Abstract
Background
Speckle tracking echocardiography (STE) is reported as a useful method to predict cardiac resynchronization therapy (CRT) responders. This study aimed to identify the incremental value of a STE parameter to predict CRT responders.
Methods and Results
We enrolled 171 patients from the Speckle Tracking imaging for the Assessment of cardiac Resynchronization Therapy (START) study. CRT responders were defined as patients with ≥15% reduction of left ventricular (LV) end‐systolic volume at 6 months post‐CRT. Based on multivariable logistic regression analysis, incremental values of STE were assessed by c‐statistics, net reclassification improvement (NRI)/integrated discrimination improvement (IDI), and decision curve analysis. Six parameters (left bundle branch block or right ventricular pacing, use of beta‐blocker, blood urea nitrogen ≤3.0 mg/dL, LV end‐systolic diameter ≤50 mm, mitral regurgitation index ≤40%, and STE parameter standard deviation of time from QRS onset to first peak on the circumferential strain curves [TSD] ≥116 ms) were identified as the determinants. Compared to the multivariable logistic regression model without TSD (model 1), that with TSD (model 2) showed significant improvement to predict CRT responders: c‐statistic (0.86 vs 0.77; P<0.001), NRI=0.19, P<0.001, and IDI=0.17, P<0.001. The decision curve of model 2 was higher than that of model 1 at threshold probabilities ≥0.2. Based on model 2, a START score was constructed. Compared to the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT‐CRT) score, the decision curve of the START score was higher than that of the MADIT‐CRT score at threshold probabilities ≥0.2.
Conclusions
Based on various statistical methods, this study revealed that STE had an incremental value to predict CRT responders.
Keywords: cardiac resynchronization therapy, heart failure, speckle training echocardiography, statistics
Subject Categories: Echocardiography, Heart Failure, Pacemaker
Introduction
Cardiac resynchronization therapy (CRT) is an established nonpharmacological therapy for patients with advanced heart failure characterized by left ventricular (LV) dysfunction with LV ejection fraction (LVEF) ≤35% and LV dyssynchrony with QRS duration ≥120 ms.1, 2, 3 The responses for CRT have been widely assessed by LV reverse remodeling, which is usually defined as a ≥15% reduction of LV end‐systolic volume post‐CRT. Based on the current guideline, the rate of CRT responders is from 60% to 70%.1, 2, 3 The usefulness of echocardiographic parameters to improve the response rate to CRT has been controversial.4, 5 Recently, speckle tracking echocardiography (STE) has been reported as a useful imaging method to detect CRT responders.6, 7, 8 In our Speckle Tracking imaging for the Assessment of cardiac Resynchronization Therapy (START) study, a STE parameter was revealed to be an independent predictor of a CRT responder and was associated with the clinical endpoints by multivariable studies.8 However, whether a STE parameter has incremental value to the preceding factors associated with CRT responses has not been well studied.9 Therefore, we hypothesized that a multivariable diagnostic model consisting of significant parameters associated with CRT responses might be helpful to quantify the incremental value of a STE parameter for the prediction of CRT responders. Although the difference in the area under the curve (AUC) of receiver operating characteristic (ROC) analysis or concordance (c) statistics have been widely used to express the incremental value of a new test/biomarker, they have been criticized because of several limitations.9 To overcome the problems of ROC analysis, the reclassification table has been popular using some measures, including net reclassification improvement (NRI) and integrated discrimination improvement (IDI).10 In addition, decision curve analysis (DCA) has been proposed to quantify the added value of a parameter as a better method than NRI and IDI.9, 11, 12, 13, 14 Thus, the aim of this study was to identify the incremental value of a STE parameter to predict CRT responders by these statistical methods.
Methods
Study Population
The START study was a prospective multicenter study to assess the feasibility of STE to predict CRT responders. The study design and results were published in December 2014.8 Patients were enrolled based on criteria that included presence of congestive heart failure refractory to optimal medical therapy and QRS duration ≥120 ms; New York Heart Association (NYHA) class II, III, or IV; and LVEF ≤35%. Finally, 180 patients were enrolled from 17 Japanese centers between September 2009 and August 2011, and clinical follow‐up was completed in September 2012. The study was approved by the institutional review committee of the University of Tsukuba Hospital (Tsukuba, Japan), and all subjects gave informed consent.
CRT Volume Responders and Clinical Outcome
A volume responder to CRT was defined as a patient with reverse remodeling as indicated by a ≥15% reduction of LV end‐systolic volume at 6 months post‐CRT. LV volume measurements at 6 months were completed in 171 (95%) of the 180 patients. Six‐month data in the remaining 9 patients could not be assessed because of death from heart failure in 4, sudden cardiac death in 3, and noncardiac death in 2. Of the remaining 171 patients, 109 (63.7%) were identified as volume responders to CRT. Clinical outcomes were assessed as the composite endpoint of cardiac death or unplanned hospitalization for heart failure. Cardiac death was defined as death from worsening of heart failure, coronary artery disease, cardiac arrhythmia, or sudden cardiac death. Unplanned hospitalization for heart failure was defined as a hospital admission by the occurrence of increasing symptoms and the need for treatment with intravenous or oral medications for heart failure.
Echocardiographic Studies
E/E′, left atrial volume index (LAVI), mitral regurgitation (MR) index, interventricular mechanical delay, and septal‐to‐posterior wall motion delay on the M‐mode image were measured by previously reported methods.8, 15 STE studies were performed at a LV short‐axis plane at the papillary muscle level and at the apical 4‐chamber, 2‐chamber, and long‐axis planes. Images were recorded and analyzed using workstations with vendor software packages (EchoPac PC v.7.0.1; GE Healthcare, Little Chalfont, UK; 2D Wall Motion Tracking; Toshiba Medical Systems Corporation, Tokyo, Japan).
In the START study, 6 parameters in each radial strain, circumferential strain (CS), and longitudinal strain component were measured. We measured and compared 2 strain peak points: Time from QRS onset to maximum strain (Tmax) and that to first peak in the multiple strain peaks (Tfirst) were measured in each segment. Three dyssynchrony parameters in each of Tmax and Tfirst were calculated as follows: first, the standard deviation of Tmax (Tmax−SD) and Tfirst (Tfirst−SD); second, the time difference (TD) between the smallest Tmax and largest Tmax (Tmax−TD) and between the smallest Tfirst and largest Tfirst (Tfirst−TD); and third, TD between the septum and lateral wall (Tmax−TDSL, Tfirst−TDSL). Among the 18 dyssynchrony parameters derived from STE, Tfirst−SD of CS (TSD) showed the best predictive value for volume responders in a single‐parameter approach (Figure 1). Therefore, standard deviation of time from QRS onset to first peak on the circumferential strain curves (TSD) was used as the surrogate parameter of dyssynchrony in this study.
Figure 1.
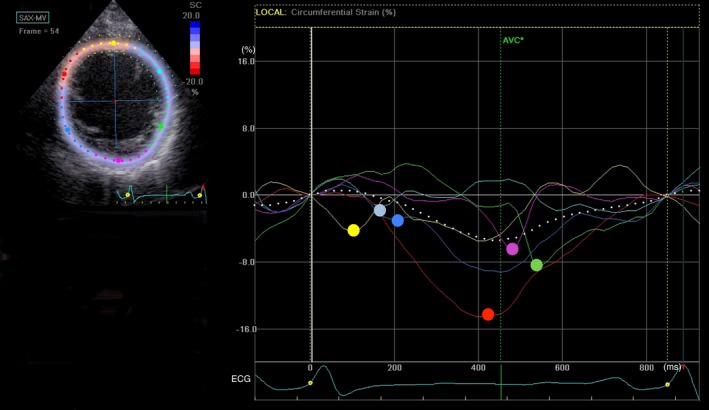
Time‐circumferential strain (CS) curves. The figure shows time‐CS curves on the midventricular short‐axis view obtained from a patient with idiopathic dilated cardiomyopathy. Each color curve is corresponding to the same color segment of short‐axis view as shown in the left upper panel; yellow=anteroseptal wall, red=septal wall, blue=inferior wall, purple=posterior wall, green=lateral wall, and light blue=anterior wall. The color point on the curve is the surrogate peak point in each segment. Time from QRS onset (white perpendicular line) to the color point (Tfirst) is measured in each segment, and the SD of Tfirst in 6 segments is calculated as TSD. AVC indicates aortic valve closure; ECG, electrocardiogram.
Statistical Analysis
Multivariable diagnostic model
The probability of being a CRT responder was calculated by a multivariable logistic regression analysis with forward selection method based on a likelihood ratio. The initially included covariates are listed in Table 1. Each numerical parameter was categorized as a binary based on the best cut‐off value point, which was defined as the point with the highest sum of sensitivity and specificity by ROC curve analysis. A calculated value (Y) was obtained by a multiple regression equation, which consisted of selected factors from a multivariable logistic regression analysis with forward selection method. Because Y is equal to log (probability/1−probability), the probability of being a CRT responder was calculated by the following formula:
Table 1.
Baseline Patient Characteristics by Tertile of the START Score
| START Score | Total (N=171) | T1 (N=56) | T2 (N=55) | T3 (N=60) | P Value |
|---|---|---|---|---|---|
| 0 to 9 | 10 to 13 | 14 to 17 | |||
| Age, y | 66±12 | 66±13 | 67±13 | 66±12 | 0.90 |
| Male | 113 (66) | 39 (70) | 33 (60) | 41 (68) | 0.51 |
| Ischemic etiology | 33 (19) | 15 (27) | 10 (18) | 8 (13) | 0.18 |
| NYHA class II/III/IV | 62/101/8 (36/59/5) | 19/36/1 (34/64/2) | 25/26/4 (46/47/7) | 18/39/3 (30/65/5) | 0.22 |
| Heart rate, bpm | 68±14 | 69±15 | 69±13 | 65±12 | 0.35 |
| SBP, mm Hg | 107±18 | 105±22 | 107±14 | 109±17 | 0.45 |
| QRS duration, ms | 159±30 | 144±28 | 159±31a | 171±25b | <0.001 |
| LBBB or RV pacing | 86 (50) | 18 (32) | 43 (78) | 57 (95) | <0.001 |
| Sustained VT/VF | 40 (23) | 13 (23) | 11 (20) | 16 (27) | 0.23 |
| Hypertension | 63 (37) | 21 (38) | 16 (29) | 26 (43) | 0.28 |
| Diabetes mellitus | 54 (32) | 20 (36) | 17 (31) | 17 (28) | 0.68 |
| Laboratory data | |||||
| Hb, g/dL | 12.7±2.2 | 12.5±2.3 | 12.6±2.2 | 12.9±2.0 | 0.74 |
| Albumin, g/dL | 3.9±0.5 | 3.9±0.4 | 3.8±0.5 | 4.0±0.4 | 0.12 |
| BUN, mg/dL | 27.9±23.3 | 36.5±33.4 | 26.1±14.4 | 21.6±12.6b , a | 0.002 |
| Cre, mg/dL | 1.5±1.7 | 2.0±2.5 | 1.4±1.3 | 1.1±0.9c | 0.02 |
| Sodium, mEq/L | 138±3.7 | 137±3.8 | 138±3.2 | 139±3.7c | 0.04 |
| BNP, pg/m | 700±950 | 965±989 | 626±805c | 520±620b | 0.03 |
| Medication | |||||
| ACE‐I/ARB | 130 (76) | 41 (73) | 39 (71) | 50 (83) | 0.25 |
| Beta‐blocker | 138 (81) | 37 (66) | 44 (80) | 57 (95) | <0.001 |
| Loop diuretics | 142 (83) | 51 (91) | 43 (78) | 48 (80) | 0.14 |
| Spironolactone | 105 (61) | 36 (64) | 33 (60) | 36 (60) | 0.86 |
| Echocardiography | |||||
| LVEDV, mL | 189±90 | 194±91 | 186±68 | 185±107 | 0.84 |
| LVESV, mL | 141±81 | 144±79 | 140±58 | 139±99 | 0.94 |
| LVEF, % | 27±6.9 | 27±6.7 | 25±6.5 | 27±7.3 | 0.18 |
| LVDd, mm | 64±9.5 | 67±9.3 | 64±7.4 | 61±11b | 0.01 |
| LVDs, mm | 55±10 | 58±9.9 | 56±8.5 | 52±10b | 0.008 |
| LVFS, % | 14±5.7 | 14±5.9 | 13±5.6 | 15±5.4 | 0.09 |
| E/E′ | 16±9.3 | 16±7.1 | 16±10 | 16±11 | 0.99 |
| MR index, % | 24±19 | 31±21 | 22±18c | 17±14b | <0.001 |
| TSD, ms | 135±52 | 92±41 | 139±47b | 171±32b , a | <0.001 |
Values are means±SD or numbers (%). ACE‐I indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blocker; BNP, B‐type natriuretic peptide; BUN, blood urea nitrogen; Cre, creatinine; E, early diastolic peak velocity of Doppler transmitral flow; E′, early diastolic mitral annular velocity; Hb, hemoglobin; LBBB, left bundle branch block; LVDd, left ventricular dimension at end diastole; LVDs, left ventricular dimension at end systole; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVFS, left ventricular fractional shortening; MR, mitral regurgitation; NYHA, New York Heart Association; RV, right ventricular; T, tertile; TSD, standard deviation of time from QRS onset to first peak on the circumferential strain curves; START, Speckle Tracking imaging for the Assessment of cardiac Resynchronization Therapy; VT/VF, ventricular tachycardia/fibrillation.
P<0.01 versus T2.
P<0.001.
P<0.05 versus T1.
To assess the incremental value of TSD, the probability by a logistic regression analysis model, in which TSD was not included (model 1), was compared with that by the other model including TSD (model 2). A probability of 0.5 was used as the threshold for responders; if the probability value was more than 0.5, the patient was classified as a responder.
Assessment of incremental value of the strain parameter
First, a c‐statistic, which was calculated as an AUC, was compared between models 1 and 2. Second, the incremental effects of adding TSD to predict volume responders were assessed by NRI and IDI.10 NRI and IDI are superior methods for assessing the incremental value of performing a presumably better technique in place of a less‐accurate technique. Compared to c‐statistic values, NRI and IDI can reveal the percentage of a population that is accurately reclassified, providing clinically meaningful results rather than only statistically significant differences between c‐statistic values. The reclassification methods are especially useful to address questions related to cost‐effectiveness.
NRI was calculated by the following formula:
where P is the proportion of patients, upward movement (up) is defined as a change into a higher probability of CRT responder category based on model 1, and downward movement (down) is defined as a change in the opposite direction. D denotes the response classification: responder=1 or nonresponder=0.
IDI was calculated by the following formula:
In this equation, Pextended|D=1 and Pextended|D=0 are the means of the predicted CRT responder probability by model 2 for, respectively, the CRT responder and the CRT nonresponder, whereas Pbasic|D=1 and Pbasic|D=0 are the means of the predicted CRT responder probability by model 1 for, respectively, the CRT responder and the CRT nonresponder.
Third, the net benefit of DCA was evaluated to assess the clinical usefulness of TSD.9, 12 DCA is an approach that even more explicitly quantifies the clinical usefulness of a new test when added to existing ones. In reclassifications including NRI and IDI, a single predefined probability threshold is chosen; a probability of 0.5 was used as the threshold for responders in our series to address the c‐statistic value, NRI, and IRI. In contrast, DCA allows each physician or patient to determine his or her own desired threshold for further actions and judge the corresponding net benefits without explicitly assigning weights or utilities to the false classifications. The expected benefit is represented by the true‐positive rate, which is the ratio of the number of CRT responders among patients who will be predicted to be responders at a threshold probability to all subjects. Contrastingly, the false‐positive rate is the ratio of the number of CRT nonresponders among patients who will be predicted to be responders at a threshold probability to all subjects, and the expected harm is represented by a false‐positive rate multiplied by a weighting factor based on the patient's threshold probability. Then, net benefit is calculated by the following formula:
where N=number of all subjects and weighting factor=threshold probability/1−threshold probability.
A net benefit was calculated from 0.0 to a zero cross‐point at an interval of 0.05 of threshold probability. Based on the net benefit at each threshold probability, DCA generates a graph of net benefit as a function of a threshold probability of a CRT responder at which an individual considers the potential benefit and harm of CRT to be equivalent.
Clinical scoring system
Each of the binary parameters was assigned a score based on the regression coefficient in the predictive formula of model 2. The parameter with the lowest regression coefficient among the selected parameters in the model was assigned the lowest integer number, and the others were assigned scores of integer numbers based on the values of their regression coefficients relative to that of the lowest value.16 The sum of each parameter point was calculated as a START score. In addition, based on the previous report from the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT‐CRT),16 the MADIT score was assessed A point for pasts hospitalization for chronic heart failure was assigned a numeric value of 1; female sex, nonischemic etiology, QRS duration ≥150 ms, left bundle branch block (LBBB), and LV end‐systolic volume index ≥125 mL/m2 were each assigned a value of 2; and LAVI <40 mL/m2 was assigned a value of 3. An individual MADIT score was calculated as a sum of each factor point. C‐statistics for CRT responders were compared between the START score and MADIT score. NRI and IDI also were assessed between both scoring systems. DCA was also used to assess the clinical usefulness of the START score.
Results are expressed as number (%) or as mean±SD. Comparisons between 2 groups were performed using the Student t test for continuous variables and the χ2 test for categorical variables. One‐way ANOVA with the post‐hoc Tukey‐Kramer test was used to compare variables between 3 or more groups.
Kaplan–Meier analysis was done to determine the influence of START scores on the endpoints. The risk of clinical endpoints was determined with Cox proportional hazard models. The univariate factors with a value of P<0.05 were entered into the multivariable model adjusted for age and sex to assess the effect of the parameters on the endpoints. A P<0.05 was considered to indicate statistical significance. Analyses were performed with SPSS software (version 17.0; SPSS Inc., Chicago, IL). In addition, comparisons of c‐statistics were performed with Analyse‐it (Analyse‐it Software, Ltd., UK).
Results
Selected parameters and the statistical results are summarized in Table 2. In model 1, other than TSD, the same 5 factors as in model 2 were selected by a multivariable logistic regression analysis with forward selection method based on a likelihood ratio using the covariates listed in Table 1, except for TSD. The c‐statistics of models 1 and 2 were 0.86 (95% CI, 0.80–0.93; P<0.001) and 0.77 (95% CI, 0.7–0.84; P<0.001), respectively. The c‐statistic of model 1 was significantly higher than that of model 2 (P<0.001). NRI and IDI were calculated between models 1 and 2. The reclassification table for our CRT responder example is shown in Table 3, with the probability threshold at 0.5. Of the CRT responders, 17.4% (18 of 109+1 of 109) were reclassified between models 1 and 2. For CRT nonresponders, this percentage was 22.6% (6 of 62+8 of 62). The NRI was (18 of 109−1 of 109)−(6 of 62−8 of 62)=0.16+0.03=0.19 (95% CI, 0.16–0.22; P<0.001). Furthermore, the IDI for our CRT example was (0.83−0.80)−(0.22−0.36)=0.17 (95% CI, 0.11–0.22; P<0.001).
Table 2.
Multivariate Logistic Regression Analysis for CRT Responders
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| R | OR (95% CI) | P Value | R | OR (95% CI) | P Value | |
| LBBB or RV pacing | 1.57 | 4.81 (2.20–10.5) | <0.001 | 1.04 | 2.82 (1.17–6.84) | 0.02 |
| Use of beta‐blocker | 1.69 | 5.40 (2.10–13.9) | <0.001 | 1.65 | 5.20 (1.91–14.1) | 0.001 |
| BUN ≤30 mg/dL | 1.42 | 4.15 (1.78–9.68) | 0.001 | 1.37 | 3.95 (1.60–9.72) | 0.003 |
| LVDs ≤50 mm | 1.42 | 4.13 (1.56–10.9) | 0.004 | 1.25 | 3.48 (1.26–9.66) | 0.02 |
| MR index ≤40% | 1.32 | 3.74 (1.48–9.46) | 0.005 | 1.08 | 2.95 (1.05–7.30) | 0.01 |
| TSD ≥116 ms | — | — | — | 1.99 | 7.28 (3.14–16.9) | <0.001 |
| Intercept | −4.14 | 0.02 | <0.001 | −4.63 | 0.01 | <0.001 |
BUN indicates blood urea nitrogen; CRT, cardiac resynchronization therapy; LBBB, left bundle branch block; LVDs, left ventricular dimension at end systole; MR, mitral regurgitation; OR, odds ratio; R, regression coefficient; RV, right ventricular; TSD, standard deviation of time from QRS onset to first peak on the circumferential strain curves.
Table 3.
Reclassification Table From Model 1 and Model 2 at an Arbitrary Cut‐off Value of 0.5
| CRT Responders (N=109) | ||||
|---|---|---|---|---|
| Model 2 | ||||
| >0.5 | ≤0.5 | Total | ||
| Model 1 | >0.5 | 82 | 1 | 83 |
| ≤0.5 | 18 | 8 | 26 | |
| Total | 100 | 9 | 109 | |
| CRT Nonresponders (N=62) | ||||
|---|---|---|---|---|
| Model 2 | ||||
| >0.5 | ≤0.5 | Total | ||
| Model 1 | >0.5 | 12 | 8 | 20 |
| ≤0.5 | 6 | 36 | 42 | |
| Total | 18 | 44 | 62 | |
A patient with a model probability of >0.5 is considered with high probability to be a CRT responder. Blue and red areas indicate reclassifications by model 2. CRT indicates cardiac resynchronization therapy.
The decision curves for models 1 and 2 are shown in Figure 1. Two additional decision guidance approaches (CRT for no one and CRT for everyone based on the current guideline, regardless of risk) were also incorporated for comparison. Figure 2 shows that the net benefit by the model 2 approach was higher than that by the model 1 approach with threshold probabilities ≥0.2, which means the optimal approach to guide decision making was model 2.
Figure 2.
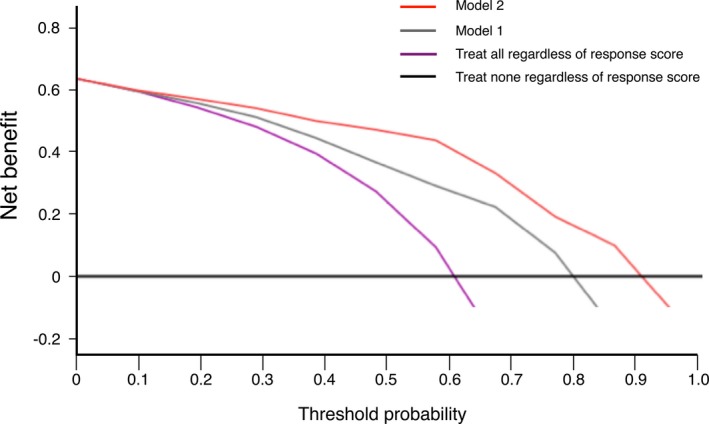
Decision curve analysis for multivariable logistic regression models to predict responders of cardiac resynchronization therapy. The thick black line is the net benefit of referring none of the patients for reference testing. The purple curve is the net benefit of referring all patients for reference testing, the gray curve is the basic prediction model (model 1), and the red curve is the extended prediction model (model 2), depending on the choice of probability threshold.
START Score
Based on model 2 (Table 2), each point in the START score was assigned a value as follows: a point for LBBB or right ventricular (RV) pacing and MR index ≤40% was assigned a numeric value of 2; use of beta‐blocker, blood urea nitrogen (BUN) ≤30 mg/dL, and LV dimension at end systole (LVDs) ≤50 mm were each assigned a numeric value of 3; and CS‐SD ≥116 ms was assigned a numeric value of 4. The c‐statistic of the START score was 0.86 (95% CI, 0.79–0.92; P<0.001). The relation between the probability of CRT responders and the START score is shown in Figure 3. A probability >0.5 corresponded to a START score ≥10, and a probability >0.9 corresponded to a score of ≥14. Tertiles of the START can be compared in Table 1.
Figure 3.
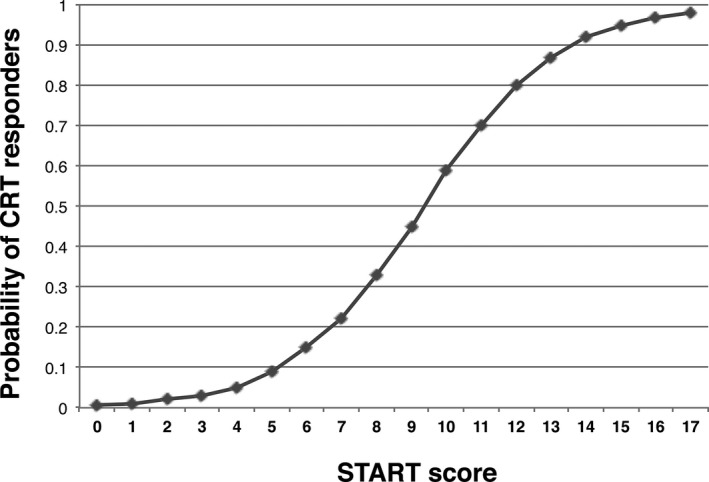
The relation between the probability of being a responder to cardiac resynchronization therapy (CRT) and the Speckle Tracking imaging for the Assessment of cardiac Resynchronization Therapy (START) score.
The c‐statistic of the MADIT score was 0.66 (95% CI, 0.58–0.75; P<0.001), which was significantly lower than that of the START score (P<0.001). The NRI of the START score compared with the MADIT score was (9 of 109−6 of 109)−(2 of 62−28 of 62)=0.03+0.42=0.45 (95% CI, 0.41–0.49; P<0.001), and the IDI was (0.84−0.80)−(0.65−0.76)=0.15 (95% CI, 0.10–0.20; P<0.001; Table 4). Decision curves from the START score and the MADIT score are shown in Figure 4. Net benefit was higher with threshold probabilities ≥0.2 by the START score approach than by the MADIT score approach.
Table 4.
Reclassification Table From the MADIT Score and the START Clinical Score at an Arbitrary Cut‐off Value of 0.5
| CRT Responders (N=109) | ||||
|---|---|---|---|---|
| START Score | ||||
| >0.5 | ≤0.5 | Total | ||
| MADIT Score | >0.5 | 91 | 6 | 97 |
| ≤0.5 | 9 | 3 | 12 | |
| Total | 100 | 9 | 109 | |
| CRT Nonresponders (N=61) | ||||
|---|---|---|---|---|
| START Score | ||||
| >0.5 | ≤0.5 | Total | ||
| MADIT Score | >0.5 | 16 | 28 | 44 |
| ≤0.5 | 2 | 16 | 18 | |
| Total | 18 | 44 | 62 | |
A patient with a model probability of >0.5 is considered with high probability to be a CRT responder. Blue and red areas indicate reclassifications by model 2. CRT indicates cardiac resynchronization therapy; MADIT, Multicenter Automatic Defibrillator Implantation Trial; START, START, Speckle Tracking imaging for the Assessment of cardiac Resynchronization Therapy.
Figure 4.
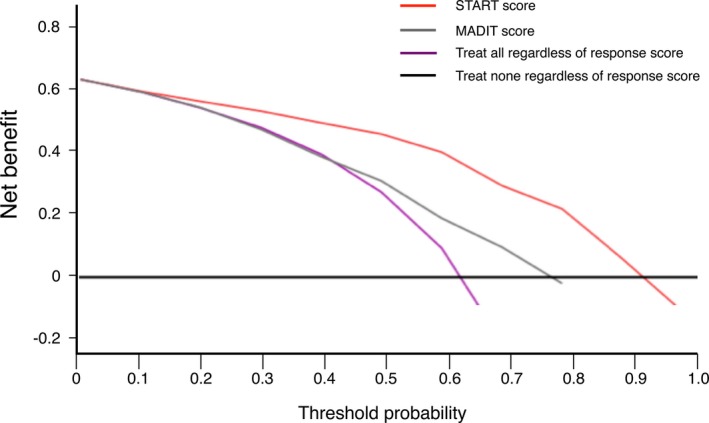
Decision curve analysis for the Speckle Tracking imaging for the Assessment of cardiac Resynchronization Therapy (START) score and the Multicenter Automatic Defibrillator Implantation Trial (MADIT) score to predict responders of cardiac resynchronization therapy. The red curve is the net benefit of treating patients according to the START score, and the gray curve is the net benefit of treating patients according to the MADIT score. As in Figure 1, the thin black line is the net benefit of referring none of the patients for reference testing, and the purple curve is that of referring all patients for reference testing.
The cut‐off value, which was determined as the point with the highest sum of sensitivity and specificity by ROC analysis, was identified as a START score of 12 to predict clinical outcomes. Kaplan–Meier estimates of the time to endpoint are shown in Figure 5. There were significant differences between groups (log rank, P<0.01). In the multivariable Cox proportional hazard model analysis adjusted for age, sex, ischemic cardiomyopathy, use of loop diuretics, and left ventricular end‐diastolic volume ≥250 mL, a START clinical score ≤12 was identified as a significant predictor of the endpoints (P=0.001; hazard ratio [HR], 3.9; 95% CI, 1.2–5.0) as was a serum creatinine level ≥1.0 mg/dL (P=0.02; HR, 2.4; 95% CI, 1.8–8.7).
Figure 5.
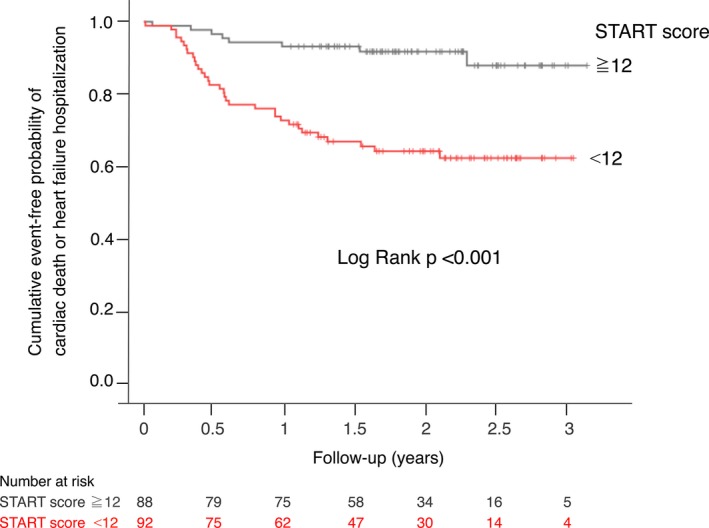
Kaplan–Meier curves for the probability of freedom from cardiac death and unplanned hospitalizations for heart failure. START indicates Speckle Tracking imaging for the Assessment of cardiac Resynchronization Therapy.
Discussion
The present study using data from the START study revealed the incremental value of a STE parameter to predict CRT responders using various statistical analyses. In the comparison of the multivariable logistic regression models, model 2, consisting of QRS morphology, degree of LV dilatation measured by LVDs, use of beta‐blocker, BUN level, and mechanical dyssynchrony assessed by TSD with the other model; model 1, in which TSD was excluded; c‐statistics to compare overall diagnostic accuracy between models 1 and 2; NRI and IDI to assess overall improvement of reclassifications from models 1 to 2; and DCA to quantify the clinical usefulness of TSD, when added to model 1, all revealed that model 2 including TSD improved the accuracy of the prediction of CRT responders. On the basis of model 2, we created a clinical scoring system, the START score, which was more reliable in predicting CRT responders than the MADIT score in our series. To our knowledge, this is the first study using DCA to examine the clinical usefulness of the dyssynchrony parameter to predict CRT responders.
Combined Assessments to Predict CRT Responders
In the START study,8 we showed that the strain parameter, TSD, was the best predictor of CRT responders in a single‐parameter approach. However, our previous study did not reveal the incremental value of TSD when added to the other clinical determinants of CRT responses. Based on the current guideline, 30% to 40% of patients do not show significant LV reverse remodeling post‐CRT, which was also observed in previous studies.1, 2, 3 Therefore, various factors, including types of intraventricular conduction delays, sex, etiology of LV dysfunction, severity of mitral regurgitation, LV diastolic dysfunction, and presence of intra‐, inter‐, and atrioventricular mechanical dyssynchrony, were reported as the novel factors that improve the accuracy of predicting CRT responders.16, 17 However, a single parameter is limited in its ability to predict responses accurately because multivariable factors affect the responses to CRT.9 Therefore, combined assessment of factors that are associated with favorable reverse remodeling post‐CRT can be used to predict clinical response to the device as was shown in the MADIT‐CRT study.16
START Score
Because the main subjects in the MADIT‐CRT study were patients classified as NYHA class I or II, it is unknown whether the MADIT scoring system can be used to make extrapolations in patients classified as NYHA class III or IV.16 Park et al18 reported that the echocardiographic score more accurately predicted LV reverse remodeling with CRT, and, compared to the MADIT score, it predicts clinical outcomes in 90% of patients classified as NYHA class III or IV. However, the echocardiographic score of these patients does not even include LBBB, which is the established clinical parameter. We hypothesized that other factors, including medications, other organ failure, and structural heart diseases, also could affect LV reverse remodeling post‐CRT. Therefore, we performed a multivariable logistic regression analysis to select significant variables among various clinical covariates to construct our original scoring system to predict CRT responders. Compared to the other scoring systems, our START score includes unique parameters, which are BUN level and the use of beta‐blocker. An elevated BUN level in heart failure is associated with enhanced proximal and distal tubular reabsorption of urea, being linked to the reabsorption of sodium and water under the effects of the renin‐angiotensin‐aldosterone and beta‐adrenergic systems.19, 20, 21 Because neurohormonal factors aggravate myocardial dysfunction,21 a lower BUN level means a more‐favorable condition for the myocardium and neurohormonal circumstances to respond more readily to CRT. As well, the use of beta‐blockers, which are the first‐line medications for heart failure, provides a better condition for CRT, but also synergistically improves myocardial function.22, 23 Considering the complex pathophysiology of heart failure, it is important to include neurohormonal factors in the START score.
However, as aforementioned, our START score was created based on the multivariable logistic regression analysis. Because each parameter was obtained from post‐hoc definitions, the score should be considered as preliminary findings. Therefore, the reliability of the START score needs to be validated in the other cohort prospectively.
Reclassification and DCA
Reclassification measures (NRI and IDI) have been of major importance in assessing the added value estimations of a biomarker or a test.24 However, NRI and IDI provide no information about whether the diagnostic probabilities calculated with a diagnostic model are in agreement with the observed CRT responder rate.9, 11 In the present study, the probability to calculate NRI and IDI, and c‐statistics, was fixed at 0.5. In contrast, DCA, which has already been used in clinical studies, is a novel method with the following concepts.9, 12, 13, 14 The probability threshold—a level of diagnostic certainty above which the patient would choose to be treated—is the key in DCA. Unlike reclassification measures, the incremental value of the diagnostic method can be clarified at an arbitrary probability. Second, DCA can take into account risk threshold, weighting benefits, and harms and is useful in evaluating the clinical utility of a prediction model. In this study, the expected harm is represented by the number of nonresponders who would be treated in error (false positives) multiplied by a weighting factor based on the patient's threshold probability. In a case that a physician strongly expects to be a CRT responder, the probability threshold will be higher. As shown in Figure 3, the DCA curve of model 2 is highest at probability thresholds >0.2, which means a multivariable approach including the mechanical dyssynchrony measure by STE would be the best decision approach for all patients in our series. The differences between the curves are wider as the probability thresholds increase, indicating the more‐incremental value of the mechanical dyssynchrony measure by STE if the intent is to avoid nonresponders. As well, the START score may be more useful compared to the MADIT score. The present results were consistent with a study that evaluated the usefulness of a multiparametric echocardiographic score method for CRT responders.18 In addition, we found that the START score was an independent predictor of clinical outcomes, which supports the finding in previous studies that patients with reverse remodeling have more‐favorable long‐term survival than those without reverse remodeling post‐RT.6, 7, 8, 16
Limitations
The START study is a multicenter study, but the number of patients is small compared to a large‐scale study like the MADIT‐CRT study.16 Thus, the statistical power might not be adequate to identify the determinants of CRT responders. In addition, this study consisted of Japanese participants only. The number of patients with ischemic heart disease in Japan is less than that in Western developed countries. In this study, only 19% of patients had ischemic heart disease, which might make the START score specific to our series. Thus, in the future, large‐scale, prospective studies will be needed to confirm the clinical usefulness of the START score.
Myocardial scar burden or presence of scar at a pacemaker lead implantation site on the LV free wall is well known to be a determinant of CRT responses. However, we did not assess myocardial scar or myocardial viability using cardiac magnetic resonance imaging, nuclear imaging, or stress echocardiography. Therefore, the START clinical score might be modified if myocardial scar or myocardial viability assessments were also included in multivariable logistic regression analyses.
Conclusions
Based on various statistical methods, including DCA, mechanical dyssynchrony assessment by STE was revealed to have an incremental value to predict CRT responders. STE may contribute to decision making for CRT indications, particularly if nonresponders are to be avoided in the clinical setting. Given that this study was performed based on the post‐hoc analyses, the findings including our clinical score need to be validated in the other cohort prospectively.
Sources of Funding
This work was supported by Japan Society for the Promotion of Science (JSPS KAKENHI) grant number 22590768.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003882 doi: 10.1161/JAHA.116.003882)
References
- 1. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators . Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 2. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L; Cardiac Resynchronization‐Heart Failure (CARE‐HF) Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA III, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W; MADIT‐CRT Trial Investigators . Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med. 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 4. Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J III, St John Sutton M, De Sutter J, Murillo J. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. [DOI] [PubMed] [Google Scholar]
- 5. Seo Y, Ito H, Nakatani S, Takami M, Naito S, Shiga T, Ando K, Wakayama Y, Aonuma K; J‐CRT investigators . The role of echocardiography in predicting responders to cardiac resynchronization therapy. Circ J. 2011;75:1156–1163. [DOI] [PubMed] [Google Scholar]
- 6. Tanaka H, Nesser HJ, Buck T, Oyenuga O, Jánosi RA, Winter S, Saba S, Gorcsan J III. Dyssynchrony by speckle‐tracking echocardiography and response to cardiac resynchronization therapy: results of the Speckle Tracking and Resynchronization (STAR) study. Eur Heart J. 2010;31:1690–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O'Halloran D, Elsik M, Read PA, Begley D, Fynn SP, Dutka DP. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59:1509–1518. [DOI] [PubMed] [Google Scholar]
- 8. Maruo T, Seo Y, Yamada S, Arita T, Ishizu T, Shiga T, Dohi K, Toide H, Furugen A, Inoue K, Daimon M, Kawai H, Tsuruta H, Nishigami K, Yuda S, Ozawa T, Izumi C, Fumikura Y, Wada Y, Doi M, Okada M, Takenaka K, Aonuma K. The speckle tracking imaging for the assessment of cardiac resynchronization therapy (START) study. Circ J. 2015;79:613–622. [DOI] [PubMed] [Google Scholar]
- 9. Moons KG, de Groot JA, Linnet K, Reitsma JB, Bossuyt PM. Quantifying the added value of a diagnostic test or marker. Clin Chem. 2012;58:1408–1417. [DOI] [PubMed] [Google Scholar]
- 10. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new biomarker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 11. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamoto S, Yamazaki S, Shimizu T, Takeshima T, Fukuma S, Yamamoto Y, Tochitani K, Tsuchido Y, Shinohara K, Fukuhara S. Prognostic utility of serum CRP levels in combination with CURB‐65 in patients with clinically suspected sepsis: a decision curve analysis. BMJ Open. 2015;5:e007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siddiqui MM, Rais‐Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL, Linehan WM, Merino MJ, Simon RM, Choyke PL, Wood BJ, Pinto PA. Comparison of MR/ultrasound fusion‐guided biopsy with ultrasound‐guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gorcsan J III, Abraham T, Agler DA, Bax JJ, Derumeaux G, Grimm RA, Martin R, Steinberg JS, Sutton MS, Yu CM; American Society of Echocardiography Dyssynchrony Writing Group . Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting–a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191–213. [DOI] [PubMed] [Google Scholar]
- 16. Goldenberg I, Moss AJ, Hall WJ, Foster E, Goldberger JJ, Santucci P, Shinn T, Solomon S, Steinberg JS, Wilber D, Barsheshet A, McNitt S, Zareba W, Klein H; MADIT‐CRT Executive Committee . Predictors of response to cardiac resynchronization therapy in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT‐CRT). Circulation. 2011;124:1527–1536. [DOI] [PubMed] [Google Scholar]
- 17. Yamamoto M, Seo Y, Ishizu T, Kawamatsu N, Sato K, Sugano A, Atsumi A, Harimura Y, Machino‐Ohtsuka T, Sakamaki F, Aonuma K. Prognostic significance of persistent restrictive filling pattern after cardiac resynchronization therapy. J Echocardiogr. 2015;13:20–26. [DOI] [PubMed] [Google Scholar]
- 18. Park JH, Negishi K, Grimm RA, Popovic Z, Stanton T, Wilkoff BL, Marwick TH. Echocardiographic predictors of reverse remodeling after cardiac resynchronization therapy and subsequent events. Circ Cardiovasc Imaging. 2013;6:864–872. [DOI] [PubMed] [Google Scholar]
- 19. Klein L, Massie BM, Leimberger JD, O'Connor CM, Piña IL, Adams KF Jr, Califf RM, Gheorghiade M; OPTIME‐CHF Investigators . Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF). Circ Heart Fail. 2008;1:25–33. [DOI] [PubMed] [Google Scholar]
- 20. Testani JM, Cappola TP, Brensinger CM, Shannon RP, Kimmel SE. Interaction between loop diuretic‐associated mortality and blood urea nitrogen concentration in chronic heart failure. J Am Coll Cardiol. 2011;58:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–585. [DOI] [PubMed] [Google Scholar]
- 22. Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. [DOI] [PubMed] [Google Scholar]
- 23. Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El AD, Vitovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Janosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled‐release metoprolol on total mortality, hospitalizations, and well‐being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT‐HF). MERIT‐HF Study Group. JAMA. 2000;283:1295–1302. [DOI] [PubMed] [Google Scholar]
- 24. Miller TD, Askew JW. Net reclassification improvement and integrated discrimination improvement: new standards for evaluating the incremental value of stress imaging for risk assessment. Circ Cardiovasc Imaging. 2013;6:496–498. [DOI] [PubMed] [Google Scholar]


