Abstract
Background
Troponin elevation with electrocardiography changes is not uncommon in patients with acute ischemic stroke; however, it is still unclear whether the mechanism of these changes is due to cardiac problems or neurally mediated myocytic damage. Thus, we investigated cardiac and neurological predictors of troponin elevation in those patients.
Methods and Results
We retrospectively analyzed medical data of the prospectively registered ischemic stroke patients on stroke registry who were admitted and underwent a serum cardiac troponin I and 12‐lead electrocardiography within 24 hours of symptom onset. However, patients with well‐known troponin‐elevating comorbidities were excluded from the analysis. Among 1404 ischemic stroke patients, 121 (8.7%) had elevated troponin, which was defined as more than 0.04 mg/mL. Multivariable analysis identified electrocardiography abnormalities such as QTc‐prolongation (odds ratio [OR]: 1.52, 95% CI: 1.02–2.28), left ventricular hypertrophy (OR: 2.14, 95% CI 1.43–3.19), Q‐wave (OR: 2.53, 95% CI: 1.48–4.32), and ST elevation (OR: 2.74, 95% CI: 1.12–6.72) as cardiac variables associated with troponin elevation, and higher National Institutes of Health Stroke Scale score (OR: 1.04, 95% CI: 1.01–1.07) and insular cortical lesions (OR: 2.78, 95% CI: 1.85–4.19) as neurological variables associated with troponin elevation. Incidence of troponin elevation as well as QTc‐prolongation was increased further in combination with cardiac and neurological factors.
Conclusions
Certain cardiac and neurological conditions in acute ischemic stroke may contribute to troponin elevation. The proposed concept of cardiac vulnerability to cerebrogenic stress can be a practical interpretation of troponin elevation and electrocardiography abnormalities in stroke patients.
Keywords: cardiac disease, electrocardiography, infarction, insular, troponin
Subject Categories: Ischemic Stroke, Biomarkers
Introduction
Stroke and myocardial infarction share common risk factors and pathological mechanisms;1 therefore, a higher proportion of patients with ischemic stroke have concomitant coronary artery disease, which in turn leads to an increased risk of death as well as development of ischemic stroke.2 Thus, since 2007, the guidelines for the management of ischemic stroke recommend to perform a 12‐lead electrocardiography and cardiac enzyme test,3, 4 particularly cardiac troponin, which is a highly sensitive and specific biomarker of myocardial damage.5
However, it still remains unclear whether the pathomechanism of troponin elevation during the acute stage of ischemic stroke is due to the concomitant cardiac problems,6 noncardiac comorbidities,7 or neurally mediated myocytic damage.8 Furthermore, ECG abnormalities are often challenging to interpret because various ECG changes can be developed by neurally mediated autonomic dysregulation after stroke (eg, particularly when stroke involves the insular cortex or is severe),9, 10 in addition to the previously developed ECG abnormalities before stroke.
Therefore, the aim of our study was to investigate cardiac and neurological predictors of troponin elevation and relationship between them during the acute stage of ischemic stroke. We excluded patients with well‐known troponin‐elevating comorbidities, and then ECG abnormalities, specific lesion locations, and stroke severity were evaluated as possible cardiac and neurological factors affecting troponin elevation during the acute stage of ischemic stroke.
Methods
Study Population
The consecutive patients who were admitted to the Asan Medical Center due to acute ischemic stroke within 24 hours of symptom onset between May 2007 and December 2011 were retrospectively evaluated. At admission, measurement of serum cardiac troponin I and ECG were routinely performed in all patients following the guidelines since 2007.3 After cardiac investigation, additional cardiac evaluations of patients were performed by cardiologists if the tests were suspected of indicating acute coronary syndrome.4 Among patients who were admitted to the stroke center, patients were excluded if they were diagnosed with troponin‐elevating conditions, including acute coronary syndrome, impaired renal function (estimated glomerular filtration rate <60 mL/min per 1.73 m2), and (3) congestive heart failure (having a history of heart failure or reduced ejection fraction ≤40%) at admission.
Demographic features and conventional risk factors were recorded, including hypertension (defined as receiving medication for hypertension, or blood pressure >140/90 mm Hg on repeated measurements), diabetes mellitus (defined as receiving medication for diabetes mellitus, fasting blood sugar ≥126 mg/dL, or 2‐hour postprandial glucose [PP2 ≥200 mg/dL]), hypercholesterolemia (defined as receiving cholesterol‐reducing agents, or overnight fasting cholesterol level >200 mg/dL), and current or recent (<6 months) history of cigarette smoking. This study was approved by the Institutional Review Board of Asan Medical Center, and written informed consent was waived because of its retrospective design.
Assessment of Ischemic Stroke
Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) and classified into 3 groups according to NIHSS scores (severe: ≥16 points, moderate: <16 and ≥7 points, mild: <7 points).11 The cause of stroke was classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria.12 The location of ischemic lesion was assessed on diffusion‐weighted magnetic resonance images with special regard to neural structures known to be involved in cardio‐autonomic control, such as the insular cortex.13
Cardiac Investigation
The lower limit of detection of serum cardiac troponin I (Abbott Laboratories, Abbott Park, IL) was 0.006 ng/mL, with significant elevation defined as a concentration >0.04 ng/mL. All patients underwent 12‐lead ECG (GE Healthcare, Waukesha, WI) at admission, with the results processed by the Marquette 12SL ECG Analysis Program. The resultant 12‐lead ECG waveforms were uploaded in digital form and interpreted by a cardiologist according to a modified version of the Minnesota code.14 Corrected QT intervals (QTc) were calculated by Bazett's formula and defined as prolonged if the QTc in lead II was ≥460 ms in women and ≥450 ms in men.
Two‐dimensional transthoracic echocardiography was conducted in patients who were suspected of having cardiogenic embolic or unknown etiologies of stroke and having cardiac diseases. Wall motion abnormality (WMA) was defined as wall motion score index >1 using a standard 16‐segment model. Regional WMA was determined by ≥2 akinetic segments corresponding to a major epicardial coronary artery. Hypertrophic myocardium (HM) was defined as left ventricular mass index >95 g/m2 for women and >115 g/m2 for men. Left atrial enlargement (LAE) was defined as an anteroposterior LA diameter >40 mm on M‐mode echocardiography.15, 16
Statistical Analysis
Continuous variables were compared using unpaired Student t tests, and categorical variables using χ2 tests. Multivariable logistic regression analyses were performed to identify predictors of troponin elevation. Age, sex, and all clinical variables with P<0.20 in the univariate analysis were included in the multivariable logistic regression model. Because of known existing collinearity between the involvement of the insular cortical lesion and stroke severity,17 we implemented 2 models to identify cardiac and neurological predictors of troponin elevation (eg, model 1 included NIHSS score as a stroke severity and model 2 included insular cortical lesion instead of NIHSS score). The results of the multivariable logistic regression analysis are reported as odds ratio (OR) and 95% CI. All reported P values were 2‐sided, and a P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS for Windows version 17.0 (SPSS Inc, Chicago, IL).
Results
Baseline Characteristics
Of the 1823 patients with acute ischemic stroke admitted to our center between May 2007 and December 2011, 419 patients were excluded from analyses, as follows: acute coronary syndrome during hospitalization in the stroke center (n=18), renal impairment (n=210), and congestive heart failure (n=56) or both (n=22), or because of inadequate quality of data (n=113) at admission. The remaining 1404 patients were included in this analysis. Their mean age was 65.0±12.4 years (range, 24–95 years), and 850 (60.0%) were male. According to the ischemic stroke subtype, 470 (33.5%) patients were classified as having a large‐artery atherosclerotic stroke and 351 (25.0%) patients were classified as having a cardiogenic–embolic stroke mainly due to atrial fibrillation (AF, n=281), valvular disease (n=23), or both (n=19) and other causes with high/medium risk of cardio‐embolic source (n=28).
Prevalence and Predictors of Troponin Elevation
Troponin elevation was identified in 121 (8.7%) patients. Patients with elevated troponin had more severe neurological deficit, and higher percentage of insular cortical lesion and more cardiogenic embolic subtype of stroke than other etiologies than patients without troponin elevation. In terms of ECG abnormalities, patients with troponin elevation had higher rates of QTc‐prolongation, left ventricular hypertrophy (LVH), Q‐waves, and ST elevation than those without troponin elevation (Table 1).
Table 1.
Characteristics of the Study Groups
| Variable | Cardiac Troponin I | P Valuea | |
|---|---|---|---|
| Elevated (n=121) | Non‐Elevated (n=1283) | ||
| Age, y | 67.2±13.9 | 64.8±12.2 | 0.07 |
| Male | 68 (56.2) | 782 (61.0) | 0.31 |
| Heart rate, beats per minute | 81.7±18.8 | 77.3±17.6 | 0.01 |
| Hypertension | 75 (62.0) | 773 (60.2) | 0.71 |
| Diabetes mellitus | 23 (19.0) | 289 (22.5) | 0.37 |
| Hyperlipidemia | 21 (17.4) | 281 (21.9) | 0.25 |
| Current smoking | 31 (25.6) | 414 (32.3) | 0.13 |
| History of ischemic heart disease | 19 (15.7) | 132 (10.3) | 0.07 |
| NIHSS score on admission, median [IQR] | 7 [4–13.5] | 4 [2–8] | <0.01 |
| Insular cortical lesion | 54 (44.6) | 281 (21.9) | <0.01 |
| Stroke subtypes | <0.01 | ||
| Large artery disease | 27 (22.1) | 443 (34.6) | |
| Cardiogenic embolism | 45 (36.9) | 306 (23.9) | |
| Small vessel disease | 8 (6.6) | 317 (24.7) | |
| Undetermined etiology | 18 (14.8) | 161 (12.6) | |
| Other determined etiology | 24 (19.7) | 55 (4.3) | |
| ECG abnormalities | |||
| QTc‐prolongation | 57 (47.1) | 401 (31.3) | <0.01 |
| LVH | 49 (40.5) | 308 (24.0) | <0.01 |
| Nonelevated ST‐T change | 38 (31.4) | 305 (23.8) | 0.06 |
| AF | 27 (22.3) | 223 (17.4) | 0.18 |
| Q‐wave | 22 (18.2) | 98 (7.6) | <0.01 |
| ST elevation | 7 (5.8) | 31 (2.4) | 0.03 |
Variables are presented as mean±SD, median (interquartile range [IQR]), or number (%). AF indicates atrial fibrillation; ECG, electrocardiography; LVH, left ventricular hypertrophy; NIHSS, National Institutes of Health Stroke Scale.
P values are calculated by Pearson χ2 test or Student t test as appropriate.
Multivariable logistic regression analyses were performed to identify independent predictors of troponin elevation. Age, sex, and all clinical variables including NIHSS score as a stroke severity and ECG abnormalities with P<0.20 in the univariate analysis were included in model 1. In model 2, an insular cortical lesion was additionally included into the baseline model 1 instead of the NIHSS score. In multivariable model 1, QTc‐prolongation (OR: 1.52, 95% CI 1.02–2.28), LVH (OR: 2.14, 95% CI 1.43–3.19), Q‐wave (OR: 2.53, 95% CI: 1.48–4.32), ST elevation (OR: 2.74, 95% CI: 1.12–6.72), and higher NIHSS score (OR: 1.04, 95% CI: 1.01–1.07) were identified as predictors of troponin elevation. In model 2, an insular cortical lesion (OR: 2.78, 95% CI: 1.85–4.19) was identified as an additional predictor of troponin elevation (Table 2).
Table 2.
Multivariable Logistic Regression Analysis for Predictors of Troponin Elevation
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age, per 1‐year increase | 1.01 | 0.99 to 1.02 | 1.01 | 0.99 to 1.03 |
| Male | 0.98 | 0.63 to 1.54 | 1.04 | 0.67 to 1.64 |
| Heart rate, per 10‐beats increase | 1.09 | 0.99 to 1.21 | 1.09 | 0.99 to 1.21 |
| Current smoking | 0.79 | 0.48 to 1.31 | 0.78 | 0.47 to 1.28 |
| History of ischemic heart disease | 1.31 | 0.75 to 2.30 | 1.20 | 0.68 to 2.11 |
| QTc‐prolongation | 1.52 | 1.02 to 2.28 | 1.54 | 1.03 to 2.30 |
| LVH | 2.14 | 1.43 to 3.19 | 2.08 | 1.39 to 3.12 |
| Nonelevated ST‐T change | 1.30 | 0.82 to 2.06 | 1.31 | 0.82 to 2.09 |
| AF | 0.74 | 0.44 to 1.26 | 0.70 | 0.41 to 1.19 |
| Q‐wave | 2.53 | 1.48 to 4.32 | 2.66 | 1.55 to 4.57 |
| ST elevation | 2.74 | 1.12 to 6.72 | 3.31 | 1.35 to 8.12 |
| Admission NIHSS, per 1‐point increase | 1.04 | 1.01 to 1.07 | N/A | |
| Insular cortical lesion | N/A | 2.78 | 1.85 to 4.19 | |
AF indicates atrial fibrillation; LVH, left ventricular hypertrophy; N/A, not applicable; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio.
Interaction of Cardiac and Neurological Factors for Troponin Elevation and QTc‐Prolongation
To investigate a relationship between cardiac and neurological factors for troponin elevation, NIHSS score was transformed as categorical variables and classified into 2 groups (eg, severe to moderate: ≥7 points; mild: <7 points).
The incidence of troponin elevation increased linearly with increased number of cardiac factors, both in patients with mild (P<0.01) and moderate‐to‐severe (P=0.01) neurological deficits, or both in patients with and without insular cortical lesion (P=0.01 and P=0.01), with rates being higher in patients with moderate‐to‐severe than mild neurological deficits (OR=1.67, 95% CI: 1.13–2.46) or higher in patients with insular cortical lesion than those without insular cortical lesion (OR=2.50, 95% CI: 1.69–3.70) even after adjusting for number of cardiac factors (Figure 1A and 1B).
Figure 1.
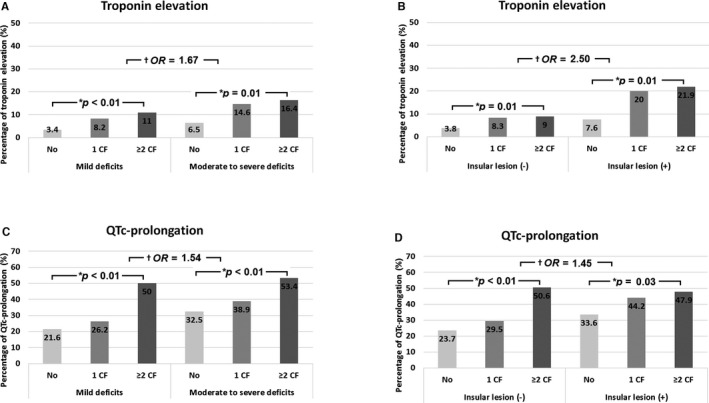
Incidence of troponin elevation (A and B) and QTc‐prolongation (C and D) as a function of the number of cardiac factors and stroke severity, or the presence of insular cortical lesion. Cardiac factors (CF) included left ventricular hypertrophy, nonelevated ST‐T change, atrial fibrillation, Q‐wave and ST elevation, but not QTc‐prolongation. *P<0.05 by linear‐by‐linear association χ2 test. † P<0.05 after adjusting for number of cardiac factors. OR indicates odds ratio.
The incidence of QTc‐prolongation increased linearly with increased number of cardiac factors, both in patients with mild (P<0.01) and moderate‐to‐severe (P<0.01) neurological deficits, or both in patients with and without insular cortical lesion (P<0.01 and P=0.03), with rates being higher in patients with moderate‐to‐severe than mild neurological deficits (OR=1.54, 95% CI: 1.21–1.96) or higher in patients with insular cortical lesion than those without insular cortical lesion (OR=1.45, 95% CI: 1.12–1.88) even after adjusting for number of cardiac factors (Figure 1C and 1D).
Incidence Troponin Elevation and QTc‐Prolongation With Adjustment for ECG Changes and Drugs Affecting QTc‐Interval
The incidence of troponin elevation was higher in patients with moderate‐to‐severe than mild neurological deficits (OR=1.76, 95% CI: 1.13–2.72) among those without Q‐waves or ST elevation on ECG, and higher in both patients with insular cortical lesion than those without insular cortical lesion (OR=2.66, 95% CI: 1.71–4.14 and OR=3.48, 95% CI: 1.34–9.00, respectively) regardless of the presence of Q‐wave or ST elevation on ECG, even after adjusting for covariables (Figure 2A and 2B).
Figure 2.
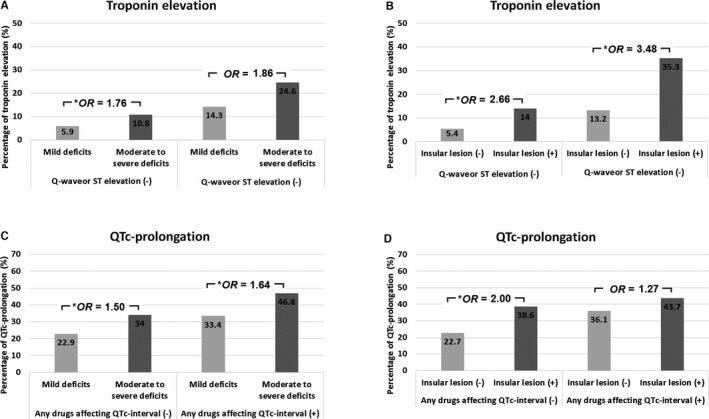
Incidence of troponin elevation after adjusting the presence of Q‐waves or ST elevation on ECG (A and B) and incidence of QTc‐prolongation after adjusting the presence of †any drugs affecting QTc‐interval (C and D) according to the stroke severity, or the presence of insular cortical lesion. OR indicates odds ratio. *P<0.05 after adjusting for age, sex, heart rate, hypertension, diabetes mellitus, hyperlipidemia, smoking, and history of ischemic heart disease. †Any drugs affecting QTc‐interval are listed in Table S2.
The incidence of QTc‐prolongation was higher in both patients with moderate‐to‐severe than mild neurological deficits (OR=1.50, 95% CI: 1.02–2.23 and OR=1.64, 95% CI: 1.20–2.24, respectively) regardless of the presence of any drugs affecting QTc‐interval (Table S1), and higher in patients with insular cortical lesion than those without insular cortical lesion (OR=2.00, 95% CI: 1.32–3.04) among those without any drugs affecting QTc‐interval, even after adjusting for covariables (Figure 2C and 2D).
Echocardiographic Abnormalities as HM, WMA, and LAE and ECG Changes
Among 1404 patients, 792 (56.4%) patients underwent transthoracic echocardiography (Table S2). HM, WMA, and LAE were identified in 292 (36.9%), 118 (14.9%), and 326 (41.2%) patients, respectively.
The incidence of echocardiographic HM was higher in patients with troponin elevation than in those without elevation (25 of 47 patients [53.2%] versus 150 of 536 patients [28%], respectively; P<0.01) among those without positive LVH on ECG (Figure 3A). The incidence of echocardiographic WMA was higher in patients with troponin elevation than in those without elevation (21.6% versus 11.2%; P<0.01) among those without positive Q‐wave or ST elevation on ECG, and showed a similar trend of higher incidence in patients with troponin elevation than in those without elevation (47.4% versus 26.1%; P=0.08) among those with positive Q‐wave or ST elevation (Figure 3B). The incidence of echocardiographic LAE was not significantly different between patients with and without troponin elevation (45.6% versus 40.7%; P=0.40), and total number of AF was not significantly different between patients with and without troponin elevation regardless of the presence of the LAE (Figure 3C).
Figure 3.
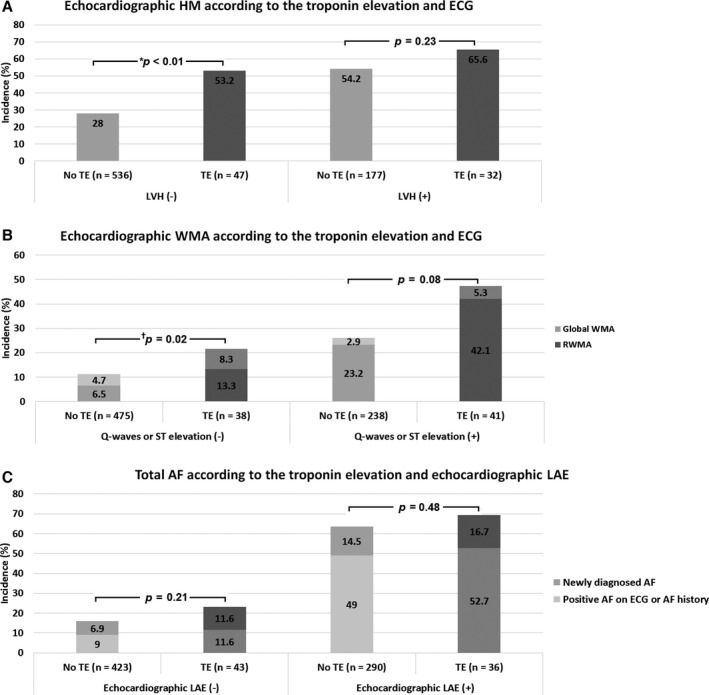
Incidence of echocardiographic HM (A) and WMA (B) according to the related ECG change and troponin elevation, and incidence of total AF (C) according to the presence of echocardiographic LAE and troponin elevation. AF indicates atrial fibrillation; ECG, electrocardiography; HM, hypertrophic myocardium; LAE, left atrial enlargement; LVH, left ventricular hypertrophy. RWMA/WMA, regional/wall motion abnormalities; TE, troponin elevation. *P<0.05 by χ2 test. † P<0.05 by χ2 test for difference of total number of abnormalities.
Discussion
This study examined the prevalence and predictors of troponin elevation during the acute stage of ischemic stroke with reference to cardiac and neurological factors. The percentage of patients with elevated troponin (8.6%) was lower than in previous studies (7.8–33%),18 because patients with troponin‐elevating comorbidities were excluded. After multivariable analysis, several ECG abnormalities, as well as specific lesion location and stroke severity, were identified as predictors of troponin elevation. In addition, we could identify the synergistic effect of the combinations of cardiac and neurological factors leading to the troponin elevation as well as QTc‐prolongation because the incidence of troponin elevation and QTc‐prolongation was sequentially increased by an increasing number of cardiac factors, and further amplified by the combination of more severe neurological deficits or insular cortical lesion (Figure 1). Furthermore, we could reconfirm the above relationship even after adjustment for a potential confounder such as an underlying cardiac problem with ischemic ECG changes (eg, indicating a prior or recent ischemia or infarction leading to troponin elevation) or drugs affecting the QTc‐interval (Figure 2).
We identified ECG abnormalities, such as LVH, Q‐wave, and ST elevation, as cardiac factors affecting troponin elevation during the acute stage of ischemic stroke. LVH represents an increase in left ventricular mass,19 and may result in troponin elevation due to mismatch of supply and demand in myocardial oxygen.20 Abnormal Q‐wave is indicative of myocardial necrosis after myocardial infarction and is associated with the extent of myocardial injury.21 ST elevation is indicative notably of myocardial infarction along with various cardiac conditions, such as LVH, bundle branch block, coronary vasospasm, and Takotsubo cardiomyopathy.22 Therefore, electrocardiographic LVH (hypertrophically remodeled myocardium), Q‐wave (damaged myocardium by ischemia), and ST elevation may reflect cardiac vulnerability to ischemia and may consequently lead to troponin elevation. This hypothesis is in agreement with currently suggested definition of type 2 myocardial infarction (eg, injury related to supply/demand imbalance of myocardial ischemia including tachy‐/bradyarrhythmia, hypertrophic cardiomyopathy, hypertension with or without LVH, and coronary spasm or endothelial dysfunction)5 in that vulnerable myocardial conditions are important sources of troponin elevation. Actually, the incidence of echocardiographic HM and WMA, which represented more severely deformed/damaged myocardium than single ECG change alone, was also higher in patients with troponin elevation than in those without elevation regardless of the related ECG changes (eg, LVH and Q‐wave or ST elevation). Thus, troponin elevation can indicate vulnerable cardiac status comprising detectable and undetectable structural abnormalities beyond the single ECG abnormalities.
The present study also showed that insular cortical lesion and severe stroke were independently associated with troponin elevation, and that troponin elevation was amplified by a combination of cardiac factors. The presence of insular cortical lesion is known as a risk factor for cardiac complications.8, 23 The pathomechanism may be due to the loss of central inhibitory control, leading to autonomic derangement and increased sympathetic tone,8 and consequently accompanied by elevated troponin.9 These reactions are also frequently found in patients with severe stroke.10, 24 However, patients with extensive hemispheric infarction often have more insular lesion,17 thus multicollinearity should be considered between these factors.
We found that QTc‐prolongation was the most common ECG abnormality and one of the cardiac factors associated with troponin elevation. In addition, QTc‐prolongation was closely associated with both cardiac and neurological factors the same as troponin elevation (Figures 1 and 2). QTc‐prolongation represents a ventricular repolarization delay and is related to diverse etiologies including LVH, ischemic heart disease, certain drugs, dyselectrolytemia, hypertension, diabetes mellitus, and stroke.25 Neuromediated autonomic dysregulation has also been suggested as a potential mechanism of QTc‐prolongation in stroke similarly to troponin elevation.24 Therefore, QTc‐prolongation is a composite cardiac marker reflecting both neurological and cardiac conditions. Taken together, our findings point to 2 different clinical implications of troponin elevation and ECG abnormalities in the acute stage of ischemic stroke as representing (1) primary changes by predisposed cardiac problems and (2) secondary changes superimposed on the primary changes before and after stroke (Figure 4).
Figure 4.
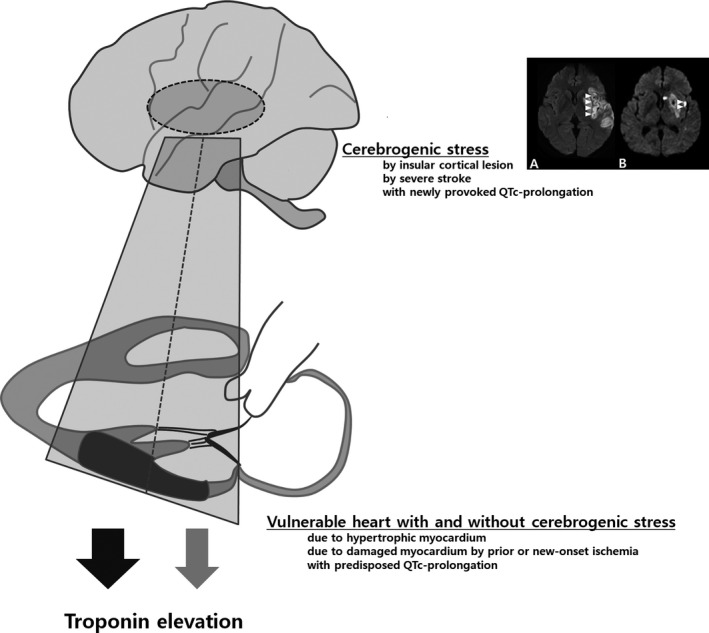
Suggested explanation of troponin elevation in acute stage of ischemic stroke. Troponin elevation may be synergistically induced by a combination of provocative cerebrogenic stress (eg, after insular cortical lesion [major or minor involvement; A and B] or severe stroke) and vulnerable heart (eg, hypertrophic or damaged myocardium). QTc‐prolongation may be a composite marker for reflecting both predisposed and newly provoked prolongation due to the underlying cardiac problems and provocative cerebrogenic stress.
Interestingly, the incidence of LAE and total AF was not different between patients with and without troponin elevation, otherwise the incidence of total AF was closely associated with the presence of LAE despite initial exclusion of patients with suspected heart failure (Figure 3C). Recently, cardiac troponins have been known to be associated with incident AF in the general population,26 and are associated with delayed diagnosis of AF after stroke.27 However, it is still unclear whether the pathomechanism of troponin elevation in AF is due to AF itself (eg, accompanying tachycardia with a fast ventricular response) or due to pre‐existing heart failure with or without LAE.28 Thus, our results could suggest that structural heart problems may play an important role in the identification of newly detected AF in stroke patients. Future studies are needed to clarify whether troponin elevation in AF is related to the structural heart problem or to AF itself in a large number of patients.
This study had several limitations. First, the design of the study was retrospective and the study was performed in a single center. Second, we were unable to perform intensive cardiac investigations for all stroke patients because these investigations were not routinely performed. Thus, in our study, there was still a remaining chance of including patients with undetected nonelevated ST elevation myocardial infarction or stress‐induced cardiomyopathy because we could only exclude patients showing definite evidence of acute coronary syndrome during their hospital stay. However, after adjusting for the potential confounder such as concomitant cardiac ischemia or infarction (eg, patients with Q‐wave or ST elevation), the impact of cardiac factors and neurologic factors on troponin elevation with synergistic interaction still existed. Third, the mechanism of troponin elevation and related ECG change is still somewhat unclear and tentative since troponin and ECG test results obtained from a single time point were used in our study. To overcome these problems, we are currently conducting a prospective trial with serial troponin and ECG tests in acute ischemic stroke patients (Clinical implications of elevated cardiac troponin‐I elevation in acute stroke patients; KCT0000682; https://cris.nih.go.kr/cris).
This study investigated cardiac and neurological factors as predictors of troponin elevation during the acute stage of ischemic stroke, and suggested an explanatory model for troponin elevation and ECG abnormalities for practical interpretation of these phenomena. The proposed concept of cardiac vulnerability to cerebrogenic stress could be explained as a synergistic effect on troponin elevation in combination with cardiac and neurological factors that could show the possible interaction between heart and brain during the acute stage of ischemic stroke. Future research and management strategies should focus on the identification of undetected or subclinical cardiac problems in ischemic stroke patients with elevated serum troponin concentration.
Sources of Funding
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare Republic of Korea (HI10C2020 and HI14C1731).
Disclosures
None.
Supporting information
Table S1. Previous Medications Affecting QTc‐Interval in Patients With and Without Troponin Elevation
Table S2. Comparison of Clinical Variables Between Patients With or Without Echocardiography
(J Am Heart Assoc. 2016;5:e004135 doi: 10.1161/JAHA.116.004135)
References
- 1. Adams RJ, Chimowitz MI, Alpert JS, Awad IA, Cerqueria MD, Fayad P, Taubert KA. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association. Circulation. 2003;108:1278–1290. [DOI] [PubMed] [Google Scholar]
- 2. Greaves SC, Zhi G, Lee RT, Solomon SD, MacFadyen J, Rapaport E, Menapace FJ, Rouleau JL, Pfeffer MA. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol. 1997;80:442–448. [DOI] [PubMed] [Google Scholar]
- 3. Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115:e478–e534. [DOI] [PubMed] [Google Scholar]
- 4. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 5. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Apple FS, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Torbicki A, Vahanian A, Windecker S, Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. [DOI] [PubMed] [Google Scholar]
- 6. Fure B, Bruun Wyller T, Thommessen B. Electrocardiographic and troponin T changes in acute ischaemic stroke. J Intern Med. 2006;259:592–597. [DOI] [PubMed] [Google Scholar]
- 7. Jensen JK, Kristensen SR, Bak S, Atar D, Hoilund‐Carlsen PF, Mickley H. Frequency and significance of troponin T elevation in acute ischemic stroke. Am J Cardiol. 2007;99:108–112. [DOI] [PubMed] [Google Scholar]
- 8. Sander D, Winbeck K, Klingelhofer J, Etgen T, Conrad B. Prognostic relevance of pathological sympathetic activation after acute thromboembolic stroke. Neurology. 2001;57:833–838. [DOI] [PubMed] [Google Scholar]
- 9. Christensen H, Boysen G, Christensen AF, Johannesen HH. Insular lesions, ECG abnormalities, and outcome in acute stroke. J Neurol Neurosurg Psychiatry. 2005;76:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kallmunzer B, Breuer L, Kahl N, Bobinger T, Raaz‐Schrauder D, Huttner HB, Schwab S, Kohrmann M. Serious cardiac arrhythmias after stroke: incidence, time course, and predictors—a systematic, prospective analysis. Stroke. 2012;43:2892–2897. [DOI] [PubMed] [Google Scholar]
- 11. Adams HP Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MD. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126–131. [DOI] [PubMed] [Google Scholar]
- 12. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 13. Palma JA, Benarroch EE. Neural control of the heart: recent concepts and clinical correlations. Neurology. 2014;83:261–271. [DOI] [PubMed] [Google Scholar]
- 14. Prineas RJ, Crow RS, Zhang Z‐M. The Minnesota Code Manual of Electrocardiographic Findings. 2nd ed London: Springer‐Verlag; 2010. [Google Scholar]
- 15. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 16. de Simone G, Daniels SR, Kimball TR, Roman MJ, Romano C, Chinali M, Galderisi M, Devereux RB. Evaluation of concentric left ventricular geometry in humans: evidence for age‐related systematic underestimation. Hypertension. 2005;45:64–68. [DOI] [PubMed] [Google Scholar]
- 17. Fink JN, Selim MH, Kumar S, Voetsch B, Fong WC, Caplan LR. Insular cortex infarction in acute middle cerebral artery territory stroke: predictor of stroke severity and vascular lesion. Arch Neurol. 2005;62:1081–1085. [DOI] [PubMed] [Google Scholar]
- 18. Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. 2009;28:220–226. [DOI] [PubMed] [Google Scholar]
- 19. Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, Bosch J, Sussex B, Probstfield J, Yusuf S. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin‐converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–1621. [DOI] [PubMed] [Google Scholar]
- 20. Chin CW, Shah AS, McAllister DA, Joanna Cowell S, Alam S, Langrish JP, Strachan FE, Hunter AL, Maria Choy A, Lang CC, Walker S, Boon NA, Newby DE, Mills NL, Dweck MR. High‐sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35:2312–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Florian A, Slavich M, Masci PG, Janssens S, Bogaert J. Electrocardiographic Q‐wave “remodeling” in reperfused ST‐segment elevation myocardial infarction: validation study with CMR. JACC Cardiovasc Imaging. 2012;5:1003–1013. [DOI] [PubMed] [Google Scholar]
- 22. Brady WJ, Perron AD, Martin ML, Beagle C, Aufderheide TP. Cause of ST segment abnormality in ED chest pain patients. Am J Emerg Med. 2001;19:25–28. [DOI] [PubMed] [Google Scholar]
- 23. Song HS, Back JH, Jin DK, Chung PW, Moon HS, Suh BC, Kim YB, Kim BM, Woo HY, Lee YT, Park KY. Cardiac troponin T elevation after stroke: relationships between elevated serum troponin T, stroke location, and prognosis. J Clin Neurol. 2008;4:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dutsch M, Burger M, Dorfler C, Schwab S, Hilz MJ. Cardiovascular autonomic function in poststroke patients. Neurology. 2007;69:2249–2255. [DOI] [PubMed] [Google Scholar]
- 25. Soliman EZ, Howard G, Cushman M, Kissela B, Kleindorfer D, Le A, Judd S, McClure LA, Howard VJ. Prolongation of QTc and risk of stroke: the REGARDS (REasons for Geographic and Racial Differences in Stroke) study. J Am Coll Cardiol. 2012;59:1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scheitz JF, Erdur H, Haeusler KG, Audebert HJ, Roser M, Laufs U, Endres M, Nolte CH. Insular cortex lesions, cardiac troponin, and detection of previously unknown atrial fibrillation in acute ischemic stroke: insights from the troponin elevation in acute ischemic stroke study. Stroke. 2015;46:1196–1201. [DOI] [PubMed] [Google Scholar]
- 28. van den Bos EJ, Constantinescu AA, van Domburg RT, Akin S, Jordaens LJ, Kofflard MJ. Minor elevations in troponin I are associated with mortality and adverse cardiac events in patients with atrial fibrillation. Eur Heart J. 2011;32:611–617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Previous Medications Affecting QTc‐Interval in Patients With and Without Troponin Elevation
Table S2. Comparison of Clinical Variables Between Patients With or Without Echocardiography


