Abstract
Background
Recent randomized data suggest that calcium supplements may be associated with increased risk of cardiovascular disease (CVD) events. Using a longitudinal cohort study, we assessed the association between calcium intake, from both foods and supplements, and atherosclerosis, as measured by coronary artery calcification (CAC).
Methods and Results
We studied 5448 adults free of clinically diagnosed CVD (52% female; aged 45–84 years) from the Multi‐Ethnic Study of Atherosclerosis. Baseline total calcium intake was assessed from diet (using a food frequency questionnaire) and calcium supplements (by a medication inventory) and categorized into quintiles. Baseline CAC was measured by computed tomography, and CAC measurements were repeated in 2742 participants ≈10 years later. At baseline, mean calcium intakes across quintiles were 313.3, 540.3, 783.0, 1168.9, and 2157.4 mg/day. Women had higher calcium intakes than men. After adjustment for potential confounders, among 1567 participants without baseline CAC, the relative risk (RR) of developing incident CAC over 10 years, by quintile 1 to 5 of calcium intake, were 1 (reference), 0.95 (0.79–1.14), 1.02 (0.85–1.23), 0.86 (0.69–1.05), and 0.73 (0.57–0.93). After accounting for total calcium intake, calcium supplement use was associated with increased risk for incident CAC (RR=1.22 [1.07–1.39]). No relation was found between baseline calcium intake and 10‐year changes in log‐transformed CAC among those participants with baseline CAC >0.
Conclusions
High total calcium intake was associated with a decreased risk of incident atherosclerosis over long‐term follow‐up, particularly if achieved without supplement use. However, calcium supplement use may increase the risk for incident CAC.
Keywords: calcium, cardiovascular imaging, coronary artery calcium, diet, epidemiology
Subject Categories: Diet and Nutrition, Atherosclerosis
Excessive dietary calcium intake, particularly from overconsumption of calcium supplements taken to prevent or treat osteoporosis, may have unintended health consequences. The well‐known “milk”‐alkali syndrome1 has been increasing in incidence attributed to the widespread use of over‐the‐counter calcium supplements.2 Supplements contribute to calcium loading (ie, excessive calcium amounts in a single dose or bolus), which leads to an increase in urinary calcium excretion in adults with normal renal function, with or without hypercalcemia, and possibly to soft tissue or ectopic calcification.3 Gallagher et al recently found that 9% of women taking calcium supplements had evidence of hypercalcemia and 31% had hypercalcuria.4
A direct relationship between total calcium intake (diet plus supplements) and cardiovascular disease (CVD), however, has not been established, and this issue remains controversial.5, 6, 7, 8, 9, 10, 11, 12, 13 Recent evidence derived from randomized, controlled trials, including the Women's Health Initiative, have raised a concern for an association between calcium supplement use and increased risk for CVD events.12, 13, 14 Among calcium supplement users, a high intake of calcium greater than 1400 mg/day has been reported to be associated with higher death rates from all causes, including from CVD.15
The purported CVD risk associated with total calcium intake may depend on the source of calcium intake.3 Intake of calcium from food sources has not been shown to increase CVD risk, whereas a signal for increased risk of myocardial infarction (MI) among calcium supplement users has been reported.7 In a similar fashion, dietary calcium intake may decrease risk of kidney stones, whereas calcium supplementation may increase risk.16 One explanation for this apparent paradox may be that large boluses of calcium intake through supplements may transiently elevate serum calcium concentrations,17, 18 which, in turn, may lead to vascular calcification and other adverse health effects.
One potential mechanism underlying the association between calcium intake and CVD risk may be through progression of atherosclerosis. The coronary artery calcium (CAC) score is a well‐established surrogate marker for burden of atherosclerosis and is prognostic for CVD risk.19 A few published reports have not demonstrated any association between calcium intake and a single evaluation of CAC.5, 20 However, little is known about the association of calcium intake with incident CAC or CAC progression, particularly in a population‐based cohort, and whether any associations with CAC differ by source of calcium intake (diet vs supplements).
In a multiethnic cohort of men and women, we hypothesized that no associations would be found between dietary calcium intake and CAC progression over 10 years of follow‐up. We also hypothesized that calcium supplement use would be associated with increased CAC progression attributed to unfavorable calcium balance.
Methods
Study Design
The Multi‐Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study investigating risk factors and progression of subclinical CVD, whose study design has previously been reported on.21 Briefly, the MESA baseline information was collected between 2000 and 2002 from 6814 individuals (52% women), aged ≥45 to 84, of 4 race/ethnicities (non‐Hispanic white, non‐Hispanic black, Hispanic, and Chinese), who were enrolled at 6 US field centers: Baltimore City and County, Maryland, Chicago, Illinois, Forsyth County, North Carolina, New York City, New York, Los Angeles County, California, and St. Paul, Minnesota. The study was conducted under the guidelines of the Declaration of Helsinki and approved by the institutional review boards at each site. Written informed consent of all participants was obtained.
Participants
Of the 6814 participants enrolled at baseline, participants were excluded from this analysis if complete information on dietary intake (n=283) was not available. Those with implausibly high calcium intakes (>5000 mg/day) were excluded (n=347). Participants with abnormal renal function, that is, estimated glomerular filtration rate (eGFR) values of 60 mL/min or less, were also excluded (n=622) given that impaired renal function could influence calcium metabolism. In addition, participants with daily energy intakes <600 or >6000 kcal/day were excluded (n=114). This left 5448 participants available for cross‐sectional analysis at the baseline exam (2000–2002).
There were 3305 subjects who participated in the MESA Air ancillary study and were eligible for a second computed tomography (CT) scan 10 years later. Of these participants, 2742 (83%) had complete covariate data and returned for a second follow‐up CAC scan at MESA Exam 5 (2010–2012) enabling them to be included in longitudinal analysis. Of these, 1567 were free of CAC at baseline and included in the incident CAC analysis, and 1175 had a baseline CAC >0 and were included in the change in CAC score analysis.
A flow diagram of participant inclusion/exclusion of our substudy is shown in Figure. Additionally, Table 1 compares the baseline characteristics of the overall MESA cohort (n=6814), the MESA Air participants with CAC measured at Exam 1 and Exam 5 (n=3305), and the sample used for longitudinal analyses in this article (n=2742).
Figure 1.
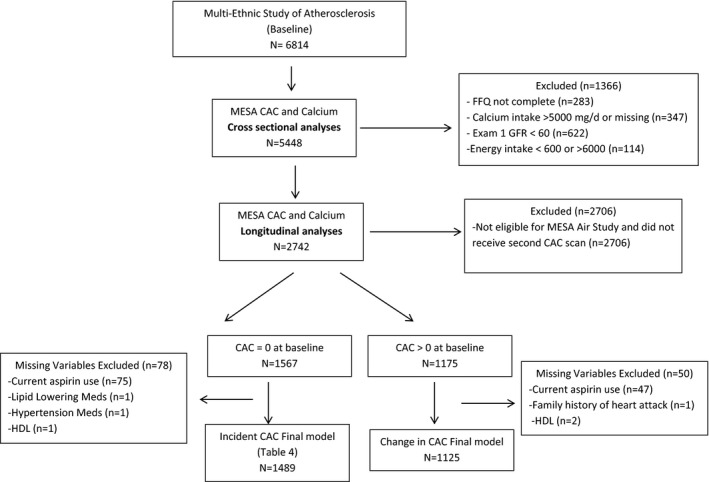
Flow diagram of study inclusion and exclusion criteria: the Multi‐Ethnic Study of Atherosclerosis (MESA; 2000–2012). CAC indicates coronary artery calcium; FFQ, food frequency questionnaire; GFR, estimated glomerular filtration rate; HDL, high density lipoprotein.
Table 1.
Clinical Characteristicsa at the MESA Baseline Exam (2000–2002) Comparing the Overall MESA Cohort, the MESA Air Ancillary Participants (With CAC Measured at Exam 1 and Exam 5), and the Sample Used for the Longitudinal Analyses of This Article
| MESA | MESA Air | Calcium and CAC Longitudinal | |
|---|---|---|---|
| N | 6814 | 3305 | 2742 |
| Total calcium intake, mg | 1150.8 | 1128.1 | 992.9 |
| Calcium supplement use, % | 42.1 | 43.2 | 45.8 |
| Age, y | 62.2 | 60.1 | 59.7 |
| Sex, male % | 47.2 | 47.5 | 49.0 |
| Race/ethnic groups, % | |||
| White | 38.5 | 39.4 | 40.5 |
| Black | 27.8 | 26.7 | 25.6 |
| Hispanic | 22.0 | 22.2 | 21.7 |
| Chinese | 11.8 | 11.7 | 12.3 |
| Education, % | |||
| ≤High school | 18.3 | 13.9 | 13.3 |
| Some college | 18.1 | 17.8 | 17.4 |
| ≥College | 63.6 | 68.3 | 69.3 |
| Gross family income <$50 000, % | 62.0 | 56.9 | 55.4 |
| Body mass index | 28.3 | 28.4 | 28.3 |
| Waist circumference, cm | 98.2 | 97.8 | 97.5 |
| Hip circumference, cm | 105.6 | 105.9 | 105.6 |
| Intentional physical activity, METs/week | 1552.8 | 1638.5 | 1661.0 |
| Smoking status | |||
| Never smoker, % | 50.3 | 51.4 | 51.5 |
| Former smoker, % | 36.6 | 36.8 | 36.6 |
| Current smoker, % | 13.1 | 11.9 | 11.9 |
| Pack/year of cigarette | 11.3 | 10.5 | 10.5 |
| Alcohol consumed, drinks/week | 4.0 | 3.9 | 4.1 |
| Systolic BP, mm Hg | 126.6 | 124.4 | 123.7 |
| Diastolic BP, mm Hg | 71.9 | 72.1 | 72.0 |
| Antihypertensive medication use, % | 34.9 | 32.4 | 30.9 |
| Hypertension, % | 45.0 | 41.2 | 39.4 |
| Cholesterol, mg/dL | |||
| Total cholesterol | 194.2 | 194.5 | 193.6 |
| HDL cholesterol | 51.0 | 50.7 | 50.5 |
| Lipid‐lowering medication, % | 16.2 | 16.0 | 15.2 |
| Diabetes mellitus, % | 12.6 | 10.1 | 9.5 |
| Diabetes medication, % | 9.8 | 8.1 | 7.3 |
| Family history of CHD, % | 42.8 | 43.8 | 42.9 |
| hs‐CRP, mg/dL | 3.8 | 3.5 | 3.5 |
| Homocysteine, mg/dL | 9.3 | 9.0 | 8.8 |
| Serum triglycerides, mg/dL | 131.6 | 130.9 | 129.6 |
| eGFR, mL/min | 81.2 | 82.0 | 83.9 |
| Framingham risk score | 14.5 | 13.0 | 12.7 |
| ASA use, % | 19.9 | 20.3 | 19.8 |
| Baseline CAC score >0 | 49.9 | 44.0 | 42.9 |
| Baseline CAC score | 146.1 | 100.2 | 98.6 |
ASA indicates acetylsalicylic acid; BP indicates blood pressure; CAC, coronary artery calcium; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; MESA, the Multi‐Ethnic Study of Atherosclerosis; MET, metabolic equivalent task.
Data are means or %.
Dietary Assessment
At the MESA baseline exam, participants’ usual dietary intake over the previous year was assessed by a modified, validated 120‐item quantitative food frequency questionnaire (FFQ).22, 23 The MESA diet questionnaire for the current population, with its designed sampling of varied ethnic groups (independent of validation in general cohort studies), was validated.24 Consumption frequency and serving size of each food or beverage were recorded. Using the Block FFQ design,22 serving sizes were quantified as small, medium, or large, with corresponding weights (g) imputed according to National Health and Nutrition Examination Survey (NHANES) data. Nutrients were calculated for each FFQ line item according to a weighted recipe using the Nutrition Data System for Research (NDS‐R database; Nutrition Coordinating Center, Minneapolis, MN). Complete information about the MESA diet data is available at https://www.mesa-nhlbi.org.
Use of calcium supplements by participants at the baseline exam was estimated using a medication inventory approach in which participants brought in all medication containers used in the past 2 weeks to be assessed and recorded.25, 26 Total daily calcium intake for each participant was determined by adding the intake from daily supplements and total daily calcium intakes. Total daily calcium intake was categorized into quintiles based on overall population distribution as follows: Q1: <434.9, Q2: 434.9 to 650.7, Q3: 650.7 to 936.5, Q4: 936.5 to 1453.5, Q5: ≥1453.5 mg.
Blood and Other Measurements
Demographic characteristics, smoking status, physical activity, medical history, and medication use (including aspirin, diabetes mellitus medications, antihypertensive medications, and lipid‐lowering medications) were collected through standardized questionnaires at the MESA baseline exam. Level of education was defined as <high school, some college, or college/graduate/professional school. Physical activity was estimated as the total amount of intentional exercise performed in a usual week and measured in metabolic equivalent task (MET)–minutes per week. Smoking status was categorized into never, former, or current smoker.
Physical examination variables (height, weight, blood pressure [BP], etc) were assessed by trained staff using standard MESA procedures.21 Body mass index (BMI) was calculated as weight (kg)/height (m2). After a 5‐minute rest, BP was measured 3 times in the seated position using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, FL) with the average of the last 2 measurements used as the measure of BP.
Before the exam, participants were instructed to fast for 12 hours and refrain from smoking or strenuous exercise. Blood samples were drawn and stored at −80°C. Blood lipid variables were measured by standard chemical methods. C‐reactive protein (CRP) was measured by a high‐sensitivity assay (N High‐Sensitivity C‐reactive protein; Dade Behring, Deerfield, IL). Homocysteine was measured by fluorescence polarization immunoassay with an IMx Analyzer (IMx Homocysteine Assay; Axis Biochemicals ASA, Oslo, Norway).
Diabetes mellitus was classified as having a fasting blood glucose ≥126 mg/dL and/or the self‐reported history of a physician diagnosis of diabetes mellitus, or the use of diabetes mellitus medications. Hypertension was diagnosed as systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or use of antihypertensive medications. eGFR was calculated based on the Modification of Diet in Renal Disease (MDRD) equation.27
CAC Assessment
At the MESA baseline exam, CAC was measured by electron‐beam computed tomography at 3 field centers and by multidetector computed tomography at the other 3 field centers,28, 29 and these scans were read independently at a centralized reading center. The methodology for acquisition and interpretation of the scans has been documented previously.30 Amount of CAC was quantified using the Agatston scoring method.31, 32 Interobserver agreement and intraobserver agreement were found to be very high (κ=0.93 and 0.90, respectively). Validation of phantom‐adjusted CAC measurement was made to adjust for attenuation differences.31, 32 At Exam 5, participants of the MESA Air ancillary study underwent repeat CT scanning, allowing for a 10‐year assessment of incident CAC and change in CAC.
At each visit (MESA baseline and Exam 5), each participant was scanned twice consecutively, and the average CAC from the 2 scans from that respective visit was used in the analysis.
Statistical Analyses
For our primary analyses, total calcium intake from diet and supplements was parameterized into quintiles to allow for examination of possible nonlinear relationships between calcium intake and CAC. Presence of CAC was defined as a detectable Agatston score of >0.
Baseline characteristics of study participants were described using means (SDs) and proportions stratified by the calcium intake quintile groups. Mean calcium intakes from total, dietary, or supplemental sources were tabulated by sex.
Relative risk regression using a generalized linear model and binomial error distribution was used to estimate prevalence ratios (PRs) and 95% CIs for the cross‐sectional association of total calcium intake with a CAC score >0 at the baseline exam.33, 34 Similar methods were used to assess the relationship of calcium intake with the relative risk (RR) and 95% CI for incident CAC at follow‐up, for those without baseline CAC (57.1%).
For participants with CAC >0 at the baseline exam (42.9%), we used linear regression methods to evaluate the cross‐sectional association of calcium intake with extent of CAC burden at baseline as well as changes in amount of CAC over 10 years of follow‐up. We also retested this relationship after log transformation of CAC score because of evidence from previous reports of the possibility of influential levels of skew.32
For both the cross‐sectional and prospective analyses, we considered 2 progressively adjusted models. Model 1 was adjusted for demographic and lifestyle factors, including age, sex, race/ethnicity, study site, BMI, exercise, smoking, pack‐years, alcohol use, education, income, health insurance, and total caloric energy intake. Model 2 was further adjusted for CVD risk markers, including systolic BP, diastolic BP, family history of heart disease, total cholesterol, high‐density lipoprotein (HDL)‐cholesterol, lipid‐lowering medication use, diabetes mellitus, eGFR, total homocysteine, current aspirin use, and calcium supplement use. Separate analyses were performed for women and men, as well as for all participants combined. Interaction terms for calcium intake with both sex and race/ethnicity were used to test for any possible effect‐measurement modification of the association by these characteristics.
Given that risk of atherosclerosis may differ by source of calcium intake (dietary vs supplementation), we examined the impact of calcium supplement use in several ways. First, we examined risk of incident CAC associated with calcium supplement use (vs nonuse) in our fully adjusted model (model 2) that was also adjusted for total daily calcium intake. Next, we examined risk for incident CAC for calcium supplement use in models adjusted for confounders, but not adjusted for total calcium intake. Finally, we created dummy variables for calcium supplement users and nonusers by total calcium intake quintiles and compared all groups to quintile 1 of calcium intake among nonsupplement users. We also checked for interaction of the calcium intake quintiles with calcium supplement use.
For all primary analyses, we used a complete case approach to missing data. However, a sensitivity analysis was performed using inverse probability of censoring weighting (IPCW) to account for incomplete follow‐up to estimate the impact of attrition of participants.33 Two‐sided P≤0.05 was considered statistically significant. Models were developed in SAS software (version 9.4; SAS Institute Inc., Cary, NC).
Results
MESA participant characteristics at the baseline exam by quintiles of total calcium intake are given in Table 2. Total calcium intake varied by key demographic characteristics, including sex, race/ethnicity, education, income, physical activity, current smoking, BP, cholesterol, diabetes mellitus, family history of heart disease, homocysteine, eGFR, Framingham Risk Score, and aspirin use. Use of calcium supplements was greater in the higher quintiles of total calcium intake. The distributions of total, dietary, and supplemental calcium intake by sex are listed in Table 3. Table 4 shows the breakdown of supplement use by quintile, separately for women and men. Women had a higher mean total calcium intake, which was driven by their higher use of calcium supplementation.
Table 2.
Baseline Characteristicsa of the Study Population (n=5448) by Quintiles of Total Daily Calcium Intake; Data From MESA 2000–2002; Calcium Intake Above 5000 mg Excluded
| Characteristics | Quintiles of Total Daily Calcium Intake | P Value | ||||
|---|---|---|---|---|---|---|
| Q1 (N=1052) | Q2 (N=1097) | Q3 (N=1105) | Q4 (N=1106) | Q5 (N=1088) | ||
| Total calcium intake, mg | 313.3 | 540.3 | 783.0 | 1168.9 | 2157.4 | |
| Calcium supplement use, % | 12.93 | 29.3 | 46.4 | 59.6 | 75.0 | <0.0001 |
| Age, yr | 61.6 | 61.5 | 61.2 | 61.0 | 62.0 | 0.54 |
| Sex, male % | 50.0 | 55.2 | 54.0 | 49.1 | 35.8 | <0.0001 |
| Race/ethnic groups, % | ||||||
| White | 27.8 | 34.2 | 38.3 | 44.2 | 48.5 | <0.0001 |
| Black | 37.0 | 32.1 | 25.6 | 20.1 | 18.5 | <0.0001 |
| Hispanic | 18.0 | 20.7 | 24.3 | 24.6 | 23.5 | 0.0005 |
| Chinese | 17.3 | 13.0 | 11.9 | 11.1 | 9.5 | <0.0001 |
| Education, % | ||||||
| ≤High school | 40.5 | 33.8 | 33.5 | 33.9 | 33.9 | 0.005 |
| Some college | 26.9 | 27.8 | 30.2 | 27.4 | 27.6 | 0.45 |
| ≥College | 32.4 | 38.3 | 36.2 | 38.6 | 38.3 | 0.01 |
| Gross family income <$50 000, % | 59.8 | 54.2 | 56.83 | 56.1 | 57.4 | 0.02 |
| BMI | 28.2 | 28.5 | 28.2 | 27.9 | 28.2 | 0.33 |
| Waist circumference, cm | 97.4 | 98.5 | 98.0 | 97.2 | 97.7 | 0.53 |
| Hip circumference, cm | 105.0 | 105.7 | 105.2 | 104.8 | 106.0 | 0.27 |
| Intentional physical activity, METs*minutes/week | 1341.8 | 1493.6 | 1704.1 | 1572.7 | 1747.6 | 0.0006 |
| Smoking status | ||||||
| Never smoker, % | 50.6 | 50.1 | 47.4 | 49.0 | 53.5 | 0.06 |
| Former smoker, % | 34.4 | 37.6 | 38.1 | 37.3 | 35.9 | 0.39 |
| Current smoker, % | 14.9 | 12.3 | 14.4 | 13.6 | 10.4 | 0.014 |
| Pack/year of cigarette | 11.3 | 11.2 | 11.9 | 10.9 | 10.7 | 0.55 |
| Alcohol consumed, drinks/week | 3.7 | 4.4 | 4.7 | 4.3 | 3.6 | 0.57 |
| Systolic BP, mm Hg | 127.5 | 126.1 | 125.8 | 124.0 | 124.6 | 0.0003 |
| Diastolic BP, mm Hg | 73.3 | 72.9 | 72.3 | 71.2 | 70.2 | <0.0001 |
| Antihypertensive medication use, % | 36.7 | 33.8 | 32.1 | 29.7 | 29.4 | 0.003 |
| Hypertension, % | 46.6 | 45.2 | 41.4 | 36.5 | 40.7 | <0.0001 |
| Cholesterol, mg/dL | ||||||
| Total cholesterol | 193.4 | 190.9 | 193.7 | 195.0 | 195.1 | 0.02 |
| HDL cholesterol | 50.0 | 50.1 | 49.6 | 51.1 | 53.6 | <0.0001 |
| Lipid‐lowering medication, % | 16.3 | 17.9 | 13.7 | 14.4 | 13.1 | 0.011 |
| Diabetes mellitus, % | 14.0 | 12.1 | 13.1 | 10.0 | 9.3 | 0.002 |
| Diabetes mellitus medication, % | 10.8 | 9.4 | 10.0 | 7.8 | 7.0 | 0.01 |
| Family history of CHD, % | 38.8 | 39.4 | 39.6 | 36.9 | 42.9 | 0.02 |
| hs‐CRP, mg/dL | 4.0 | 3.5 | 3.6 | 3.5 | 3.8 | 0.95 |
| Homocysteine, mg/dL | 9.5 | 9.2 | 9.0 | 8.7 | 8.6 | <0.0001 |
| Serum triglycerides, mg/dL | 128.0 | 123.7 | 136.4 | 130.5 | 131.6 | 0.09 |
| eGFR, mL/min | 85.0 | 84.9 | 84.7 | 83.7 | 82.5 | 0.0002 |
| Framingham risk score | 15.1 | 14.5 | 14.8 | 13.5 | 12.4 | <0.0001 |
| Aspirin use, % | 14.9 | 18.1 | 18.8 | 21.0 | 19.8 | 0.003 |
| Baseline CAC score >0 | 51.7 | 49.2 | 49.5 | 46.0 | 45.7 | 0.25 |
BMI indicates body mass index; BP, blood pressure; CAC, coronary artery calcium; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; MESA, the Multi‐Ethnic Study of Atherosclerosis; MET, metabolic equivalent task.
Data are means or %.
Table 3.
Comparison of Mean Level of Calcium, by Sex, Comparing Calcium From Diet, Calcium From Supplements and Total Calcium (mg); Calcium Intakes Above 5000 mg/day Excluded From Study
| Sex | Variable | No. of Subjects | Mean (mg) | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Women | Total calcium | 2788 | 1080.52 | 778.35 | 105.75 | 4927.47 |
| Dietary calcium | 2788 | 704.61 | 514.05 | 105.75 | 4368.42 | |
| Calcium from supplements | 2788 | 712.00 | 649.74 | 2.00 | 4200.00 | |
| Men | Total calcium | 2660 | 907.92 | 642.92 | 73.76 | 4780.57 |
| Dietary calcium | 2660 | 756.08 | 527.29 | 73.76 | 4780.57 | |
| Calcium from supplements | 2660 | 415.53 | 524.77 | 1.00 | 4200.00 |
Table 4.
Calcium Intake (Dietary and Supplemental) by Overall Total Calcium Intake Quintile
| Quintile of Calcium Intake | No. | % Supplement Users | Mean Dietary Calcium (mg/day) | Mean Supplementary Calcium (mg/day) | Mean Total Calcium (mg/day) |
|---|---|---|---|---|---|
| (A) Women | |||||
| Q1 | 526 | 14 | 300 [SD=83] | 94 [SD=60] | 314 [SD=83] |
| Q2 | 492 | 32 | 481 [SD=108] | 177 [SD=78] | 539 [SD=60] |
| Q3 | 508 | 49 | 636 [SD=193] | 299 [SD=187] | 784 [SD=81] |
| Q4 | 563 | 69 | 792 [SD=361] | 567 [SD=307] | 1186 [SD=147] |
| Q5 | 699 | 83 | 1146 [SD=739] | 1212 [SD=713] | 2169 [SD=692] |
| (B) Men | |||||
| Q1 | 526 | 11 | 302 [SD=78] | 97 [SD=63] | 312 [SD=78] |
| Q2 | 605 | 26 | 500 [SD=93] | 156 [SD=71] | 541 [SD=62] |
| Q3 | 597 | 43 | 693 [SD=139] | 204 [SD=125] | 782 [SD=83] |
| Q4 | 543 | 49 | 963 [SD=283] | 380 [SD=274] | 1151 [SD=144] |
| Q5 | 389 | 57 | 1574 [SD=788] | 966 [SD=804] | 2136 [SD=685] |
Table 5 shows the adjusted risk of prevalent CAC at the baseline exam. In cross‐sectional analysis adjusted for demographics and lifestyle factors (model 1), quintile 2 (PR=0.92 [95% CI, 0.85–1.00]) and quintile 4 (0.90 [0.83–0.99]) of calcium intake were statistically significantly associated with a lower prevalence of CAC >0 when compared to participants in quintile 1, although this association was attenuated with further adjustments of CVD risk factors. Calcium supplement use was not significantly associated with prevalent CAC (PR=0.96 [0.91–1.02]). For those with CAC >0 at baseline exam, there was no cross‐sectional association of calcium intake with extent of CAC burden (Table 6).
Table 5.
Adjusted Regression Models for the Risk of Prevalent CAC (Agatston Score >0) by Quintiles of Total Calcium Intake (mg) at the MESA Baseline Exam
| Calcium Intake Quintile at Baseline | Median Ca Intake | Prevalence Ratio | 95% CI | P Value |
|---|---|---|---|---|
| Model 1a | ||||
| Q1: <434.9 | 323.3 | 1 | Reference | — |
| Q2: 434.9–650.7 | 541.8 | 0.92 | 0.85–1.00 | 0.04 |
| Q3: 650.7–936.5 | 783.0 | 0.94 | 0.87–1.02 | 0.14 |
| Q4: 936.5–1453.5 | 1160.4 | 0.90 | 0.83–0.99 | 0.02 |
| Q5: ≥1453.5 | 1919.0 | 0.92 | 0.84–1.01 | 0.10 |
| Model 2b | ||||
| Q1: <434.9 | 323.3 | 1 | Reference | — |
| Q2: 434.9–650.7 | 541.8 | 0.94 | 0.87–1.02 | 0.12 |
| Q3: 650.7–936.5 | 783.0 | 0.96 | 0.88–1.05 | 0.40 |
| Q4: 936.5–1453.5 | 1160.4 | 0.93 | 0.85–1.02 | 0.13 |
| Q5: ≥1453.5 | 1919.0 | 0.98 | 0.88–1.09 | 0.72 |
BMI indicates body mass index; BP, blood pressure; Ca, calcium; CAC, coronary artery calcium; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; MESA, the Multi‐Ethnic Study of Atherosclerosis.
Model 1 adjusted for age, sex, race/ethnicity, site, BMI, exercise, smoking, pack‐years, alcohol, education, income, health insurance, and total dietary caloric intake.
Model 2: adjusted for model 1 variables+systolic BP, diastolic BP, family history of heart disease, total cholesterol, HDL‐cholesterol, lipid‐lowering medication use, diabetes mellitus, eGFR, total homocysteine, current aspirin use, and calcium supplement use.
Table 6.
Adjusted Regression Models Assessing Extent of CAC Burden in Participants With CAC >0 by Quintiles of Total Calcium Intake at the MESA Baseline Exam
| Calcium Intake Quintile | Difference in Agatston Unitsa | 95% CI Limits | P Value |
|---|---|---|---|
| Model 1b | |||
| Q1: <434.9 | 0 | Reference | — |
| Q2: 434.9–650.7 | −15.45 | −76.5 to 45.6 | 0.62 |
| Q3: 650.7–936.5 | −19.69 | −80.7 to 41.3 | 0.53 |
| Q4: 936.5–1453.5 | −31.78 | −94.3 to 30.7 | 0.32 |
| Q5: ≥1453.5 | −7.84 | −71.4 to 55.7 | 0.81 |
| Model 2c | |||
| Q1: <434.9 | 0 | Reference | — |
| Q2: 434.9–650.7 | −31.95 | −95.2 to 31.3 | 0.32 |
| Q3: 650.7–936.5 | −45.16 | −113.7 to 23.4 | 0.20 |
| Q4: 936.5–1453.5 | −59.33 | −133.3 to 14.6 | 0.12 |
| Q5: ≥1453.5 | −51.00 | −133.6 to 31.6 | 0.23 |
BMI indicates body mass index; BP, blood pressure; CAC, coronary artery calcium; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; MESA, the Multi‐Ethnic Study of Atherosclerosis.
Log transformed.
Model 1 adjusted for age, sex, race/ethnicity, site, BMI, exercise, smoking, pack‐years, alcohol, education, income, health insurance, and total caloric intake.
Model 2 adjusted for model 1 variables+systolic BP, diastolic BP, family history of heart disease, total cholesterol, HDL‐cholesterol, lipid‐lowering medication use, diabetes mellitus, eGFR, total homocysteine, current aspirin use, and calcium supplement use.
Table 7 shows the longitudinal associations between baseline calcium intake and incident CAC over 10‐year follow‐up, among those without baseline CAC. In the fully adjusted model, which included adjustment for calcium supplement use (model 2), the highest quintile of total calcium intake compared to the lowest was associated with decreased risk of incident CAC (RR=0.73 [0.57–0.93]).
Table 7.
Adjusted Estimates of Risk for Incident CAC (n=707 Instances of Incident CAC) by Total Calcium Intake Among the 1567 Participants With No Baseline CAC and No Missing Covariate Information
| Calcium Intake Quintile at Baseline | Relative Risk | 95% CI Limits | P Value |
|---|---|---|---|
| Model 1a | |||
| Q1: <434.9 | 1 | Reference | — |
| Q2: 434.9–650.7 | 0.96 | 0.80–1.16 | 0.69 |
| Q3: 650.7–936.5 | 1.13 | 0.95–1.34 | 0.17 |
| Q4: 936.5–1453.5 | 0.92 | 0.76–1.12 | 0.41 |
| Q5: ≥1453.5 | 0.83 | 0.67–1.03 | 0.09 |
| Model 2b | |||
| Q1: <434.9 | 1 | Reference | — |
| Q2: 434.9–650.7 | 0.95 | 0.79–1.14 | 0.59 |
| Q3: 650.7–936.5 | 1.02 | 0.85–1.23 | 0.84 |
| Q4: 936.5–1453.5 | 0.86 | 0.69–1.05 | 0.15 |
| Q5: ≥1453.5 | 0.73 | 0.57–0.93 | 0.01 |
Estimates are grouped by quintile of baseline calcium intake, indexed to the cross‐sectional cut points for ease of comparison. BMI indicates body mass index; BP, blood pressure; CAC, coronary artery calcium; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein.
Model 1 adjusted for age, sex, race/ethnicity, site, BMI, exercise, smoking, pack‐years, alcohol, education, income, health insurance, and total dietary caloric intake.
Model 2: adjusted for model 1 variables+systolic BP, diastolic BP, family history of heart disease, total cholesterol, HDL‐cholesterol, lipid‐lowering medication use, diabetes mellitus, eGFR, total homocysteine, current aspirin use, and calcium supplement use.
On the other hand, in this same fully adjusted model, also adjusted for total calcium intake, calcium supplement use was associated with a 22% increase in risk in incident CAC (RR=1.22 [1.07–1.39]). Given that this risk associated with calcium supplement use was conditioned on total calcium intake, we also explored a model of dietary calcium only and evaluated the association of supplement use with CAC without adjustment for total calcium intake (Table 8). In this particular model, calcium supplement use was associated with a barely significant, slight increase risk of incident CAC (RR=1.12 [1.00–1.26], P=0.047).
Table 8.
Adjusted Estimates for Incident CAC for Dietary Calcium Intake Only Among the 1567 Participants With No Baseline CAC and No Missing Covariate Information
| Dietary Calcium Intake Quintile at Baseline | Relative Risk | 95% CI Limits | P Value |
|---|---|---|---|
| Model 1a | |||
| Q1: <349.2 | 1 | Reference | — |
| Q2: 349.2–499.6 | 0.91 | 0.76–1.09 | 0.30 |
| Q3: 499.6–680.9 | 0.91 | 0.76–1.09 | 0.31 |
| Q4: 680.9–1022.0 | 1.00 | 0.83–1.21 | 1.00 |
| Q5: ≥1022.0 | 0.89 | 0.70–1.12 | 0.31 |
| Model 2b | |||
| Q1: <349.2 | 1 | Reference | — |
| Q2: 349.2–499.6 | 0.93 | 0.78–1.12 | 0.46 |
| Q3: 499.6–680.9 | 0.92 | 0.77–1.10 | 0.35 |
| Q4: 680.9–1022.0 | 1.03 | 0.85–1.24 | 0.75 |
| Q5: ≥1022.0 | 0.91 | 0.72–1.16 | 0.46 |
| Supplement use | 1.12 | 1.00–1.26 | 0.047 |
BMI indicates body mass index; BP, blood pressure; CAC, coronary artery calcium; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein.
Model 1 adjusted for age, sex, race/ethnicity, site, BMI, exercise, smoking, pack‐years, alcohol, education, income, health insurance, and total dietary caloric intake.
Model 2: adjusted for model 1 variables+systolic BP, diastolic BP, family history of heart disease, total cholesterol, HDL‐cholesterol, lipid‐lowering medication use, diabetes mellitus, eGFR, total homocysteine, current aspirin use, and calcium supplement use (yes/no).
Therefore, we further examined the association of quintiles of calcium intake with risk of incident CAC stratified by nonusers and users of calcium supplements, comparing nonsupplement users in quintile 1 as reference (Table 9). We found that there was a signal for increased risk of incident CAC among users of calcium supplements across the first 4 quintiles of calcium intake, with greatest risk noted among calcium supplement users with the lowest total calcium intake (quintile 1; RR, 1.41 [1.02–1.97]). For quintile 5, the previously noted inverse association of high calcium intake with incident CAC was attenuated among calcium supplement users (0.91 [0.72–1.15]) versus quintile 5 of intake among nonsupplement users (0.74 [0.51–1.07]). However, there was no statistically significant interaction of calcium supplement use with total calcium intake for quintiles 2 to 5 (P interaction, >0.05 for all).
Table 9.
Adjusteda Estimates Using Model 4 Adjustments for Dietary and Supplement Calcium Intake by Overall Calcium Quintile Among the 1567 Participants With No Baseline CAC and No Missing Covariate Information
| Quintile of Calcium Intake | N | Average Calcium Intake From Diet | Average Calcium Intake From Supplements | % Supplement Users | RR Calcium (No Sup) | RR Calcium (w/Sup) |
|---|---|---|---|---|---|---|
| Q1 | 521 |
306.0 (76.9) N=521 |
90.6 (60.5) N=70 |
13 | Reference (1) |
1.41 (1.02, 1.97) P=0.038 |
| Q2 | 544 |
491.9 (100.2) N=544 |
165.0 (70.9) N=162 |
30 |
0.96 (0.77, 1.19) P=0.71 |
1.22 (0.96, 1.56) P=0.10 |
| Q3 | 570 |
670.0 (170.7) N=570 |
248.6 (167.4) N=268 |
46 |
1.08 (0.87, 1.36) P=0.46 |
1.22 (0.99, 1.51) P=0.063 |
| Q4 | 573 |
870.8 (329.5) N=573 |
492.6 (329.5) N=346 |
60 |
0.90 (0.69, 1.17) P=0.43 |
1.06 (0.85, 1.31) P=0.60 |
| Q5 | 534 |
1280.5 (779.7) N=534 |
1123.3 (717.7) N=410 |
75 |
0.74 (0.51, 1.07) P=0.11 |
0.91 (0.72, 1.15) P=0.45 |
BMI indicates body mass index; BP, blood pressure; CAC, coronary artery calcium; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; Sup, supplement.
Adjusted for age, sex, race/ethnicity, site, BMI, exercise, smoking, pack‐years, alcohol, systolic BP, diastolic BP, family history of heart disease, total cholesterol, HDL‐cholesterol, lipid‐lowering medication use, diabetes mellitus medication, education, income, health insurance, family history of heart attacks, eGFR, total homocysteine, current aspirin use, and total caloric intake.
Among those with prevalent CAC at the baseline exam, calcium intake was not associated with an increase in CAC progression over an average of 10 years of follow‐up (Table 10). This lack of association persisted even when we considered calcium as a continuous variable and log‐transformed CAC (Δlog CAC=−0.0004 [−0.047 to 0.046]) per gram of calcium consumed per day.
Table 10.
Adjusted Change in CAC Over Follow‐up for the 1175 Participants With Baseline CAC >0, a Follow‐up CT Scan, and No Missing Covariate Information
| Calcium Intake Quintile | Change in Agatston unitsa | 95% CI Limits | P Value |
|---|---|---|---|
| Model 1b | |||
| Q1: <434.9 | 0 | Reference | — |
| Q2: 434.9–650.7 | +10.12 | −70.1 to 90.4 | 0.80 |
| Q3: 650.7–936.5 | −17.12 | −99.1 to 64.8 | 0.68 |
| Q4: 936.5–1453.5 | −58.66 | −145.7 to 28.4 | 0.19 |
| Q5: ≥1453.5 | −37.43 | −130.7 to 55.9 | 0.43 |
| Model 2c | |||
| Q1: <434.9 | 0 | Reference | — |
| Q2: 434.9–650.7 | +25.62 | −55.7 to 107.0 | 0.54 |
| Q3: 650.7–936.5 | −14.35 | −101.4 to 72.7 | 0.75 |
| Q4: 936.5–1453.5 | −32.88 | −127.1 to 61.3 | 0.49 |
| Q5: ≥1453.5 | −17.33 | −122.5 to 87.8 | 0.74 |
BMI indicates body mass index; BP, blood pressure; CAC, coronary artery calcium; CT, computed tomography; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein.
Log transformed.
Model 1 adjusted for age, sex, race/ethnicity, site, BMI, exercise, smoking, pack‐years, alcohol, education, income, health insurance, and total caloric intake.
Model 2 adjusted for model 1 variables+systolic BP, diastolic blood BP, family history of heart disease, total cholesterol, HDL‐cholesterol, lipid‐lowering medication use, diabetes mellitus, eGFR, total homocysteine, current aspirin use, and calcium supplement use.
We conducted a sensitivity analysis using IPCW to account for participant loss to follow‐up and compared these to the primary analysis. Using IPCW, estimates of RR of incident CAC among participants with baseline CAC=0: were Q2: 0.95 (0.78–1.16); Q3: 1.16 (0.97–1.40); Q4: 0.96 (0.79–1.18); and Q5: 0.85 (0.68–1.07). RRs did not vary from the adjusted complete case estimates, using the lowest intake group (Q1) as the reference.
Effect modification was tested by sex and race/ethnicity, and no significant interactions were found.
Discussion
In this large, multiethnic study of men and women without past history of clinical CVD, our results suggest a possible protective association against risk for incident CAC over a mean follow‐up of 10 years for those with the highest daily calcium intake, particularly among those who achieved this without calcium supplements. On the other hand, calcium supplement use, conditioned on total calcium intake, was actually associated with an increased risk of incident CAC.
Previous calcium balance studies suggest that healthy nongrowing adults require ≈550 to 1200 mg of dietary calcium per day to maintain zero balance.34 Other balance studies have shown that calcium intakes greater than 1400 mg/day result in positive calcium balance both in individuals with normal renal function as well as in patients with end‐stage renal disease.35, 36 Little of the additional calcium provided by calcium supplements, however, is incorporated in bone by adults,37, 38 but it may lead to a positive calcium balance and contribute to ectopic calcification.
Because of the widespread awareness and treatment of osteoporosis with calcium supplements among older adults, this population would appear to be at greater risk of developing the adverse consequences of positive calcium balance, including vascular calcification. Calcium supplements are used by 43% of US adults according to NHANES data.39 The current Institute of Medicine recommendations of calcium intake for adults 51 years and older in the United States and Canada are 1200 mg/day for women and 1000 mg/day for men,40 but a substantial percentage of adults are consuming total amounts of calcium in excess of 1200 mg/day.12, 15, 38 In our MESA sample, the overall mean calcium intake of participants was slightly less than US guidelines, with mean intakes of 1081 and 908 mg for women and men, respectively. However, among the highest quintile of calcium intake, mean intake was 2157 mg, nearly double the recommended daily allowance.
Calcium may be involved in pathogenesis of CVD through multiple pathways, including through influences in lipid metabolism, insulin secretion and sensitivity, inflammation, thrombosis, regulation of body weight, and vascular calcification.41 However, only a few past studies have investigated the relationship between dietary calcium and subclinical atherosclerosis, as assessed by CAC. A past study from the Framingham Study did not find any association of total dietary calcium with CAC measured on a single CT 4 years later,5 but they did not have a baseline measure of CAC to evaluate for change. An ancillary study of the Women's Health Initiative did not find that women randomized to calcium/vitamin D supplements had increased burden of CAC on a single CT obtained 7 years after treatment, but, again, there was no baseline CAC to assess for change.20 One study (n=144 women) did not find any significant progression of CAC among older women taking calcium supplements.42 However, that study did not factor calcium supplement use in the context of total calcium intake and was a small study of only women.
To our knowledge, this is the first study that evaluated total dietary intake of calcium with progression of CAC scores in a large multiethnic population of men and women. After full adjustment for demographics, lifestyle factors, CVD risk factors, and use of calcium supplements, we found that among participants with a baseline CAC of zero, the highest calcium intake (≥1453 mg) compared to the lowest intake (<434 mg) was associated with a 27% decreased risk for incident CAC, suggesting a protective effect of total calcium intake in the highest consumers of overall calcium. However, when considering supplement use, the risk of developing incident CAC was 22% higher in those who used supplements than those who did not. When stratified by supplement users versus nonusers, the highest risk for incident CAC was found among supplement users with the lowest intake of total calcium (Q1); conversely, the lowest risk of incident CAC was noted among nonsupplement users with the highest intake of total calcium (Q5). These results suggest that any protective association of calcium intake and incident CAC occurs in the participants with high dietary calcium intake (excluding supplemental calcium), which could be a proxy for overall healthier diets. Approximately 75% of participants in quintile 5 and 60% in quintile 4 were supplement users. Without clarifying the method of calcium intake, increasing total daily calcium intake through supplement use might be considered protective of heart disease.
We were prompted to conduct this analysis because several recent reports have suggested that an association exists between high calcium intakes in older adults (ie, calcium supplement loading), and an increase in the risk of CVD, including MI,12, 13, 14 but this is not without controversy.8, 10 Our findings add further support to previously published reports by suggesting that the relationship between calcium intake and CVD risk is complex and appears to depend on the source of calcium intake, with dietary calcium generally showing a protective effect, but calcium supplement use being associated with increased risk.
Rather than promoting bone health, excess calcium from the diet and supplements is postulated to accrue in vascular tissues. Pathological changes, presumably resulting from atheromas, initiate conversions of smooth muscle cells to bone‐forming cells or osteroblasts.43 Excessive calcium loading also has the potential to decrease parathyroid hormone (PTH) to suboptimal levels and thus increase the risk for adynamic or low bone turnover.44 To date, long‐term evidence that calcium loading from excessive dietary and supplement sources may accelerate pre‐existent arterial calcification has been lacking. CAC scoring is now recognized as a reliable biomarker of total atherosclerotic plaque burden and prognostic of risk for all‐cause mortality and coronary heart disease.19, 32 Although widely prevalent, CAC typically occurs in the absence of positive calcium balance. It is uncertain whether CAC that occurs in the setting of a positive calcium balance has the same association with CVD risk as CAC that occurs in the absence of a positive calcium balance.
Low calcium intake (ie, less than 800 mg/day) has also been suggested to be associated with increased CVD risk.45 This mechanism may be related to excess phosphorus intake because of a low dietary calcium‐to‐phosphorus ratio.46 Our results suggest that a wide range of calcium intakes between ≈400 and 1400 mg/day are not associated with CAC over a period of 10 years.
Low bone mineral density (BMD) has been linked to vascular calcification in past studies.47, 48, 49 Those results suggest that older women and, possibly, men may be transferring calcium ions from extracellular bone fluid compartments to vascular sites, even in the absence of calcium loading from supplements. This phenomenon may result from a chronically elevated serum PTH concentration because of calcium intakes that are too low relative to high dietary phosphorus, but this scenario has not been established. Under these conditions, calcium ions are thought to be shunted from bone to arteries and other soft tissue sites that have previously been signaled by phosphate ions to convert medial arterial cells to osteoblasts and subsequent bone formation.
When low BMD is identified in older osteoporotic patients, they are typically treated with additional calcium and vitamin D as supplements. Rather than increase skeletal mass, excessive calcium consumption may contribute to cardiovascular calcification, especially smooth muscle calcification.44, 45 A leading risk factor for stimulating arterial calcification that is supported by laboratory data is an elevation of serum phosphate ions that have been shown to induce calcification in animal models and cells.44, 45 Better understanding of the mechanisms of vascular calcification, which have not yet been established, may generate better insight into the long‐term development of this process.
A key strength of our study is the ability to evaluate the association of calcium intake, source of calcium intake, and CAC in a large, multiethnic sample of US men and women at both baseline and with repeat longitudinal estimates of incident CAC up to 10 years. However, our findings should be placed in the context of several limitations. First, we used an FFQ for the assessment of dietary calcium intake. Although the quantitative tool used in this assessment has been previously validated, the daily variability of dietary calcium intakes remains high and therefore an issue of potential measurement error. Calcium supplement intake recall may also be questionable despite use of a validated questionnaire.25 For example, a very small percentage of participants (1.2%) self‐reported implausibly low mg values for their calcium supplements (0–34 mg), where 1 to 2 mg might have been intended to be 1 to 2 g. However, study findings were consistent even when supplemental calcium was not considered. Our study did not control for vitamin D intake or seasonal ultraviolet exposure of skin. We did not have measures of BMD at Exams 1 and 5 to consider this possible confounder. Other study concerns may relate to accuracy of self‐reported data attained by questionnaires that elicited information on drug usage, concurrent diseases, physical activity, and other personal health issues, despite validation of the questionnaires.
Additionally, study participants who took the recommended amounts of dietary calcium may have been engaging in other unmeasurable health‐promoting behaviors (ie, the healthy user effect), which could potentially explain why a decrease in risk of CAC development was observed in the highest quintile of calcium intake.50 Calcium‐rich foods are associated with a healthy diet, and many of the participants with a high dietary calcium intake may be consuming vegetables, dairy, nuts, and fish—that provide cardioprotective benefits. Associations between calcium intake and cardiovascular events observed in previous research may possibly have resulted from completely different mechanisms than an increase in CAC. Even though 50% of our study participants had CAC at baseline, they were unusually healthy as a result of stringent MESA recruitment protocols and the current results may not necessarily generalize to other populations. Another consideration of our study is that although the prognostic value of CAC is well established, it is only a surrogate marker for clinical CVD. Finally, we did perform multiple testing, and it is possible that associations found may be attributed to chance. Thus, our findings should be considered hypothesis generating to stimulate further investigation in this area. A type 2 statistical error (ie, a false‐negative result) is also always possible.
In summary, results from this long‐term study of 10 years showed a protective relationship between total calcium intake and incident coronary atherosclerosis, particularly among nonsupplement users. Even though mean total calcium intake in quintile 5 was greater than the upper limits of current recommendations, no increased risk of CAC progression was found, and the highest quintile of calcium intake actually had decreased risk of incident CAC among those without prevalent CAC at baseline. However, we found evidence that calcium supplement use was independently associated with incident CAC, whether or not we adjusted for total calcium intake. This finding suggests that calcium loading with supplements may not be entirely free of undesirable side effects, especially considering evidence for events in randomized trials of calcium supplementation like the Women's Health Initiative. Finally, our findings should reassure individuals who are following dietary calcium recommendations by eating high‐calcium foods that consuming calcium from diet alone at these levels or higher is not associated with incident CAC.
Sources of Funding
This research was supported by contracts N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐TR‐000040 and UL1‐TR‐001079 from the National Center for Research Resources (NCRR). The authors thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This publication was also developed under a STAR research assistance agreement, No. RD831697 (MESA Air), awarded by the US Environmental Protection Agency (EPA). It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors, and the EPA does not endorse any products or commercial services mentioned in this publication. Dr Delaney received support from R21HL120394‐01. Dr Michos is supported by a grant (R01NS072243) from NIH/NINDS and the Blumenthal Scholars Award in Preventive Cardiology.
Disclosures
The authors have nothing to disclose related to this article. Dr Michos reports receiving an honorarium from Siemens Diagnostics (modest) for work unrelated to this study.
Acknowledgments
The authors thank the staff and participants of the MESA study for their important contributions. The contributions to the initiation of this study by Dr Philip J. Klemmer, MD, are duly recognized. The data analysis was conducted by Ms Kruszka, MS, in consultation with Dr Delaney, PhD. We appreciate the help in literature searches from the staff of the Health Sciences Library at the University of North Carolina, especially Max Bowman.
(J Am Heart Assoc. 2016;5:e003815 doi: 10.1161/JAHA.116.003815)
References
- 1. Felsenfeld AJ, Levine BS. Milk alkali syndrome and the dynamics of calcium homeostasis. Clin J Am Soc Nephrol. 2006;1:641–654. [DOI] [PubMed] [Google Scholar]
- 2. Patel AM, Goldfarb S. Got calcium? Welcome to the calcium‐alkali syndrome. J Am Soc Nephrol. 2010;21:1440–1443. [DOI] [PubMed] [Google Scholar]
- 3. Reid IR, Bristow SM, Bolland MJ. Cardiovascular complications of calcium supplements. J Cell Biochem. 2015;116:494–501. [DOI] [PubMed] [Google Scholar]
- 4. Gallagher JC, Smith LM, Yalamanchili V. Incidence of hypercalciuria and hypercalcemia during vitamin D and calcium supplementation in older women. Menopause. 2014;21:1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Samelson EJ, Booth SL, Fox CS, Tucker KL, Wang TJ, Hoffmann U, Cupples LA, O'Donnell CJ, Kiel DP. Calcium intake is not associated with increased coronary artery calcification: the Framingham Study. Am J Clin Nutr. 2012;96:1274–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lutsey PL, Michos ED. Vitamin D, calcium, and atherosclerotic risk: evidence from serum levels and supplementation studies. Curr Atheroscler Rep. 2013;15:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li K, Kaaks R, Linseisen J, Rohrmann S. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC‐Heidelberg). Heart. 2012;98:920–925. [DOI] [PubMed] [Google Scholar]
- 8. Lewis JR, Calver J, Zhu K, Flicker L, Prince RL. Calcium supplementation and the risks of atherosclerotic vascular disease in older women: results of a 5‐year RCT and a 4.5‐year follow‐up. J Bone Miner Res. 2011;26:35–41. [DOI] [PubMed] [Google Scholar]
- 9. Paik JM, Curhan GC, Sun Q, Rexrode KM, Manson JE, Rimm EB, Taylor EN. Calcium supplement intake and risk of cardiovascular disease in women. Osteoporos Int. 2014;25:2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, Trevisan M; Women's Health Initiative I . Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846–854. [DOI] [PubMed] [Google Scholar]
- 11. Nordin BE, Lewis JR, Daly RM, Horowitz J, Metcalfe A, Lange K, Prince RL. The calcium scare—what would Austin Bradford Hill have thought? Osteoporos Int. 2011;22:3073–3077. [DOI] [PubMed] [Google Scholar]
- 12. Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta‐analysis. BMJ. 2011;342:d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, Reid IR. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta‐analysis. BMJ. 2010;341:c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A, Ames R, Gamble GD, Grey A, Reid IR. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008;336:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michaelsson K, Melhus H, Warensjo Lemming E, Wolk A, Byberg L. Long term calcium intake and rates of all cause and cardiovascular mortality: community based prospective longitudinal cohort study. BMJ. 2013;346:f228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497–504. [DOI] [PubMed] [Google Scholar]
- 17. Karp HJ, Ketola ME, Lamberg‐Allardt CJ. Acute effects of calcium carbonate, calcium citrate and potassium citrate on markers of calcium and bone metabolism in young women. Br J Nutr. 2009;102:1341–1347. [DOI] [PubMed] [Google Scholar]
- 18. Bristow SM, Gamble GD, Stewart A, Horne L, House ME, Aati O, Mihov B, Horne AM, Reid IR. Acute and 3‐month effects of microcrystalline hydroxyapatite, calcium citrate and calcium carbonate on serum calcium and markers of bone turnover: a randomised controlled trial in postmenopausal women. Br J Nutr. 2014;112:1611–1620. [DOI] [PubMed] [Google Scholar]
- 19. Blaha MJ, Blumenthal RS, Budoff MJ, Nasir K. Understanding the utility of zero coronary calcium as a prognostic test: a Bayesian approach. Circ Cardiovasc Qual Outcomes. 2011;4:253–256. [DOI] [PubMed] [Google Scholar]
- 20. Manson JE, Allison MA, Carr JJ, Langer RD, Cochrane BB, Hendrix SL, Hsia J, Hunt JR, Lewis CE, Margolis KL, Robinson JG, Rodabough RJ, Thomas AM; Women's Health I and Women's Health Initiative‐Coronary Artery Calcium Study I . Calcium/vitamin D supplementation and coronary artery calcification in the Women's Health Initiative. Menopause. 2010;17:683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 22. Block G, Woods M, Potosky A, Clifford C. Validation of a self‐administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–1335. [DOI] [PubMed] [Google Scholar]
- 23. Mayer‐Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S. Validity and reproducibility of a food frequency interview in a Multi‐Cultural Epidemiology Study. Ann Epidemiol. 1999;9:314–324. [DOI] [PubMed] [Google Scholar]
- 24. Nettleton JA, Schulze MB, Jiang R, Jenny NS, Burke GL, Jacobs DR Jr. A priori‐defined dietary patterns and markers of cardiovascular disease risk in the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2008;88:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45:683–692. [DOI] [PubMed] [Google Scholar]
- 26. Smith NL, Psaty BM, Heckbert SR, Tracy RP, Cornell ES. The reliability of medication inventory methods compared to serum levels of cardiovascular drugs in the elderly. J Clin Epidemiol. 1999;52:143–146. [DOI] [PubMed] [Google Scholar]
- 27. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 28. Carr JJ, Nelson JC, Wong ND, McNitt‐Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population‐based studies: standardized protocol of Multi‐Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 29. Nelson JC, Kronmal RA, Carr JJ, McNitt‐Gray MF, Wong ND, Loria CM, Goldin JG, Williams OD, Detrano R. Measuring coronary calcium on CT images adjusted for attenuation differences. Radiology. 2005;235:403–414. [DOI] [PubMed] [Google Scholar]
- 30. Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi‐Ethnic Study of Atherosclerosis (MESA). Circulation. 2007;115:2722–2730. [DOI] [PubMed] [Google Scholar]
- 31. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 32. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 33. Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log‐rank tests. Biometrics. 2000;56:779–788. [DOI] [PubMed] [Google Scholar]
- 34. Hunt CD, Johnson LK. Calcium requirements: new estimations for men and women by cross‐sectional statistical analyses of calcium balance data from metabolic studies. Am J Clin Nutr. 2007;86:1054–1063. [DOI] [PubMed] [Google Scholar]
- 35. Kopple JD, Coburn JW. Metabolic studies of low protein diets in uremia. II. Calcium, phosphorus and magnesium. Medicine (Baltimore). 1973;52:597–607. [DOI] [PubMed] [Google Scholar]
- 36. Spiegel DM, Brady K. Calcium balance in normal individuals and in patients with chronic kidney disease on low‐ and high‐calcium diets. Kidney Int. 2012;81:1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Warensjo E, Byberg L, Melhus H, Gedeborg R, Mallmin H, Wolk A, Michaelsson K. Dietary calcium intake and risk of fracture and osteoporosis: prospective longitudinal cohort study. BMJ. 2011;342:d1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson JJ, Roggenkamp KJ, Suchindran CM. Calcium intakes and femoral and lumbar bone density of elderly U.S. men and women: National Health and Nutrition Examination Survey 2005–2006 analysis. J Clin Endocrinol Metab. 2012;97:4531–4539. [DOI] [PubMed] [Google Scholar]
- 39. Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140:817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo‐Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rautiainen S, Wang L, Manson JE, Sesso HD. The role of calcium in the prevention of cardiovascular disease–a review of observational studies and randomized clinical trials. Curr Atheroscler Rep. 2013;15:362. [DOI] [PubMed] [Google Scholar]
- 42. Bhakta M, Bruce C, Messika‐Zeitoun D, Bielak L, Sheedy PF, Peyser P, Sarano M. Oral calcium supplements do not affect the progression of aortic valve calcification or coronary artery calcification. J Am Board Fam Med. 2009;22:610–616. [DOI] [PubMed] [Google Scholar]
- 43. Leopold JA. Vascular calcification: mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med. 2015;25:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anderson JJ, Klemmer PJ. Risk of high dietary calcium for arterial calcification in older adults. Nutrients. 2013;5:3964–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang X, Chen H, Ouyang Y, Liu J, Zhao G, Bao W, Yan M. Dietary calcium intake and mortality risk from cardiovascular disease and all causes: a meta‐analysis of prospective cohort studies. BMC Med. 2014;12:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adatorwovor R, Roggenkamp K, Anderson JJ. Intakes of calcium and phosphorus and calculated calcium‐to‐phosphorus ratios of older adults: NHANES 2005–2006 data. Nutrients. 2015;7:9633–9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jensky NE, Hyder JA, Allison MA, Wong N, Aboyans V, Blumenthal RS, Schreiner P, Carr JJ, Wassel CL, Ix JH, Criqui MH. The association of bone density and calcified atherosclerosis is stronger in women without dyslipidemia: the Multi‐Ethnic Study of Atherosclerosis. J Bone Miner Res. 2011;26:2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Uyama O, Yoshimoto Y, Yamamoto Y, Kawai A. Bone changes and carotid atherosclerosis in postmenopausal women. Stroke. 1997;28:1730–1732. [DOI] [PubMed] [Google Scholar]
- 49. Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68:271–276. [DOI] [PubMed] [Google Scholar]
- 50. Brookhart MA, Patrick AR, Dormuth C, Avorn J, Shrank W, Cadarette SM, Solomon DH. Adherence to lipid‐lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166:348–354. [DOI] [PubMed] [Google Scholar]


