Abstract
Background
Timely reperfusion after ST‐elevation myocardial infarction (STEMI) improves survival. Guidelines recommend primary percutaneous coronary intervention (PPCI) within 90 minutes of arrival at a PCI‐capable hospital. The alternative is fibrinolysis within 30 minutes for those in those for whom timely transfer to a PCI‐capable hospital is not feasible.
Methods and Results
We identified STEMI patients receiving reperfusion therapy at 229 hospitals participating in the Get With the Guidelines—Coronary Artery Disease (GWTG‐CAD) database (January 1, 2003 through December 31, 2008). Temporal trends in the use of fibrinolysis and PPCI, its timeliness, and in‐hospital mortality outcomes were assessed. We also assessed predictors of fibrinolysis versus PPCI and compliance with performance measures. Defect‐free care was defined as 100% compliance with all performance measures. We identified 29 190 STEMI patients, of whom 2441 (8.4%) received fibrinolysis; 38.2% of these patients achieved door‐to‐needle times ≤30 minutes. Median door‐to‐needle times increased from 36 to 60 minutes (P=0.005) over the study period. Among PPCI patients, median door‐to‐balloon times decreased from 94 to 64 minutes (P<0.0001) over the same period. In‐hospital mortality was higher with fibrinolysis than with PPCI (4.6% vs 3.3%, P=0.001) and did not change significantly over time. Patients receiving fibrinolysis were less likely to receive defect‐free care compared with their PPCI counterparts.
Conclusions
Use of fibrinolysis for STEMI has decreased over time with concomitant worsening of door‐to‐needle times. Over the same time period, use of PPCI increased with improvement in door‐to‐balloon times. In‐hospital mortality was higher with fibrinolysis than with PPCI. As reperfusion for STEMI continues to shift from fibrinolysis to PPCI, it will be critical to ensure that door‐to‐needle times and outcomes do not worsen.
Keywords: fibrinolysis, myocardial infarction, outcome and process assessment, primary percutaneous coronary intervention
Subject Categories: Myocardial Infarction, Percutaneous Coronary Intervention, Quality and Outcomes, Epidemiology, Secondary Prevention
Introduction
The 2013 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines for ST‐elevation myocardial infarction (STEMI) recommend emergent reperfusion of patients presenting with STEMI with a goal first medical contact (FMC)‐to‐device time of ≤90 minutes for patients undergoing primary percutaneous coronary intervention (PPCI) and presenting to a PCI‐capable hospital.1 For patients presenting to a non‐PCI‐capable hospital, the goal FMC to device time is ≤120 minutes. The other alternative to PPCI is fibrinolysis, when timely transfer from a non‐PCI‐capable to PCI‐capable hospital is not feasible, with a recommended door‐to‐needle (DTN) time of ≤30 minutes. These recommendations have evolved from 20042 with goal door‐to‐balloon (DTB) time for PPCI being ≤90 minutes and have become a focus of regional and national quality improvement initiatives.3 However, many hospitals in the United States still do not have the capacity to perform PPCI or do so during regular hours only, and many of them are located in geographical areas where timely transfer for PPCI is not feasible, resulting in failure to meet guideline‐recommended timely reperfusion with PPCI.4 A study from the Euro Heart Survey ACS‐III database examined temporal trends in reperfusion in STEMI patients and found an overall decrease in fibrinolytic use with an increase in timely fibrinolysis from 61.7% to 71.1% with a concomitant decrease in DTN time from 20 to 15 minutes between 2006 and 2008.5 It is unclear whether a parallel improvement in the timeliness of reperfusion with fibrinolytic therapy has been observed in the United States and whether it has influenced in‐hospital mortality. Furthermore, compliance with acute myocardial infarction (AMI) performance measures6 in patients receiving fibrinolysis is not known.
Therefore, we aimed to examine the frequency and temporal pattern of fibrinolytic use compared to PPCI in patients with STEMI enrolled in the Get With the Guidelines—Coronary Artery Disease (GWTG‐CAD) database. We also evaluated the predictors of fibrinolytic use and the timeliness of reperfusion with either strategy and compared in‐hospital mortality and performance measures in patients receiving fibrinolysis versus PPCI.
Methods
Study Population
The GWTG‐CAD database was launched in 2000 and represents a national, prospective, observational registry and quality improvement initiative established by the American Heart Association (AHA).7, 8, 9, 10 It is a collaborative effort among researchers, professional organizations, and hospitals to provide feedback on performance and strategies to improve the care of patients with CAD. The details of the program have been described elsewhere.10 The database includes teaching and nonteaching, rural and urban, large and small hospitals, and community and tertiary referral hospitals from all census regions of the United States. Clinical information on patient demographics, medical history, symptoms on arrival, results of laboratory testing, in‐hospital treatment and events, discharge treatment and counseling, and on patient disposition is submitted using an online, interactive case report form and patient management tool (Patient Management Tool, Outcome Sciences Inc, Cambridge, MA). Hospitals are encouraged to enroll all eligible patients consecutively with case finding preferentially based on clinical identification of patients.11 All participating institutions were required to comply with local regulatory and privacy guidelines and to submit the GWTG‐CAD protocol for review and approval by their institutional review boards. Because data were used primarily at each local site for quality improvement, sites were granted a waiver of informed consent under the common rule.
Cohort Development and Definitions
Between January 1, 2003 and December 31, 2008, data were available on 238 465 patients enrolled from 415 hospitals participating in the GWTG‐CAD program who were hospitalized with a confirmed clinical diagnosis of CAD—including patients with acute coronary syndromes, those with stable CAD hospitalized for revascularization, and those with documented CAD hospitalized for reasons other than heart failure. Hospitals with >25% missing from the medical history panel and patients with unrecorded sex were excluded from the analyses. Patients were excluded if they were not diagnosed with STEMI, did not receive fibrinolysis or primary PCI, had missing discharge status, were transferred to another acute care facility, or left against medical advice (Figure 1). Sites with fewer than 5 patients after the prior exclusions were also excluded. Following these exclusions, our study population consisted of 29 190 STEMI patients from 229 sites.
Figure 1.
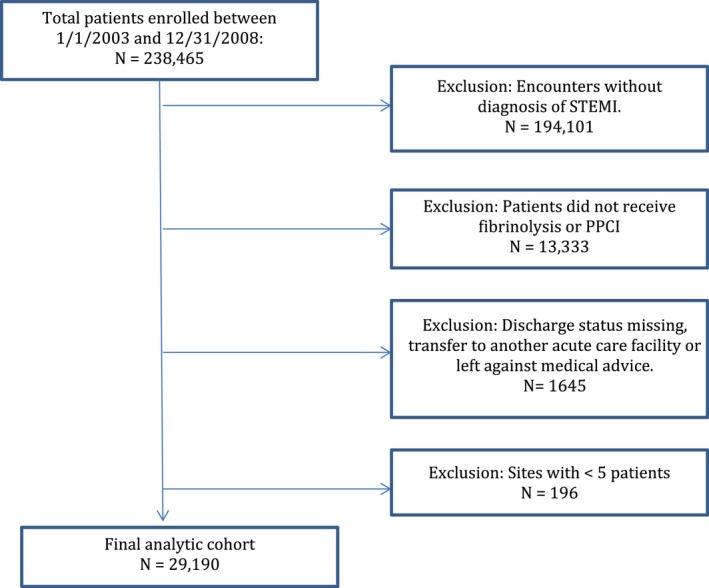
Study cohort development and exclusions. STEMI indicates ST elevation myocardial infarction.
Patients who had procedures listed as PCI, PCI with stent, or percutaneous transluminal angioplasty (PTCA) and had not received fibrinolytics were classified as patients receiving PPCI.
Study Outcomes and Measures
Modality of reperfusion (fibrinolytic therapy vs PPCI) was the primary independent variable. We studied the following outcomes: (1) the overall frequency of fibrinolysis, (2) the frequency of timely reperfusion with fibrinolysis (DTN ≤30 minutes) and PPCI (door‐to‐balloon (DTB) ≤90 minutes), and (3) the association of fibrinolysis with in‐hospital mortality and compliance with AMI performance measures of the GWTG‐CAD program in patients presenting with STEMI. We also assessed the temporal trends in fibrinolytic use, timeliness of reperfusion, in‐hospital mortality, and performance measure compliance during the study period.
The performance measures of the GWTG‐CAD program have been previously described8 and included (1) aspirin therapy within 24 hours of acute myocardial infarction (AMI), (2) aspirin therapy at discharge, (3) smoking cessation counseling for eligible patients, (4) angiotensin‐converting enzyme inhibitor (ACE‐I) or angiotensin receptor blocker (ARB) therapy in patients with left ventricular systolic dysfunction at discharge, (5)β‐blocker (BB) therapy at discharge, and (6) lipid‐lowering therapy for patients with low‐density lipoprotein (LDL) cholesterol levels >100 mg/dL. A composite performance measure of compliance termed “defect‐free care” was also assessed and defined as achievement of all 6 GWTG‐CAD performance measures.12 Length of stay was also examined.
Statistical Analyses
For the descriptive analyses, patients' sociodemographic, insurance type and medical history variables, hospital characteristics, clinical performance measures, and in‐hospital mortality rates were compared between fibrinolytic therapy and PPCI. Data were reported as mean±SD values for continuous variables (or medians and interquartile ranges, 25th‐75th percentiles) and as percentages for categorical variables. Categorical and continuous variables were compared with the use of the Pearson χ2 and Wilcoxon rank‐sum tests, respectively.
A Cochran‐Armitage test was performed to assess the temporal trend in the timeliness of reperfusion during the study period. Multivariable logistic regression analyses were used to examine the predictors of fibrinolytic use and delayed therapy (DTN >30 minutes or DTB >90 minutes) as well as to evaluate the association of fibrinolysis and timely reperfusion with in‐hospital outcomes. The generalized estimating equation method with exchangeable working correlation structure was used to account for within‐hospital clustering because patients at the same hospital are more likely to have similar treatment relative to patients in other hospitals. Sites not capable of performing PCI were excluded from the analysis of therapy type. Transfer‐in patients and sites not capable of PCI were also excluded from the analysis of delayed DTB time. The regression models adjusted for the following covariates: patient demographics (age, sex, race, height, weight, blood pressure, insurance), medical history variables (anemia, atrial fibrillation, atrial flutter, CAD, chronic obstructive pulmonary disease [COPD] or asthma, cardiac resynchronization therapy—defibrillator [CRT‐D], CRT‐pacing [CRT‐P], cerebrovascular accident [CVA]/transient ischemic attack [TIA], depression, diabetes, dialysis, heart failure, hyperlipidemia, hypertension, ICD only, pacemaker, peripheral vascular disease [PVD], coronary artery bypass graft [CABG], prior myocardial infarction [MI], prior PCI, renal insufficiency, valvular heart disease, prior CABG, smoking), arrival time (off hours vs regular hours), hospital characteristics (region, hospital type, number of beds, rural vs urban, capability of cardiothoracic surgery), and admission year. Additional analyses included comparisons of in‐hospital outcomes and performance measure compliance between patients who received timely therapy and those who did not (DTN ≤30 minutes vs DTN >30 minutes, and DTB ≤90 minutes vs DTB >90 minutes). Most variables had complete data or had a missing rate <3%, with the exception of insurance, weight, and BMI, which had 7% missing. We used multiple imputation for missing values when performing logistic regression.
A P<0.05 was considered statistically significant for all tests, and all tests of statistical significance were 2‐tailed. All statistical analyses were performed centrally at the Duke Clinical Research Institute (DCRI, Durham, NC) with SAS software (version 9.1, SAS Institute, Cary, NC).
Results
Study exclusions are shown in Figure 1. We evaluated a total of 238 465 patients from 415 hospitals between January 1, 2003 and December 31, 2008 from the GWTG‐CAD database. After excluding patients not diagnosed with STEMI, those not undergoing reperfusion therapy, patients with missing discharge data, transfer‐out patients, and those leaving against medical advice, the final study cohort included 29 190 STEMI patients from 229 hospitals. Of those, 2441 (8.4%) patients received fibrinolysis in 180 hospitals. Overall, 38.2% of patients achieved timely fibrinolysis with a median DTN of 37 minutes (IQR 21‐65). During hospitalization, 74.4% of these patients underwent coronary angiography, 58.8% underwent PCI, and 7.2% underwent CABG surgery.
Baseline characteristics of patients receiving fibrinolysis compared to PPCI are summarized in Table 1. Patients receiving fibrinolysis had a lower prevalence of prior CAD and dyslipidemia, higher prevalence of diabetes and hypertension, and presented to rural hospitals more frequently. Patients receiving PPCI were less likely to receive CABG during their hospitalization. Overall, 54.8% of patients undergoing PPCI received timely PPCI (DTB ≤90 minutes) with a median DTB of 80 (IQR 51‐120) minutes.
Table 1.
Baseline Characteristics of Patients With STEMI Receiving Fibrinolysis vs PPCI
| Variable, n (%) Unless Otherwise Indicated | Fibrinolysis n=2441 (%)a | PPCI n=26 749 (%)a | P Value |
|---|---|---|---|
| Demographics | |||
| Age (mean±standard deviation), y | 60.4±13.2 | 60.8±13.2 | 0.19 |
| Male | 1729 (70.8) | 18 991 (71.0) | 0.86 |
| Race | |||
| White | 1748 (73.2) | 20 875 (79.4) | <0.001 |
| Black | 192 (8.0) | 1643 (6.3) | |
| Hispanic | 290 (12.1) | 1814 (6.9) | |
| BMI, mean±SD | 28.7±6.3 | 28.9±6.0 | 0.19 |
| Insurance typeb | |||
| Medicare | 481 (22.1) | 5611 (22.6) | <0.001 |
| Other | 1118 (51.4) | 13 969 (56.3) | |
| None | 415 (19.1) | 3908 (15.8) | |
| Medical history | |||
| CAD | 158 (7.2) | 2636 (10.4) | <0.001 |
| Atrial fibrillation | 73 (3.3) | 803 (3.2) | 0.71 |
| Diabetes | 598 (27.2) | 5762 (22.8) | <0.001 |
| Hypertension | 1331 (60.6) | 14 704 (58.2) | 0.03 |
| Dyslipidemia | 851 (38.8) | 11 884 (47.0) | <0.001 |
| Heart failure | 138 (6.3) | 1252 (5.0) | 0.01 |
| Prior PCI | 19 (0.9) | 539 (2.1) | <0.001 |
| Prior CABG | 10 (0.5) | 158 (0.6) | 0.33 |
| COPD or asthma | 223 (10.2) | 2203 (8.7) | 0.02 |
| Renal insufficiency | 88 (4.0) | 793 (3.1) | 0.03 |
| CVA/TIA | 90 (4.1) | 1069 (4.2) | 0.77 |
| Smoking | 999 (46.9) | 10 748 (43.3) | 0.001 |
| Hospital characteristics | |||
| Region | |||
| West | 525 (21.5) | 6021 (22.5) | <0.001 |
| South | 1068 (43.8) | 7947 (29.7) | |
| Midwest | 504 (20.7) | 7466 (27.9) | |
| Northeast | 344 (14.1) | 5315 (19.9) | |
| Teaching hospital | 1246 (51.1) | 17 324 (64.8) | <0.0001 |
| Number of beds (mean±SD) | 441.8±279.7 | 456.3±274.7 | <0.0001 |
| Rural location | 223 (9.1) | 1153 (4.7) | <0.001 |
BMI indicates body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; PCI, percutaneous coronary intervention; PPCI, primary percutaneous coronary intervention; SD, standard deviation; STEMI, ST elevation myocardial infarction; TIA, transient ischemic attack.
Percentage for each characteristic calculated out of available data.
Patients can have more than 1 type of insurance.
During the study period, fibrinolysis decreased from 20.5% in 2003 to 3.7% in 2008 (P<0.0001) (Figure 2A). There was an increase in median DTN (36 to 60 minutes; P=0.005), and median DTB decreased (94 to 64 minutes; P<0.0001). As a result, there was a decrease in the proportion of patients with timely fibrinolysis and an increase in the proportion of patients with timely PPCI (Figure 2B). In‐hospital mortality with fibrinolysis was higher than that with PPCI (4.6% vs 3.3%, P=0.001) and was consistent during the study period (Figure 2C). Median length of stay was longer for patients receiving fibrinolysis compared to PPCI (4 [IQR 3‐6] vs 3 [IQR 2‐5] days, P<0.0001) (Table 2).
Figure 2.
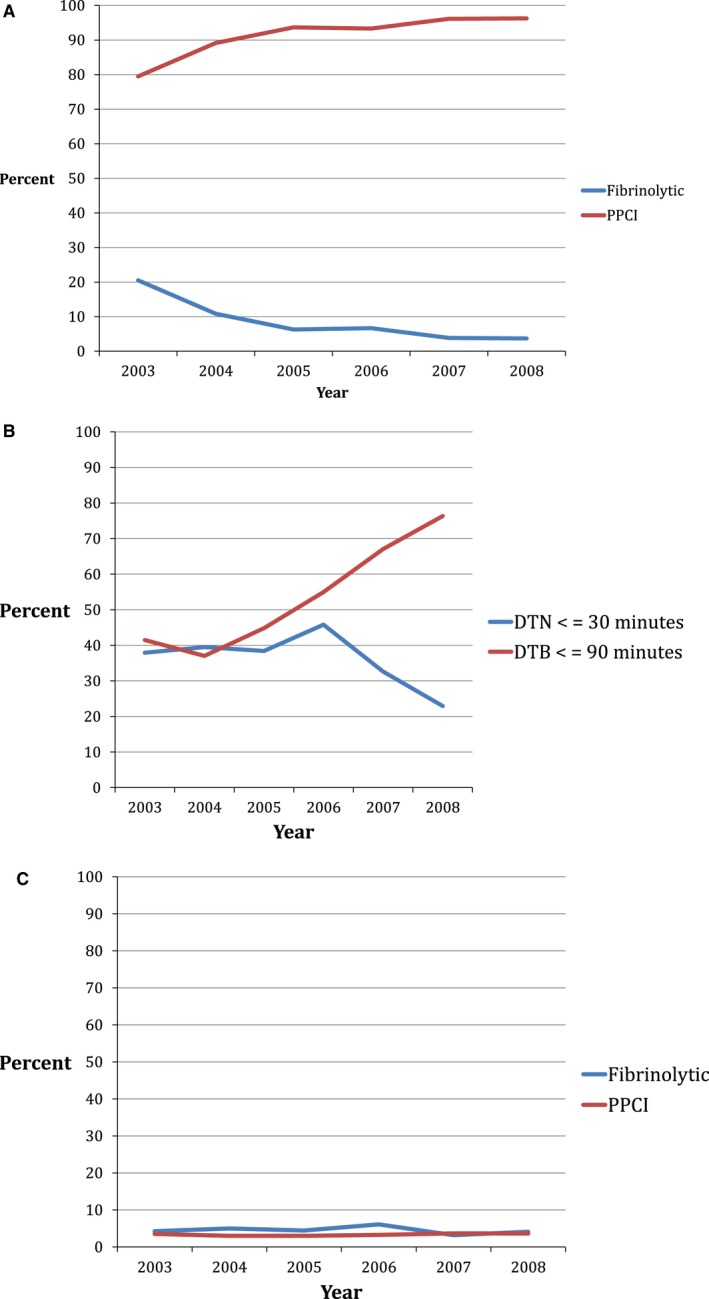
A, Annual trends in fibrinolysis and PPCI for patients with STEMI in the GWTG‐CAD database. The frequency of fibrinolysis decreased from 20.5% in 2003 to 3.7% in 2008 while PPCI increased from 79.5% in 2003 to 96.3% in 2008 (P value from Cochran‐Armitage test <0.0001). B, Annual trends in the proportion of patients receiving timely reperfusion with fibrinolysis (DTN ≤30 minutes) and PPCI (DTB ≤90 minutes) for patients with STEMI in the GWTG‐CAD database. The frequency of timely fibrinolysis peaked at 45.8% in 2006 and decreased to 22.9% in 2008 while timely PPCI increased from 37.0% in 2003 to 76.3% in 2008. (P value from Cochran‐Armitage test=0.2769 for DTN ≤30 minutes; P<0.0001 for DTB ≤90 minutes.) C, Annual trends of in‐hospital mortality for patients receiving fibrinolysis and PPCI. In‐hospital mortality was 4.6% in patients receiving fibrinolysis and 3.3% in patients receiving PPCI and did not change significantly over time. (P value from Cochran‐Armitage test=0.9473 for fibrinolytics patients, P=0.1474 for PPCI patients.) CAD indicates coronary artery disease; DTB, door to balloon; DTN, door to needle; GWTG, Get With the Guidelines; PPCI, primary percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
Table 2.
In‐Hospital Outcomes Between Patients With STEMI Receiving Fibrinolysis vs PPCI
| Variable | Fibrinolytics n=2441 (%) | PPCI n=26 749 (%) | P Value |
|---|---|---|---|
| In‐hospital mortality | 112 (4.6) | 890 (3.3) | 0.001 |
| Length of stay (median, IQR), days | 4.0 (3‐6) | 3.0 (2‐5) | <0.0001 |
| Length of stay >4 days | 663 (38.9) | 5268 (27.8) | <0.0001 |
| Door‐to‐reperfusion time | |||
| DTN, median (IQR), minutes | 37 (21–65) | n/a | |
| DTN ≤30 minutes (%) | 731 (38.2) | n/a | |
| DTB, median (IQR), minutes | n/a | 80 (51‐120) | |
| DTB ≤90 minutes (%) | n/a | 10 907 (54.8) | |
DTB indicates door to balloon; DTN, door to needle; IQR, interquartile range; PPCI, primary percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
Independent predictors of fibrinolytic use included off‐hour presentation, Midwest and southern regions versus northeast, rural location, smoking, and no hyperlipidemia. Independent predictors of delayed fibrinolysis and delayed PPCI are summarized in Tables S1 through S3.
Performance measures and defect‐free care were lower among patients receiving fibrinolysis compared to PPCI (defect‐free care 81.1% vs 90.1%, P<0.0001) (Table 3). However, in patients receiving fibrinolysis, defect‐free care increased over the study period (74% to 91%, P<0.0001). No difference in compliance with performance measures was noted between patients with timely versus delayed fibrinolysis, although patients with timely PPCI were more likely to receive defect‐free care than patients with delayed PPCI (93.3% vs 87.7%, P<0.0001).
Table 3.
Adherence to CAD Performance Measures Between Patients With STEMI Receiving Fibrinolysis vs PPCI
| Performance Measure | Fibrinolytics N=2441 (%) | Primary PCI n=26 749 (%) | P Value |
|---|---|---|---|
| ACE‐I or ARB for LVSD | 342 (83.2) | 4440 (90.2) | <0.0001 |
| Aspirin at discharge | 2175 (97.0) | 24 685 (98.6) | <0.0001 |
| Aspirin within 24 hours | 1510 (93.5) | 17 707 (96.7) | <0.0001 |
| Β‐Βlockers at discharge | 2061 (95.0) | 23 616 (97.9) | <0.0001 |
| Patients with LDL‐C >100 mg/dL who received statins or lipid‐lowering drugs | 783 (90.2) | 9090 (95.3) | <0.0001 |
| Smoking cessation for CAD | 980 (88.9) | 10 773 (94.7) | <0.0001 |
| Defect‐free care (100% compliance with performance measures) | 1932 (81.1) | 23 631 (90.1) | <0.0001 |
ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; LDL, low‐density lipoprotein; LVSD, left ventricular systolic dysfunction; PPCI, primary percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
After multivariable adjustment, there was no significant association of reperfusion therapy type or timely fibrinolysis (DTN ≤30 minutes) with in‐hospital mortality. However, an association between timely PPCI (DTB ≤90 minutes) and in‐hospital mortality was seen (OR 0.63, 95% CI 0.49‐0.79; P=0.0001) (Table 4).
Table 4.
Association of Outcomes With Therapy Type and Timely Reperfusion
| Outcome | Fibrinolytics vs PPCI | DTN ≤30 vs DTN >30 | DTB <90 vs DTB >90 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | |
| In‐hospital mortality | 1.26 (0.94‐1.70) | 0.12 | 1.22 (0.94‐1.58) | 0.14 | 0.59 (0.37‐0.95) | 0.03 | 0.79 (0.47‐1.33) | 0.37 | 0.52 (0.41‐0.66) | <0.0001 | 0.63 (0.49‐0.79) | 0.0001 |
| Length of stay ≥4 days | 1.51 (1.28‐1.77) | <0.0001 | 1.53 (1.29‐1.83) | <0.0001 | 0.76 (0.61‐0.95) | 0.01 | 0.87 (0.68‐1.10) | 0.23 | 0.65 (0.60‐0.70) | <0.0001 | 0.71 (0.65‐0.78) | <0.0001 |
| Aspirin within 24 hours | 0.71 (0.51‐0.98) | 0.04 | 0.69 (0.51‐0.93) | 0.01 | 0.77 (0.54‐1.10) | 0.14 | 0.79 (0.55‐1.14) | 0.21 | 1.42 (1.15‐1.75) | 0.001 | 1.37 (1.06‐1.76) | 0.02 |
| Aspirin at discharge | 0.85 (0.60‐1.21) | 0.37 | 0.92 (0.63‐1.34) | 0.65 | 1.03 (0.61‐1.76) | 0.91 | 1.02 (0.58‐1.79) | 0.96 | 1.26 (0.86‐1.84) | 0.24 | 1.12 (0.74‐1.69) | 0.61 |
| Smoking cessation advice given | 0.87 (0.64‐1.18) | 0.37 | 0.86 (0.62‐1.20) | 0.38 | 1.09 (0.62‐1.91) | 0.76 | 1.09 (0.66‐1.81) | 0.73 | 1.16 (0.91‐1.48) | 0.23 | 1.15 (0.88‐1.51) | 0.30 |
| ACE‐I/ARB for LVSD at discharge | 0.92 (0.60‐1.40) | 0.69 | 0.91 (0.59‐1.40) | 0.66 | 0.75 (0.40‐1.40) | 0.36 | 0.58 (0.25‐1.39) | 0.23 | 2.00 (1.50‐2.65) | <0.0001 | 1.73 (1.26‐2.38) | 0.0008 |
| β‐Blockers at discharge | 0.69 (0.47‐1.00) | 0.05 | 0.75 (0.49‐1.13) | 0.16 | 0.86 (0.53‐1.39) | 0.53 | 0.88 (0.54‐1.42) | 0.60 | 1.41 (1.05‐1.90) | 0.02 | 1.36 (0.97‐1.89) | 0.07 |
| Statins or lipid‐lowering drugs at discharge for patients with LDL‐C >100 mg/dL | 0.81 (0.58‐1.13) | 0.21 | 0.83 (0.59‐1.17) | 0.29 | 1.24 (0.79‐1.93) | 0.36 | 1.21 (0.72‐2.05) | 0.48 | 1.08 (0.79‐1.46) | 0.64 | 1.01 (0.71‐1.42) | 0.98 |
Adjusted for age, sex, race, insurance, body mass index (BMI), height, medical history including anemia, atrial fibrillation, atrial flutter, coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD) or asthma, cardiac resynchronization therapy‐defibrillator (CRT‐D), cardiac resynchronization therapy‐pacemaker (CRT‐P), cerebrovascular accident (CVA)/transient ischemic attack (TIA), diabetes, heart failure, hyperlipidemia, hypertension, implantable cardioverter defibrillator (ICD), pacemaker, peripheral vascular disease (PVD), coronary artery bypass graft (CABG), prior myocardial infarction (MI), prior percutaneous coronary intervention (PCI), renal insufficiency, valvular heart disease, smoking; hospital characteristics (region, hospital type, number of beds, rural vs urban, admission year‐quarter). ACE‐I indicates angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; CI, confidence interval; DTB, door to balloon; DTN, door to needle; LDL, low‐density lipoprotein; LVSD, left ventricular systolic dysfunction; OR, odds ratio; PPCI, primary percutaneous coronary intervention.
Discussion
Our data provide an insight into the use of fibrinolytic therapy for acute reperfusion treatment of STEMI in a large national registry of hospitals in the United States. We have shown that 8.4% of patients presenting with STEMI received fibrinolysis, of whom only 38.2% achieved timely DTN ≤30 minutes. The use of fibrinolytic therapy decreased from 20.5% in 2003 to 3.7% in 2008, and its timeliness worsened, with nearly doubling in median DTN times from 36 to 60 minutes (P=0.005). DTB significantly improved during the same time period. Performance measures and defect‐free care improved in patients receiving fibrinolysis over the study period. Therefore, it is important to continue to monitor timeliness and outcomes of fibrinolysis in eligible patients as use of fibrinolysis becomes more infrequent.
PPCI is associated with improved mortality compared to fibrinolytic therapy in patients presenting with STEMI and is the preferred reperfusion strategy when feasible in a timely manner.1, 13 It has been shown that for every 10‐minute delay in performing PCI, the mortality benefit of PCI compared to fibrinolysis is decreased by 1%, and both reperfusion strategies become almost equivalent at 62 minutes.14 However, only one third of acute care hospitals in the United States have around‐the‐clock PCI capability.15, 16 Further, despite investment of effort and resources, limited reductions in interhospital transfer times have occurred.17 The guidelines for management of patients with STEMI have evolved with the 1996 and 1999 recommendations18 stating that PPCI was an alternative to fibrinolytic therapy for reperfusion and 2004 recommendations2 stating that PPCI was the preferred reperfusion strategy with fibrinolysis being used when timely PPCI could not be delivered. This likely influenced practice patterns and is consistent with the temporal trend we found in our study with increasing use of PPCI and declining fibrinolytic use. The current guidelines for STEMI recommend fibrinolysis for eligible patients presenting to non‐PCI‐capable hospitals who would not receive FMC‐to‐device time ≤120 minutes on transfer. Therefore, fibrinolysis plays a key role in the management of eligible STEMI patients unable to receive timely PPCI.
A recent report from the National Cardiovascular Data Registry (NCDR)4 showed that, among patients presenting to a non‐PCI‐capable hospital and transferred for PPCI, only 51.3% of patients achieved DTB ≤120 minutes. Among patients eligible to receive fibrinolysis with estimated drive time over 60 minutes, only 52.7% received fibrinolysis. Median DTN was 34 minutes, and only 43.8% achieved DTN ≤30 minutes, which is consistent with our results. The in‐hospital mortality of patients receiving fibrinolysis was 3.7% in that report,4 which is also similar to our findings. In‐hospital mortality of patients undergoing PPCI in that report was 3.9%, which is higher than the 3.3% seen in our study and may have been due to delay in transfer with median DTB time of 126 minutes. Possible reasons for the worsening in the timeliness of fibrinolysis include the overall decrease in the frequency of fibrinolytic use, resulting in unfamiliarity with its administration protocols, complex care coordination between transferring and receiving centers resulting in delays and cancellation of some transfers, and the perceived hazards of fibrinolytic therapy (eg, intracranial hemorrhage) despite delayed transfer resulting in reluctance in its use.
Another report from the NCDR examined temporal trends in DTB time.19 They found that between 2005 and 2009, median DTB time had decreased from 83 to 67 minutes while the percentage of patients achieving timely PPCI (DTB ≤90 minutes) had increased from 59.7% to 83.1%. Despite these significant improvements in DTB times, they noted no significant change in in‐hospital mortality at 4.7%. Our results corroborate these findings. We previously argued that some of the time differential in DTB times observed in clinical studies may be too small to exert a meaningful clinical impact, especially when short‐term outcomes are examined.20, 21 A prior report from the National Registry of Myocardial Infarction (NRMI)22 evaluating reperfusion strategies in STEMI patients in 1990 through 2006 found a decrease in fibrinolysis from 52.5% to 27.6% with a nearly linear decline in DTN from 59 to 29 minutes and a corresponding decrease in mortality from 7.0% to 6.0% over the study period. The relative improvement in mortality attributable to improvements in DTN time was 16.3%. The median DTN in our study was lowest in 2006 (31 minutes), similar to the results from this study. This was followed by an increase to 43.5 minutes in 2007 and 60 minutes in 2008. Overall, the mortality rates in our study were among the lowest reported with both reperfusion strategies. The lack of significant change in in‐hospital mortality is consistent with other reports and can be attributed to already marked improvement in in‐hospital mortality rates, making it difficult to achieve further incremental benefit. One may also argue that the total ischemic time from onset of arterial occlusion to reperfusion, rather than medical contact to reperfusion times, may play a more important role in short‐term in‐hospital outcomes. This may also be related to a low event rate among a small number of patients who received fibrinolysis in our study.
Further, there has been some renewed interest in fibrinolysis and pharmacoinvasive strategies in which cardiac catheterization is routinely performed within 6 hours of fibrinolytic administration. The Trial of Routine Angioplasty and Stenting After Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction (TRANSFER‐AMI)23 showed that high‐risk STEMI patients receiving fibrinolysis and routine early PCI had improved short‐term outcomes compared to patients receiving standard therapy and delayed PCI. A report of 73 Belgian hospitals24 found that modern fibrinolytic strategies substantially attenuated the mortality benefit of PPCI over fibrinolysis except for patients in the highest‐risk subgroup with thrombolysis in myocardial infarction (TIMI) scores of 7 to 14. The Strategic Reperfusion Early After Myocardial Infarction (STREAM)25 study showed that in patients with STEMI unable to undergo PPCI within 1 hour, prehospital fibrinolysis and angiography within 6 to 24 hours resulted in effective reperfusion compared to PPCI alone. However, fibrinolysis was associated with a slight increase in intracranial bleeding.26 Therefore, given the delays in transfer times from non‐PCI‐capable hospitals and the possible lack of mortality benefit of PPCI over fibrinolysis in low‐risk patients, the role of timely reperfusion with fibrinolysis followed by routine angiography needs to reconsidered.
The ACC/AHA emphasis on care of patients with STEMI has resulted in the public reporting of these performance measures,3 which are also now tied to reimbursement from the Centers for Medicare and Medicaid Services27 and therefore have financial implications for institutions. Our results, as well as those of others, depict the significant improvements seen in DTB times and timely PPCI. However, as our study has shown, this improvement has not occurred in DTN times and timely fibrinolysis. Additionally, the improvement noted in defect‐free care and adherence to performance measures in patients receiving either reperfusion strategy over the study period indicates the important role played by quality improvement programs. However, defect‐free care was found to be significantly lower in patients receiving fibrinolysis and should raise concerns in patients receiving fibrinolysis as use continues to decrease.
Study Limitations
Our study has several limitations. First, the GWTG program is a quality improvement program, and participation is voluntary. Therefore, these hospitals may be highly motivated for quality improvement, and results may not be fully representative of national care patterns and clinical outcomes. Second, data on prehospital delay or treatments, 24‐hour PCI capability of hospitals, bleeding outcomes, or postdischarge mortality and morbidity were not available. Third, eligibility, type, and time of treatment received were based on documentation in the medical record and were thus dependent on the accuracy of this documentation. Fourth, there might be other measured or unmeasured confounding variables that, had they been adjusted for, would have altered outcomes. Finally, trends in reperfusion strategies were assessed between 2003 and 2008, and therefore, contemporary patterns of fibrinolysis may be different. However, our study demonstrates the importance of tracking and improving DTN times in hospitals that do not perform PPCI around the clock as well as the need for contemporary data in trends and outcomes of fibrinolytic use for STEMI.
Conclusions
The use of fibrinolytic therapy for STEMI has decreased from 2003 to 2008, in contradistinction to the use of PPCI. Despite improvements in timely PPCI and defect‐free care, a significant delay in timely fibrinolysis was noted over time. In‐hospital mortality among STEMI patients receiving fibrinolysis remained unchanged over the study period and worse than that of their PPCI counterparts. Our findings highlight important opportunities to improve the use of fibrinolytic therapy and its timeliness among eligible STEMI patients.
Sources of Funding
Hira was the recipient of a Database Research Seed Grant from the American Heart Association Council on Clinical Cardiology.
Disclosures
Bhatt discloses the following relationships: Advisory Board of Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors of Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair of American Heart Association Quality Oversight Committee; Data Monitoring Committee of Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria from American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), others including Clinical Cardiology (Deputy Editor); Research Funding from Amarin, Amgen, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties from Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co‐Investigator at Biotronik and St. Jude Medical; Trustee of American College of Cardiology; unfunded research for FlowCo, PLx Pharma, Takeda. Fonarow is a member of the GWTG Steering Committee. Virani receives grant/research support (all significant and paid to the institution, not individual) from the Department of Veterans Affairs, American Diabetes Association, and American Heart Association. Eapen discloses the following relationships: Novartis (consultant, advisory board), Amgen (consultant, advisory board), SHL Telemedicine (consultant), Janssen (honorarium), MyoKardia (consultant). Albert was the Immediate Past Chair of the GWTG Executive Database Steering Committee. Peterson is the codirector of the GWTG Data Analytic Center at the Duke Clinical Research Institute. All other authors have no relevant disclosures.
Supporting information
Table S1. Predictors of Fibrinolytic Use vs PPCI
Table S2. Predictors of Delayed Fibrinolysis (DTN >30 Minutes) Versus Timely Fibrinolysis (DTN <30 Minutes)
Table S3. Predictors of Delayed PPCI (DTB >90 Minutes) vs Timely PPCI (DTB <90 Minutes)
(J Am Heart Assoc. 2016;5:e004113 doi: 10.1161/JAHA.116.004113)
This manuscript was handled independently by Saket Girotra, MD, as a guest editor. The editors had no role in the evaluation of this manuscript or in the decision about its acceptance.
References
- 1. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 2. Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). Circulation. 2004;110:588–636. [DOI] [PubMed] [Google Scholar]
- 3. Krumholz HM, Anderson JL, Bachelder BL, Fesmire FM, Fihn SD, Foody JM, Ho PM, Kosiborod MN, Masoudi FA, Nallamothu BK; American College of Cardiology/American Heart Association Task Force on Performance Measures, American Academy of Family Physicians, American College of Emergency Physicians, American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Cardiovascular Angiography and Interventions, and Society of Hospital Medicine . ACC/AHA 2008 performance measures for adults with ST‐elevation and non‐ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (writing committee to develop performance measures for ST‐elevation and non‐ST‐elevation myocardial infarction) developed in collaboration with the American Academy of Family Physicians and American College of Emergency Physicians endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Cardiovascular Angiography and Interventions, and Society of Hospital Medicine. J Am Coll Cardiol. 2008;52:2046–2099. [DOI] [PubMed] [Google Scholar]
- 4. Vora AN, Holmes DN, Rokos I, Roe MT, Granger CB, French WJ, Antman E, Henry TD, Thomas L, Bates ER, Wang TY. Fibrinolysis use among patients requiring interhospital transfer for ST‐segment elevation myocardial infarction care: a report from the US National Cardiovascular Data Registry. JAMA Intern Med. 2015;175:207–215. [DOI] [PubMed] [Google Scholar]
- 5. Schiele F, Hochadel M, Tubaro M, Meneveau N, Wojakowski W, Gierlotka M, Polonski L, Bassand JP, Fox KA, Gitt AK. Reperfusion strategy in Europe: temporal trends in performance measures for reperfusion therapy in ST‐elevation myocardial infarction. Eur Heart J. 2010;31:2614–2624. [DOI] [PubMed] [Google Scholar]
- 6. Xian Y, Pan W, Peterson ED, Heidenreich PA, Cannon CP, Hernandez AF, Friedman B, Holloway RG, Fonarow GC; GWTG Steering Committee and Hospitals . Are quality improvements associated with the Get With the Guidelines‐Coronary Artery Disease (GWTG‐CAD) program sustained over time? A longitudinal comparison of GWTG‐CAD hospitals versus non‐GWTG‐CAD hospitals. Am Heart J. 2010;159:207–214. [DOI] [PubMed] [Google Scholar]
- 7. Cohen MG, Fonarow GC, Peterson ED, Moscucci M, Dai D, Hernandez AF, Bonow RO, Smith SC Jr. Racial and ethnic differences in the treatment of acute myocardial infarction: findings from the Get With the Guidelines‐Coronary Artery Disease program. Circulation. 2010;121:2294–2301. [DOI] [PubMed] [Google Scholar]
- 8. LaBresh KA, Ellrodt AG, Gliklich R, Liljestrand J, Peto R. Get With the Guidelines for cardiovascular secondary prevention: pilot results. Arch Intern Med. 2004;164:203–209. [DOI] [PubMed] [Google Scholar]
- 9. LaBresh KA, Fonarow GC, Smith SC Jr, Bonow RO, Smaha LC, Tyler PA, Hong Y, Albright D, Ellrodt AG; Get With The Guidelines Steering Committee . Improved treatment of hospitalized coronary artery disease patients with the Get With the Guidelines program. Crit Pathw Cardiol. 2007;6:98–105. [DOI] [PubMed] [Google Scholar]
- 10. LaBresh KA, Gliklich R, Liljestrand J, Peto R, Ellrodt AG. Using “get with the guidelines” to improve cardiovascular secondary prevention. Jt Comm J Qual Saf. 2003;29:539–550. [DOI] [PubMed] [Google Scholar]
- 11. Qian F, Ling FS, Deedwania P, Hernandez AF, Fonarow GC, Cannon CP, Peterson ED, Peacock WF, Kaltenbach LA, Laskey WK, Schwamm LH, Bhatt DL; Get With The Guidelines Steering Committee, Investigators . Care and outcomes of Asian‐American acute myocardial infarction patients: findings from the American Heart Association Get With the Guidelines‐Coronary Artery Disease program. Circ Cardiovasc Qual Outcomes. 2012;5:126–133. [DOI] [PubMed] [Google Scholar]
- 12. Ambardekar AV, Fonarow GC, Dai D, Peterson ED, Hernandez AF, Cannon CP, Krantz MJ; Get With The Guidelines Steering Committee, Hospitals . Quality of care and in‐hospital outcomes in patients with coronary heart disease in rural and urban hospitals (from Get With the Guidelines‐Coronary Artery Disease program). Am J Cardiol. 2010;105:139–143. [DOI] [PubMed] [Google Scholar]
- 13. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. [DOI] [PubMed] [Google Scholar]
- 14. Nallamothu BK, Bates ER. Percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: is timing (almost) everything? Am J Cardiol. 2003;92:824–826. [DOI] [PubMed] [Google Scholar]
- 15. Concannon TW, Nelson J, Goetz J, Griffith JL. A percutaneous coronary intervention lab in every hospital? Circ Cardiovasc Qual Outcomes. 2012;5:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacobs AK, Antman EM, Faxon DP, Gregory T, Solis P. Development of systems of care for ST‐elevation myocardial infarction patients: executive summary. Circulation. 2007;116:217–230. [DOI] [PubMed] [Google Scholar]
- 17. Wang TY, Peterson ED, Ou FS, Nallamothu BK, Rumsfeld JS, Roe MT. Door‐to‐balloon times for patients with ST‐segment elevation myocardial infarction requiring interhospital transfer for primary percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. Am Heart J. 2011;161:76–83.e71. [DOI] [PubMed] [Google Scholar]
- 18. Ryan TJ, Antman EM, Brooks NH, Califf RM, Hillis LD, Hiratzka LF, Rapaport E, Riegel B, Russell RO, Smith EE 3rd, Weaver WD, Gibbons RJ, Alpert JS, Eagle KA, Gardner TJ, Garson A Jr, Gregoratos G, Ryan TJ, Smith SC Jr. 1999 update: ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee on management of acute myocardial infarction). J Am Coll Cardiol. 1999;34:890–911. [DOI] [PubMed] [Google Scholar]
- 19. Menees DS, Peterson ED, Wang Y, Curtis JP, Messenger JC, Rumsfeld JS, Gurm HS. Door‐to‐balloon time and mortality among patients undergoing primary PCI. N Engl J Med. 2013;369:901–909. [DOI] [PubMed] [Google Scholar]
- 20. Jneid H. Interplay between time of presentation, timeliness of reperfusion, and outcome after ST‐segment‐elevation myocardial infarction. Circ Cardiovasc Qual Outcomes. 2014;7:637–639. [DOI] [PubMed] [Google Scholar]
- 21. Kumbhani DJ, Cannon CP, Fonarow GC, Liang L, Askari AT, Peacock WF, Peterson ED, Bhatt DL; Get With the Guidelines Steering Committee Investigators . Association of hospital primary angioplasty volume in ST‐segment elevation myocardial infarction with quality and outcomes. JAMA. 2009;302:2207–2213. [DOI] [PubMed] [Google Scholar]
- 22. Gibson CM, Pride YB, Frederick PD, Pollack CV Jr, Canto JG, Tiefenbrunn AJ, Weaver WD, Lambrew CT, French WJ, Peterson ED, Rogers WJ. Trends in reperfusion strategies, door‐to‐needle and door‐to‐balloon times, and in‐hospital mortality among patients with ST‐segment elevation myocardial infarction enrolled in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1035–1044. [DOI] [PubMed] [Google Scholar]
- 23. Cantor WJ, Fitchett D, Borgundvaag B, Ducas J, Heffernan M, Cohen EA, Morrison LJ, Langer A, Dzavik V, Mehta SR, Lazzam C, Schwartz B, Casanova A, Goodman SG; TRANSFER‐AMI Trial Investigators . Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360:2705–2718. [DOI] [PubMed] [Google Scholar]
- 24. Claeys MJ, de Meester A, Convens C, Dubois P, Boland J, De Raedt H, Vranckx P, Coussement P, Gevaert S, Sinnaeve P, Evrard P, Beauloye C, Renard M, Vrints C. Contemporary mortality differences between primary percutaneous coronary intervention and thrombolysis in ST‐segment elevation myocardial infarction. Arch Intern Med. 2011;171:544–549. [DOI] [PubMed] [Google Scholar]
- 25. Armstrong PW, Gershlick AH, Goldstein P, Wilcox R, Danays T, Lambert Y, Sulimov V, Rosell Ortiz F, Ostojic M, Welsh RC, Carvalho AC, Nanas J, Arntz HR, Halvorsen S, Huber K, Grajek S, Fresco C, Bluhmki E, Regelin A, Vandenberghe K, Bogaerts K, Van de Werf F; STREAM Investigative Team . Fibrinolysis or primary PCI in ST‐segment elevation myocardial infarction. N Engl J Med. 2013;368:1379–1387. [DOI] [PubMed] [Google Scholar]
- 26. Bhatt DL. Timely PCI for STEMI—still the treatment of choice. N Engl J Med. 2013;368:1446–1447. [DOI] [PubMed] [Google Scholar]
- 27. QualityNet Specifications Manual, Version 4.4a. Available at: http://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1228773989482. Accessed January 18, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Predictors of Fibrinolytic Use vs PPCI
Table S2. Predictors of Delayed Fibrinolysis (DTN >30 Minutes) Versus Timely Fibrinolysis (DTN <30 Minutes)
Table S3. Predictors of Delayed PPCI (DTB >90 Minutes) vs Timely PPCI (DTB <90 Minutes)


