Abstract
Background
Clinical studies implicate trimethylamine N‐oxide (TMAO; a gut microbiota‐dependent nutrient metabolite) in cardiovascular disease risk. There is a lack of population‐based data on the role of TMAO in advancing early atherosclerotic disease. We tested the prospective associations between TMAO and coronary artery calcium (CAC) and carotid intima‐media thickness (cIMT).
Methods and Results
Data were from the Coronary Artery Risk Development in Young Adults Study (CARDIA), a biracial cohort of US adults recruited in 1985–1986 (n=5115). We randomly sampled 817 participants (aged 33–55 years) who attended examinations in 2000–2001, 2005–2006, and 2010–2011, at which CAC was measured by computed tomography and cIMT (2005–2006) by ultrasound. TMAO was quantified using liquid chromotography mass spectrometry on plasma collected in 2000–2001. Outcomes were incident CAC, defined as Agatston units=0 in 2000–2001 and >0 over 10‐year follow‐up, CAC progression (any increase over 10‐year follow‐up), and continuous cIMT. Over the study period, 25% (n=184) of those free of CAC in 2000–2001 (n=746) developed detectable CAC. In 2000–2001, median (interquartile range) TMAO was 2.6 (1.8–4.2) μmol/L. In multivariable‐adjusted models, TMAO was not associated with 10‐year CAC incidence (rate ratio=1.03; 95% CI: 0.71–1.52) or CAC progression (0.97; 0.68–1.38) in Poisson regression, or cIMT (beta coefficient: −0.009; −0.03 to 0.01) in linear regression, comparing the fourth to the first quartiles of TMAO.
Conclusions
In this population‐based study, TMAO was not associated with measures of atherosclerosis: CAC incidence, CAC progression, or cIMT. These data indicate that TMAO may not contribute significantly to advancing early atherosclerotic disease risk among healthy early‐middle‐aged adults.
Keywords: atherosclerosis, biomarker, epidemiology, follow‐up study, risk factor
Subject Categories: Epidemiology, Risk Factors, Computerized Tomography (CT), Atherosclerosis, Biomarkers
Introduction
Recent studies implicate trimethylamine N‐oxide (TMAO; a gut microbiota‐dependent nutrient metabolite) in advancing cardiovascular disease (CVD) risk.1, 2, 3 In the apoE−/− mouse, direct TMAO administration resulted in increased foam cell formation, decreased reverse cholesterol transport, and progression of aortic plaque lesions.1, 3 In a large sample of patients undergoing elective coronary angiography, plasma TMAO concentrations were positively associated with the rate of major CVD events over a 3‐year follow‐up.2 Production of TMAO from dietary precursors relies, in part, on gut bacteria, and atherosclerosis susceptibility has been transferred through gut microbial transplantation in a mouse model.4 These findings have generated interest given that they may point to an important new prognostic variable that is potentially modifiable through targeted diet or gut microbial interventions.
Although animal models indicate that TMAO influences CVD risk through atherosclerosis, there have been no prospective studies of early atherosclerosis progression in population‐based samples. Previous human studies of TMAO and atherosclerosis have been small or cross‐sectional and have yielded mixed results.5, 6 Studies of TMAO and CVD events have been conducted in older clinic‐based samples with a high prevalence of comorbidities,1, 6 and the relationship between TMAO and atherosclerotic disease in younger and healthier individuals is not known. Furthermore, previous data indicate that significantly increased CVD risk may be restricted to higher concentrations of TMAO.2 TMAO has been shown to increase with age,2 and there is a need to understand the relevance of lower TMAO concentrations that are typical among younger individuals.
We tested the hypothesis that plasma TMAO is positively associated with CAC incidence and progression using data from CARDIA (Coronary Artery Risk Development in Young Adults), a prospective, population‐based cohort study of CVD risk evolution in adulthood. Coronary artery calcium (CAC) is a measure of atherosclerosis in the coronary arteries7 and is predictive of fatal and nonfatal coronary heart disease and CVD events.8 In a study of 4 racial/ethnic groups, participants with a CAC score between 1 and 100 had a hazard rate of major coronary events of 3.89, and those with a score between 101 and 300 had a hazard rate of 7.08, as compared to individuals with a CAC score of 0.8 CAC accelerates in early‐middle adulthood,9 before many comorbidities become prevalent in the general population. We measured TMAO and quantified prospective associations between TMAO and 10‐year (1) CAC incidence and (2) CAC progression. Our analysis tests the role of TMAO in the advancement of atherosclerosis in a population‐based sample of adults at a critical life period for CVD prevention.
Materials and Methods
Study Sample
CARDIA, a longitudinal study of cardiometabolic disease risk over adulthood, began in 1985–1986 with 5115 black and white adults aged 18 to 30 years recruited from 4 metropolitan areas (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA).10 There have been 7 follow‐up examinations (years 2, 5, 7, 10, 15, 20, and 25) with the majority of survivors participating (91%, 86%, 81%, 79%, 74%, 72%, and 72%, respectively). CARDIA was approved by institutional review boards of each field center; each study participant provided informed written consent.
The 10‐year study period for the present analysis was 2000–2001 through 2010–2011, years 15, 20, and 25 of CARDIA. We randomly selected 860 individuals from within race‐sex strata of participants who attended the 2000–2001 exam; had CAC data from 2000 to 2001, 2005 to 2006, and 2010 to 2011; and complete covariate data from 2000 to 2001 (Figure). Of these participants, 817 had stored plasma for analysis (Figure). The analytic sample comprised 211 black men, 194 black women, 213 white men, and 199 white women, representing 30%, 19%, 23%, and 19% of the respective stratum‐specific 2000–2001 exam totals.
Figure 1.
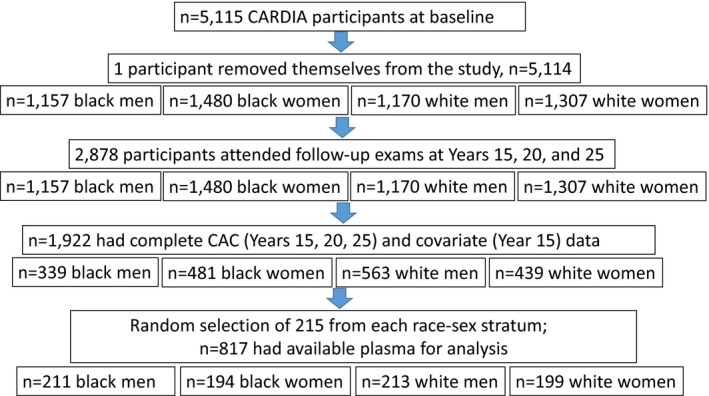
Flow diagram for eligibility and selection of study sample. CAC indicates coronary artery calcium; CARDIA, Coronary Artery Risk Development in Young Adults Study.
TMAO Measurement
Participants were asked to fast ≥12 hours and avoid heavy physical activity and smoking for the 2 hours before the exam. Blood was drawn by venipuncture and stored at 4°C. Within 90 minutes of collection, blood samples were separated through centrifugation, aliquoted into airtight vials, flash‐frozen, and stored at −70°C.
TMAO was quantified using liquid chromatography/stable‐isotope dilution/multiple‐reaction monitoring/mass spectrometry by the University of North Carolina Nutrition Obesity Research Center.11, 12 Using 3 volume units of internal standard‐spiked acetonitrile (40 μmol/L of TMAO‐d9, DLM‐4779‐1; Cambridge Isotope Laboratories, Inc., Tewksbury, MA), samples were extracted for TMAO. Samples were chromatographed on an Atlantis HILIC Silica 3‐μm 4.6×50 mm column (Waters Corporation, Milford, MA) in junction with a Waters ACQUITY UPLC system. The 5‐minute sequencing time consisted of a gradient of 5% A for 0.05 minutes, to 15% A at 0.40 minutes, to 20% A at 1.00 minutes, to 30% A at 2.00 minutes, to 45% A at 2.55 minutes, to 55% A at 2.60 minutes, at 55% A for 0.90 minutes, to 5% A at 3.55 minutes, at 5% A for the remainder of the sequence. Mobile phase A was composed of 10% acetonitrile and 90% water with 10 mmol/L of ammonium formate and 0.125% formic acid; mobile phase B was 90% acetonitrile and 10% water with 10 mmol/L of ammonium formate and 0.125% formic acid. Flow rate was kept constant at 1 mL/min, and the column manager was set at 40°C for the duration of the sequence. A Waters TQ detector equipped with an electrospray ionization probe operating in positive‐ion mode was used for mass spectrometric analysis. TMAO specificity was maintained by monitoring ion transitions from precursor to product ions for both TMAO (75→58) and the internal standard, TMAO‐d9 (85→66). Quantification was achieved by using a calibration curve constructed from the peak area ratios of TMAO to its internal standard. Assay quality assurance was monitored by routine analysis of pooled quality control (QC) plasma at 2 concentrations of TMAO: a low quality control (LQC) consisted of normal pooled human plasma with endogenous TMAO and a high quality control (HQC) prepared by spiking a stock solution of TMAO into pooled LQC plasma sample for a final concentration of 50.0 μmol/L. Two of each QC sample were extracted simultaneously with CARDIA samples and standards per each assay. Coefficients of variation were 6.1% for TMAO, 5.8% for LQC, and 4.11% for HQC. Limits of detection and quantification for TMAO were 0.03 and 0.06 μmol/L, respectively; the linear range was 0.06 to 500 μmol/L.13
CAC Measurement
In 2000–2001 and 2005–2006, an electron‐beam computed tomography (CT) scanner (Imatron C‐150; GE Imatron, San Francisco, CA) was used at the Chicago and Oakland field centers, and a multidetector CT scanner (GE Lightspeed; General Electric, Fairfield, CT; Siemens VZ; Siemens AG, Munich, Germany) was used at the Birmingham and Minneapolis centers to obtain contiguous 2.5‐ to 3.0‐mm‐thick transverse images from the root of the aorta to the apex of the heart in 2 sequential scans; in 2010–2011, multidetector CT scanners were used at all field centers.14 Scan data were transmitted electronically to an independent CT reading center, where a trained technician examined each image and identified potential foci of CAC. An expert investigator adjudicated all discordant scan pairs. There was high interobserver (k=0.89) and intraobserver (k=0.95) agreement for the presence of CAC.14 A total calcium score (Agatston units)7 was calculated by summing the scores of all lesions within and across arteries (left anterior descending, left main, circumflex, and right coronary). Means of the 2 scans were used in analysis.
Other Measurements
Standard questionnaires were used to obtain demographic (age, sex, race, and education) and behavioral (physical activity, cigarette smoking, and alcohol consumption) data. The validated interview‐administered CARDIA Physical Activity History queried past‐year engagement in 13 activities, from which a total activity score was calculated.15 Participants reported their medication use for hypertension, lipid lowering, and diabetes mellitus. Diet was assessed with an interviewer‐administered, validated dietary history in 1985–1986, 1992–1993, and 2005–2006.16
Standardized protocols were used by trained staff for all clinic measures. Height and weight were measured to the nearest 0.50 cm and 0.20 kg, respectively, for body mass index (BMI; kg/m2). Resting systolic and diastolic blood pressure values were calculated as the mean of the second and third of 3 measurements taken with a random‐zero sphygmomanometer. Plasma concentrations of total cholesterol, high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), and triglycerides were determined using enzymatic procedures or estimated by the Friedewald equation. Serum glucose was measured using hexokinase coupled to glucose‐6‐phosphate dehydrogenase. Insulin was measured with the use of a radioimmunoassay. Diabetes mellitus was defined as having fasting glucose level ≥126 mg/dL (7 mmol/L), 2‐hour oral glucose tolerance test (OGTT) ≥200 mg/dL (11.1 mmol/L), glycated hemoglobin (HbA1c) ≥6.5% (48 mmol/mol), or use of hypoglycemic medications. Plasma C‐reactive protein (CRP) was measured using high‐sensitivity, nephelometry‐based methods, soluble intracellular adhesion molecule 1 (sICAM‐1) by enzyme‐linked immunosorbent assay, and F2‐isoprostanes by gas chromatography/mass spectrometry. Urine albumin was measured using a nephelometric procedure with a specific antialbumin monoclonal antibody and creatinine using the Jaffe method. Carotid intima‐media thickness (cIMT) was assessed in 2005–2006 from high‐resolution B‐mode ultrasound images at 3 levels: common carotid artery, carotid artery bulb, and internal carotid artery. We used the average maximum common cIMT (4 measurements) in analysis.
Statistical Analysis
Educational status was modeled as the maximum (at any visit) reported years completed. Smoking was classified as current versus former/never. To account for typical dietary consumption, we averaged consumption of TMAO precursor food groups, including eggs and red meat, from years 7 (1992–1993) and 20 (2005–2006) of CARDIA, as well as a measure of diet quality.17
We natural log‐transformed CRP and triglycerides to normalize data. Estimated glomerular filtration rate (eGFR) (mL/min per 1.73 m2) was calculated from serum creatinine using the 2009 CKD‐EPI (CKD Epidemiology Collaboration) equation.18 Urine albumin‐creatinine ratios (UACRs) were standardized to sex and race and expressed in milligrams per gram of creatinine. We estimated insulin resistance using homeostasis model assessment for insulin resistance (HOMA‐IR).19
Multivariable‐adjusted regression models controlled for 2000–2001 covariate values. Variables were included in the model as continuous, unless noted. A minimally adjusted model (model 1) controlled for participant sex (male, female), study center (Birmingham, Chicago, Minneapolis, or Oakland), race (white, black), and age. Additional adjustment (model 2) included years of education, current cigarette smoking (current, never/former), intensity units of physical activity, systolic blood pressure, LDL‐C, HDL‐C, natural log‐transformed triglycerides, HOMA‐IR, BMI, natural log‐transformed high‐sensitivity CRP, eGFR, and UACR. We tested the sensitivity of models additionally adjusted for the use of lipid‐lowering or blood pressure mediation, sICAM‐1 and F2‐isoprostanes, and dietary variables.
Incident CAC was defined as having an Agatston score of 0 in 2000–2001 (Year 15) and a score >0 at follow‐up. We used Poisson regression with an offset of time to incident CAC to estimate the effect of TMAO on 10‐year CAC incidence. To account for CT error in detection of CAC, we reran models based on a ≥10 score increase. We also defined 10‐year CAC progression as any CAC increase over follow‐up (including prevalent CAC in 2000–2001) and estimated relative rates of 10‐year CAC progression with respect to TMAO using Poisson regression.
We assessed the sensitivity of results to the specification of TMAO in regression models. In addition to our primary analysis of sample‐based quartiles, we included linear and quadratic specifications of continuous TMAO, and TMAO categories with cutpoints from a previous study of TMAO and CVD (<2.43, 2.43–3.66, 3.67–6.18, and >6.18 μmol/L).2
We additionally considered prospective associations between TMAO and 10‐year incidence of nonfatal CVD events using Cox proportional hazards regression, association between TMAO in 2000–2001 and level of common cIMT in 2005–2006 using linear regression, and the prospective association between TMAO and 10‐year changes in eGFR and UACR using linear regression. We assessed potential selectivity of our study sample by comparing those eligible for our analysis (required to attend all 3 exams in the study period) and all participants who attended the 2000–2001 exam. Statistical analyses were completed with SAS (v9.4; SAS Institute Inc., Cary, NC) and Stata/MP (v14.0; StataCorp LP, College Station, TX) software.
Results
TMAO had an overall median (interquartile range; IQR) of 2.6 μmol/L (1.8–4.2), with medians of 1.3 μmol/L (IQR, 1.1, 1.5) in the lowest quartile and 6.6 μmol/L (5.1, 10.1) in the highest quartile (Table 1). Quartile cutpoints of TMAO were <1.8, 1.8 to 2.5, 2.6 to 4.2, and >4.2 μmol/L, respectively. Across TMAO quartiles, there were significant differences in age, education, sex, HDL‐C, triglycerides, and eGFR (Table 1). TMAO was positively associated with egg consumption (P=0.02) and had a marginal positive association with red meat consumption (P=0.07), but was not associated with other dietary variables (Table 2).
Table 1.
Participant Characteristicsa According to Quartile of Plasma TMAO (n=817): CARDIA, 2000–2001
| Quartiles of Plasma TMAO | P Valueb | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| N | 203 | 199 | 214 | 201 | |
| TMAO, μmol/L, median (IQR) | 1.3 (1.1, 1.5) | 2.1 (1.9, 2.3) | 3.1 (2.8, 3.5) | 6.6 (5.1, 10.1) | <0.001 |
| Age, y | 39.8 (3.5) | 39.7 (3.6) | 40.8 (3.5) | 40.3 (3.8) | 0.002 |
| Education, y | 15.5 (2.4) | 15.4 (2.4) | 16.2 (2.6) | 15.7 (2.5) | 0.013 |
| White race, % | 44.8 | 48.2 | 55.1 | 52.7 | 0.141 |
| Female, % | 61.6 | 49.3 | 40.8 | 40.9 | <0.001 |
| Current smoking, % | 14.8 | 21.6 | 21.6 | 21.7 | 0.240 |
| Study center, % | |||||
| Birmingham, Alabama (n=234) | 27.8 | 24.4 | 23.9 | 23.9 | |
| Chicago, Illinois (n=248) | 26.2 | 23.4 | 21.8 | 28.6 | |
| Minneapolis, Minnesota (n=165) | 17.6 | 27.9 | 32.1 | 22.4 | |
| Oakland, California (n=170) | 25.9 | 22.4 | 30.0 | 21.8 | 0.129 |
| Physical activity unitsc, median (IQR) | 300 (138, 498) | 286 (150, 476) | 339 (174, 533) | 295 (157, 472) | 0.230 |
| BMI, kg/m2 | 28.8 (6.8) | 28.1 (5.2) | 28.0 (5.2) | 28.7 (5.7) | 0.719 |
| HOMA‐IRd, median (IQR) | 1.75 (1.26, 2.38) | 1.75 (1.32, 2.53) | 1.86 (1.31, 2.65) | 1.91 (1.42, 2.71) | 0.502 |
| LDL cholesterol, mg/dL | 115 (31) | 117 (32) | 112 (29) | 118 (34) | 0.267 |
| HDL cholesterol, mg/dL | 53 (14) | 50 (13) | 49 (14) | 49 (15) | 0.014 |
| Triglycerides, mg/dL, natural log‐transformed | 4.4 (0.5) | 4.5 (0.5) | 4.5 (0.5) | 4.5 (0.5) | 0.017 |
| Systolic blood pressure, mm Hg | 112 (14) | 111 (13) | 113 (15) | 112 (13) | 0.714 |
| CRP, μg/mL, natural log‐transformed, median (IQR) | 0.18 (−0.18, 0.83) | 0.16 (−0.19, 0.62) | 0.09 (−0.14, 0.55) | 0.18 (−0.17, 0.66) | 0.342 |
| F2‐isoprostanes, ng/L, median (IQR) | 49.03 (40.9, 64.2) | 49.4 (39.7, 66.7) | 47.8 (37.9, 60.6) | 53.2 (41.4, 71.0) | 0.154 |
| Soluble ICAM‐1, μg/L | 150 (37) | 157 (41) | 156 (67) | 155 (41) | 0.256 |
| Estimated glomerular filtration rate (eGFR)e, mL/min per 1.73 m2 | 106 (16) | 102 (16) | 100 (17) | 101 (17) | 0.002 |
| eGFR <60 mL/min per 1.73 m2, % | 0 | 0 | 1.40 | 1.00 | 0.161 |
| eGFR <100 mL/min per 1.73 m2, % | 37.9 | 48.7 | 52.3 | 48.3 | 0.023 |
| Urine albumin/creatinine ratio (UACR), mg/g, median (IQR) | 3.88 (3.18, 5.94) | 3.76 (2.84, 5.37) | 3.85 (3.01, 6.3) | 3.88 (3.12, 6.96) | 0.554 |
| UACR <30 mg/g, % | 2.46 | 1.51 | 4.21 | 3.98 | 0.337 |
| History of diabetes mellitusf, % | 3.45 | 4.02 | 5.14 | 2.49 | 0.553 |
| Blood pressure medication useg, % | 8.9 | 10.1 | 8.3 | 5.9 | 0.509 |
| Lipid‐lowering medication useh, % | 0.49 | 3.02 | 3.21 | 0.99 | 0.097 |
ACE indicates angiotensin‐converting enzyme; BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults Study; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CRP, C‐reactive protein; Hb1Ac, glycated hemoglobin; HDL, high‐density lipoprotein; HMG‐CoA, 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A; HOMA‐IR, homeostasis model assessment for insulin resistance; ICAM‐1, intracellular adhesion molecule 1; IQR, interquartile range; LDL, low‐density lipoprotein; OGTT, oral glucose tolerance test; TMAO, trimethylamine N‐oxide.
Mean (SD) unless noted.
Comparisons across TMAO quartiles were from chi‐square test for categorical variables, the Kruskal–Wallis test for means, and the Brown–Mood test (multisample median test) for medians.
Physical activity units derived from the CARDIA physical activity questionnaire and reflect frequency and intensity of engagement in 13 activities.15
HOMA‐IR defined according to Matthews et al.19
eGFR was calculated from serum creatinine using the 2009 CKD‐EPI equation.18
Any history of diabetes mellitus from 1985 to 1986 (CARDIA baseline), defined as having at least 1 of the following: fasting glucose ≥126 mg/dL, 2‐hour OGTT ≥200 mg/dL, HbA1c ≥6.5%, or using hypoglycemic medications.
Blood pressure medications include: ACE inhibitors, alpha‐adrenergic blockers, beta‐adrenergic blockers, calcium‐channel blockers, loop diuretics, potassium‐sparing diuretics, thiazide diuretic use.
Lipid‐lowering medication use include: HMG‐CoA reductase inhibitors (statins), gemfibrozil.
Table 2.
Dietary Variablesa (Mean [SD]) According to Quartiles of TMAO
| Quartiles of Plasma TMAO | P Value | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Fast food consumption, times/weekb | 2.11 (2.91) | 2.18 (2.72) | 1.87 (2.11) | 2.30 (3.02) | 0.477 |
| Diet quality scorec | 62.9 (11.0) | 62.2 (10.8) | 63.2 (10.2) | 62.5 (10.3) | 0.693 |
| Eggs | 0.49 (0.46) | 0.64 (0.79) | 0.57 (0.47) | 0.67 (0.79) | 0.008 |
| Processed meat | 0.83 (0.81) | 0.92 (0.88) | 0.95 (0.96) | 0.92 (0.91) | 0.372 |
| Lean red meat | 0.44 (0.49) | 0.47 (0.50) | 0.51 (0.51) | 0.52 (0.74) | 0.396 |
| Regular (nonlean) red meat | 1.60 (1.44) | 1.81 (1.62) | 1.69 (1.29) | 1.88 (1.37) | 0.071 |
| Poultry | 1.46 (1.14) | 1.57 (1.56) | 1.45 (1.15) | 1.47 (1.25) | 0.985 |
| Fish | 0.04 (0.12) | 0.03 (0.09) | 0.05 (0.17) | 0.04 (0.14) | 0.952 |
| Total red meatd | 2.87 (2.22) | 3.21 (2.41) | 3.14 (2.25) | 3.32 (2.30) | 0.120 |
| Total precursorse | 3.42 (2.46) | 3.9 (2.88) | 3.76 (2.45) | 4.04 (2.72) | 0.056 |
CARDIA indicates Coronary Artery Risk Development in Young Adults Study; TMAO, trimethylamine N‐oxide.
Unless otherwise noted, mean consumption, in servings per day, of food groups reported on dietary assessments at CARDIA exams in 1992–1993 and 2005–2006.
Self‐reported fast food consumption from 2000 to 2001.
Mean diet quality scores from dietary assessments at CARDIA exams in 1992–1993 and 2005–2006. CARDIA diet quality score derived as previously described.17 Higher scores reflect greater consumption of food groups hypothesized to be beneficial to health, relative to consumption of food groups considered adverse to health.
Total red meat is sum of servings per day of processed meat, lean red meat, and regular (nonlean) red meat.
Total precursors is the sum of servings per day of eggs, processed meat, lean red meat, regular (nonlean) red meat, poultry, and fish.
CAC Incidence
Over the 10‐year period, 184 individuals developed detectable CAC among those who were free of CAC at baseline (n=746). In multivariable‐adjusted Poisson regression analysis, TMAO measured in 2000–2001 was not significantly associated with 10‐year CAC incidence, among the 746 individuals free of CAC at baseline (rate ratio [RR]=1.03; 95% CI: 0.71–1.52, for the fourth vs first quartile; Table 3). These findings were not affected by adjustment for an extensive set of covariates, including CVD risk factors, kidney function, or egg and red meat consumption, or by alternative specifications of TMAO. In addition, further adjustment for blood pressure or lipid‐lowering medication use did not materially change findings (RR for CAC incidence=1.06; 95% CI: 0.72–1.55, comparing the fourth to the first quartile of TMAO).
Table 3.
Multivariable‐Adjusted Effect Estimates (95% CI) for Plasma TMAO and 10‐Year CAC Incidencea
| Sample‐Based Quartiles of Plasma TMAO, μmol/L | ||||
|---|---|---|---|---|
| Q1 (Ref) | Q2 | Q3 | Q4 | |
| TMAO, μmol/L | <1.71 | 1.71 to 2.50 | 2.60 to 4.20 | >4.20 |
| n (cases) | 190 (51) | 186 (49) | 192 (53) | 178 (60) |
| Rate ratios (95% CIs) for TMAO and 10‐year CAC incidenceb | ||||
| Model 1c | 1 | 0.89 (0.60, 1.31) | 0.83 (0.56, 1.23) | 1.06 (0.73, 1.54) |
| Model 2d | 1 | 0.88 (0.59, 1.30) | 0.82 (0.55, 1.23) | 1.03 (0.71, 1.52) |
| Categories of TMAO (μmol/L) Presented in Tang et al2 | ||||
|---|---|---|---|---|
| C1 (Ref) | C2 | C3 | C4 | |
| TMAO, μmol/L | <2.43 | 2.43 to 3.66 | 3.67 to 6.18 | >6.18 |
| n (cases) | 355 (90) | 174 (52) | 118 (38) | 99 (33) |
| Rate ratios (95% CIs) for TMAO and 10‐year CAC incidence | ||||
| Model 1 | 1 | 1.05 (0.74, 1.49) | 1.13 (0.77, 1.66) | 1.12 (0.75, 1.68) |
| Model 2 | 1 | 1.08 (0.75, 1.54) | 1.13 (0.77, 1.67) | 1.09 (0.73, 1.64) |
BMI indicates body mass index; CAC, coronary artery calcium; CARDIA, Coronary Artery Risk Development in Young Adults Study; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment for insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; TMAO, trimethylamine N‐oxide; UACR, urine albumin/creatinine ratio.
CAC incidence was defined as having CAC score=0 in 2000–2001 and CAC score >0 in 2005–2006 or 2010–2011. CAC cases are incident CAC over the 10‐year study period.
Rate ratios (RR) were obtained from Poisson regression using SAS PROC GENMOD. TMAO was measured in 2000–2001 and CAC was assessed at 2000–2001, 2005–2006, and 2010–2011.
Model 1 was adjusted for age, race (black/white), sex (male/female), and CARDIA field center (Birmingham, AL; Chicago, IL; Minneapolis, MN; Oakland, CA).
Model 2 was additionally adjusted for physical activity (CARDIA physical activity units), smoking (never or former/current), BMI, CRP (natural log‐transformed), HOMA‐IR, eGFR, UACR, LDL‐C, HDL‐C, systolic blood pressure, and triglycerides (natural log‐transformed).
We were limited in our ability to reliably examine associations according to higher levels of TMAO: 46% of our sample had a TMAO value that fell within the first quartile of TMAO concentration reported by Tang et al in an older clinic‐based sample.2 Effect estimates for incident CAC were higher using the cutpoints of Tang et al, though did not achieve statistical significance (RRs [95% CIs] for second, third, and fourth category, respectively, compared to the first: 1.08 [0.75–1.54], 1.13 [0.77–1.67], and 1.09 [0.73–1.64]; Table 3). We tested other specifications of TMAO as well. Linear TMAO from a continuous specification was not statistically significantly associated with incident CAC (RR=1.01; 95% CI: 0.99–1.02). A quadratic TMAO specification also did not support an association between TMAO and CAC (data not shown).
CAC Progression
In multivariable‐adjusted Poisson regression analysis, TMAO was also not associated with CAC progression over the 10‐year follow‐up period (RR=0.97; 95% CI: 0.68–1.38, for the fourth vs first quartile; Table 4). Results did not change with additional adjustment for lipid‐lowering or blood pressure medication use (RR for CAC progression=0.99; 95% CI: 0.70, 1.41, for the fourth vs first quartile). CAC progression was also not statistically significantly associated with TMAO using the cutpoints of Tang et al (Table 4) or with specifications of continuous TMAO as linear or quadratic (data not shown).
Table 4.
Multivariable‐Adjusted Effect Estimates (95% CI) for TMAO and 10‐Year CAC Progressiona
| Sample‐Based Quartiles of Plasma TMAO, μmol/L | ||||
|---|---|---|---|---|
| Q1 (Ref) | Q2 | Q3 | Q4 | |
| TMAO, μmol/L | <1.71 | 1.71 to 2.50 | 2.60 to 4.20 | >4.20 |
| n (cases) | 203 (58) | 199 (54) | 214 (65) | 201 (74) |
| Rate ratios (95% CIs) for TMAO and 10‐year CAC progressionb | ||||
| Model 1c | 1 | 0.85 (0.59, 1.24) | 0.81 (0.56, 1.16) | 1.01 (0.71, 1.43) |
| Model 2d | 1 | 0.85 (0.58, 1.23) | 0.79 (0.55, 1.15) | 0.97 (0.68, 1.38) |
| Categories of TMAO (μmol/L) Presented in Tang et al2 | ||||
|---|---|---|---|---|
| C1 (Ref) | C2 | C3 | C4 | |
| TMAO, μmol/L | <2.43 | 2.43 to 3.66 | 3.67 to 6.18 | >6.18 |
| n (cases) | 378 (100) | 195 (66) | 136 (66) | 108 (38) |
| Rate ratios (95% CIs) for TMAO and 10‐year CAC progression | ||||
| Model 1 | 1 | 1.05 (0.77, 1.45) | 1.06 (0.75, 1.51) | 1.09 (0.75, 1.59) |
| Model 2 | 1 | 1.08 (0.78, 1.49) | 1.02 (0.72, 1.46) | 1.06 (0.72, 1.56) |
BMI indicates body mass index; CAC, coronary artery calcium; CARDIA, Coronary Artery Risk Development in Young Adults Study; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment for insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; TMAO, trimethylamine N‐oxide; UACR, urine albumin/creatinine ratio.
CAC progression was defined as any increase in detectable CAC over the 10‐year period, including incident CAC (CAC score=0 in 2000–2001 and CAC score >0 in 2005–2006 or 2010–2011) among those with CAC=0 in 2000–2001 or any increase in CAC in 2005–2006 or 2010–2011 among those with CAC >0 in 2000–2001.
Rate ratios (RR) were obtained from Poisson regression using SAS PROC GENMOD. TMAO was measured in 2000–2001 and CAC was assessed at 2000–2001, 2005–2006, and 2010–2011.
Model 1 was adjusted for age, race (black/white), sex (male/female), and CARDIA field center (Birmingham, AL; Chicago, IL; Minneapolis, MN; Oakland, CA).
Model 2 was additionally adjusted for physical activity (CARDIA physical activity units), smoking (never or former/current), BMI, CRP (natural log‐transformed), HOMA‐IR, eGFR, UACR, LDL‐C, HDL‐C, systolic blood pressure, and triglycerides (natural log‐transformed).
Other CVD‐Related Outcomes
TMAO was not associated with cIMT measured in 2005–2006 (beta coefficients [95% CI] from a linear regression model was −0.009 [−0.03, 0.01], comparing the fourth to the first quartiles of TMAO; Table 5). TMAO was not associated with the 10‐year incidence of nonfatal CVD events, although power for this analysis was low, with only 22 CVD events in our sample over the 10‐year study period (data not shown). TMAO was also not associated with 10‐year changes in eGFR or UACR (Table 6). In cross‐sectional analysis (2000–2001), there was a suggestion of higher TMAO at lower eGFR, although few participants had low eGFR values (n=5 had eGFR <60 mL/min per 1.73 m2) and differences were not statistically significant (data not shown).
Table 5.
Multivariable‐Adjusteda Effect Estimates (95% CI) for the Association Between TMAO (2000–2001) and Carotid Intima‐Media Thickness (2005–2006)
| Quartiles of Plasma TMAO | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| cIMTb | ||||
| nc | 193 | 180 | 200 | 193 |
| Mean (SD) cIMT | 0.80 (0.12) | 0.79 (0.11) | 0.81 (0.15) | 0.80 (0.13) |
| Beta coefficient (95% CI)d | Ref | −0.014 (−0.04, 0.01) | −0.006 (−0.03, 0.02) | −0.009 (−0.03, 0.01) |
cIMT indicates carotid intima‐media thickness; TMAO, trimethylamine N‐oxide.
Regression models adjusted for age, sex, race, study center, educational attainment, current smoking status, physical activity, and body mass index. All covariates measured in 2000–2001.
Mean common carotid artery based on 4 measurements taken in 2005–2006.
Analytic sample size.
Beta coefficient from multivariable‐adjusted linear regression for the association between TMAO (2000–2001) and cIMT (2005–2006).
Table 6.
Multivariable‐Adjusteda Effect Estimates (95% CI) for Prospective Associations Between TMAO (2000–2001) and 10‐Year Changes in Measures of Kidney Function
| Quartiles of Plasma TMAO | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| 10‐year changesb | ||||
| 10‐year change in eGFR | −8.53 (14.3) | −5.71 (13.9) | −7.09 (14.0) | −8.10 (13.5) |
| Beta‐coefficients (95% CI) | Ref | 2.80 (0.07, 5.53) | 1.05 (−1.66, 3.77) | 0.22 (−2.53, 2.96) |
| 10‐year change in UACR | 0.63 (14.8) | 3.30 (13.5) | 3.24 (26.8) | 1.04 (19.7) |
| Beta‐coefficients (95% CI) | Ref | 2.63 (−1.25, 6.50) | 2.34 (−1.51, 6.19) | 0.21 (−3.68, 4.11) |
eGFR indicates estimated glomerular filtration rate; TMAO, for trimethylamine N‐oxide; UACR, urine albumin/creatinine ratio.
Regression models adjusted for age, sex, race, study center, educational attainment, current smoking status, physical activity, and body mass index. All covariates measured in 2000–2001.
Beta coefficients from linear regression for the association between TMAO (2000–2001) and 10‐year changes in eGFR or UACR. Change variables were defined as 10‐year difference in each continuous measure (eg, [2010–2011 eGFR]−[2000–2001 eGFR]).
Individuals who were eligible for our study were somewhat healthier, as compared to those who were ineligible, as expected, given our requirements of survival and participation in the 3 CARDIA exams over the 10‐year study period. However, CVD event rates were not appreciably different among those eligible and ineligible for our study. Furthermore, we note that a large percentage of participants had incident or progressive CAC over follow‐up; in addition, measures predictive of CAC in previous work were significantly associated in our sample, including sICAM‐120 and duration of adiposity21 (data not shown).
Discussion
In a cohort of adults, aged 33 to 45, plasma TMAO concentration was not associated with CAC incidence or progression over 10 years of follow‐up, after accounting for an extensive set of potential confounders. Nor was TMAO associated with other measures of CVD risk, including cIMT, insulin resistance, inflammatory markers, and lipids. TMAO concentrations in our sample were notably lower than in previous work that showed a positive association between TMAO and CVD risk.2 Our results contribute to refining our understanding of population subgroups that may be most susceptible to the adverse effects of TMAO. In particular, our findings suggest that TMAO levels may not significantly influence progression of early atherosclerosis.
Our results are not consistent with our hypothesis that TMAO is positively associated with CAC in an early‐middle‐age sample; however, our results are not necessarily inconsistent with previous reports that support a role for higher TMAO concentrations in advancing CVD risk in other groups. In a cohort of 4007 patients undergoing elective coronary angiography, Tang et al found that TMAO significantly predicted 3‐year major CVD events (n=513) among individuals at the highest quartile of TMAO (>6.18 μmol/L), as compared to the lowest quartile, in adjusted analysis (hazard rate [HR]=1.49; 95% CI, 1.10–2.03), but not at lower TMAO concentrations (HR=1.08, 0.79–1.48 and 1.15, 0.85–1.56 for second and third quartiles, respectively).2 Using the same TMAO cutpoints, we observed similar point estimates for the second and third categories (respectively, 1.08 and 1.13; Table 3), but we had limited power to estimate the effect of TMAO among individuals (n=99; 33 CAC) with TMAO >6.18 μmol/L (at which we observed an estimate of 1.09 [0.73–1.64]). It is possible that the adverse effect of TMAO is limited to higher concentrations than we observed in our sample.
It is also possible that TMAO may be etiologically relevant at later stages of the disease process, within high‐risk subgroups, or among older individuals. We and others2 have found that TMAO increases significantly with age. Strong support for a role of TMAO in CVD risk came initially from a sample of individuals receiving diagnostic angiography with a mean age of 63 years.2 Study investigators controlled for an extensive set of risk factors in regression analysis and showed that TMAO was also predictive among lower‐risk individuals in a subgroup analysis.2 These analytic approaches will not, however, compensate for meaningful differences between studies, most notably the younger age distribution and lower overall risk burden in our sample, if there are differential subgroup effects. For example, some studies show that TMAO may be most predictive of CVD among individuals with known comorbidities, including diabetes mellitus,22 heart failure,23, 24, 25 or chronic kidney disease,26 though such results have been inconsistently reported.27
High TMAO reflects higher consumption of TMAO precursors, some of which are also hypothesized to influence CVD risk. In the present analysis, statistical adjustment for dietary precursors, including red meat, eggs, and fish, did not materially impact effect estimates. The role of dietary precursors for TMAO in CVD risk is mixed, with red meat shown to increase CVD risk, whereas fish is considered cardioprotective.28 In addition, recent data suggest that oral supplementation with TMAO precursor l‐carnitine may contribute to lowering plasma lipoprotein(a).29
We assessed the role of TMAO in advancing early atherosclerotic disease. One consideration is the relevance of studying a relatively young and healthy sample. However, this is a critical life period for primary prevention activities and 25% of our study sample developed detectable CAC over the 10‐year study period. In addition, many studies have documented strong associations between risk factors measured from young adulthood to early middle age and CAC. For example, in previous CARDIA analysis, CAC at ages 33 to 55 has been associated with obesity,21, 30 F2‐isoprostanes,20 and nonoptimal lipids31 measured in young adulthood or early middle age. Other studies have reported associations between traditional CVD risk factors and CAC measured at even younger ages.32
We studied CAC because it is a strong predictor of future CVD events among asymptomatic individuals8, 33 and because of compelling data that support an etiological role for TMAO in atherosclerosis.1, 3, 4 In the apoE−/− mouse model, TMAO increased foam cell formation and accelerated progression of aortic plaque lesions.1 Furthermore, in cross‐sectional analysis of 1020 patients, there was a positive dose‐response relationship between plasma TMAO and the number of coronary vessels (0–3) with ≥50% stenosis on diagnostic coronary angiography.1 It is possible that our measures of atherosclerosis—CAC and carotid IMT—do not reflect TMAO‐related atherosclerotic processes. The precise mechanism through which TMAO may impact CVD risk remains under study, with reported data supporting potential effects on reverse cholesterol transport3 and platelet activation.34 More work is needed to determine the underlying mechanisms by which TMAO may affect disease risk, but published findings underscore the range of possible pathways through which TMAO may influence CVD risk. Still, our results were not expected, given that individuals with higher CAC scores have been shown to have greater total coronary plaque (calcified and noncalcified),35 and calcification is associated with high‐risk plaques and with acute coronary syndrome lesions.36, 37, 38
A strength of our study was the ability to study the prospective association between TMAO and CAC in a relatively young and healthy population‐based sample. We know of no other prospective study of TMAO and atherosclerosis in a population‐based sample. Cross‐sectional studies have yielded inconsistent support for an association between TMAO and atherosclerosis and have been limited by small samples or patient‐based selection.5, 25, 39, 40, 41
In addition, our study limited the potential for confounding by comorbidities, such as kidney function, an independent risk factor for CVD.42 TMAO is excreted in urine,43, 44 and circulating TMAO increases as kidney function declines.45 The consideration that circulating TMAO may reflect kidney function, however, does not refute the possibility that high TMAO concentrations may adversely affect cardiovascular health, if, for example, TMAO influences atherosclerosis through a pathway related to declining kidney function. In C57BL/6J male mice, administration of TMAO promoted renal fibrosis and dysfunction.26 Among 1434 Framingham Offspring Study participants, TMAO was 1 of 16 metabolites identified to be positively associated with incident chronic kidney disease (n=123 cases) over 8 years of follow‐up.46 We found some evidence that TMAO was associated with decreased kidney function in our analysis, though findings were not statistically significant.
Additional strengths of the CARDIA study include extensive covariate data on traditional CVD risk factors, diet, kidney function, and inflammatory markers. In addition, our study captured a period of significant CAC progression—the prevalence of individuals with any CAC increased from 9% to 30% over the 10‐year follow‐up—and an important period for CVD prevention activities.
Along with the relative youth of our sample, eligibility criteria for our study selected for a generally healthy sample. Specifically, our requirement that individuals have all 3 CAC measurements eliminated participants who died or were lost to follow‐up over the 10‐year study period. However, as noted, CVD event rates were not appreciably different among those eligible and ineligible for our study, and significant associations have been found in previous studies of incident CAC with this same sample restriction.
Our study was not powered for the analysis of CVD events or subgroup differences, though, importantly, we had sufficient power to replicate previously reported associations between risk factors and CAC progression in the full CARDIA sample.20, 21 Our analysis relied on analysis of TMAO from plasma that had been stored at −70°C from the time of fasting blood collection at the 2000–2001 exam. TMAO has been shown to be stable when stored at −80°C over 5 years, despite multiple freeze‐thaw cycles,47 but we know of no data on the stability of TMAO over 15 years. TMAO concentrations (median=2.6 μmol/L [IQR, 1.8–4.2]) in our sample were comparable to age‐matched levels in a community‐based sample (n=349) of healthy individuals.47
In conclusion, in this population‐based sample of early‐middle‐aged adults, plasma TMAO was not associated with 10‐year CAC incidence or progression or cIMT. Our results support the need for further research to define the role of TMAO in CVD‐related outcomes across the distribution of circulating TMAO concentrations and among population groups with variable underlying CVD risk or risk factors such as diet or gut microbiota.
Sources of Funding
This work was supported by grants from the National Heart, Lung and Blood Institute (NHLBI) for statistical analysis and manuscript preparation (K01‐HL127159; Meyer) and for measurement and analysis of CAC (R01‐HL098445; Carr); Egg Nutrition Center (Meyer) and UNC Nutrition Research Institute (Meyer) for TMAO assays; and National Institute of Diabetes and Digestive and Kidney Diseases (P30‐DK056350; Zeisel) for general support. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the NHLBI, the Intramural Research Program of the National Institute on Aging (NIA), and an intra‐agency agreement between the NIA and NHLBI (AG0005).
Disclosures
This work was partially funded by a research grant from the Egg Nutrition Center (Meyer). The Egg Nutrition Center was not involved in study conceptualization, implementation, or conduct; interpretation or presentation of data; or manuscript preparation, review, or approval. There are no other disclosures to report.
(J Am Heart Assoc. 2016;5:e003970 doi: 10.1161/JAHA.116.003970)
References
- 1. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L‐carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, Lusis AJ, Hazen SL. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mente A, Chalcraft K, Ak H, Davis AD, Lonn E, Miller R, Potter MA, Yusuf S, Anand SS, McQueen MJ. The relationship between trimethylamine‐N‐oxide and prevalent cardiovascular disease in a multiethnic population living in Canada. Can J Cardiol. 2015;31:1189–1194. [DOI] [PubMed] [Google Scholar]
- 6. Haissman JM, Knudsen A, Hoel H, Kj AA, Kristoffersen US, Berge RK, Katzenstein TL, Svardal A, Ueland T, Aukrust P, Lebech AM, Nielsen SD, Trøseid M. Microbiota‐dependent marker TMAO is elevated in silent ischemia but is not associated with first‐time myocardial infarction in HIV infection. J Acquir Immune Defic Syndr. 2016;71:130–136. [DOI] [PubMed] [Google Scholar]
- 7. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 8. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 9. Hoff JA, Chomka EV, Krainik AJ, Daviglus M, Rich S, Kondos GT. Age and gender distributions of coronary artery calcium detected by electron beam tomography in 35,246 adults. Am J Cardiol. 2001;87:1335–1339. [DOI] [PubMed] [Google Scholar]
- 10. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 11. daCosta KA, Vrbanac JJ, Zeisel SH. The measurement of dimethylamine, trimethylamine, and trimethylamine N‐oxide using capillary gas chromatography‐mass spectrometry. Anal Biochem. 1990;187:234–239. [DOI] [PubMed] [Google Scholar]
- 12. Miller CA, Corbin KD, da Costa KA, Zhang S, Zhao X, Galanko JA, Blevins T, Bennett BJ, O'Connor A, Zeisel SH. Effect of egg ingestion on trimethylamine‐N‐oxide production in humans: a randomized, controlled, dose‐response study. Am J Clin Nutr. 2014;100:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao X, Zeisel SH, Zhang S. Rapid LC‐MRM‐MS assay for simultaneous quantification of choline, betaine, trimethylamine, trimethylamine N‐oxide, and creatinine in human plasma and urine. Electrophoresis. 2015;36:2207–2214. [DOI] [PubMed] [Google Scholar]
- 14. Carr JJ, Nelson JC, Wong ND, McNitt‐Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population‐based studies: standardized protocol of Multi‐Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 15. Jacobs DR Jr, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and Minnesota Heart Health Program. J Cardiopulm Rehabil. 1989;9:448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McDonald A, Van Horn L, Slattery M, Hilner J, Bragg C, Caan B, Jacobs D Jr, Liu K, Hubert H, Gernhofer N, Betz E, Havlik D. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc. 1991;91:1104–1112. [PubMed] [Google Scholar]
- 17. Sijtsma FP, Meyer KA, Steffen LM, Shikany JM, Van Horn L, Harnack L, Kromhout D, Jacobs DR Jr. Longitudinal trends in diet and effects of sex, race, and education on dietary quality score change: the Coronary Artery Risk Development in Young Adults Study. Am J Clin Nutr. 2012;95:580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD EPI . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 20. Gross MD, Bielinski SJ, Suarez‐Lopez JR, Reiner AP, Bailey K, Thyagarajan B, Carr JJ, Duprez DA, Jacobs DR Jr. Circulating soluble intercellular adhesion molecule 1 and subclinical atherosclerosis: the Coronary Artery Risk Development in Young Adults Study. Clin Chem. 2012;58:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reis JP, Loria CM, Lewis CE, Powell‐Wiley TM, Wei GS, Carr JJ, Terry JG, Liu K. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;310:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, McEntyre CJ, Elmslie JL, Atkinson W, Molyneux SL, Troughton RW, Frampton CM, Richards AM, Chambers ST. Betaine and trimethylamine‐N‐oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS One. 2014;9:e114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic value of elevated levels of intestinal microbe‐generated metabolite trimethylamine‐N‐oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang WH, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, Klein AL, Hazen SL. Intestinal microbiota‐dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trøseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, Aakhus S, Gude E, Bjorndal B, Halvorsen B, Karlsen TH, Aukrust P, Gullestad L, Berge RK, Yndestad A. Microbiota‐dependent metabolite trimethylamine‐N‐oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277:717–726. [DOI] [PubMed] [Google Scholar]
- 26. Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa‐Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota‐dependent trimethylamine N‐oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalim S, Clish CB, Wenger J, Elmariah S, Yeh RW, Deferio JJ, Pierce K, Deik A, Gerszten RE, Thadhani R, Rhee EP. A plasma long‐chain acylcarnitine predicts cardiovascular mortality in incident dialysis patients. J Am Heart Assoc. 2013;2:e000542 doi: 10.1161/JAHA.113.000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; American Heart Association Strategic Planning Task F and Statistics C . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 29. Serban MC, Sahebkar A, Mikhailidis DP, Toth PP, Jones SR, Muntner P, Blaha MJ, Andrica F, Martin SS, Borza C, Lip GY, Ray KK, Rysz J, Hazen SL, Banach M. Impact of L‐carnitine on plasma lipoprotein(a) concentrations: a systematic review and meta‐analysis of randomized controlled trials. Sci Rep. 2016;6:19188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee CD, Jacobs DR Jr, Schreiner PJ, Iribarren C, Hankinson A. Abdominal obesity and coronary artery calcification in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2007;86:48–54. [DOI] [PubMed] [Google Scholar]
- 31. Pletcher MJ, Bibbins‐Domingo K, Liu K, Sidney S, Lin F, Vittinghoff E, Hulley SB. Nonoptimal lipids commonly present in young adults and coronary calcium later in life: the CARDIA (Coronary Artery Risk Development in Young Adults) Study. Ann Intern Med. 2010;153:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mahoney LT, Burns TL, Stanford W, Thompson BH, Witt JD, Rost CA, Lauer RM. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J Am Coll Cardiol. 1996;27:277–284. [DOI] [PubMed] [Google Scholar]
- 33. Lakoski SG, Greenland P, Wong ND, Schreiner PJ, Herrington DM, Kronmal RA, Liu K, Blumenthal RS. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the Multi‐Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2007;167:2437–2442. [DOI] [PubMed] [Google Scholar]
- 34. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WH, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron‐beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. [DOI] [PubMed] [Google Scholar]
- 36. Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, Nakamura Y, Yamashita H, Yamagishi H, Takeuchi K, Naruko T, Haze K, Becker AE, Yoshikawa J, Ueda M. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–3429. [DOI] [PubMed] [Google Scholar]
- 37. Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND, Virmani R, Kondo T, Ozaki Y, Narula J. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. [DOI] [PubMed] [Google Scholar]
- 38. Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Genereux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63:1703–1714. [DOI] [PubMed] [Google Scholar]
- 39. Mueller DM, Allenspach M, Othman A, Saely CH, Muendlein A, Vonbank A, Drexel H, von Eckardstein A. Plasma levels of trimethylamine‐N‐oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis. 2015;243:638–644. [DOI] [PubMed] [Google Scholar]
- 40. Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TD, Spertus JA, Yu AS. Serum trimethylamine‐N‐oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaysen GA, Johansen KL, Chertow GM, Dalrymple LS, Kornak J, Grimes B, Dwyer T, Chassy AW, Fiehn O. Associations of trimethylamine N‐oxide With nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J Ren Nutr. 2015;25:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW; American Heart Association Councils on Kidney in Cardiovascular Disease HBPRCC, Epidemiology and Prevention . Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. [DOI] [PubMed] [Google Scholar]
- 43. Zeisel SH, daCosta KA, Youssef M, Hensey S. Conversion of dietary choline to trimethylamine and dimethylamine in rats: dose‐response relationship. J Nutr. 1989;119:800–804. [DOI] [PubMed] [Google Scholar]
- 44. de la Huerga J, Popper H. Urinary excretion of choline metabolites following choline administration in normals and patients with hepatobiliary diseases. J Clin Invest. 1951;30:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine‐N‐oxide in end‐stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant. 2006;21:1300–1304. [DOI] [PubMed] [Google Scholar]
- 46. Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, Yang Q, Cheng S, Pierce K, Deik A, Souza AL, Farrell L, Domos C, Yeh RW, Palacios I, Rosenfield K, Vasan RS, Florez JC, Wang TJ, Fox CS, Gerszten RE. A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol. 2013;24:1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine‐N‐oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


