Abstract
Background
The possibility that lifestyle factors such as diet, specifically potassium intake, may modify the risk of stroke has been suggested by several observational cohort studies, including some recent reports. We performed a systematic review and meta‐analysis of existing studies and assessed the dose–response relation between potassium intake and stroke risk.
Methods and Results
We reviewed the observational cohort studies addressing the relation between potassium intake, and incidence or mortality of total stroke or stroke subtypes published through August 6, 2016. We carried out a meta‐analysis of 16 cohort studies based on the relative risk (RR) of stroke comparing the highest versus lowest intake categories. We also plotted a pooled dose–response curve of RR of stroke according to potassium intake. Analyses were performed with and without adjustment for blood pressure. Relative to the lowest category of potassium intake, the highest category of potassium intake was associated with a 13% reduced risk of stroke (RR=0.87, 95% CI 0.80–0.94) in the blood pressure–adjusted analysis. Summary RRs tended to decrease when original estimates were unadjusted for blood pressure. Analysis for stroke subtypes yielded comparable results. In the spline analysis, the pooled RR was lowest at 90 mmol of potassium daily intake (RRs=0.78, 95% CI 0.70–0.86) in blood pressure–adjusted analysis, and 0.67 (95% CI 0.57–0.78) in unadjusted analysis.
Conclusions
Overall, this dose–response meta‐analysis confirms the inverse association between potassium intake and stroke risk, with potassium intake of 90 mmol (≈3500 mg)/day associated with the lowest risk of stroke.
Keywords: cohort studies, meta‐analysis, potassium, stroke
Subject Categories: Diet and Nutrition, Epidemiology, Primary Prevention, Risk Factors
Introduction
In addition to the well‐recognized determinants of stroke such as hypertension, diabetes, and atrial fibrillation, lifestyle risk factors such as diet, obesity, and smoking are hypothesized to play a significant role in stroke etiology.1 The identification of modifiable risk factors for stroke such as diet quality is of key importance as potential targets for stroke prevention strategies.1, 2, 3, 4 Among the dietary factors that have been associated with the risk of stroke, potassium has received attention because of substantial evidence from randomized controlled trials of a blood pressure–lowering effect of potassium supplementation in hypertensive subjects.5, 6 Some, but not all, observational cohort studies have found an inverse association between potassium intake and stroke risk. This association, together with the effect of potassium on blood pressure, have been considered when setting dietary reference values and recommendations for this nutrient.5, 7, 8, 9
Since the latest reviews of the relation between potassium and stroke risk,6, 10, 11 new cohort studies have been published, all with observational design. We therefore undertook a new meta‐analysis including all relevant studies published to date. Using a different method from previous meta‐analyses, we included studies based on dietary assessment methods as well as on measurements of urinary potassium excretion. We also aimed at exploring features of this association, such as the presence and the shape of a dose–response relation, any sex‐related differences, and eventually blood pressure as a potential mechanism mediating the association. Specific associations between potassium intake and stroke subtypes (ischemic versus hemorrhagic) were also investigated.
Methods
We followed the “Preferred reporting items for systematic reviews and meta‐analyses” (PRISMA) statement for reporting of this systematic review.12 We performed a systematic literature search on potassium intake and stroke into the PubMed/Medline database on August 6, 2016. The search strategy is reported in Box 1 of Figure 1. “Stroke” and “potassium” were used both as MeSH terms and title/abstract keywords. Only cohort studies were selected, provided that they investigated stroke incidence or stroke mortality, and that they assessed dietary potassium intake through dietary questionnaires or determination of urinary potassium excretion. We did not apply any restriction based on the length of follow‐up. The extracted data (made in duplicates by M.V. and T.F.) included the population characteristics (sex, total study sample, location of the study), the length of follow‐up and the percentage loss at follow‐up, the baseline median/mean potassium intake for each exposure category, the type of stroke and number of cases for total stroke and stroke subtypes (including person‐years within each exposure category), the covariates adjusted for in the multivariable analysis and eventually the relative risk (RR) estimates with their 95% CI for all exposure categories. We assessed the overall quality of studies using the Newcastle‐Ottawa quality assessment scale13 in duplicate (T.F. and A.S.), and publication bias through Egger's test.14
Figure 1.
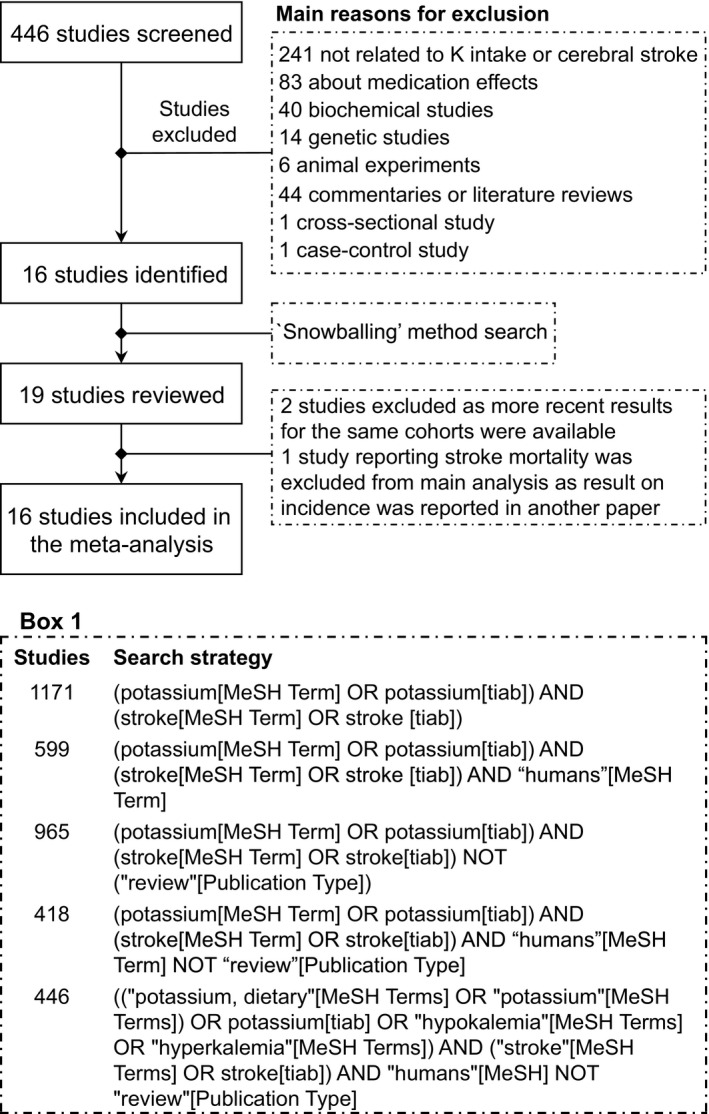
Flow chart summarizing study identification and selection. Box 1 shows details of research strategy.
In our meta‐analyses, we used the RRs adjusted for the greatest number of potential confounders in the first place (“most adjusted model”). To assess the potential for overadjustment when controlling for blood pressure, we also computed alternative analyses based on most adjusted RRs without adjustment for blood pressure level or hypertension status (“blood pressure unadjusted model”). When the study provided results by stroke subtype or by population subgroup but no overall RR estimate, the individual RRs were used. In addition, we performed analyses restricted to the studies that reported results both with and without adjustment for blood pressure.
We used random‐effect models to account for heterogeneity in study‐specific results. We first included in this analysis the RR and its 95% CI of the highest versus lowest categories of potassium intake. When studies provided RR only for potassium intake expressed as continuous variable, this value was used. We always included in the meta‐analyses overall incidence data (ie, fatal and nonfatal stroke) except when only mortality data for the study cohort were available. We performed stratified analyses according to sex, stroke type (ischemic versus hemorrhagic) and outcome (fatal and nonfatal versus fatal only), method of assessment of potassium intake (dietary questionnaires versus urinary excretion), and category of baseline potassium intake. For the latter analysis, we defined 3 exposure subgroups using dietary potassium intakes of 90 and 120 mmol/day as cut points, similar to those previously used to investigate the relation of potassium with blood pressure6 and stroke15 and also corresponding to proposed dietary reference intakes of this mineral.5, 7 A study could contribute to this analysis more than once, when its intake categories fell into more than 1 of the defined subgroups. When several intake categories fell in the same subgroup, we used the RR related to the highest category. In cases where the baseline potassium exposure assessment was expressed as 24‐hour urinary potassium excretion, we multiplied that value by 1.3 to convert it to daily dietary intake.15, 16, 17
We also performed a dose–response meta‐analysis to assess a potential nonlinear relation between potassium intake and stroke risk, using the methodology developed by Greenland and Longnecker, and Orsini et al.18, 19 Restricted cubic spline models with 3 knots were fitted in each study taking into account the covariance among log RR, and the regression coefficients were then combined using multivariate meta‐analysis. For each potassium category, we assigned the mean intake (or median if mean was not available), along with RR with its 95% lower and upper bounds, the number of cases, and amount of person‐years. If neither means nor medians were available, we used the midpoint within each exposure category. For open (highest and lowest) categories for which no mean or median value was provided by the authors, we entered a 20% higher or lower value departing from the closest cut point, based on what was observed in studies with complete data.20, 21, 22, 23 We excluded from analysis the studies not reporting the number of subjects and/or the person‐years of follow‐up in each intake category, to avoid biases in the estimates for the variances.19 Studies that provided a RR analyzed as a continuous variable were also excluded from this dose–response analysis. In stratified analysis, we assessed the dose–response according to stroke subtype. We finally carried out several sensitivity analyses by including studies with missing category‐specific number of cases and person‐year, by excluding the 2 studies based on single unit increase,24, 25 by using a shift of 10% instead of 20% to assign the typical potassium intake for the highest and lowest exposure categories, by excluding studies based on urinary potassium excretion measurement, by considering a lower multiplication factor (1.16) based on data from the Trial of Nonpharmacologic Intervention in the Elderly (TONE)26 to estimate dietary potassium intake from urinary excretion measurement, and eventually by removing the studies that reported fatal stroke only.
We used the metan, glst, mkspline, and mvmeta routines in the Stata statistical software (version 14.1, 2016; Stata Corp, College Station, TX).
Results
In the first‐stage search, we identified 446 potentially eligible studies, of which 430 were subsequently excluded on the basis of title/abstract or after full‐text reading when abstracts were not available in PubMed. Three studies were further identified with backward and forward citations method (“snowballing” method). Among the 19 studies reviewed, 3 were excluded: 227, 28 because more recent results for the same cohorts were available29, 30 and 1 because it reported on stroke mortality31 in a cohort for which overall incidence data were subsequently reported20 (yet this article was included in the subgroup analysis for stroke mortality). Thus, a final number of 16 studies published between 1987 and 2016 were available for overall analyses (Figure 1).20, 21, 22, 23, 24, 25, 29, 30, 32, 33, 34, 35, 36, 37, 38, 39 These studies involved 19 522 stroke events among 639 440 participants (full details are reported in Table S1). Six studies were conducted in the United States,20, 25, 29, 30, 33, 38 5 in Europe,23, 24, 34, 37, 39 3 in Asia,32, 35, 36 and 2 large cohorts with subjects recruited from several countries.21, 22 All studies used internal comparison groups. Fourteen studies reported comparison between intake categories,20, 21, 22, 23, 29, 30, 32, 33, 34, 35, 36, 37, 38, 39 2 reported RR by continuous increase of potassium intake,24, 25 and 2 reported both continuous and categorical RR.23, 39 In studies reporting comparison between potassium intake categories with blood pressure–adjusted RR estimates, median intake was 103.0 mmol/day (range 68–149.8) in the highest category and 50.1 mmol/day (24–100.1) in the lowest one, while corresponding figures for studies reporting blood pressure–unadjusted estimates were 103.0 mmol/day (60–149.8) and 52.5 mmol/day (24–100.1). Nine studies assessed potassium intake through food frequency questionnaires,24, 29, 30, 33, 34, 36, 37, 38, 39 4 using structured dietary recall administered by a dietitian,20, 25, 32, 35 and 4 measured urinary potassium excretion by using either an overnight urine sample,24 a single morning fasting urine collection,21, 22 or multiple 24‐hour urine collection.23 Most studies involved subjects without personal history of stroke at baseline, with some apparent exceptions.21, 22, 39 Outcome was ascertained by an independent blind assessment or by record linkage in 2 and 9 studies, respectively, while it was self‐reported in 4 studies. Median follow‐up was >10 years in 12 of the studies, ranging from 3.7 to 25.8 years. Only 10 studies reported a follow‐up not lower than 95% of the cohort. All studies controlled for age and all but 2 adjusted for smoking status.32, 33 Seven and 5 studies, respectively, included the adjustment for history of hypertension21, 29, 30, 35, 36, 37, 38 or for various types of blood pressure measurements20, 21, 25, 33, 34 in 1 regression model. Other covariates frequently considered were body mass index20, 21, 22, 23, 24, 29, 30, 35, 36, 37, 38, 39 or presence of obesity,25, 36 level of physical activity,20, 21, 29, 30, 34, 35, 36, 37, 38, 39 total energy intake,20, 24, 34, 37, 39 serum cholesterol level* and/or cholesterol intake,20, 37 saturated fat intake,20, 24 and alcohol intake.20, 22, 23, 24, 29, 30, 34, 35, 36, 37, 38, 39
Overall quality of studies is reported in Table S2. Newcastle‐Ottawa scale score ranged from 4 to 9, with a median value of 7. No substantial evidence of publication bias emerged from the funnel plot (Figure 2), as also confirmed by the Egger asymmetry test, whose intercept based on all retrieved studies was −0.32 (95% CI −1.82 to 1.19, P=0.663).
Figure 2.
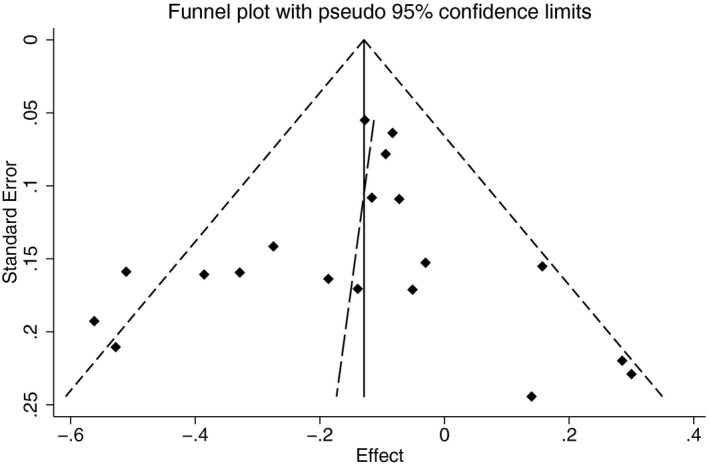
Funnel plot for publication bias for the observational cohort studies included in the meta‐analysis. Long‐dash line shows fitted line corresponding to the regression test for funnel‐plot asymmetry proposed by Egger.
Forest plots of the meta‐analysis using adjusted RR estimates comparing the highest versus the lowest potassium category, either including blood pressure level or hypertension status among the covariates or not, are reported in Figure 3.20, 21, 22, 23, 24, 25, 29, 30, 32, 33, 34, 35, 36, 37, 38, 39 Heterogeneity, expressed as I2, was 45.5% in the “most adjusted model” and 53.2% in the “blood pressure–unadjusted model.” The pooled RR for stroke was 0.87 (95% CI 0.80–0.94) in the “most adjusted model,” while this estimate was slightly lower (0.85, 95% CI 0.79–0.91) when estimates were not adjusted for blood pressure. Restricting the meta‐analysis to studies that provided RRs both with and without adjustment for blood pressure, the difference between pooled estimates was further increased, with summary RR of 0.90 (95% CI 0.84–0.96) in the adjusted analysis and 0.85 (95% CI 0.79–0.92) in the unadjusted one.
Figure 3.
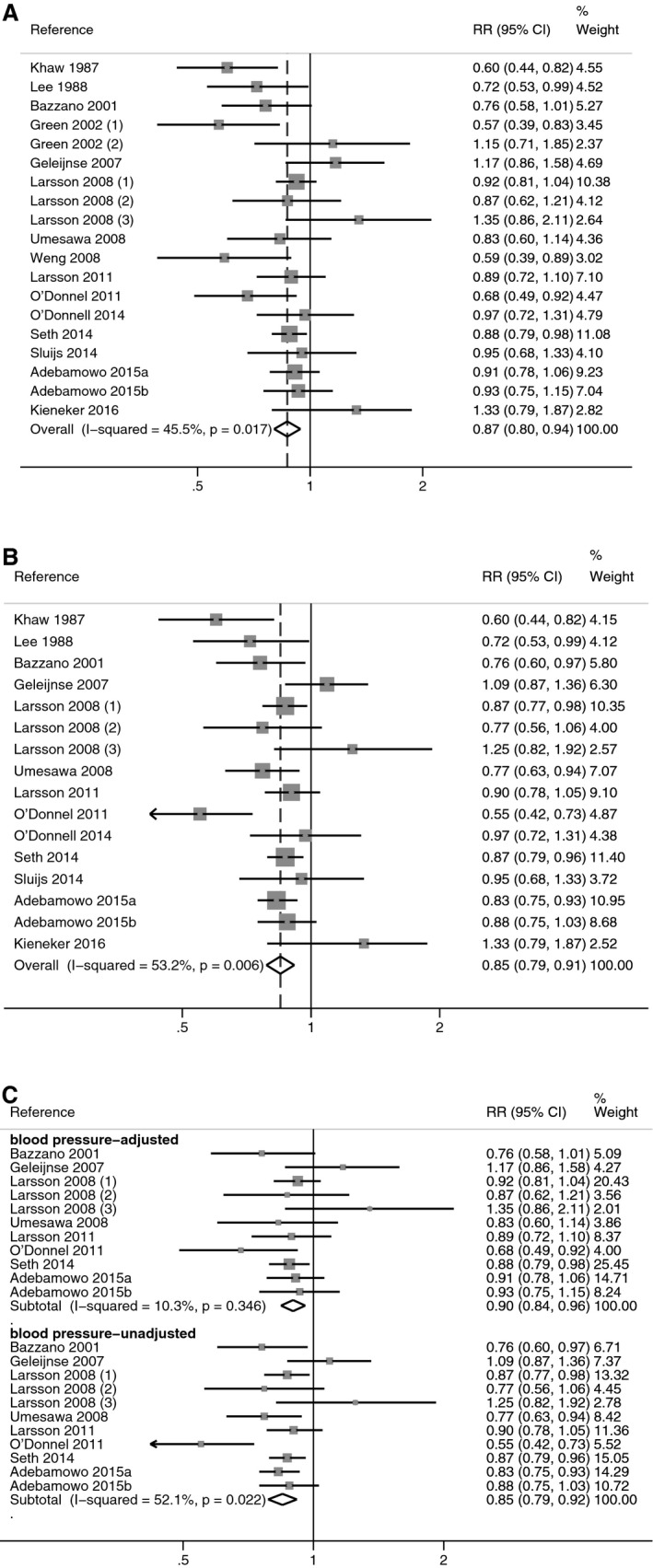
Meta‐analysis of the relative risk (RR), with 95% CI of stroke in observational cohort studies, according to the covariates included in multivariate modeling. RRs from all studies using the “most adjusted model” (A) and the “blood pressure–unadjusted model” (B), and from the studies reporting both blood pressure–adjusted and unadjusted estimates (C).
In stratified analyses by subgroups of dietary potassium intake (<90, 90–120, and ≥120 mmol/day), the summary RRs for the highest versus the lowest potassium exposure category increased with increasing exposure, as did the statistical imprecision of the estimate: 0.87 (95% CI 0.82–0.93), 0.92 (95% CI 0.82–1.04), and 1.02 (95% CI 0.83–1.24) for the respective subgroups (Figure 4).20, 21, 22, 23, 29, 30, 32, 33, 34, 35, 36, 37, 38, 39 Corresponding figures for “blood pressure–unadjusted model” were 0.85 (95% CI 0.81–0.89), 0.90 (95% CI 0.79–1.01), and 0.97 (95% CI 0.77–1.21). In the analysis restricted to studies reporting estimates both with and without adjustment for blood pressure (Figure S1), the blood pressure–adjusted estimates were higher in all exposure categories compared to the unadjusted ones. Comparing adjusted with unadjusted estimates, overall RRs decreased from 0.88 (95% CI 0.82–0.95) to 0.85 (95% CI 0.80–0.90) in the lowest subgroup of potassium intake, from 0.85 (95% CI 0.82–0.95) to 0.78 (95% CI 0.61–0.99) in the intermediate subgroup of potassium intake, and from 0.96 (95% CI 0.80–1.14) to 0.90 (95% CI 0.74–1.09) in the highest subgroup of potassium intake.
Figure 4.
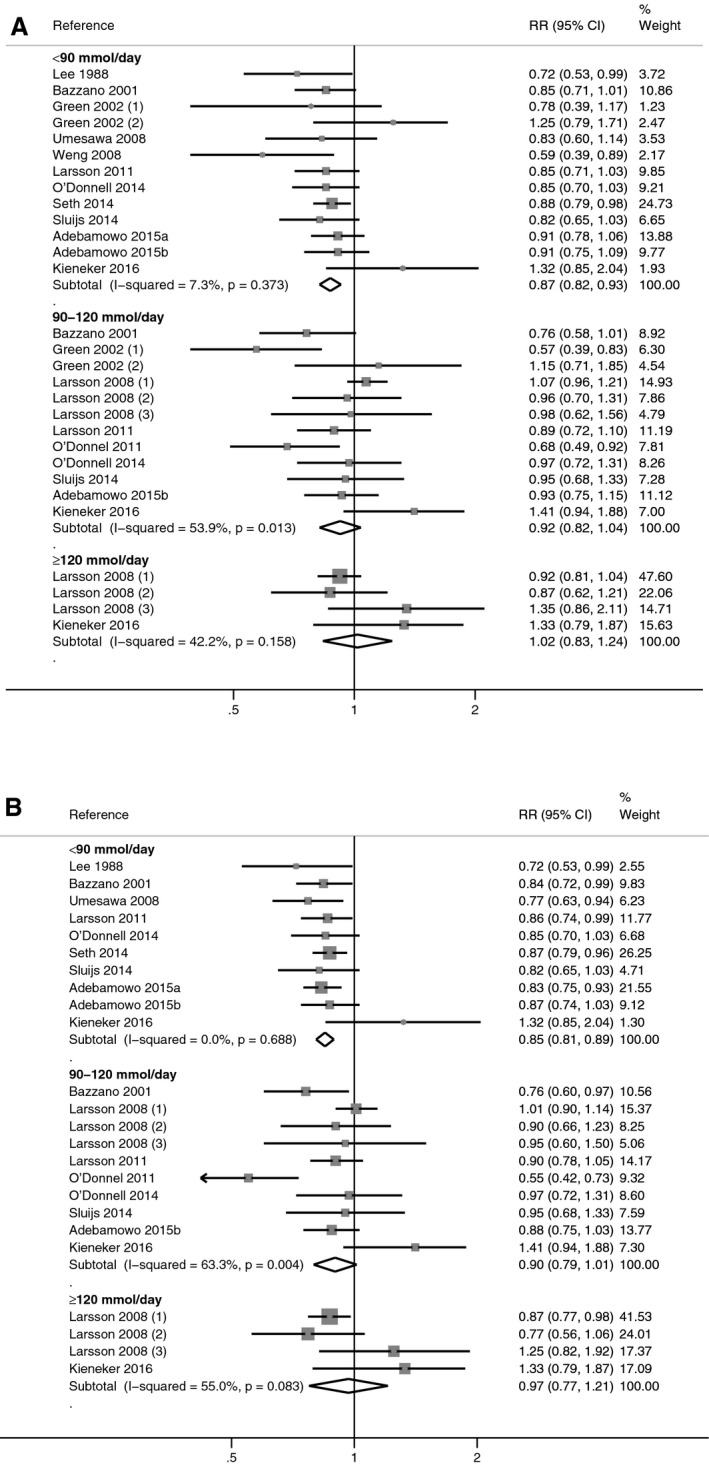
Meta‐analysis of the relative risk (RR), with 95% CI of stroke in observational cohort studies according to category of baseline dietary potassium intake (<90, ≥90 to <120, ≥120 mmol/day). Estimates are from the most‐adjusted multivariate models with (A) and without (B) adjustment for blood pressure.
In stratified analysis by outcome definition, a considerably lower summary RR estimate was found for fatal stroke than for overall stroke incidence, though the RR was based on only 4 studies (Figure S2).25, 31, 32, 35 When stratifying by stroke subtype, summary RRs were 0.87 (95% CI 0.81–0.94) for ischemic stroke (7 studies) and 0.93 (95% CI 0.81–1.07) for hemorrhagic stroke (7 studies) for the “most adjusted model” (Figure 5).29, 30, 32, 34, 35, 37, 38 The use of the “blood pressure–unadjusted model” had little effect on the estimate for ischemic stroke, while the summary RR for hemorrhagic stroke was lower (0.85, 95% CI 0.75–0.95). By conducting separate analyses according to potassium intake assessment methods, summary RRs obtained when pooling studies based on urinary assessment methods (4 studies) were higher and more statistically unstable (wider confidence intervals) than when pooling studies based on dietary questionnaires (12 studies), both when the “most adjusted models” and “blood pressure–unadjusted models” were applied (data not shown).
Figure 5.
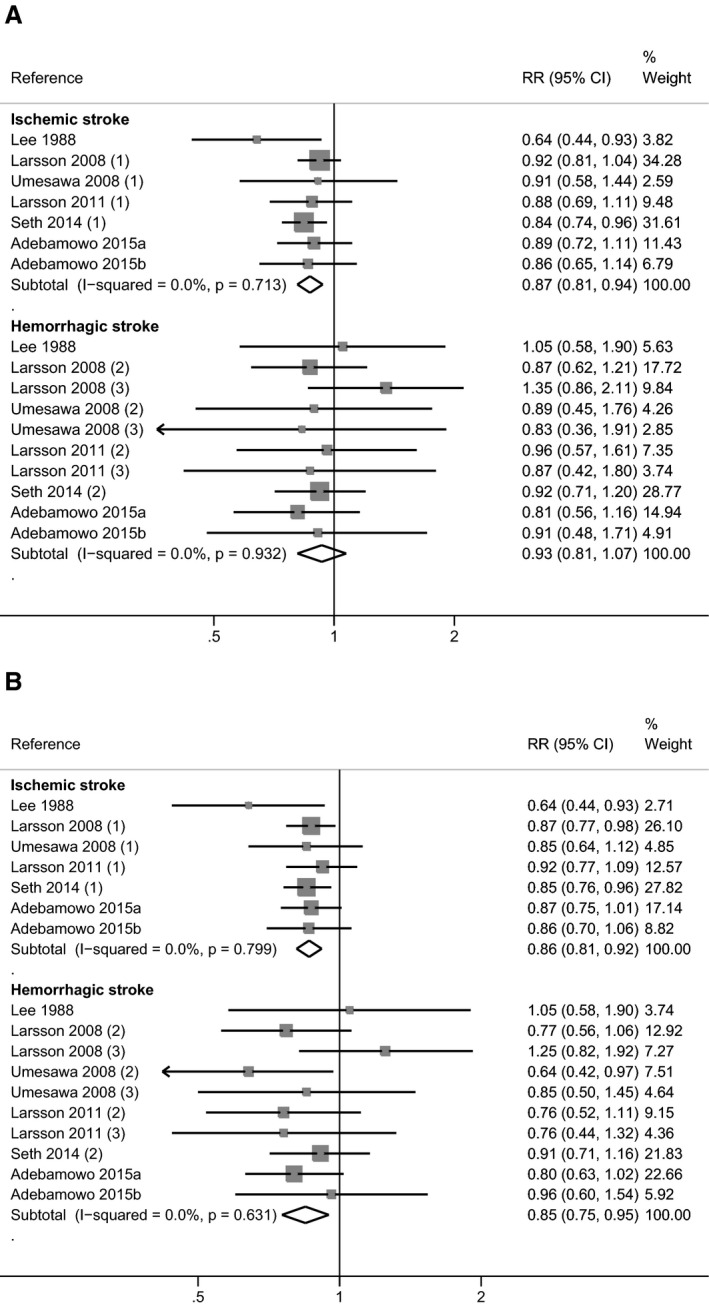
Meta‐analysis of the relative risk (RR) with 95% CI of stroke in observational cohort studies according to stroke subtype (ischemic and hemorrhagic). Estimates are from the “most adjusted model” (A) and from the “blood pressure–unadjusted model” (B).
In sex‐specific analyses, summary RRs for stroke were similar for males and females in both the “most adjusted models” and “blood pressure–unadjusted models.” Values obtained with the blood pressure–unadjusted models tended to be lower than those from the most adjusted models for both sexes (Figure S3). This tendency was enhanced when the analysis was restricted to studies providing both RRs with and without blood pressure adjustment.
In spline regression analysis (Figure 6A), we observed a decrease in the pooled RR up to around 90 mmol/day potassium intake (≈3500 mg/day), based on the most adjusted model. At this cut point of intake, the RR was 0.78 (95% CI 0.70–0.86), while above it the RR flattened and if any slightly increased above 130 mmol/day, though there was substantial uncertainty in this upper range of the distribution. Based on RRs not adjusted for blood pressure, a U‐shaped dose–response curve was observed (Figure 6B). Similarly, pooled RR for stroke decreased up to a potassium intake of around 90 mmol/day (RR 0.67, 95% CI 0.57–0.78). However, the trend was reversed above this cut point. In stratified analysis according to stroke subtype, based on considerably fewer cases, a linear dose–response relation was confirmed up to around 90 mmol/day of potassium intake, while little evidence of an increased risk at higher levels of intake emerged in both the BP‐adjusted and unadjusted analyses (Figure S4). Finally, the various sensitivity analyses we performed on this dose–response relation had little effect on the results (data not shown).
Figure 6.
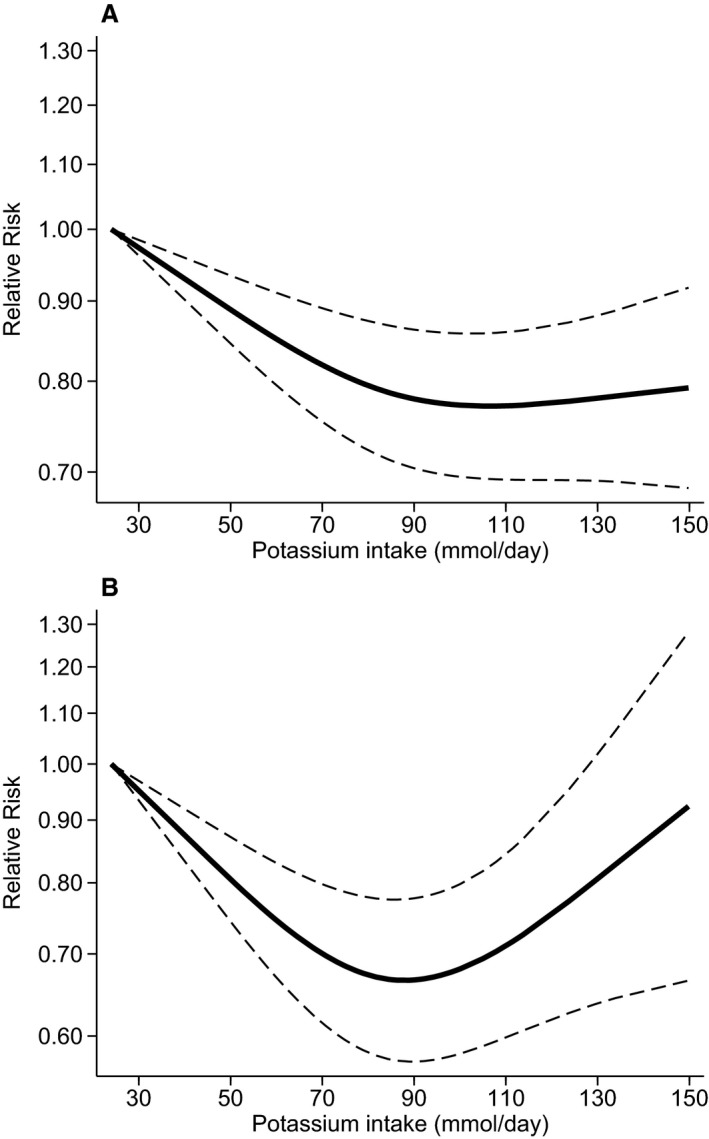
Pooled dose–response association between potassium intake and risk of stroke (solid line) in a meta‐analysis modeling potassium intake with restricted cubic splines in a multivariate random‐effects model, with (A) and without (B) adjustment for blood pressure. The relative risks are plotted on the log scale, with 25 mmol of daily potassium intake serving as referent category. Dashed lines represent the 95% CI for the spline model.
Discussion
As compared with the latest meta‐analyses of prospective cohort studies on potassium intake and stroke risk,6, 10, 11 the present systematic review includes 5 recently published studies22, 23, 29, 30, 38 and an older eligible study that had previously gone undetected.32 We also explored the dose–response relation between potassium intake and stroke risk by stratifying results into 3 subgroups of potassium intake and updating a previously performed spline regression analysis.10 A dilution of the effect by overadjustment for blood pressure, the role of sex as a potential effect‐modifier, and a possible differential effect of potassium intake on stroke subtypes was also investigated. A preliminary assessment showed little evidence for publication bias, thus not supporting the occurrence of this potential source of bias.
Overall, the summary estimates computed in the present meta‐analysis confirm the previously observed inverse association between potassium intake and risk of stroke, though our summary RRs were slightly closer to the unity than those computed in the World Health Organization report15 and the D'Elia et al review,11 but on the converse they had higher precision. In addition, we found evidence that the association may not be linear, and that potassium intake of 90 mmol (≈3500 mg) per day may be associated with the lowest risk of stroke.
Summary RRs were generally lower when we used estimates unadjusted for blood pressure compared with adjusted estimates. This difference was enhanced when we restricted our analyses to the studies that provided RRs both with and without adjustment for blood pressure in addition to other variables. This may suggest that at least part of a beneficial effect of potassium on stroke risk is mediated by a beneficial effect on blood pressure,15 and therefore the risk for overadjustment when blood pressure is included among the covariates in multivariate analysis. Still, the inverse association between potassium intake and stroke risk remained when estimates were adjusted for blood pressure, consistent with the hypothesis of an involvement of other mechanisms, such as a protective effect of potassium against thrombus formation, atherosclerotic lesion progression, endothelial dysfunction, and free radical generation.29, 40, 41, 42, 43 Blood pressure may also be an effect modifier of the potassium–stroke relation, although results of the 2 studies that investigated this issue are entirely contradictory.37, 38 However, it must be noted that blood pressure was assessed at baseline, and generally with a single clinic measurement or a simple assessment of hypertensive status, thus precluding the possibility of taking into account variations of blood pressure during the follow‐up and their possible role in modifying stroke risk. In addition, the multivariate regression models including blood pressure might not be entirely comparable with the blood pressure–unadjusted ones, since in the various studies other covariates, in addition to blood pressure, were considered in the “most adjusted analysis.” Therefore, our indication for a blood pressure–independent effect of potassium intake on stroke risk must be interpreted with caution.
We found evidence of a dose–response relation between potassium intake and stroke risk, both in stratified analysis and in spline regression analysis. Forest plots were consistent with a protective effect of potassium intake in the lowest (<90 mmol/day) and intermediate categories (90–120 mmol/day) of baseline daily potassium intake, but not in the highest subgroup. The spline analysis allowed us to better model the shape of the dose–response relation between potassium intake and stroke risk. This analysis indicated a decrease of RRs for daily potassium intake from around 40 mmol up to 90 mmol, above which the curve tended to remain stable. When RRs unadjusted for blood pressure were used in the model, a U‐shaped dose–response was observed, although the RRs estimates had very large confidence intervals at high levels of potassium intakes, and the increase at higher levels of potassium intake was not observed in the analysis by stroke subtypes (based on fewer number of cases compared with total stroke). Therefore, the possibility that high intakes of potassium may have detrimental effects on stroke risk cannot be entirely ruled out, also in line with recent observation on hypertension risk.44 Overall, these results suggest that a potassium intake on the order of 90 mmol/day may be adequate for stroke prevention. This supports the guidelines for a minimum adequate potassium intake in adults and children of 90 mmol/day issued by the World Health Organization,5 and the dietary reference values of 90 mmol/day for adults proposed by the European Food Safety Authority.9
We did not identify sex‐related differences concerning the potassium–stroke association using blood pressure–adjusted estimates, while analyses based on blood pressure–unadjusted RRs suggested an increased protective effect of potassium in males. We could not explore potential effects of ethnic or genetic factors due to the lack of data collected from these studies. We found a lower summary RR for ischemic stroke than for hemorrhagic stroke when based on the “most adjusted model.” However, estimates for hemorrhagic stroke were more statistically imprecise, due to a greater inconsistency among studies estimates and to the lower number of cases recorded for this outcome, as expected. Interestingly, adjustment for blood pressure appeared to affect mainly the hemorrhagic subtype. This is consistent with the hypothesis of an effect of potassium mediated by its hypotensive effect, as hemorrhagic stroke is more strongly associated with raised blood pressure than ischemic stroke.45 Overall, taking into account a potential overadjustment for blood pressure in the “most adjusted model,” the effect of potassium on both stroke subtypes appears to be similar, though possibly mediated by partially different mechanisms.
A limitation of the evidence yielded by the present meta‐analysis is that it generates from studies with observational and not experimental design, thus potentially subject to biases due to (nondifferential) exposure misclassification or to unmeasured confounding.
The first issue affects particularly studies that relied on the less‐reliable methods to assess potassium intake, such as those based on concentration in spot urine samples, and is expected to lead to a dilution of the RR estimates towards the unity. In the present analysis, and contrary to the previous analysis by Larsson et al,10 we have included studies that used potassium urinary excretion as a marker of potassium intake. This allowed the inclusion of a significantly higher number of cases and of populations with rather “high” potassium intake,22, 23 thus allowing a better and more statistically stable assessment of the risk of stroke in the higher range of potassium intake, as compared with the previous meta‐analysis.10 Yet, out of the 4 studies that measured potassium urinary excretion, only 1 collected multiple 24‐hour urine samples,23 while the others used cruder methods (overnight urine collection24 and morning sport urine collection),21, 22 which are more prone to exposure misclassification.46 In addition, there are uncertainties over the multiplication factor to be used to estimate potassium intake from potassium urinary excretion. Still, exclusion of these 4 studies or use of an alternative factor did not appreciably change the results of both the meta‐analysis and the pooled dose–response analysis.
The second concern is of greater importance for study validity and was outlined as a major methodological limitation in most studies. In fact, a certain degree of collinearity between minerals and more generally nutrient intakes has been reported,29, 30 and no observational study could clearly assess all the remaining dietary variables and other potential confounders. This is a major difference with the literature linking potassium intake and blood pressure, in which a large number of intervention studies are available, particularly in hypertensives,6, 47 in addition to a few observational cohort studies.48, 49
In conclusion, despite the limitations of observational studies and of pooling results from studies carried out in different populations, with some differences in design and data analysis, our meta‐analysis indicates an inverse association between potassium intake and risk of total, hemorrhagic, and ischemic stroke, with the lowest risk occurring at a potassium intake of around 90 mmol/day. The mechanisms by which potassium intake may affect stroke risk require further investigation, as it can only partially be explained by an effect on blood pressure, particularly for ischemic stroke and in females.
Sources of Funding
Financial support for this study was provided by the National Health Service ‐ Local Health Unit of Reggio Emilia.
Disclosures
Agnès de Sesmaisons is employed with the European Food Safety Authority (EFSA) in the Nutrition Unit that provides scientific and administrative support to the Panel on Dietetics Products, Nutrition and Allergies in the area of Dietary Reference Values for minerals. Marco Vinceti is a member of the EFSA Panel on Dietetics Products, Nutrition and Allergies as an independent scientific expert. However, the present article is published under the sole responsibility of the authors and may not be considered as an EFSA scientific output. The positions and opinions presented in this article are those of the authors alone and do not necessarily represent the views or scientific work of EFSA. To learn about the views or scientific outputs of EFSA, please consult its website under http://www.efsa.europa.eu.
Supporting information
Table S1. Number of Participants, Number of Events, Years of Follow‐Up and Adjustment Factors of Included Studies (SBP, Systolic Blood Pressure; BP, Blood Pressure; BMI, Body Mass Index; LDL, Low‐Density Lipoprotein; HDL, High‐Density Lipoprotein)
Table S2. Newcastle–Ottawa Quality Assessment Scale for Included Studies (A), With Details of Score Assignment With Number of Stars in Parentheses (B)
Figure S1. Meta‐analysis of the relative risk (RR, with 95% CI) of stroke in observational cohort studies according to categories of baseline dietary potassium intake (<90, ≥90 to <120, ≥120 mmol/day) restricted to studies reporting both blood pressure–adjusted and unadjusted estimates.6,7,9,10,12,14,15
Figure S2. Meta‐analysis of the relative risk (RR, with 95% CI) of stroke in observational cohort studies according to stroke outcome (mortality or incidence). Estimates are from the “most adjusted model” (A) and from the “blood pressure–unadjusted model” (B).1–17
Figure S3. Meta‐analysis of the relative risk (RR, with 95% CI) of stroke in observational cohort studies according to sex of study participants. Estimates are from the “most adjusted model” (A), from the “blood pressure–unadjusted model” (B), and from studies using both methods (C).1,2,6,9,12,14,15
Figure S4. Pooled dose–response association between potassium intake and risk of stroke (solid line) in a meta‐analysis modeling potassium intake with restricted cubic splines in a multivariate random‐effects model, according to stroke subtype: ischemic type with (A) and without (B) for blood pressure adjustment, and hemorrhagic subtype with (C) and without (D) blood pressure adjustment. Dashed lines represent the 95% CIs for the spline model.
(J Am Heart Assoc. 2016;5:e004210 doi: 10.1161/JAHA.116.004210)
Note
References
- 1. Boden‐Albala B, Southwick L, Carman H. Dietary interventions to lower the risk of stroke. Curr Neurol Neurosci Rep. 2015;15:15. [DOI] [PubMed] [Google Scholar]
- 2. Larsson SC, Akesson A, Wolk A. Healthy diet and lifestyle and risk of stroke in a prospective cohort of women. Neurology. 2014;83:1699–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haring B, Misialek JR, Rebholz CM, Petruski‐Ivleva N, Gottesman RF, Mosley TH, Alonso A. Association of dietary protein consumption with incident silent cerebral infarcts and stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2015;46:3443–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsivgoulis G, Psaltopoulou T, Wadley VG, Alexandrov AV, Howard G, Unverzagt FW, Moy C, Howard VJ, Kissela B, Judd SE. Adherence to a Mediterranean diet and prediction of incident stroke. Stroke. 2015;46:780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO . Guideline: Potassium Intake for Adults and Children. Geneva: World Health Organization (WHO); 2012. [PubMed] [Google Scholar]
- 6. Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta‐analyses. BMJ. 2013;346:f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Institute of Medicine . Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- 8. Whelton PK. Sodium, potassium, blood pressure, and cardiovascular disease in humans. Curr Hypertens Rep. 2014;16:465. [DOI] [PubMed] [Google Scholar]
- 9. EFSA Panel on Dietetic Products Nutrition and Allergies (NDA) . Public Consultation on the Draft Scientific Opinion on Dietary Reference Values for Potassium. Parma: European Food Safety Authority; 2016. [Google Scholar]
- 10. Larsson SC, Orsini N, Wolk A. Dietary potassium intake and risk of stroke: a dose‐response meta‐analysis of prospective studies. Stroke. 2011;42:2746–2750. [DOI] [PubMed] [Google Scholar]
- 11. D'Elia L, Iannotta C, Sabino P, Ippolito R. Potassium‐rich diet and risk of stroke: updated meta‐analysis. Nutr Metab Cardiovasc Dis. 2014;24:585–587. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses. Ottawa: Ottawa Hospital Research Institute; 2004. [Google Scholar]
- 14. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. WHO . Effect of Increased Potassium Intake on Cardiovascular Disease, Coronary Heart Disease and Stroke. Geneva: World Health Organization; 2012. [Google Scholar]
- 16. Holbrook JT, Patterson KY, Bodner JE, Douglas LW, Veillon C, Kelsay JL, Mertz W, Smith JC Jr. Sodium and potassium intake and balance in adults consuming self‐selected diets. Am J Clin Nutr. 1984;40:786–793. [DOI] [PubMed] [Google Scholar]
- 17. Tasevska N, Runswick SA, Bingham SA. Urinary potassium is as reliable as urinary nitrogen for use as a recovery biomarker in dietary studies of free living individuals. J Nutr. 2006;136:1334–1340. [DOI] [PubMed] [Google Scholar]
- 18. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol. 1992;135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 19. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, Whelton PK. Dietary potassium intake and risk of stroke in US men and women: National Health and Nutrition Examination Survey I epidemiologic follow‐up study. Stroke. 2001;32:1473–1480. [DOI] [PubMed] [Google Scholar]
- 21. O'Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, McQueen M, Sleight P, Sharma AM, Dans A, Probstfield J, Schmieder RE. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–2238. [DOI] [PubMed] [Google Scholar]
- 22. O'Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, Rosengren A, Lopez‐Jaramillo P, Diaz R, Avezum A, Lanas F, Yusoff K, Iqbal R, Ilow R, Mohammadifard N, Gulec S, Yusufali AH, Kruger L, Yusuf R, Chifamba J, Kabali C, Dagenais G, Lear SA, Teo K, Yusuf S, Investigators P. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. [DOI] [PubMed] [Google Scholar]
- 23. Kieneker LM, Gansevoort RT, de Boer RA, Brouwers FP, Feskens EJ, Geleijnse JM, Navis G, Bakker SJ, Joosten MM; Group PS . Urinary potassium excretion and risk of cardiovascular events. Am J Clin Nutr. 2016;103:1204–1212. [DOI] [PubMed] [Google Scholar]
- 24. Geleijnse JM, Witteman JC, Stijnen T, Kloos MW, Hofman A, Grobbee DE. Sodium and potassium intake and risk of cardiovascular events and all‐cause mortality: the Rotterdam Study. Eur J Epidemiol. 2007;22:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khaw KT, Barrett‐Connor E. Dietary potassium and stroke‐associated mortality. A 12‐year prospective population study. N Engl J Med. 1987;316:235–240. [DOI] [PubMed] [Google Scholar]
- 26. Espeland MA, Kumanyika S, Wilson AC, Reboussin DM, Easter L, Self M, Robertson J, Brown WM, McFarlane M; Group TCR . Statistical issues in analyzing 24‐hour dietary recall and 24‐hour urine collection data for sodium and potassium intakes. Am J Epidemiol. 2001;153:996–1006. [DOI] [PubMed] [Google Scholar]
- 27. Ascherio A, Rimm EB, Hernan MA, Giovannucci EL, Kawachi I, Stampfer MJ, Willett WC. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation. 1998;98:1198–1204. [DOI] [PubMed] [Google Scholar]
- 28. Iso H, Stampfer MJ, Manson JE, Rexrode K, Hennekens CH, Colditz GA, Speizer FE, Willett WC. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke. 1999;30:1772–1779. [DOI] [PubMed] [Google Scholar]
- 29. Adebamowo SN, Spiegelman D, Flint AJ, Willett WC, Rexrode KM. Intakes of magnesium, potassium, and calcium and the risk of stroke among men. Int J Stroke. 2015;10:1093–1100. [DOI] [PubMed] [Google Scholar]
- 30. Adebamowo SN, Spiegelman D, Willett WC, Rexrode KM. Association between intakes of magnesium, potassium, and calcium and risk of stroke: 2 cohorts of US women and updated meta‐analyses. Am J Clin Nutr. 2015;101:1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fang J, Madhavan S, Alderman MH. Dietary potassium intake and stroke mortality. Stroke. 2000;31:1532–1537. [DOI] [PubMed] [Google Scholar]
- 32. Lee CN, Reed DM, MacLean CJ, Yano K, Chiu D. Dietary potassium and stroke. N Engl J Med. 1988;318:995–996. [DOI] [PubMed] [Google Scholar]
- 33. Green DM, Ropper AH, Kronmal RA, Psaty BM, Burke GL; Cardiovascular Health S . Serum potassium level and dietary potassium intake as risk factors for stroke. Neurology. 2002;59:314–320. [DOI] [PubMed] [Google Scholar]
- 34. Larsson SC, Virtanen MJ, Mars M, Mannisto S, Pietinen P, Albanes D, Virtamo J. Magnesium, calcium, potassium, and sodium intakes and risk of stroke in male smokers. Arch Intern Med. 2008;168:459–465. [DOI] [PubMed] [Google Scholar]
- 35. Umesawa M, Iso H, Date C, Yamamoto A, Toyoshima H, Watanabe Y, Kikuchi S, Koizumi A, Kondo T, Inaba Y, Tanabe N, Tamakoshi A; Group JS . Relations between dietary sodium and potassium intakes and mortality from cardiovascular disease: the Japan Collaborative Cohort Study for Evaluation of Cancer Risks. Am J Clin Nutr. 2008;88:195–202. [DOI] [PubMed] [Google Scholar]
- 36. Weng LC, Yeh WT, Bai CH, Chen HJ, Chuang SY, Chang HY, Lin BF, Chen KJ, Pan WH. Is ischemic stroke risk related to folate status or other nutrients correlated with folate intake? Stroke. 2008;39:3152–3158. [DOI] [PubMed] [Google Scholar]
- 37. Larsson SC, Virtamo J, Wolk A. Potassium, calcium, and magnesium intakes and risk of stroke in women. Am J Epidemiol. 2011;174:35–43. [DOI] [PubMed] [Google Scholar]
- 38. Seth A, Mossavar‐Rahmani Y, Kamensky V, Silver B, Lakshminarayan K, Prentice R, Van Horn L, Wassertheil‐Smoller S. Potassium intake and risk of stroke in women with hypertension and nonhypertension in the Women's Health Initiative. Stroke. 2014;45:2874–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sluijs I, Czernichow S, Beulens JW, Boer JM, van der Schouw YT, Verschuren WM, Grobbee DE. Intakes of potassium, magnesium, and calcium and risk of stroke. Stroke. 2014;45:1148–1150. [DOI] [PubMed] [Google Scholar]
- 40. Hunt BD, Cappuccio FP. Potassium intake and stroke risk: a review of the evidence and practical considerations for achieving a minimum target. Stroke. 2014;45:1519–1522. [DOI] [PubMed] [Google Scholar]
- 41. Gijsbers L, Dower JI, Schalkwijk CG, Kusters YH, Bakker SJ, Hollman PC, Geleijnse JM. Effects of sodium and potassium supplementation on endothelial function: a fully controlled dietary intervention study. Br J Nutr. 2015;114:1419–1426. [DOI] [PubMed] [Google Scholar]
- 42. Palmer BF, Clegg DJ. Achieving the benefits of a high‐potassium, paleolithic diet, without the toxicity. Mayo Clin Proc. 2016;91:496–508. [DOI] [PubMed] [Google Scholar]
- 43. Ekmekcioglu C, Elmadfa I, Meyer AL, Moeslinger T. The role of dietary potassium in hypertension and diabetes. J Physiol Biochem. 2016;72:93–106. [DOI] [PubMed] [Google Scholar]
- 44. Xi L, Hao YC, Liu J, Wang W, Wang M, Li GQ, Qi Y, Zhao F, Xie WX, Li Y, Sun JY, Liu J, Qin LP, Zhao D. Associations between serum potassium and sodium levels and risk of hypertension: a community‐based cohort study. J Geriatr Cardiol. 2015;12:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao‐Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez‐Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S; Investigators I . Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case‐control study. Lancet. 2010;376:112–123. [DOI] [PubMed] [Google Scholar]
- 46. Hooft van Huysduynen EJ, Hulshof PJ, van Lee L, Geelen A, Feskens EJ, van‘t Veer P, van Woerkum CJ, de Vries JH. Evaluation of using spot urine to replace 24 h urine sodium and potassium excretions. Public Health Nutr. 2014;17:2505–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Food Standards Australia New Zealand . Systematic Review of the Evidence for a Relationship Between Potassium and Blood Pressure. FSANZ; 2014. Available at: https://www.foodstandards.gov.au/publications/Documents/EU health claims reviews/Systematic Review Potassium and blood pressure.pdf. Accessed September 26, 2016. [Google Scholar]
- 48. Chien KL, Hsu HC, Chen PC, Su TC, Chang WT, Chen MF, Lee YT. Urinary sodium and potassium excretion and risk of hypertension in Chinese: report from a community‐based cohort study in Taiwan. J Hypertens. 2008;26:1750–1756. [DOI] [PubMed] [Google Scholar]
- 49. Kieneker LM, Gansevoort RT, Mukamal KJ, de Boer RA, Navis G, Bakker SJ, Joosten MM. Urinary potassium excretion and risk of developing hypertension: the prevention of renal and vascular end‐stage disease study. Hypertension. 2014;64:769–776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Number of Participants, Number of Events, Years of Follow‐Up and Adjustment Factors of Included Studies (SBP, Systolic Blood Pressure; BP, Blood Pressure; BMI, Body Mass Index; LDL, Low‐Density Lipoprotein; HDL, High‐Density Lipoprotein)
Table S2. Newcastle–Ottawa Quality Assessment Scale for Included Studies (A), With Details of Score Assignment With Number of Stars in Parentheses (B)
Figure S1. Meta‐analysis of the relative risk (RR, with 95% CI) of stroke in observational cohort studies according to categories of baseline dietary potassium intake (<90, ≥90 to <120, ≥120 mmol/day) restricted to studies reporting both blood pressure–adjusted and unadjusted estimates.6,7,9,10,12,14,15
Figure S2. Meta‐analysis of the relative risk (RR, with 95% CI) of stroke in observational cohort studies according to stroke outcome (mortality or incidence). Estimates are from the “most adjusted model” (A) and from the “blood pressure–unadjusted model” (B).1–17
Figure S3. Meta‐analysis of the relative risk (RR, with 95% CI) of stroke in observational cohort studies according to sex of study participants. Estimates are from the “most adjusted model” (A), from the “blood pressure–unadjusted model” (B), and from studies using both methods (C).1,2,6,9,12,14,15
Figure S4. Pooled dose–response association between potassium intake and risk of stroke (solid line) in a meta‐analysis modeling potassium intake with restricted cubic splines in a multivariate random‐effects model, according to stroke subtype: ischemic type with (A) and without (B) for blood pressure adjustment, and hemorrhagic subtype with (C) and without (D) blood pressure adjustment. Dashed lines represent the 95% CIs for the spline model.


