Abstract
Background
The extent to which angina is associated with future cardiovascular events in patients with coronary artery disease has long been debated.
Methods and Results
Included were outpatients with established coronary artery disease who were enrolled in the REACH registry and were followed for 4 years. Angina at baseline was defined as necessitating episodic or permanent antianginal treatment. The primary end point was the composite of cardiovascular death, myocardial infarction, or stroke. Secondary end points included heart failure, cardiovascular hospitalizations, and coronary revascularization. The independent association between angina and first/total events was examined using Cox and logistic regression models. Out of 26 159 patients with established coronary artery disease, 13 619 (52%) had angina at baseline. Compared with patients without angina, patients with angina were more likely to be older, female, and had more heart failure and polyvascular disease (P<0.001 for each). Compared with patients without angina, patients with angina had higher rates of first primary end‐point event (14.2% versus 16.3%, unadjusted hazard ratio 1.19, CI 1.11–1.27, P<0.001; adjusted hazard ratio 1.06, CI 0.99–1.14, P=0.11), and total primary end‐point events (adjusted risk ratio 1.08, CI 1.01–1.16, P=0.03). Patients with angina were at increased risk for heart failure (adjusted odds ratio 1.17, CI 1.06–1.28, P=0.002), cardiovascular hospitalizations (adjusted odds ratio 1.29, CI 1.21–1.38, P<0.001), and coronary revascularization (adjusted odds ratio 1.23, CI 1.13–1.34, P<0.001).
Conclusions
Patients with stable coronary artery disease and angina have higher rates of future cardiovascular events compared with patients without angina. After adjustment, angina was only weakly associated with cardiovascular death, myocardial infarction, or stroke, but significantly associated with heart failure, cardiovascular hospitalization, and coronary revascularization.
Keywords: angina, cardiovascular events, coronary artery disease
Subject Categories: Coronary Artery Disease, Angina, Myocardial Infarction
Introduction
Stable angina affects more than 8 million people in the United States each year.1 Patients with stable angina have reduced quality of life and utilize greater healthcare resources.2 The extent to which angina is independently associated with future cardiovascular events or is just a marker of disease severity has long been debated.3, 4, 5, 6, 7, 8, 9, 10, 11 While several studies have demonstrated that angina is independently associated with cardiovascular outcome including cardiovascular death and myocardial infarction (MI),6, 7, 8, 9 others have not found a compelling association between angina and “hard” cardiovascular end points.10, 12 In addition, large‐scale trials have shown that the alleviation of anginal symptoms by pharmacological treatment or by coronary revascularization improves quality of life measures but does not tend to decrease the rates of future MI or mortality.13 Furthermore, in outpatients with stable coronary artery disease (CAD), most cardiovascular events actually occur in patients without prior angina.9 We therefore aimed to examine the independent association between angina at baseline and future cardiovascular events in patients with stable CAD who were included in a large international outpatient registry.
Methods
Study Design
The design of the Reduction of Atherothrombosis for Continued Health (REACH) registry has been previously published.14, 15 In brief, REACH is an outpatient registry of patients with either stable symptomatic vascular disease (CAD, cerebrovascular disease, or peripheral artery disease) or with multiple atherosclerotic risk factors. Patients from 3647 centers in 29 countries were enrolled between 2003 and 2004 and treated according to best judgment and practices of their primary care physicians. Detailed information was collected at baseline, with subsequent annual follow‐up on a longitudinal outpatient basis at 1, 2, 3, and 4 years. Final database lock was in April 2009. In each country, 10% of all sites underwent data control audits and were monitored for source documentation and accuracy of all case report forms. The protocol was approved by local institutional review boards, and each enrolled patient was required to provide a signed informed consent.
In the current analysis, we included patients with documented CAD at baseline who completed at least 1 postbaseline follow‐up visit and were enrolled at centers that participated in the 4‐year REACH follow‐up study.16 CAD was defined as having 1 or more of the following: stable angina, history of unstable angina (UAP), previous MI, history of percutaneous coronary intervention (PCI), or history of coronary artery bypass graft surgery (CABG). Patients without CAD at baseline were excluded from the current analysis. A sensitivity analysis included only patients who had a previous MI, history of PCI, or a history of CABG.
Stable Angina
Patients were stratified by the presence of stable angina symptoms at baseline. Stable angina at baseline was documented by the treating physician using the case report form and was defined as angina necessitating episodic or permanent medication use. The last episode of angina was documented in the case report form as either occurring ≤1 year prior to baseline or >1 year prior to baseline.
Clinical End Points
Data regarding events were collected locally and forwarded to the central research organization. The primary end point was a composite of cardiovascular death, MI, or stroke. The rate of each end point was calculated and stratified by angina status at baseline. End points were not adjudicated. Cardiovascular death included fatal stroke, fatal MI, or other cardiovascular death. Other cardiovascular death included other death of cardiac origin; pulmonary embolism; any sudden death including unobserved and unexpected death (eg, death while sleeping) unless proven otherwise by autopsy; death following a vascular operation, vascular procedure, or amputation; death attributed to heart failure; death following a visceral or limb infarction; and any other death that could not be definitely attributed to a nonvascular cause or hemorrhage. Any MI or stroke followed by a death whatever the cause in the next 28 days was considered to be a fatal MI or fatal stroke.
Secondary end points included all‐cause death, heart failure, UAP, cardiovascular hospitalizations, and coronary revascularization. Heart failure was defined as symptoms of heart failure leading to hospitalization. Cardiovascular hospitalization consisted of hospitalization for UAP, transient ischemic attack, worsening of claudication related to peripheral artery disease, other ischemic arterial event, coronary revascularization (PCI or CABG), carotid surgery, carotid angioplasty/stenting, amputation affecting lower limbs, peripheral bypass graft, or angioplasty/stenting for peripheral artery disease. Complete definitions of other clinical end points have been previously described.15
The total number of the primary end‐point events (total cardiovascular death, MI, or stroke), as well as the total cardiovascular hospitalizations and total coronary revascularizations during follow‐up, were also examined and stratified by angina status at baseline.
Statistical Analysis
Continuous variables are presented as mean± SD, and categorical variables as frequencies and percentages. Cumulative incidence for cardiovascular death, MI, or stroke was examined using the Kaplan–Meier approach. Cox proportional hazard models were used to examine whether angina is associated with cardiovascular death, MI, or stroke. All‐cause death was also examined using the Kaplan–Meier approach. Incidence of heart failure, UAP, cardiovascular hospitalizations, and coronary revascularizations are presented as crude rates at 45 months. Logistic regression models were used to examine the association between angina and heart failure, UAP, cardiovascular hospitalizations, and coronary revascularizations. The variables included in all multivariate models were based on the previously validated REACH model for recurrent cardiovascular events.17 This model included the following variables: age, sex, current smoker, history of diabetes mellitus, body mass index <20 (calculated as weight in kg/m2), ischemic event (≤1 year, >1 year, or no ischemic event), vascular disease status (polyvascular disease defined as CAD with concomitant cerebrovascular disease/peripheral artery disease, or single vascular disease [ie, only CAD]), congestive heart failure, atrial fibrillation/flutter, aspirin (at baseline), statins (at baseline), and Eastern Europe and Middle East, or Japan versus other regions (geographic regions were collapsed into higher [Eastern Europe and Middle East] and lower [Japan] risk locations). The total event counts of the composite of cardiovascular death, MI, or stroke, as well as cardiovascular hospitalization and coronary revascularization were fitted by the negative binomial regression model after accounting for varying lengths of individual's total follow‐up time as an offset parameter as well as other baseline confounding factors. Results are reported in terms of adjusted incidence rate ratio (RR) and corresponding 95% CI from this model. Similar statistical methods were performed in the sensitivity analysis.
Patients were further stratified to 4 risk‐groups according to the REACH model for recurrent cardiovascular events.17 This model includes traditional risk factors, burden of disease, lack of treatment, and geographic location,17 whereas angina status was not a candidate variable in the derivation of the REACH model. Cox proportional hazard models were used to examine whether angina is associated with cardiovascular death, MI, or stroke in the different risk‐groups. Hazard ratios (HRs) are reported as unadjusted given the stratification by the REACH model, which already includes the adjustment variables.
Statistical significance was considered as a 2‐sided probability of <0.05. Statistical analyses were performed using SAS version 9 (SAS Institute, Cary, NC).
Results
Patient Population
Of the 45 227 patients who were included in the REACH 4‐year follow‐up study, 44 736 patients had data on angina status at baseline. Of these, 18 577 patients without CAD were excluded from the current analysis. Thus, 26 159 patients with CAD were included and were followed for a median of 43.5 months (interquartile range 31.3–45.0). The patients’ mean age was 68 years (SD 10) and 70.8% were men. Hypertension (79.7%) and hypercholesterolemia (75.5%) were very common. More than half of the patients had a prior MI, a quarter had polyvascular disease, and a fifth of the patients had prior heart failure (Table 1).
Table 1.
Baseline Characteristics
| Characteristic | Angina (n=13 619) | No Angina (n=12 540) | Total (n=26 159) | P‐Value |
|---|---|---|---|---|
| Age, y—mean (SD) | 68.5 (10.0) | 67.5 (10.0) | 68.0 (10.0) | <0.001 |
| >75 y | 3788 (27.9) | 3094 (24.8) | 6882 (26.4) | <0.001 |
| Men | 9061 (66.6) | 9449 (75.4) | 18 510 (70.8) | <0.001 |
| Regiona | <0.001 | |||
| North America | 4430 (47.6) | 4881 (52.4) | 9331 (35.7) | |
| Latin America | 286 (37.2) | 483 (62.8) | 769 (2.9) | |
| Western Europe | 4367 (51.6) | 4102 (48.4) | 8469 (32.4) | |
| Eastern Europe | 2276 (71.4) | 912 (28.6) | 3188 (12.2) | |
| Middle East | 147 (44.8) | 181 (55.2) | 328 (1.2) | |
| Asia Pacific | 2113 (51.6) | 1981 (48.4) | 4094 (15.7) | |
| Hypertension | 11 316 (83.1) | 9541 (76.1) | 20 857 (79.7) | <0.001 |
| Hypercholesterolemia | 10 076 (74.0) | 9666 (77.1) | 19 742 (75.5) | <0.001 |
| Diabetes mellitus | 5308 (39.0) | 4526 (36.1) | 9834 (37.6) | <0.001 |
| Obesity (BMI ≥30) | 3974 (29.4) | 3391 (27.3) | 7365 (28.4) | <0.001 |
| Current smoker at baseline | 1778 (13.5) | 1605 (13.2) | 3383 (13.3) | <0.001 |
| Prior ischemic event | 7042 (52.4) | 8298 (66.7) | 15 340 (59.3) | <0.001 |
| Prior MI | 6010 (44.6) | 7933 (63.7) | 13 943 (53.8) | <0.001 |
| Prior PCI | 5015 (37.1) | 5975 (47.9) | 10 990 (42.2) | <0.001 |
| Prior CABG | 3790 (28.0) | 4579 (36.6) | 8369 (32.1) | <0.001 |
| Heart failure | 3016 (22.5) | 2007 (16.2) | 5023 (19.5) | <0.001 |
| Atrial fibrillation | 1780 (13.3) | 1304 (10.5) | 3084 (12.0) | <0.001 |
| CVD at baseline | 2732 (20.1) | 1670 (13.3) | 4402 (16.8) | <0.001 |
| PAD at baseline | 1633 (12.0) | 1183 (9.4) | 2816 (10.8) | <0.001 |
| Polyvascular disease | 3888 (28.6) | 2622 (20.9) | 6510 (24.9) | <0.001 |
| Aortic valve stenosis | 588 (4.5) | 411 (3.4) | 999 (4.0) | <0.001 |
| ≥1 Antithrombotic drug | 8334 (92.2) | 7612 (94.8) | 15 946 (93.4) | <0.001 |
| ≥1 Lipid‐lowering drug | 7271 (80.6) | 6850 (85.3) | 14 121 (82.8) | <0.001 |
| Medication at 4 y | ||||
| Statins | 6879 (76.4) | 6562 (81.9) | 13 441 (79.0) | <0.001 |
| ACE inhibitor or ARB | 6348 (70.3) | 5670 (70.7) | 12 018 (70.5) | <0.001 |
| β‐Blocker | 5875 (65.1) | 5265 (65.7) | 11 140 (65.4) | <0.001 |
| Diuretic | 4197 (46.7) | 3359 (42.1) | 7556 (44.5) | <0.001 |
| Calcium channel blocker | 3530 (39.3) | 2620 (32.8) | 6150 (36.3) | <0.001 |
| Nitrate or other antianginalb | 3642 (40.5) | 2194 (27.6) | 5836 (34.4) | <0.001 |
| Any β‐blockers, calcium channel blocker, nitrate, or other antianginal | 7941 (87.9) | 6769 (84.3) | 14 710 (86.2) | <0.001 |
| Aspirin+another antiplatelet drug | 1248 (13.8) | 1110 (13.8) | 2358 (13.8) | <0.001 |
| Oral anticoagulant drug | 1206 (13.3) | 1113 (13.8) | 2319 (13.6) | <0.001 |
Data shown are n (%) unless otherwise indicated. ACE indicates angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; CVD, cerebrovascular disease; MI, myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention.
Percentages are for each region except for the total population. P‐value is calculated using χ2.
Nitrate as a chronic treatment and not if given episodically. Treatment could have been prescribed for other indications (eg, heart failure). Other antianginal includes molsidomine and nicorandil.
Out of the 26 159 patients with CAD, 13 619 (52%) have had angina prior to baseline and 12 540 (48%) did not have angina prior to baseline. Compared with patients without angina, patients with angina were more likely to be older, female, had more heart failure and polyvascular disease, but had fewer ischemic events and coronary revascularization procedures (PCI or CABG) prior to baseline (P<0.001 for each; Table 1). Angina was more commonly reported in Eastern Europe and less in Latin America (Table 1). Patients with angina at baseline were more likely to be treated with either β‐blockers, calcium channel blockers, or nitrates at 4 years of follow‐up, but less likely to be treated with statins (P<0.001 for each; Table 1).
Angina and Cardiovascular Events
During follow‐up, the rate of the composite primary end point of cardiovascular death, MI, or stroke was 16.3% in patients with angina and 14.2% in patients without angina (unadjusted HR 1.19, 95% CI 1.11–1.27, P<0.001; Figure 1). In a landmark analysis, this difference in the rate of the primary end point between patients with versus without angina became statistically significant after 6 months from baseline and it remained significant at 4 years (Figure 1). The rate of each individual component of the composite primary end point was also higher among patients with angina (Table 2). After adjusting for multiple variables (Tables 2 and 3), the association between angina and the composite of cardiovascular death, MI, or stroke was attenuated (adjusted HR 1.06, 95% CI 0.99–1.14, P=0.11), and so was the association between angina and each of the individual components (Table 2). An analysis of the total number of events during follow‐up demonstrated that angina was significantly, albeit weakly, associated with the total number of the primary end‐point events (rate ratio 1.08, 95% CI 1.01–1.16, P=0.03; Table 4).
Figure 1.
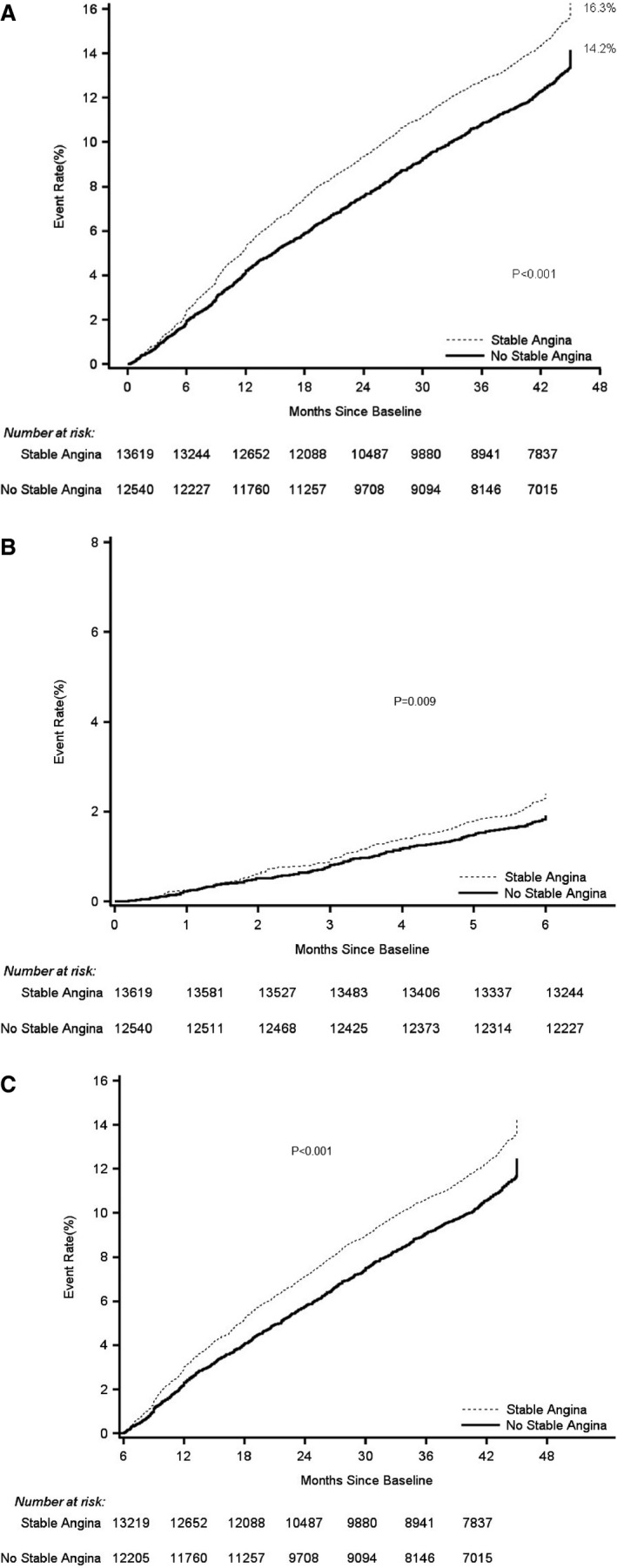
Kaplan–Meier rates of the primary composite end point of cardiovascular death, MI, or stroke by presence of angina at baseline in the overall period (A), and landmark analysis during the first 6 months (B) and during 6 months to 4 years (C). The Kaplan–Meier curves demonstrate a higher rate of the composite primary end point of cardiovascular death, MI, or stroke in patients with angina, as compared to patients without angina. MI indicates myocardial infarction.
Table 2.
Clinical End Points by Angina Status at Baseline
| End Point | Angina (n=13 619) 4‐Y KM Rate, n (%) | No Angina (n=12 540) 4‐Y KM Rate, n (%) | Unadjusted HR (95% CI) | P‐Value | Adjusted HRa (95% CI) | P‐Value |
|---|---|---|---|---|---|---|
| CVD, MI, or stroke | 1911 (16.3) | 1479 (14.2) | 1.19 (1.11–1.27) | <0.001 | 1.06 (0.99–1.14) | 0.11 |
| CVD | 964 (8.4) | 781 (7.6) | 1.12 (1.02–1.23) | 0.02 | 0.95 (0.86–1.05) | 0.33 |
| MI | 541 (4.8) | 428 (4.2) | 1.16 (1.02–1.31) | 0.03 | 1.14 (1.00–1.31) | 0.06 |
| Stroke | 606 (5.4) | 406 (4.1) | 1.37 (1.21–1.55) | <0.001 | 1.19 (1.04–1.37) | 0.01 |
| Any‐cause death | 1473 (12.6) | 1260 (12.1) | 1.06 (0.99–1.15) | 0.11 | 0.93 (0.85–1.01) | 0.07 |
| CVD, or MI | 1429 (12.3) | 1156 (11.1) | 1.13 (1.04–1.22) | 0.002 | 1.01 (0.93–1.10) | 0.83 |
| Heart failureb | 1498 (11.0) | 1006 (8.0) | 1.42 (1.30–1.54) | <0.001 | 1.17 (1.06–1.28) | 0.002 |
| CVD, or heart failureb | 2167 (15.9) | 1578 (12.6) | 1.31 (1.23–1.41) | <0.001 | 1.08 (1.00–1.17) | 0.06 |
| Unstable anginab | 2073 (15.2) | 1334 (10.6) | 1.51 (1.40–1.62) | <0.001 | 1.40 (1.29–1.52) | <0.001 |
| CVHb | 3664 (26.9) | 2637 (21.0) | 1.38 (1.31–1.46) | <0.001 | 1.29 (1.21–1.38) | <0.001 |
| Coronary revascularizationb | 1547 (11.4) | 1258 (10.0) | 1.15 (1.06–1.24) | 0.001 | 1.23 (1.13–1.34) | <0.001 |
CAD indicates coronary artery disease; CVD, cardiovascular death; CVH, cardiovascular hospitalization; HR, hazard ratio; KM, Kaplan–Meier; MI, myocardial infarction.
Adjusted for age, sex, current smoker, history of diabetes mellitus, body mass index <20, ischemic event (≤1 year, ischemic event >1 year), polyvascular disease (CAD+ cerebrovascular disease/peripheral arterial disease), congestive heart failure, atrial fibrillation/flutter, aspirin (at baseline), statins (at baseline), and region.
Event rates are crude rates at 45 months. Logistic regression models were used. Data presented are odds ratio (95% CI).
Table 3.
Cox‐Proportional Hazard Model for Predictors of Cardiovascular Death, Myocardial Infarction, or Stroke
| Variable | Adjusted HR (95% CI) | χ2 | P‐Value |
|---|---|---|---|
| Age, per 1‐y increase | 1.035 (1.031–1.039) | 284.3 | <0.001 |
| Congestive heart failure, yes vs no | 1.75 (1.62–1.89) | 191.1 | <0.001 |
| Polyvascular disease vs single vascular disease | 1.52 (1.41–1.64) | 118.5 | <0.001 |
| History of diabetes mellitus, yes vs no | 1.44 (1.34–1.56) | 100.6 | <0.001 |
| Ischemic event ≤1 y vs no ischemic event | 1.67 (1.50–1.85) | 87.7 | <0.001 |
| Ischemic event >1 y vs no ischemic event | 1.48 (1.36–1.61) | 85.6 | <0.001 |
| Statins, yes vs no | 0.74 (0.68–0.80) | 57.0 | <0.001 |
| Japan vs other regions | 0.61 (0.53–0.72) | 39.6 | <0.001 |
| Current smoker vs former or never | 1.37 (1.24–1.52) | 35.9 | <0.001 |
| Eastern Europe and Middle East vs other regions | 1.27 (1.16–1.40) | 23.1 | <0.001 |
| Atrial fibrillation/flutter, yes vs no | 1.24 (1.13–1.36) | 19.4 | <0.001 |
| Sex, male vs female | 1.12 (1.03–1.21) | 7.7 | 0.005 |
| BMI <20, yes vs no | 1.28 (1.06–1.54) | 6.8 | 0.009 |
| Aspirin, yes vs no | 0.93 (0.86–1.01) | 3.0 | 0.08 |
| History of stable angina vs no history of stable angina | 1.06 (0.99–1.14) | 2.6 | 0.11 |
BMI indicates body mass index; HR indicates hazard ratio.
Table 4.
Total Events During 4 Years by Angina Status at Baseline
| Total End Point | Angina (n=13 619) Total Events, n | No Angina (n=12 540) Total Events, n | Unadjusted RR (95% CI) | P‐Value | Adjusted RRa (95% CI) | P‐Value |
|---|---|---|---|---|---|---|
| CVD, MI, or stroke | 2176 | 1649 | 1.21 (1.12–1.29) | <0.001 | 1.08 (1.01–1.16) | 0.03 |
| CVH | 7488 | 5065 | 1.36 (1.28–1.44) | <0.001 | 1.27 (1.20–1.35) | <0.001 |
| Coronary revascularization | 1814 | 1499 | 1.11 (1.03–1.20) | 0.006 | 1.19 (1.10–1.29) | <0.001 |
BMI indicates body mass index; CAD, coronary artery disease; CVD, cardiovascular death; CVH, cardiovascular hospitalization; MI, myocardial infarction; RR, rate ratio.
Adjusted for age, sex, current smoker, history of diabetes mellitus, BMI <20, ischemic event (≤1 year, ischemic event >1 year), polyvascular disease (CAD+ cerebrovascular disease/peripheral arterial disease), congestive heart failure, atrial fibrillation/flutter, aspirin (at baseline), statins (at baseline), and region.
During follow‐up, nearly a quarter of the patients were hospitalized due to cardiovascular causes (Table 2). Compared with patients without angina, patients with angina had higher rates of heart failure (8.0% versus 11.0%, P<0.001), cardiovascular hospitalization (21.0% versus 26.9%, P<0.001), and coronary revascularization (10.0% versus 11.4%, P<0.001). After adjusting for multiple variables, the association between angina and each of these end points remained significant. Compared with patients without angina, patients with angina had a 17% higher relative risk for heart failure (P=0.002), a 29% higher relative risk for cardiovascular hospitalization (P<0.001), and a 23% higher relative risk for coronary revascularization (P<0.001; Table 2). In addition, angina was associated with the total number of cardiovascular hospitalizations, as well as the total number of coronary revascularizations (Table 4).
No significant difference in the association between angina and the primary end point was observed in subgroups by age, sex, time from ischemic event, current smoking, heart failure, or prior coronary revascularization (PCI or CABG) (Figure 2). However, a significant interaction was observed between angina and the primary end point by polyvascular disease status (adjusted HR 0.99, 95% CI 0.88–1.12, in patients with polyvascular disease; adjusted HR 1.13, 95% 1.03–1.24, in patients without polyvascular disease; P‐interaction=0.015). In addition, a marginal interaction was observed between angina and the primary end point by diabetes mellitus status (adjusted HR 0.99, 95% CI 0.89–1.10, in patients with diabetes mellitus; adjusted HR 1.13, 95% CI 1.02–1.25, in patients without diabetes mellitus; P‐interaction=0.08).
Figure 2.
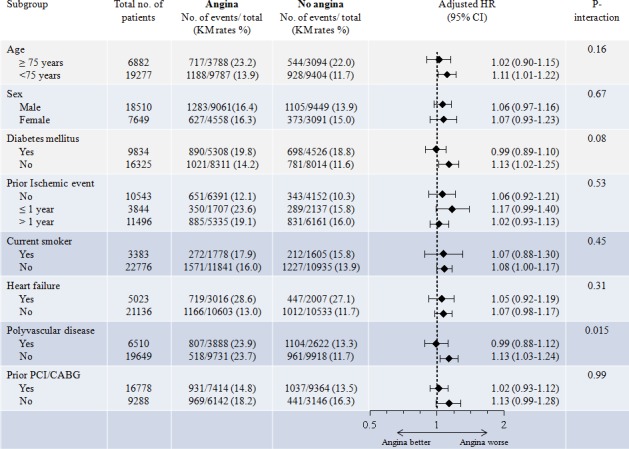
Rates and adjusted hazard ratios (95% CI) of the primary composite end point of cardiovascular death, MI, or stroke, in patients with and without angina at baseline by subgroups. Adjustment variables: age, sex, current smoker, history of diabetes mellitus, body mass index <20, ischemic event (≤1 year, ischemic event >1 year), polyvascular disease (CAD+ cerebrovascular disease/peripheral arterial disease), congestive heart failure, atrial fibrillation/flutter, aspirin (at baseline), statins (at baseline), and region. No significant interaction in the association between angina and the primary end point was observed in subgroups by age, sex, time from ischemic event, current smoking, heart failure, or prior PCI/CABG. However, a significant interaction was observed by polyvascular disease status and a marginal interaction was observed by diabetes mellitus status. CABG, coronary artery bypass graft surgery; CAD, coronary artery disease; HR, hazard ratio; KM, Kaplan–Meier; MI, myocardial infarction; PCI, percutaneous coronary intervention.
No association was observed between angina and the primary end point by the time of last angina episode. In patients who had anginal symptoms during ≤1 year prior to baseline (n=4085), the rate of the primary end point was 16.1%, whereas in patients who have had the last anginal symptoms >1 year prior to baseline (n=9534), the rate of the primary end point was 16.5% (adjusted HR angina versus no angina 1.06, 95% CI 0.96–1.18, P=0.26; 1.06, 95% CI 0.98–1.15, P=0.14, respectively).
Stratifying the patients to quartiles according to the REACH risk score for recurrent cardiovascular events,17 patients in higher quartiles had higher rates of the primary end point of cardiovascular death, MI, or stroke (Figure 3). Interestingly, angina was associated with the primary end point in lower‐risk patients (unadjusted HR angina versus no angina 1.17, 95% CI 0.98–1.41, P=0.09 in quartile I; 1.21, 95% CI 1.02–1.43, P=0.03 in quartile II), whereas it was not associated with the primary end point in patients at higher risk for recurrent cardiovascular events (unadjusted HR angina versus no angina 0.95, 95% CI 0.84–1.09, P=0.47 in quartile III; 0.98, 95% CI 0.87–1.10, P=0.69 in quartile IV; Figure 3).
Figure 3.
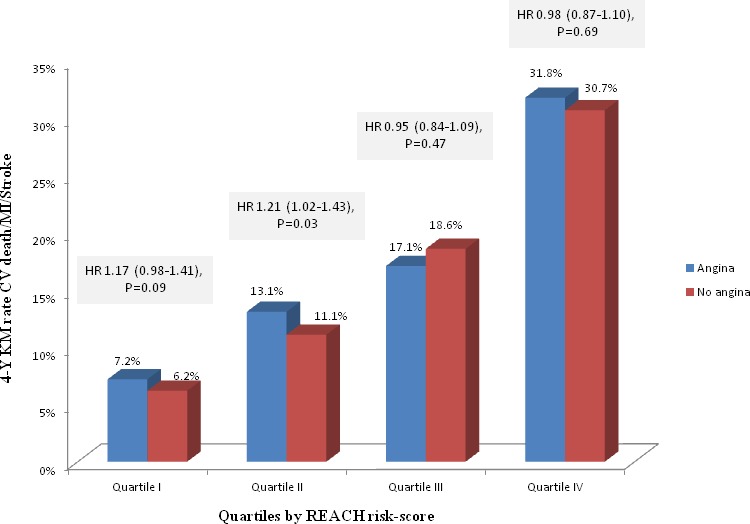
Kaplan–Meier rates and unadjusted hazard ratios (95% CI) of the primary composite end point of CVD, MI, or stroke in patients with and without angina, stratified by patients’ risk according to the REACH risk score for recurrent Cardiovascular events.17 Data were available for 24 315 patients. Stratifying the patients to quartiles according to the REACH risk score for recurrent Cardiovascular events, patients in higher quartiles had higher rates of the primary end point of CVD, MI, or stroke. Angina was associated with the primary end point in lower‐risk patients, whereas it was not associated with the primary end point in patients at higher risk of recurrent Cardiovascular events. CVD indicates cardiovascular death; HR, hazard ratio; KM, Kaplan–Meier; MI, myocardial infarction.
In a sensitivity analysis that included only patients with previous MI, history of PCI, or CABG (n=21 344), consistent qualitative results for the association between angina and future cardiovascular events were obtained (adjusted HR for the primary end point 1.08, 95% CI 1.00–1.17, P=0.07; Table 5).
Table 5.
Sensitivity Analysis–Clinical End Points by Angina Status at Baseline of Patients With Previous MI, History of PCI or CABG
| End Point | Angina (n=9415) 4‐Y KM Rate, n (%) | No Angina (n=11 929) 4‐Y KM Rate, n (%) | Unadjusted HR (95% CI) | P‐Value | Adjusted HRa (95% CI) | P‐Value |
|---|---|---|---|---|---|---|
| CVD, MI, or stroke | 1338 (16.6) | 1401 (14.4) | 1.21 (1.12–1.30) | <0.001 | 1.08 (1.00–1.17) | 0.07 |
| CVD | 691 (8.7) | 736 (7.5) | 1.18 (1.06–1.30) | 0.002 | 1.00 (0.90–1.12) | 0.94 |
| MI | 427 (5.4) | 415 (4.3) | 1.30 (1.13–1.49) | <0.001 | 1.20 (1.04–1.38) | 0.01 |
| Stroke | 366 (4.7) | 379 (4.0) | 1.22 (1.05–1.41) | 0.007 | 1.11 (0.96–1.30) | 0.17 |
| Any‐cause death | 1048 (13.1) | 1199 (12.1) | 1.09 (1.01–1.19) | 0.04 | 0.97 (0.89–1.06) | 0.48 |
| CVD, or MI | 1056 (13.2) | 1100 (11.1) | 1.21 (1.11–1.32) | <0.001 | 1.06 (0.97–1.16) | 0.19 |
| Heart failureb | 1063 (11.3) | 953 (8.0) | 1.47 (1.34–1.61) | <0.001 | 1.18 (1.06–1.31) | 0.002 |
| CVD, or heart failureb | 1534 (16.3) | 1492 (12.5) | 1.36 (1.26–1.47) | <0.001 | 1.12 (1.02–1.22) | 0.02 |
| Unstable anginab | 1573 (16.7) | 1175 (9.9) | 1.84 (1.69–1.99) | <0.001 | 1.68 (1.54–1.83) | <0.001 |
| CVHb | 2704 (28.7) | 2432 (20.4) | 1.57 (1.48–1.68) | <0.001 | 1.48 (1.39–1.59) | <0.001 |
| Coronary revascularizationb | 1258 (13.4) | 1198 (10.0) | 1.38 (1.27–1.50) | <0.001 | 1.39 (1.27–1.52) | <0.001 |
CABG indicates coronary artery bypass graft surgery; CAD, coronary artery disease; CVD, cardiovascular death; CVH, cardiovascular hospitalization; HR, hazard ratio; KM, Kaplan–Meier; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Adjusted for age, sex, current smoker, history of diabetes mellitus, body mass index <20, ischemic event (≤1 year, ischemic event >1 year), polyvascular disease (CAD+ cerebrovascular disease/peripheral arterial disease), congestive heart failure, atrial fibrillation/flutter, aspirin (at baseline), statins (at baseline), and region.
Event rates are crude rates at 45 months. Logistic regression models were used. Data presented are odds ratio (95% CI).
Discussion
This study from a large international registry demonstrates several observations. First, patients with stable CAD who have angina substantially differ in their baseline characteristics, atherosclerotic risk factors, concomitant cardiovascular diseases, and medications use from patients without angina. Second, patients with angina have higher rates of future cardiovascular events, including cardiovascular death and MI. Third, the independent association between angina and cardiovascular events was attenuated after a rigorous adjustment for baseline comorbidities. Specifically, angina was only weakly associated with cardiovascular death, MI, or stroke, but the association with heart failure, cardiovascular hospitalization, and coronary revascularizations remained significant after multivariable adjustment. Finally, stratifying the patients by their risk of recurrent cardiovascular events, angina seemed to be associated with cardiovascular death, MI, or stroke in lower‐risk patients, but not in high‐risk patients.
In patients with CAD included in the REACH registry, prior cardiovascular disease, particularly prior ischemic events, heart failure, cerebrovascular disease, and peripheral artery disease were common and found to be robust and independent markers of subsequent cardiovascular end points, even more so than traditional atherosclerotic risk factors18, 19 (Table 3). Indeed, several prior studies have examined angina‐associated risk with cardiovascular events after adjusting solely for traditional risk factors such as diabetes mellitus, hypertension, and hyperlipidemia and thus their results might be different based on the degree of multivariable modeling.6, 7, 8 Our results are consistent with findings from several prior studies such as the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial (BARI 2D)10 and the Heart and Soul study,12 in which angina was not or was very weakly associated with future cardiovascular death, MI, or stroke. Nevertheless, a recent analysis from the CLARIFY registry9 demonstrated a consistent association between angina, with or without ischemia, and cardiovascular death or MI, even after adjusting for the REACH risk score. Interestingly, compared with CLARIFY, the patients included in this analysis from REACH had a higher‐risk profile and had a 2‐fold annual rate of the composite end point of cardiovascular death, MI, or stroke and of each of the individual components. Thus, it is not clear whether angina has different prognostication according to the patient's risk: While it may be independently associated with “hard” cardiovascular events in patients at lower risk, it is perhaps only a surrogate for more advanced disease in patients at higher risk. Interestingly, by stratifying the patients by their risk of recurrent cardiovascular events, we indeed demonstrated that angina might be associated with “hard” cardiovascular events only in patients at lower risk and not in patients at higher risk. In addition, our subgroup analysis also demonstrated that angina was independently associated with cardiovascular death, MI, or stroke, in several lower‐risk groups such as patients without diabetes mellitus and without other vascular beds involved besides CAD. Nevertheless, the complex association between angina and “hard” cardiovascular end points should be further delineated in future research.
The clinical diagnosis of stable angina has been linked historically to the classic chronic condition caused by epicardial coronary stenosis.2 Nevertheless, stable angina includes other less common presentations such as microvascular angina, vasospastic angina, and angina caused by ischemic cardiomyopathy.2 In each of these conditions, myocardial ischemia is present, albeit each with a different burden and mechanism. The association between angina, ischemia, and cardiovascular outcome is complex and is probably not consistent across all patients. In the BARI 2D trial, among patients with diabetes mellitus, myocardial ischemia, rather than anginal symptoms, appeared to be prognostic for future cardiovascular events.10 These results are consistent with our findings in diabetic patients in whom angina by itself was not predictive of cardiovascular death, MI, or stroke. Interestingly, in the CLARIFY registry, patients who had only ischemia without angina had adjusted risk for cardiovascular outcomes comparable to patients without both, whereas patients who had both angina and ischemia had the worst outcome.9 Interestingly, more than half of the cardiovascular death and MI events in the CLARIFY study occurred in patients without detectable ischemia or angina at baseline. In our study, ischemia status during follow‐up was not available and could not be accounted for. Nevertheless, angina, regardless of ischemia status, was independently associated with more cardiovascular hospitalization and coronary revascularization procedures. Of interest, the association between angina and revascularization, although statistically significant, was not robust, perhaps reflecting the fact that the majority of patients with angina (70%) had their last angina symptoms >1 year prior to baseline, thus reducing the clinical impetus for revascularization. In addition, this may also reflect regional differences in the treatment of angina with revascularization.
Prior studies have demonstrated that the major benefit of optimal medical therapy or revascularization in patients with stable CAD is the relief of anginal symptoms rather than a reduction in cardiovascular death or MI.13, 20, 21 Thus, the relationship between angina, ischemia, and future cardiovascular events remains an area of debate.22, 23, 24 The ongoing International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA trial, NCT 01471522) might address this intricate relationship. Regardless, in this analysis from REACH, patients with angina have greater healthcare utilization with higher rates of total hospitalizations and revascularizations and therefore present an opportunity to improve care and reduce costs.
Limitations
This analysis is based on a registry, which has inherent limitations. The end points in the study were not adjudicated. Analysis of patients with angina might have introduced a selection bias. Angina was ascertained by the investigator's report in the electronic case report form at baseline, and patient self‐reporting data were not available. Data regarding the exact date of last anginal episode, grading of angina severity, or change in angina over time were not available. In addition, data on the presence of objective coronary ischemia at baseline, or on left ventricular ejection fraction were also not available. Logistic regression models were used to examine the secondary end points since the exact time of event was not available for all subjects.
Conclusions
Patients with stable CAD and angina have higher rates of future cardiovascular events compared with patients without angina. After accounting for baseline differences, angina was only weakly associated with cardiovascular death, MI, or stroke, but was significantly associated with heart failure, cardiovascular hospitalization, and coronary revascularization.
Sources of Funding
The REACH Registry was sponsored by Sanofi‐Aventis, Bristol‐Myers Squibb, and the Waksman Foundation (Tokyo, Japan) and is endorsed by the World Heart Federation.
Disclosures
Dr Bhatt discloses the following relationships—Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Vice‐Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, St. Jude Medical; Trustee: American College of Cardiology; Unfunded Research: FlowCo, PLx Pharma, Takeda. Dr P. Gabriel Steg discloses the following relationships: research grant from Merck, Sanofi, and Servier; speaking or consulting fees from Amarin, AstraZeneca, Bayer, Boehringer‐Ingelheim, Bristol‐Myers Squibb, CSL‐Behring, Daiichi‐Sankyo, GlaxoSmithKline, Janssen, Lilly, Merck Novartis, Pfizer, Regeneron, Sanofi, Servier, The Medicines Company. The remaining authors have no disclosures to report.
Supporting information
Appendix S1. Members of the REACH Registry Investigators.
(J Am Heart Assoc. 2016;5:e004080 doi: 10.1161/JAHA.116.004080)
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2. Task Force Members , Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ; ESC Committee for Practice Guidelines , Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers , Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner‐Banzhoff N, Erol C, Frank H, Funck‐Brentano C, Gaemperli O, Gonzalez‐Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 3. Ferrari R, Abergel H, Ford I, Fox KM, Greenlaw N, Steg PG, Hu D, Tendera M, Tardif JC; CLARIFY Investigators . Gender‐ and age‐related differences in clinical presentation and management of outpatients with stable coronary artery disease. Int J Cardiol. 2013;167:2938–2943. [DOI] [PubMed] [Google Scholar]
- 4. Steg PG, Greenlaw N, Tardif JC, Tendera M, Ford I, Kääb S, Abergel H, Fox KM, Ferrari R; CLARIFY Registry Investigators . Women and men with stable coronary artery disease have similar clinical outcomes: insights from the international prospective CLARIFY registry. Eur Heart J. 2012;33:2831–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohn PF, Harris P, Barry WH, Rosati RA, Rosenbaum P, Waternaux C. Prognostic importance of anginal symptoms in angiographically defined coronary artery disease. Am J Cardiol. 1981;47:233–237. [DOI] [PubMed] [Google Scholar]
- 6. Berecki‐Gisolf J, Humphreyes‐Reid L, Wilson A, Dobson A. Angina symptoms are associated with mortality in older women with ischemic heart disease. Circulation. 2009;120:2330–2336. [DOI] [PubMed] [Google Scholar]
- 7. Mozaffarian D, Bryson CL, Spertus JA, McDonell MB, Fihn SD. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015–1022. [DOI] [PubMed] [Google Scholar]
- 8. Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimaki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA. 2006;295:1404–1411. [DOI] [PubMed] [Google Scholar]
- 9. Steg PG, Greenlaw N, Tendera M, Tardif JC, Ferrari R, Al‐Zaibag M, Dorian P, Hu D, Shalnova S, Sokn FJ, Ford I, Fox KM; Prospective Observational Longitudinal Registry of Patients With Stable Coronary Artery Disease (CLARIFY) Investigators . Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: data from the International Observational CLARIFY Registry. JAMA Intern Med. 2014;174:1651–1659. [DOI] [PubMed] [Google Scholar]
- 10. Dagenais GR, Lu J, Faxon DP, Bogaty P, Adler D, Fuentes F, Escobedo J, Krishnaswami A, Slater J, Frye RL; BARI 2D Study Group . Prognostic impact of the presence and absence of angina on mortality and cardiovascular outcomes in patients with type 2 diabetes and stable coronary artery disease: results from the BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) trial. J Am Coll Cardiol. 2013;61:702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Everett BM, Brooks MM, Vlachos HE, Chaitman BR, Frye RL, Bhatt DL; BARI 2D Study Group . Troponin and cardiac events in stable ischemic heart disease and diabetes. N Engl J Med. 2015;373:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beatty AL, Spertus JA, Whooley MA. Frequency of angina pectoris and secondary events in patients with stable coronary heart disease (from the Heart and Soul Study). Am J Cardiol. 2014;114:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS; COURAGE Trial Research Group . Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 14. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Röther J, Wilson PW; REACH Registry Investigators . International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
- 15. Ohman EM, Bhatt DL, Steg PG, Goto S, Hirsch AT, Liau CS, Mas JL, Richard AJ, Röther J, Wilson PW; REACH Registry Investigators . The REduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events‐study design. Am Heart J. 2006;151:786. [DOI] [PubMed] [Google Scholar]
- 16. Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Röther J, Smith SC Jr, Salette G, Contant CF, Massaro JM, Steg PG; REACH Registry Investigators . Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 17. Wilson PW, D'Agostino R Sr, Bhatt DL, Eagle K, Pencina MJ, Smith SC, Alberts MJ, Dallongeville J, Goto S, Hirsch AT, Liau CS, Ohman EM, Röther J, Reid C, Mas JL, Steg PG; REACH Registry . An international model to predict recurrent cardiovascular disease. Am J Med. 2012;125:695–703. [DOI] [PubMed] [Google Scholar]
- 18. Steg PG, Bhatt DL, Wilson PW, D'Agostino R Sr, Ohman EM, Röther J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, Pencina MJ, Goto S; REACH Registry Investigators . One‐year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. [DOI] [PubMed] [Google Scholar]
- 19. Alberts MJ, Bhatt DL, Mas JL, Ohman EM, Hirsch AT, Röther J, Salette G, Goto S, Smith SC Jr, Liau CS, Wilson PW, Steg PG; REduction of Atherothrombosis for Continued Health Registry Investigators . Three‐year follow‐up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur Heart J. 2009;30:2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bangalore S, Steg G, Deedwania P, Crowley K, Eagle KA, Goto S, Ohman EM, Cannon CP, Smith SC, Zeymer U, Hoffman EB, Messerli FH, Bhatt DL; REACH Registry Investigators . β‐blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308:1340–1349. [DOI] [PubMed] [Google Scholar]
- 21. Califf RM, Mark DB, Harrell FE Jr, Hlatky MA, Lee KL, Rosati RA, Pryor DB. Importance of clinical measures of ischemia in the prognosis of patients with documented coronary artery disease. J Am Coll Cardiol. 1988;11:20–26. [DOI] [PubMed] [Google Scholar]
- 22. Gehi AK, Ali S, Na B, Schiller NB, Whooley MA. Inducible ischemia and the risk of recurrent cardiovascular events in outpatients with stable coronary heart disease: the Heart and Soul Study. Arch Intern Med. 2008;168:1423–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stone PH. Ischemia dictates outcome, not symptoms. J Am Coll Cardiol. 2013;61:712–713. [DOI] [PubMed] [Google Scholar]
- 24. Gehi AK, Rumsfeld JS, Liu H, Schiller NB, Whooley MA. Relation of self‐reported angina pectoris to inducible myocardial ischemia in patients with known coronary artery disease: the Heart and Soul Study. Am J Cardiol. 2003;92:705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Members of the REACH Registry Investigators.


