trial. Circ Cardiovasc Interv. 2011;4:456–462. [DOI] [PubMed] [Google Scholar]
- 25. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 26. Recio‐Mayoral A, Chaparro M, Prado B, Cozar R, Mendez I, Banerjee D, Kaski JC, Cubero J, Cruz JM. The reno‐protective effect of hydration with sodium bicarbonate plus N‐acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the RENO Study. J Am Coll Cardiol. 2007;49:1283–1288. [DOI] [PubMed] [Google Scholar]
- 27. Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M . Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. [DOI] [PubMed] [Google Scholar]
- 28. Brown JR, McCullough PA, Splaine ME, Davies L, Ross CS, Dauerman HL, Robb JF, Boss R, Goldberg DJ, Fedele FA, Kellett MA, Phillips WJ, Ver Lee PN, Nelson EC, Mackenzie TA, O'Connor GT, Sarnak MJ, Malenka DJ. How do centres begin the process to prevent contrast‐induced acute kidney injury: a report from a new regional collaborative. BMJ Qual Saf. 2012;21:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown JR, Solomon RJ, Sarnak MJ, McCullough PA, Splaine ME, Davies L, Ross CS, Dauerman HL, Stender JL, Conley SM, Robb JF, Chaisson K, Boss R, Lambert P, Goldberg DJ, Lucier D, Fedele FA, Kellett MA, Horton S, Phillips WJ, Downs C, Wiseman A, MacKenzie TA, Malenka DJ; for the Northern New England Cardiovascular Disease Study G . Reducing contrast‐induced acute kidney injury using a regional multicenter quality improvement intervention. Circ Cardiovasc Qual Outcomes. 2014;7:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bui KL, Horner JD, Herts BR, Einstein DM. Intravenous iodinated contrast agents: risks and problematic situations. Cleve Clin J Med. 2007;74:361–364, 367. [DOI] [PubMed] [Google Scholar]
- 31. Tumlin J, Stacul F, Adam A, Becker CR, Davidson C, Lameire N, McCullough PA. Pathophysiology of contrast‐induced nephropathy. Am J Cardiol. 2006;98:14K–20K. [DOI] [PubMed] [Google Scholar]
- 32. Brown JR, McCullough PA. Contrast nephropathy and kidney injury In: Thompson CA, ed. Textbook of Cardiovascular Intervention. London: Springer‐Verlag; 2014:53–63. [Google Scholar]
- 33. Gruberg L, Mintz GS, Mehran R, Gangas G, Lansky AJ, Kent KM, Pichard AD, Satler LF, Leon MB. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre‐existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542–1548. [DOI] [PubMed] [Google Scholar]
- 34. Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium‐induced nephropathy. Kidney Int. 2005;68:14–22. [DOI] [PubMed] [Google Scholar]
- 35. Tepel M, Aspelin P, Lameire N. Contrast‐induced nephropathy: a clinical and evidence‐based approach. Circulation. 2006;113:1799–1806. [DOI] [PubMed] [Google Scholar]
- 36. Goldenberg I, Chonchol M, Guetta V. Reversible acute kidney injury following contrast exposure and the risk of long‐term mortality. Am J Nephrol. 2009;29:136–144. [DOI] [PubMed] [Google Scholar]
- 37. Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, Lansky AJ, Moussa I, Stone GW, Moses JW, Leon MB, Mehran R. Contrast‐induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13–19. [DOI] [PubMed] [Google Scholar]
- 38. de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens. 2009;27:1333–1340. [DOI] [PubMed] [Google Scholar]
- 39. Fukumoto Y, Tsutsui H, Tsuchihashi M, Masumoto A, Takeshita A; Cholesterol Embolism Study I . The incidence and risk factors of cholesterol embolization syndrome, a complication of cardiac catheterization: a prospective study. J Am Coll Cardiol. 2003;42:211–216. [DOI] [PubMed] [Google Scholar]
- 40. Saklayen MG, Gupta S, Suryaprasad A, Azmeh W. Incidence of atheroembolic renal failure after coronary angiography. A prospective study. Angiology. 1997;48:609–613. [DOI] [PubMed] [Google Scholar]
- 41. Scolari F, Ravani P, Gaggi R, Santostefano M, Rollino C, Stabellini N, Colla L, Viola BF, Maiorca P, Venturelli C, Bonardelli S, Faggiano P, Barrett BJ. The challenge of diagnosing atheroembolic renal disease: clinical features and prognostic factors. Circulation. 2007;116:298–304.17606842 [Google Scholar]
- 42. Coca SG. Is it AKI or nonrecovery of renal function that is important for long‐term outcomes? Clin J Am Soc Nephrol. 2013;8:173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]


