Abstract
Background
Sex‐related differences in morbidity and survival in bicuspid aortic valve (BAV) adults are fundamentally unknown. Contemporary studies portend excellent survival for BAV patients identified at early echocardiographic‐clinical stages. Whether BAV adults incur a survival disadvantage throughout subsequent echocardiographic‐clinical stages remains undetermined.
Methods and Results
Analysis was done of 3 different cohorts of consecutive patients with echocardiographic diagnosis of BAV identified retrospectively: (1) a community cohort of 416 patients with first BAV diagnosis (age 35±21 years, follow‐up 16±7 years), (2) a tertiary clinical referral cohort of 2824 BAV adults (age 51±16 years, follow‐up 9±6 years), and (3) a surgical referral cohort of 2242 BAV adults referred for aortic valve replacement (AVR) (age 62±14 years, follow‐up 6±5 years). For the community cohort, 20‐year risks of aortic regurgitation (AR), AVR, and infective endocarditis were higher in men (all P≤0.04); for a total BAV‐related morbidity risk of 52±4% vs 35±6% in women (P=0.01). The cohort's 25‐year survival was identical to that in the general population (P=0.98). AR independently predicted mortality in women (P=0.001). Baseline AR was more common in men (P≤0.02) in the tertiary cohort, with 20‐year survival lower than that in the general population (P<0.0001); age‐adjusted relative death risk was 1.16 (95% confidence interval [CI] 1.05‐1.29) for men versus 1.67 (95% CI 1.38‐2.03) for women (P=0.001). AR independently predicted mortality in women (P=0.01). Baseline AR and infective endocarditis were higher in men (both ≤0.001) for the surgical referral cohort, with 15‐year survival lower than that in the general population (P<0.0001); age‐adjusted relative death risk was 1.34 (95% CI 1.22‐1.47) for men versus 1.63 (95% CI 1.40‐1.89) for women (P=0.026). AR and NYHA class independently predicted mortality in women (both P≤0.04).
Conclusions
Within evolving echocardiographic‐clinical stages, the long‐term survival of adults with BAV is not benign, as both men and women incur excess mortality. Although BAV‐related morbidity is higher in men in the community, and AR and infective endocarditis are more prevalent in men, women exhibit a significantly higher relative risk of death in tertiary and surgical referral cohorts, which is independently associated with AR.
Keywords: bicuspid aortic valve, sex‐specific, survival
Subject Categories: Mortality/Survival, Valvular Heart Disease, Echocardiography, Women
Introduction
Bicuspid aortic valve (BAV) is the most common congenital heart defect,1 and aortic valve replacement (AVR) its most frequent long‐term complication with over 50% of patients incurring AVR 25 years after BAV diagnosis.2, 3, 4 Over 25% of BAV patients also develop ascending aorta aneurysms (AA) 25 years after BAV diagnosis, with cohort risks of aortic dissection ranging from 0.5% to 1%.3, 4 In addition, infective endocarditis (IE) is observed in ~2% of contemporary BAV cohorts.2, 3, 5, 6, 7
Although the BAV condition begins at birth, the echocardiographic‐clinical evolution of most adults with BAV begins at echocardiographic diagnosis in young adulthood,2 followed by continued surveillance of BAV dysfunction and/or AA locally or via tertiary clinical referral.3 The subsequent evolving echocardiographic‐clinical stage for over 50% of BAV adults is surgical referral for AVR.4, 6
Beyond the fact that BAV is more frequent in men than women, sex‐specific morbidity and survival patterns in BAV adults remain fundamentally unknown.8 In addition, contemporary assessment of long‐term survival in BAV patients has involved young patient cohorts, frequently identified at the time of BAV diagnosis, and has included children.2, 3, 4 The resulting survival rates have been identical to those in the general population, suggesting a benign prognosis for BAV patients.2, 3, 4
Nonetheless, whether BAV cohorts identified at subsequent echocardiographic‐clinical stages beyond the diagnosis stage could incur survival penalties6 remains undetermined. Therefore, investigating long‐term survival in BAV adults requires assessment of large cohorts beyond the stage of BAV diagnosis to include the time of tertiary clinical referral and the time of AVR referral.
In addition to allowing population‐based analysis of BAV cohorts,4, 9 Mayo Clinic (Rochester) serves as a tertiary center with high cardiac diagnostic and surgical referral volumes, allowing survival analysis of large BAV patient cohorts at different stages of their condition. In order to examine sex‐specific morbidity patterns in BAV patients, we analyzed the largest population‐based community cohort with the longest reported follow‐up.4, 7 To verify those morbidity patterns and test the hypothesis that survival may be compromised in BAV adults across evolving echocardiographic‐clinical junctures, we analyzed overall and sex‐specific survival in a tertiary clinical referral cohort and in an AVR referral cohort.
Methods
We analyzed 3 different groups of consecutive BAV patients: (1) a community cohort comprising only residents of Olmsted County, MN, with a first BAV echocardiographic diagnosis, on which we previously had reported overall aorta/IE complications4, 7; (2) a tertiary clinical referral cohort of consecutive non‐Olmsted BAV adult patients with a first‐Mayo echocardiogram; and (3) a surgical referral cohort of consecutive BAV adult patients undergoing AVR at Mayo‐Rochester. The protocol was approved by the Institutional Review Board, and patients gave informed consent for clinical studies.
Echocardiography
All patients underwent baseline clinical evaluation and comprehensive transthoracic echocardiography with state‐of‐the‐art technology at diagnosis.2, 4 Left ventricular ejection fraction was assessed with 2‐dimensional echocardiography, visual estimation, or volumetric assessment.10 Diagnosis of BAV was based on short‐axis aortic valve imaging demonstrating 2 commissures delimiting only 2 aortic valve cusps.2, 3 Doppler was used to measure blood velocity and assess degree of aortic regurgitation (AR),11 with assessment of flow reversal in the aortic arch and Doppler measurement of maximal aortic jet velocity and mean gradient from multiple windows.12 Aortic regurgitation was graded as 1+ (trivial or mild), 2+ (moderate), 3+ (moderate‐severe) and 4+ (severe) with utilization of specific and supporting signs as recommended.13 Baseline and follow‐up AS (any AS) was defined as Doppler mean transaortic systolic gradient ≥20 mm Hg or peak systolic transaortic velocity ≥3.0 m/s. Severe AS was defined as a mean Doppler transaortic gradient ≥40 mm Hg or peak Doppler transaortic velocity ≥4 m/s. Two‐dimensional ascending aorta measurements were obtained in the parasternal long‐axis view at end‐diastole. Root and ascending aorta diameters were measured by the leading‐edge‐to‐leading‐edge technique.10 The largest diameter between the root and ascending aorta for each patient was used for analysis. Aortic diameters were corrected by height. The ratio of the aortic diameter cross‐sectional area by the height (ratio=r 2 π[cm2]/height [m]) was also used to correct for dissimilarities in body size.14, 15
Cohort Definition and Follow‐Up Procedures
Community Cohort
The community cohort included consecutive residents of Olmsted County of all ages with a first echocardiographic BAV diagnosis from January 1980 to December 1999 (Figure 1). The follow‐up of this cohort was obtained by review of medical records, mailed surveys, telephone interviews, and social security search.
Figure 1.
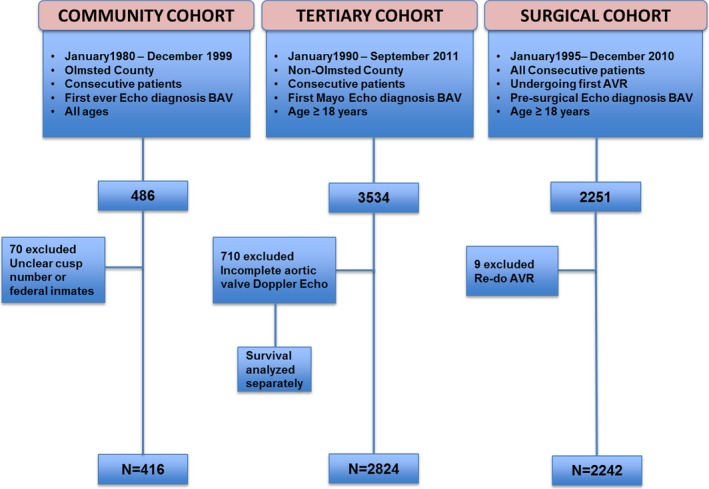
Assemblage of 3 separate cohorts of BAV patients AVR indicates aortic valve replacement; BAV, bicuspid aortic valve.
Tertiary Clinical Referral Cohort
From January 1990 to September 2011, all consecutive non‐Olmsted patients ≥18 years old with a first‐Mayo echocardiogram confirming BAV were identified from our echocardiographic database, and those patients with comprehensive Doppler aortic valve assessment were included for analysis (Figure 1). All‐cause mortality within this group was determined by electronic medical record search, social security search, and by National Death Index search (NDI number 2012‐0008). The use of these national mortality databases guarantees identification of >90% of deaths.16
Surgical Referral Cohort
From January 1995 to December 2010, all consecutive BAV patients ≥18 years old undergoing AVR at our institution were identified from our STS database, which is abstracted regularly (Figure 1). All‐cause mortality within this group was determined by electronic medical record search and social security search.
Endpoints
Community cohort endpoints were all‐cause mortality, follow‐up occurrence of AS, AR, AVR, IE, development of thoracic aorta dissection,17 AA (diameter of ≥45 mm),4 and surgery of the aorta. Infective endocarditis events were defined by modified Duke criteria.18 The community cohort total BAV‐related morbidity endpoint included AVR, AA, dissection, IE, and surgery of the aorta (for dissection, coarctation, or elective aneurysm repair).
For the tertiary clinical referral and surgical referral cohorts, the single clinical endpoint was all‐cause mortality.
Statistical Analysis
Continuous variables are expressed as mean±SD and percentage for categorical variables. T test and Fisher test or Chi‐squared test were used for comparison between continuous and categorical data, respectively. Survival and event rates were estimated with the Kaplan‐Meier method and compared between groups with the 2‐sample log‐rank test. Survival of patients was compared with that of the Minnesota white population matched for age and sex as defined by the US Census Bureau life tables and tested with a 1‐sample log‐rank test. Association of baseline clinical characteristics with the incidence of events was analyzed with the Cox proportional‐hazards method. The association of age and sex with standardized mortality relative risk of death (standardized to the US general population) was assessed using a generalized linear model with a Poisson distribution for the observed number of deaths. The functional relationship between age and the hazard ratio for death was estimated using smoothing splines at the population level (adjusting for expected survival). Statistical significance was set a priori at P<0.05. All analyses were performed with SAS version 9.2 (SAS Institute Inc, Cary, NC).
Results
Community Cohort
From 1980 to 1999, there were 416 Olmsted County residents with new echocardiographic BAV diagnoses. Indication for echocardiogram was abnormal auscultation in 295 (71%). Other indications were left ventricular function evaluation, cardiac symptoms, and other known congenital heart disease. Baseline characteristics by sex are presented in Table 1. Age at echocardiographic diagnosis was similar between sexes. Maximum aorta diameter and AA at baseline were higher in men. Any degree of AR was significantly more common in men at baseline.
Table 1.
Baseline Community Cohort Characteristics by Sex
| Variable | Total (n=416) | Men (n=288) | Women (n=128) | P Value |
|---|---|---|---|---|
| Age, y | 35±21 | 36±21 | 34±21 | 0.37 |
| Weighted Charlson index | 0.45±1 | 0.52±1.1 | 0.31±0.7 | 0.17 |
| Hypertension, n (%) | 93 (22) | 58 (20) | 35 (27) | 0.10 |
| Smoking, n (%) | 132 (32) | 95 (33) | 37 (29) | 0.40 |
| Diabetes mellitus, n (%) | 15 (4) | 11 (4) | 4 (3) | 0.76 |
| Atherosclerosis, n (%)a | 21 (5) | 15 (5) | 6 (5) | 0.83 |
| Coarctation, n (%) | 30 (7) | 18 (6) | 12 (9) | 0.26 |
| Endocarditis, n (%) | 2 (0.5) | 2 (0.7) | 0 (0) | 0.85 |
| Congenital heart disease, n (%) | 54 (13) | 34 (12) | 20 (16) | 0.29 |
| LV EDD, mm | 50±11 | 51±11 | 48±11 | 0.01 |
| LV ejection fraction, % | 62±7 | 62±8 | 63±7 | 0.22 |
| Aorta diameter, mm | 34±9 | 35±9 | 31±7 | <0.0001 |
| Aorta diameter/heightb, mm/m | 22.5±13 | 22.5±11 | 22.6±17 | 0.94 |
| Aneurysm ≥45 mm, n (%) | 32 (8) | 29 (10) | 3 (2) | 0.002 |
| R‐L cusp fusion BAV, n (%) | 350 (84) | 243 (85) | 107 (84) | 0.88 |
| Any AR, n (%) | 247 (59) | 185 (64) | 62 (48) | 0.003 |
| AR 3+ or 4+, n (%) | 34 (8) | 31 (11) | 3 (2) | 0.003 |
| Any AS, n (%) | 94 (23) | 66 (23) | 28 (22) | 0.89 |
AR indicates aortic regurgitation; AS, aortic stenosis; BAV, bicuspid aortic valve; BSA, body surface area; EDD, end‐diastolic diameter; LV, left ventricle; R‐L, right‐left.
History of stroke, transient ischemic attack, and/or myocardial infarction at baseline.
Height in meters available for 257 men and 111 women.
Community Cohort Sex‐Specific Morbidity Patterns at Follow‐Up
Community cohort mean clinical follow‐up time was 16±7 years. Sex‐specific morbidity at follow‐up is presented in Table 2. Of 291 patients with no significant aortic valve dysfunction at baseline (patients with baseline AS or AR 3+ or 4+ excluded), 232 (80%) had follow‐up echocardiograms 12±7 years after initial diagnosis. The 20‐year rates of AR ≥2+ development at follow‐up were 36±5% and 14±5% for men and women, respectively (P=0.01). The 20‐year AVR rates were 41±4% and 24±5% for men and women, respectively (P=0.01). Two male patients had acute aortic dissection during follow‐up versus no women (P=0.52). The 25‐year rates of AA formation were 36±6% and 14±4% for men and women, respectively (P=0.11). The 25‐year rates of surgery of the aorta were 30±6% and 20±5% for men and women, respectively (P=0.25). At 9±6 years of follow‐up, 9 male patients developed new definite or possible IE by modified Duke criteria. The 25‐year rates of IE were 5±2% for men and 0% for women (P=0.04).
Table 2.
Follow‐Up Community Cohort Outcomes by Sex
| Outcome During Follow‐Up | Entire Cohort (n=416) | Men (n=288) | Women (n=128) | P Value |
|---|---|---|---|---|
| AVR, n (%) | 133 (32) | 103 (36) | 30 (23) | 0.01 |
| Severe AS, n (%)a | 84 (20) | 59 (21) | 25 (20) | 0.89 |
| 84/133 (63) | 59/103 (57) | 25/30 (83) | 0.009 | |
| Severe AR, n (%)a | 38 (9) | 34 (12) | 4 (3) | 0.003 |
| 38/133 (28) | 34/103 (33) | 4/30 (13) | 0.04 | |
| Aortic dissection, n (%) | 2 (0.5) | 2 (0.7) | 0 (0) | 0.52 |
| Aorta diameter, mmb | 39±7 (n=336) | 40±7 (n=239) | 37±6 (n=97) | 0.0002 |
| Aneurysm formation, n (%)c | 49 (13) (n=384) | 38 (15) (n=259) | 11 (9) (n=125) | 0.14 |
| Aorta surgery, n (%)d | 49 (12) | 37 (13) | 12 (9) | 0.4 |
| Endocarditis, n (%) | 9 (2) | 9 (4) | 0 (0) | 0.06 |
| Total events, n % | 161 (39) | 123 (43) | 38 (30) | 0.01 |
AR indicates aortic regurgitation; AS, aortic stenosis; AVR, aortic valve replacement; BAV, bicuspid aortic valve.
Total (first row) and as cause for aortic valve replacement (second row).
There were 336 follow‐up echocardiograms (81%) with aortic diameter measurements in 416 patients.
After excluding 32 patients with aneurysms at baseline, the remaining 384 (men 259, women 125) patients remained at risk for aneurysm formation at follow‐up.
Surgery for aneurysm, coarctation, or dissection.
Community Cohort Total BAV‐Related Morbidity and Survival
The 20‐year rate of total BAV‐related morbidity was 52±4% for men and 35±6% for women (P=0.01, Figure 2A). We performed multivariate analysis of baseline variables including age, sex, hypertension, diabetes mellitus, atherosclerotic disease, aortic coarctation and other congenital heart disease as predictors of BAV‐related morbidity. Only age ≥50 years (HR 3.1; 95% CI 2.1‐4.6; P<0.0001) and male sex (HR 1.52; 95% CI 1.06‐2.2; P=0.02) were independent predictors of BAV‐related morbidity. In addition, the 25‐year rate of ischemic events (documented myocardial infarction or coronary revascularization) was also higher in men (16±3% vs 5±2%, P=0.01). Despite these morbidity differences, the 25‐year survival was not different between sexes (P=0.41, Figure 2B). Survival of the entire cohort was identical to that of the general population, P=0.98. Independent predictors of death for women were baseline age ≥50 years (HR 49.4; 95% CI 11.2‐399.9; P<0.0001) and baseline AR 3+ or 4+ (HR 44.9; 95% CI 5.2‐399.7; P=0.001). Independent predictors of death for men were baseline age ≥50 years (HR 2.8; 95% CI 1.06‐8.49; P=0.03), baseline aortic valve peak velocity ≥2.5 m/s (HR 7.9; 95% CI 2.2‐39.2; P=0.0005), and baseline LVEF (HR 0.93; 95% CI 0.90‐0.97; P=0.001, per unit increase%).
Figure 2.
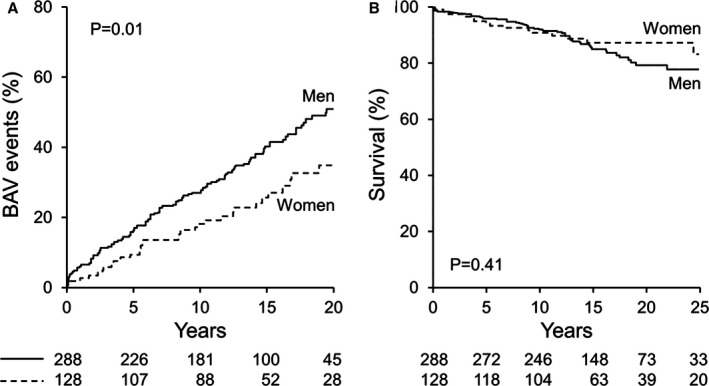
Community cohort sex‐specific BAV‐related morbidity and sex‐specific survival. A, Kaplan‐Meier 20‐year risk of BAV‐associated morbidity. B, Kaplan‐Meier 25‐year rates of survival after echocardiographic BAV diagnosis. BAV indicates bicuspid aortic valve.
Tertiary Clinical Referral Cohort
From 1990 to 2011, there were 3534 adult non‐Olmsted tertiary clinical referral consecutive patients with a first‐Mayo transthoracic echocardiogram depicting BAV (Figure 1). Of these, 2824 patients had comprehensive aortic valve Doppler‐hemodynamic echocardiographic data and were analyzed: 2089 (74%) men and 735 (26%) women. Indications for echocardiogram were known BAV or known BAV dysfunction in 1227 (43%), cardiac murmur in 439 (16%) patients, AA with BAV in 192 (7%), other congenital heart disease (most commonly history of aortic coarctation) in 127 (5%), cardiac symptoms or arrhythmias in 320 (11%), and miscellaneous in 519 (18%). Miscellaneous indications included ischemic heart disease, hypertension, dilated cardiomyopathy, congestive heart failure, preoperative evaluation, abnormal electrocardiogram, other (nonaortic) valvular dysfunction, and hypertrophic cardiomyopathy. Follow‐up for survival was complete until death or September 2012. Mean follow‐up time was 9±6 years and not different between sexes. Table 3 summarizes their baseline characteristics. Baseline weighted Charlson comorbidity index was low and identical between sexes (P=0.82), rendering them comparable for analyzing long‐term mortality. Congruent with the community cohort, maximum aorta diameters and baseline AA were greater in men but clinically comparable when corrected for height and as the ratio of aortic diameter to cross‐sectional area by height (Table 3). However, more men reached a cutoff of 10 cm2/m than women (18% vs 11%, respectively, P<0.0001) for the ratio. Congruent with the community cohort, all AR severity grades were excessive in men. Despite absolute left ventricular end‐systolic diameter (ESD) being larger in men than in women with AR ≥2+ (Table 3), ESD‐corrected BSA was the same between sexes, suggesting a similar degree of left ventricular dilatation between women and men with AR ≥2+.
Table 3.
Tertiary Referral Cohort Baseline Clinical and Echocardiographic Features by Sex
| Variable | Total (n=2824) | Men (n=2089) | Women (n=735) | P Value |
|---|---|---|---|---|
| Age, y | 51±16 | 52±16 | 49±16 | 0.0003 |
| BMI, kg/m2 | 28±10 | 28±9 | 27±12 | 0.02 |
| Weighted Charlson index | 1.39±2 | 1.40±2 | 1.38±2 | 0.82 |
| Ejection fraction, % | 61±9 | 60±10 | 63±8 | <0.0001 |
| Coarctation, n (%) | 149 (5) | 96 (5) | 53 (7) | 0.008 |
| Endocarditis, n (%) | 2 (0.07) | 2 (0.1) | 0 (0) | 0.97 |
| Aorta diameter, mma | 41±7 (n=2078) | 42±7 (n=1543) | 37±7 (n=535) | <0.0001 |
| Aneurysm (≥45 mm), n (%)a | 564 (27) | 491 (32) | 73 (14) | <0.0001 |
| Aorta diameter/height, mm/ma | 23±4 | 23.3±4 | 22.8±7 | 0.01 |
| Aorta cross‐section by height, cm2/ma | 7.6±2.5 | 7.8±2.5 | 6.9±2.5 | <0.0001 |
| Aortic peak velocity, m/s | 2.44±1.1 | 2.44±1.1 | 2.43±1.1 | 0.83 |
| Severe AS | 333 (12) | 243 (12) | 90 (12) | 0.64 |
| AR 2+, n (%) | 313 (11) | 248 (12) | 65 (9) | 0.02 |
| AR 3+, n (%) | 150 (5) | 129 (6) | 21 (3) | 0.0004 |
| AR 4+, n (%) | 227 (8) | 211 (10) | 16 (2) | <0.0001 |
| ESD for AR ≥2+, mmb | 39±8 (n=439) | 40±8 (n=371) | 35±7 (n=68) | <0.0001 |
| ESD/BSA for AR ≥2+, mm/m2 b | 19±4 | 19±4 | 20±4 | 0.44 |
AR indicates aortic regurgitation; AS, aortic stenosis; BMI, body mass index; BSA, body surface area; ESD, end‐systolic diameter.
Aorta diameter was available for 2078 (74%) (1543 men, 535 women) of 2824 patients; thus, aneurysm % are based on those denominators. Height in meters.
ESD diameter was available for 439 (64%) (371 men, 68 women) of 690 patients with AR ≥2+; thus, ESD/BSA are based on those denominators.
Tertiary Clinical Referral Cohort Survival at Follow‐Up
There were 463 deaths (16%) after 9±6 years of follow‐up. The entire cohort's 20‐year survival was 64±2% versus an age‐ and sex‐matched US population (64% vs 72%, P<0.0001, Figure 3A). The 20‐year survival rates were 70±3% and 62±3% for women and men, respectively (P=0.79, Figure 3B). However, the 20‐year survival of men was lower than that in the general male population (62% vs 69%, relative death‐risk 1.16; 95% CI 1.05‐1.29; P=0.005, Figure 3C). A more pronounced 20‐year decreased survival was observed in women compared to the general female population (74% vs 81%, relative death‐risk 1.67; 95% CI 1.38‐2.03; P<0.0001, Figure 3D). The relative difference in risk of death between men and women was 1.44 (95% CI 1.15‐1.79; P=0.001). Multivariate analysis of baseline predictor variables was performed including age ≥50 years, Charlson comorbidity index, BMI ≥30, ejection fraction ≥50%, severe AS, AR ≥2+, aortic diameter, and known aortic coarctation. Independent predictors of mortality were age ≥50 years and Charlson comorbidity index for both sexes and ejection fraction for men only (Table 4). Standardized mortality analysis showed similar independent predictor variables for both men and women. A cutoff of 10 cm2/m for the ratio of aortic diameter cross‐sectional area by height was univariately predictive of death (relative risk 1.46; 1.04‐1.99; P=0.03) for the entire cohort, but not for each sex separately (a trend observed for men only, P=0.09). This parameter lost statistical significance when incorporated into multivariate models.
Figure 3.
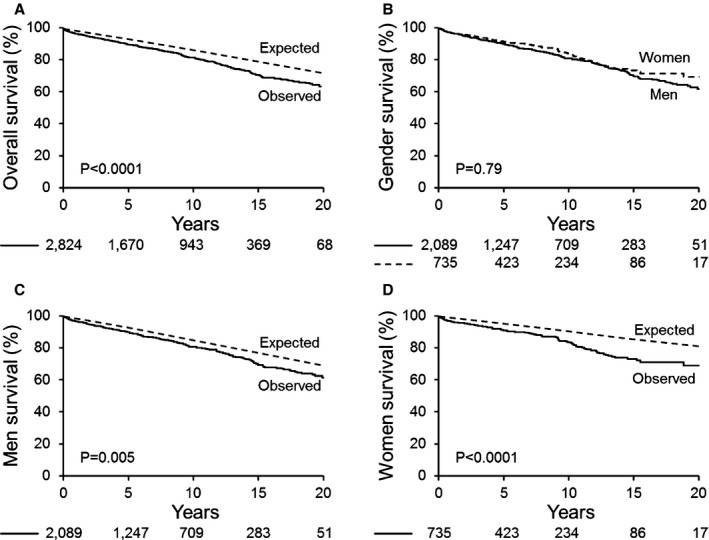
Tertiary clinical referral cohort overall and sex‐specific survival compared to the general population. A, Kaplan‐Meier 20‐year rate of survival after first‐Mayo echocardiogram for BAV in the overall cohort vs general population expected rate. B, Kaplan‐Meier 20‐year rate, direct comparison by sex. C, Kaplan‐Meier 20‐year rate of survival after first‐Mayo echocardiogram for BAV for men. D, Kaplan‐Meier 20‐year rate of survival after first‐Mayo echocardiogram for women. BAV indicates bicuspid aortic valve.
Table 4.
Independent Mortality Predictors for Entire Tertiary Clinical‐Referral Cohort by Sex
| Variable | Men | Women | ||
|---|---|---|---|---|
| Risk Ratio (95% CI) | P Value | Risk Ratio (95% CI) | P Value | |
| Age ≥50 y | 3.53 (2.37‐5.45) | <0.0001 | 2.54 (1.26‐5.34) | 0.008 |
| Charlson indexa | 1.27 (1.21‐1.34) | <0.0001 | 1.28 (1.16‐1.40) | <0.0001 |
| EF ≥50% | 0.54 (0.37‐0.81) | 0.004 | — | — |
EF indicates left ventricular ejection fraction.
Per unit change. Variables included in model: age ≥50 years, Charlson comorbidity index, BMI ≥30, ejection fraction ≥50%, severe AS, AR ≥2+, aortic diameter, and known aortic coarctation.
When the relative risk of death was analyzed as a function of age as a continuous variable, the effect of age relative to the expected survival was significantly higher for ages <45 years (P<0.0001). Therefore, we analyzed the 1010 patients <45 years old within the cohort. AR ≥2+ was more prevalent at baseline in <45‐year‐olds (18% vs 11%, P<0.0001), and the 15‐year relative death risk was increased in men (1.62; 1.16‐2.26; P=0.003) but very prominently increased in women (3.59; 2.23‐5.77; P<0.0001). Multivariate analysis of baseline predictor variables including Charlson comorbidity index, ejection fraction ≥50%, AR ≥2+, and aortic valve peak velocity was performed in <45‐year‐olds by sex. Independent predictors of mortality were Charlson comorbidity index for both sexes, ejection fraction for men only, and AR ≥2+ for women only (Table 5). When ESD/BSA was included in the multivariate analysis, AR continued to be significant for women only (P=0.01), and ESD/BSA was not significant (P=0.46). A cutoff of 10 cm2/m for the ratio of aortic diameter cross‐sectional area by height was insignificant in uni‐ and multivariable analyses.
Table 5.
Independent Mortality Predictors for Tertiary Clinical‐Referral Cohort (<45‐Year‐Olds Only) by Sex
| Variable | Men (n=698) | Women (n=312) | ||
|---|---|---|---|---|
| Risk Ratio (95% CI) | P Value | Risk Ratio (95% CI) | P Value | |
| Charlson indexa | 1.39 (1.20‐1.59) | <0.0001 | 1.44 (1.20‐1.67) | 0.0004 |
| EF ≥50% | 0.27 (0.11‐0.80) | 0.02 | — | — |
| AR ≥moderate | — | — | 3.84 (1.26‐10.8) | 0.01 |
AR indicates aortic regurgitation; EF, left ventricular ejection fraction.
Per unit change. Variables included in model: Charlson comorbidity index, ejection fraction ≥50%, AR ≥2+, and aortic valve peak velocity.
For patients ≥45 years old, the 15‐year relative death risk was not significant for men and 1.33 (1.08‐1.64, P=0.007) for women.
Survival Analysis of Initially Excluded Tertiary Clinical Referral Patients
A total of 710 patients from the tertiary clinical referral echocardiographic database were not initially analyzed because their echocardiographic aortic valve Doppler assessment was incomplete. Hence, we separately ascertained their survival by the same methods and found the same results: relative death risks for men and women of 1.87 (95% CI 1.57‐2.21) and 2.21 (95% CI 1.76‐2.78), respectively, compared to the general population (both P<0.0001).
Surgical Referral Cohort
From 1995 to 2010, 2251 consecutive BAV patients underwent AVR at our institution, of whom 461 (20%) were carryovers from the tertiary clinical referral cohort. Nine were excluded because they were redo AVRs; thus, 2242 patients remained for analysis. Follow‐up for survival was completed until death or September 2014. Mean follow‐up time was 6±5 years and not different between sexes. Age at AVR was 62±14 years, similar for both sexes (Table 6). Preoperative NYHA class 3 to 4 symptoms were present in 58% of women versus 46% of men (P<0.0001). Congruent with the community cohort, AS as cause for AVR was more common in women, and AR as cause for AVR more common in men. Congruent with the tertiary referral cohort, larger aorta diameters and more AA were present in men, but the aortic diameters corrected for patients' heights were identical between sexes (Table 6). The ratio of aortic diameter cross‐sectional area by height, although statistically different due to the large number of patients, was also clinically comparable between sexes (Table 6). However, as in the tertiary referral cohort, more men reached a cutoff of 10 cm2/m than women (21% vs 15%, respectively, P=0.006). More men also underwent aorta repair than women. As in the tertiary referral cohort, the BSA‐corrected ESD in patients with AR ≥2+ was clinically similar between the sexes.
Table 6.
STS Surgical Referral Cohort Clinical and Echocardiographic Features by sex
| Variable | Total (N=2242) | Men (N=1663) | Women (N=579) | P Value |
|---|---|---|---|---|
| Age, y | 62±14 | 62±13 | 63±14 | 0.12 |
| NYHA class III or IV, n (%) | 1099 (49) | 764 (46) | 335 (58) | <0.0001 |
| Weighted Charlson index | 1.43±1.7 | 1.48±1.7 | 1.28±1.6 | 0.01 |
| Ejection fraction, % | 59±14 | 58±14 | 62±12 | <0.0001 |
| Preop AS, n (%) | 1969 (88) | 1420 (86) | 549 (95) | <0.0001 |
| Preop severe AR, n (%) | 438 (20) | 383 (23) | 55 (10) | <0.0001 |
| Prosthesis size | 25±2.5 | 25±2.2 | 22±1.8 | <0.0001 |
| Mechanical valve | 1071 (48) | 822 (49) | 249 (43) | 0.009 |
| Coarctation, n (%) | 20 (0.9) | 14 (0.8) | 6 (1) | 0.67 |
| Aorta diameter, mma | 41±9 (n=1679) | 42±7 (n=1236) | 38±12 (n=443) | <0.0001 |
| Aorta diameter/height, mm/ma | 24±5 | 24±4 | 24±7 | 0.71 |
| Aorta cross‐sectional area by height, cm2/ma | 7.8±2.8 | 8.1±2.8 | 7.3±2.9 | <0.0001 |
| Aneurysm (≥45 mm), n (%)a | 484 (22) | 404 (24) | 80 (14) | <0.0001 |
| Concomitant ascending aorta repair, n (%) | 546 (24) | 441 (27) | 105 (18) | <0.0001 |
| Concomitant CABG, n (%) | 612 (27) | 509 (31) | 103 (18) | <0.0001 |
| Indication endocarditis | 49 (2) | 45 (3) | 4 (0.7) | 0.001 |
| ESD for AR ≥2+, mmb | 41±9 (n=234) | 42±9 (n=202) | 35±9 (n=32) | <0.0001 |
| ESD/BSA for AR ≥2+, mm/m2 b | 20±4 | 20±4 | 20±4 | 0.75 |
AR indicates aortic regurgitation; AS, aortic stenosis; CABG, coronary artery bypass surgery; ESD, end‐systolic diameter; NYHA, New York Heart Association class; Preop, preoperative.
Aorta diameter by echocardiogram was available for 1679 (75%) (1236 men, 443 women) of 2242 patients; thus, aneurysm % are based on those denominators. Height in meters.
ESD diameter was available for 234 (56%) (202 men, 32 women) of 418 patients with AR ≥2+ defined by echocardiogram; thus, ESD/BSA are based on those denominators.
Surgical Referral Cohort Survival at Follow‐Up
There were 637 deaths (28%) after 6±5 years of follow‐up. The entire cohort's 15‐year survival was 38±2% versus that in an age‐ and sex‐matched sample of the US population (38% vs 61%, P<0.0001, Figure 4A). The 15‐year survival rates were 34±4% and 39±2% for women and men, respectively (P=0.15, Figure 4B). Congruent with the tertiary clinical referral cohort, the 15‐year survival of men was significantly lower than that in the general male population (39% vs 60%, relative death risk 1.34 [95% CI 1.22‐1.47], P<0.0001, Figure 4C) and a more pronounced 15‐year decreased survival was observed for women compared to the general female population (34% vs 64%, relative death risk 1.63; 95% CI 1.40‐1.89; P<0.0001, Figure 4D). The difference in relative risk of death between sexes was 1.22 (95% CI 1.02‐1.45, P=0.026). We performed multivariate analysis of baseline predictor variables including age ≥60 years, Charlson comorbidity index, BMI ≥30, ejection fraction ≥50%, severe AS, AR4+, NYHA class 3 to 4, aortic diameter, baseline IE, and known aortic coarctation (Table 7). Independent predictors of mortality were age ≥60 years and Charlson comorbidity index for both sexes; ejection fraction ≥50% and NYHA class 3 to 4 for men only. Aortic regurgitation 4+ was associated with better survival in men and independently associated with mortality in women (Table 7). When ESD/BSA was included in the multivariate analysis for the surgical cohort, AR4+ became statistically insignificant (P=0.71), and ESD/BSA became an independent predictor (HR 1.23; 95% CI 1.1‐1.39; P=0.0004, per unit change) for women only. Independent predictors remained unchanged for men. Standardized mortality analysis showed similar independent predictor variables for men, but for women only NYHA class 3 to 4 independently predicted death (2.17; 95% CI 1.44‐3.27; P=0.0002). A cutoff of 10 cm2/m for the ratio of aortic diameter cross‐sectional area by height was statistically insignificant in univariate and multivariable analyses as a mortality predictor.
Figure 4.
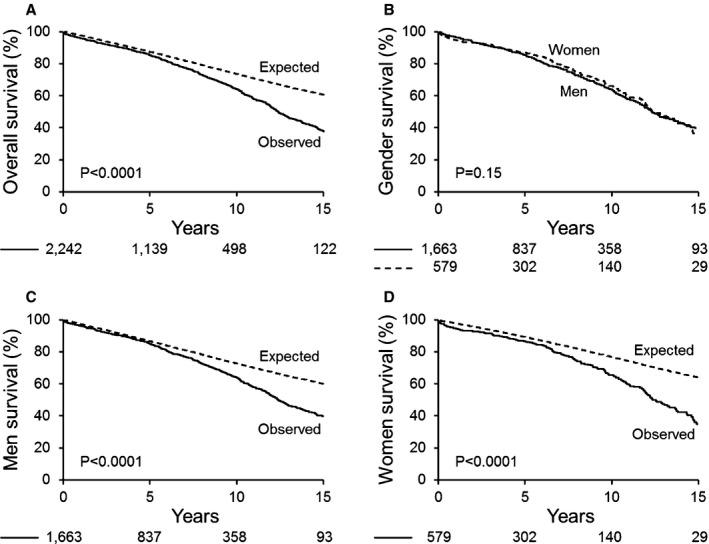
Surgical referral cohort overall and sex‐specific survival compared to the general population. A, Kaplan‐Meier 15‐year rate of survival after presurgical echocardiogram in the overall cohort vs general population expected rate. B, Kaplan‐Meier 15‐year rate of survival after presurgical echocardiogram, direct comparison by sex. C, Kaplan‐Meier 15‐year rate of survival after presurgical echocardiogram in men. D, Kaplan‐Meier 15‐year rate of survival after presurgical echocardiogram in women.
Table 7.
Independent Mortality Predictors for Entire Surgical Referral Cohort by Sexb
| Variable | Men | Women | ||
|---|---|---|---|---|
| Risk Ratio (95% CI) | P Value | Risk Ratio (95% CI) | P Value | |
| Age ≥60 y | 2.54 (1.85‐3.56) | <0.0001 | 2.99 (1.73‐5.58) | <0.0001 |
| Charlson indexa | 1.17 (1.11‐1.22) | <0.0001 | 1.12 (1.01‐1.22) | 0.02 |
| EF ≥50% | 0.68 (0.52‐0.89) | 0.006 | — | — |
| AR 4+ | 0.46 (0.22‐0.84) | 0.01 | 4.45 (1.004‐13.99) | 0.04 |
| NYHA 3 to 4 | 1.64 (1.28‐2.11) | <0.0001 | — | — |
AR indicates aortic regurgitation; EF, left ventricular ejection fraction; NYHA, New York Heart Association class.
Per unit change. Variables included in model: age ≥60 years, Charlson comorbidity index, BMI ≥30, ejection fraction ≥50%, severe AS, AR 4+, NYHA class 3 to 4, aortic diameter, baseline IE and known aortic coarctation.
When ESD/BSA was entered into the multivariate analysis, AR4+ lost statistical significance (P=0.71), and ESD/BSA became an independent predictor (HR 1.23; 95% CI 1.1‐1.39; P=0.0004 per unit change) for women only. Independent predictors remained unchanged for men.
There was no association between severe AS and NYHA 3 to 4 by sex. Conversely, NYHA 3 to 4 was present in 82% of women with AR4+ versus 33% of men (P=0.08, for a trend). The effect of age relative to the expected survival was significantly higher across all ages (P<0.0001).
Discussion
Our study examined sex‐specific morbidity and survival patterns in adults with echocardiographic diagnosis of BAV. To corroborate patterns of BAV‐related morbidity by sex and perform survival analysis in the context of BAV‐morbidity accumulation and aging, we analyzed 3 different contemporary cohorts representing evolving echocardiographic‐clinical stages of the BAV condition (Figure 5): (1) first‐time BAV echocardiographic diagnosis in the community (community cohort), where the main echocardiographic indication was abnormal auscultation; (2) first tertiary clinical referral echocardiogram (tertiary clinical referral cohort), where the principal echocardiographic indications were known BAV, known BAV dysfunction, and BAV‐related aortopathy; and (3) at the time of first AVR (surgical referral cohort), which is the most common BAV‐associated long‐term complication4 and thus inherent to the BAV condition. These 3 junctures depict initial and evolving echocardiographic‐clinical stages in BAV adults and have significant clinical and epidemiological value because they are representative of adults with echocardiographically identified BAV cared for by institutions across the United States and across the world.
Figure 5.
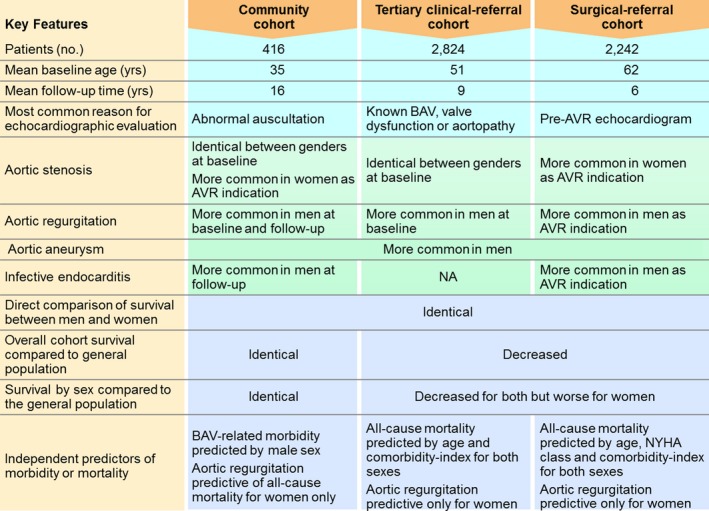
Main study findings and comparison among 3 different cohorts. AVR indicates aortic valve replacement; BAV, bicuspid aortic valve; NYHA, New York Heart Association class.
Our principal findings were these (Figure 5): (1) Sex‐specific patterns of BAV dysfunction were consistently observed across the 3 cohorts; AR was significantly more common at baseline, at echocardiographic follow‐up, and as a cause for AVR referral in men, whereas severe AS was more common for women as cause for AVR. (2) Sex‐specific BAV‐related morbidity patterns were congruent; men exhibited significantly higher risk of AR and IE in the community cohort, AR and AA in the tertiary referral cohort, and AR, AA, and IE in the surgical referral cohort. (3) Sex‐specific survival patterns were congruent; women exhibited a significantly higher relative risk of mortality than men in both the tertiary and surgical referral cohorts. (4) Aortic regurgitation ≥3+ independently predicted mortality in women in the community cohort; AR ≥2+ independently predicted mortality in <45‐year‐old women in the tertiary clinical referral cohort; and AR 4+ and NYHA class 3 to 4 were independent predictors of post‐AVR (surgical referral cohort) mortality in women.
Overall Survival in the Tertiary Referral Cohort
By virtue of including exclusively adult patients, analyzing progressing echocardiographic‐clinical junctures, and utilizing comprehensive death ascertainment, a survival disadvantage was uncovered in the tertiary and surgical referral cohorts despite low baseline Charlson comorbidity indexes19 (Tables 3 and 6). Tzemos et al3 also reported outcomes in a tertiary clinical referral cohort of 642 BAV patients. However, after 9±5 years of follow‐up, their survival was identical to that in the general population. Major baseline differences between that cohort and our tertiary clinical referral cohort are older age in our cohort (51±16 vs 35±16 years), more frequent AR in our cohort (24% vs 20%), and more frequent AA in our cohort (27% vs <20%). The difference in baseline age between our tertiary cohort and that of Tzemos et al reflects our exclusion of community patients (ie, Olmsted County) and exclusion of patients <18 years old. Indeed, Tzemos et al included patients <18 years old and excluded referred patients from outside Ontario, whereas we included all patients regardless of their referral state. Although age was independently predictive of mortality for both men and women in all cohorts, for the tertiary clinical referral cohort, the effect of age relative to expected survival was significantly higher for patients <45 years old, and women <45 years exhibited a significantly larger mortality relative risk than men (62% for men vs >200% for women), which was independently associated with AR. This enhanced effect of age for <45‐year‐olds is thus likely due to the fact that AR ≥2+ was more prevalent in <45‐year‐olds (P<0.0001). This pattern was verified in the surgical referral cohort, where the baseline age of patients presenting for AVR with AR4+ was 50±15 years versus 63±13 years for the rest (P<0.0001). Overall, these observations suggest that besides age, BAV‐associated valvular dysfunction and sex are critical determinants of overall survival in these BAV patients, such that middle‐aged patients (particularly women) exhibit an increased risk of death associated with AR.
Overall Survival in the Surgical Referral Cohort
It would seem logical to assume that a decreased survival (as compared to the general population) is expected in a cohort undergoing AVR (the surgical referral cohort). However, undergoing AVR or other cardiac surgery does not necessarily predict higher mortality as compared to the general population. This notion is exemplified in our previous Olmsted county experience, where despite a rate of 53% of AVR at 25 years of follow‐up, and a 25% rate of surgery for the aorta at 25 years, the 25‐year mortality was identical to that in the general population.4 In addition, Klodas et al20 demonstrated that the post‐AVR long‐term survival for patients with class I or II symptoms before surgery for severe aortic regurgitation (tricuspid aortic valves), was identical to that of the general population. Tribouilloy et al21 also demonstrated that the postmitral surgery survival for patients with class I or II symptoms before surgery for severe organic mitral regurgitation was identical to the general population. It may also seem attractive to hypothesize that our AVR cohort was “sicker” than other national cohorts and thus exhibited higher mortality; however, in a recent multicenter contemporary review of 54 183 patients undergoing primary AVR in the United States by Kaneko et al,22 STS‐defined NYHA class 3 to 4 was present in 60% of patients versus 49% in our surgical cohort (P<0.05). Finally, the baseline mean Charlson index was 1.43 for the surgical cohort, placing these patients at the lower spectrum of comorbidity.19 Therefore, despite having identified baseline characteristics such as age, ejection fraction, AR4+, and advanced NYHA as independent determinants of mortality for the surgical cohort (Table 7), we also hypothesize that overall accumulating BAV‐related morbidity may also contribute to that decreased survival, a proposition that needs to be studied in a BAV surgical cohort with long‐term morbidity assessment.
Sex‐Specific Morbidity
Across the 3 cohorts studied, significant AR was significantly more common in men at baseline, follow‐up, and at AVR referral. Conversely, AS was more common in women only at AVR referral, as recently observed.8 Studies have identified an aortovalvular phenotype (“root phenotype”) characterized by AR, root dilatation, and male predominance.6, 23, 24 In addition, men have larger absolute aortic root diameters, which could render aortic valves insufficient.
The community cohort total BAV‐related morbidity was excessive in men versus women (Figure 2A), driven by statistically significant excess of AVR and IE in men. Congruently, IE was also significantly greater in men undergoing AVR in the surgical referral cohort (Table 6). This is a critical finding because IE has a higher incidence than aortic dissection in BAV patients7; thus, we show this higher incidence to be driven by its occurrence in men.
Aneurysm formation and aorta surgery were greater in men but did not reach statistical significance in the community cohort. Notwithstanding, tertiary referral and surgical referral cohorts showed statistically significant excess AA formation in men (Tables 3 and 6), and more men underwent concomitant elective aorta repair within the surgical referral group. This is likely related to men reaching accepted elective aorta repair absolute diameter cutoffs (ie, 45 mm) at the time of AVR,25 although surprisingly, aortic diameters corrected for height were clinically similar between sexes.
Sex‐Specific Survival
Compared to the general population, both men and women exhibited decreased survival within evolving echocardiographic‐clinical junctures, but the relative death risk was ~40% higher for women than men in the tertiary clinical referral cohort and 20% higher for women in the surgical referral cohort, which is incongruent with the fact that men incur significantly higher AR, AA, and IE, and the baseline Charlson comorbidity index was higher in men than women in the surgical referral cohort (Table 6). This paradox is reflected in the 3 cohorts showing no difference in mortality when women and men are directly compared (Figures 2B, 3B, and 4B), as men would be expected to die more. Our study supports this mortality paradox being at least partially related to AR in women, as shown across the 3 cohorts, where AR was independently associated with mortality in women only. When referring patients with severe AR for AVR based on absolute ESD cutoffs,25 men will have larger absolute diameters because they also have larger BSA and thus will likely be referred for surgical correction earlier. However, in the tertiary and surgical referral cohorts, ESD/BSA were identical between men and women with significant AR (Tables 3 and 6), suggesting the same degree of left ventricular dilatation in both sexes. Remarkably, AR 4+ was a “protective” factor for men in the surgical referral cohort but independently predicted mortality in women (Table 7). Timely ESD‐guided surgical referral for AVR in men with significant AR, despite contributing to their excess morbidity, may be responsible for their “less severe” mortality, as their AVR becomes a preventative strategy. Klodas et al20 demonstrated that the post‐AVR long‐term survival for pure AR patients with class I or II symptoms was identical to that for the general population, as compared to patients with NYHA class 3 to 4 symptoms. Indeed, women had attained advanced NYHA symptoms more frequently than men at the time of surgical referral (Table 6), and NYHA class 3 to 4 was the single factor independently associated with mortality in women by standardized mortality analysis. In addition, when ESD/BSA was added to the multivariate analysis for the surgical cohort, ESD/BSA became an independent death predictor (P=0.0004) for women only. Furthermore, there was a trend (P=0.08) for women with AR4+ to present with NYHA 3 to 4 symptoms when compared to men. In fact, higher mortality in women with AR (versus men with AR) has been previously reported for patients with tricuspid aortic valves26 but never before in BAV patients. Finally, we hypothesize that the higher absolute aorta diameters in men, which are clinically similar to those in women after correction for height (Tables 3 and 6), could lead to earlier referral for elective aorta repair in men, potentially resulting in excess fatal aorta complications (ie, dissection) in women, a hypothesis that needs to be studied. Nonetheless, in the large cohorts analyzed in this study, the proposed cutoff (10 cm2/m)15 for the ratio of aortic diameter cross‐sectional area by height, was attained more frequently by men and not statistically predictive of worse survival for either sex.
Limitations
Despite the large size of our 3 cohorts and extended follow‐up, the study design for all cohorts was retrospective, with its inherent limitations. However, retrospective identification of patients constitutes the only feasible method to assess long‐term outcomes in BAV,4, 6 as prospective long‐term follow‐up of large BAV cohorts is unrealistic due to financial costs and the duration over multiple decades to attain outcomes. The echocardiographic follow‐up of the community cohort (Table 2) and some baseline echocardiographic features for the tertiary and surgical referral cohorts (Tables 3 and 6) were not complete; however, sex patterns of valvular dysfunction and aorta dilatation observed in the community and tertiary referral cohorts were similar, and the surgical referral cohort confirmed these patterns reflected in congruent surgical indications for AVR and aorta surgery by sex. Specific BAV phenotype analysis was not available for the tertiary and surgical referral cohorts. Subjective biases of referring physicians cannot be accounted for and could be partially responsible for the observed sex differences in AVR‐referral patterns. Although we estimate an accuracy of mortality ascertainment beyond 90%, the mortality differences between the general population and the BAV cohorts studied could still be underestimated.
We were not able to ascertain aortic dissection events in the tertiary and surgical cohorts; thus, sex differences regarding this important outcome remain unknown. We reported all‐cause mortality (instead of cardiac death) for all cohorts, but this may be an advantage rather than a limitation given the inconsistencies and biases observed in cause‐of‐death data derived from death certificates27 in retrospective studies.
Conclusions
We reported sex differences in morbidity and survival patterns throughout evolving echocardiographic‐clinical stages in adults with BAV. Compared to women, men exhibited a higher risk of BAV‐related morbidity including AR, IE, and aneurysm formation. Aortic regurgitation was significantly more common in men at all junctures but independently predicted mortality in all cohorts for women only. Overall, the long‐term survival of adults with echocardiographic diagnosis of BAV was lower than expected within evolving echocardiographic‐clinical stages. This survival limitation was more prominent in women and was associated with AR as well as NYHA at the time of AVR. This new evidence should prompt early referral of BAV adults to specialized care with careful adherence to appropriate follow‐up and referral guidelines for valvular intervention, with special attention to women with AR in whom careful symptom assessment, prevention of advanced NYHA class attainment, as well as ESD correction by BSA seem critical. Research on the genetics and biology of male‐predominant regurgitant phenotypes as well as mechanisms and prevention of IE is warranted. Research on the clinical significance of aortic diameters corrected by anthropometric parameters (ie, height) is warranted.
Disclosures
None.
Acknowledgments
The authors wish to thank David J. Huschka for his contributions in electronic research application solutions.
(J Am Heart Assoc. 2016;5:e004211 doi: 10.1161/JAHA.116.004211)
References
- 1. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 2. Michelena HI, Desjardins VA, Avierinos JF, Russo A, Nkomo VT, Sundt TM, Pellikka PA, Tajik AJ, Enriquez‐Sarano M. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117:2776–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, Webb GD, Siu SC. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300:1317–1325. [DOI] [PubMed] [Google Scholar]
- 4. Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, Eidem B, Edwards WD, Sundt TM 3rd, Enriquez‐Sarano M. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. [DOI] [PubMed] [Google Scholar]
- 5. Tribouilloy C, Rusinaru D, Sorel C, Thuny F, Casalta JP, Riberi A, Jeu A, Gouriet F, Collart F, Caus T, Raoult D, Habib G. Clinical characteristics and outcome of infective endocarditis in adults with bicuspid aortic valves: a multicentre observational study. Heart. 2010;96:1723–1729. [DOI] [PubMed] [Google Scholar]
- 6. Michelena HI, Prakash SK, Della Corte A, Bissell MM, Anavekar N, Mathieu P, Bosse Y, Limongelli G, Bossone E, Benson DW, Lancellotti P, Isselbacher EM, Enriquez‐Sarano M, Sundt TM 3rd, Pibarot P, Evangelista A, Milewicz DM, Body SC. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the international Bicuspid Aortic Valve C onsortium (BAVCon). Circulation. 2014;129:2691–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michelena HI, Katan O, Suri RM, Baddour LM, Enriquez‐Sarano M. Incidence of infective endocarditis in patients with bicuspid aortic valves in the community. Mayo Clin Proc. 2016;91:122–123. [DOI] [PubMed] [Google Scholar]
- 8. Andrei AC, Yadlapati A, Malaisrie SC, Puthumana JJ, Li Z, Rigolin VH, Mendelson M, Clennon C, Kruse J, Fedak PW, Thomas JD, Higgins JA, Rinewalt D, Bonow RO, McCarthy PM. Comparison of outcomes and presentation in men‐versus‐women with bicuspid aortic valves undergoing aortic valve replacement. Am J Cardiol. 2015;116:250–255. [DOI] [PubMed] [Google Scholar]
- 9. Melton LJ 3rd. History of the Rochester epidemiology project. Mayo Clin Proc. 1996;71:266–274. [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 11. Perry GJ, Helmcke F, Nanda NC, Byard C, Soto B. Evaluation of aortic insufficiency by Doppler color flow mapping. J Am Coll Cardiol. 1987;9:952–959. [DOI] [PubMed] [Google Scholar]
- 12. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1–25. [DOI] [PubMed] [Google Scholar]
- 13. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 14. Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross‐sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg. 2003;126:892–893. [DOI] [PubMed] [Google Scholar]
- 15. Svensson LG, Kim KH, Blackstone EH, Rajeswaran J, Gillinov AM, Mihaljevic T, Griffin BP, Grimm R, Stewart WJ, Hammer DF, Lytle BW. Bicuspid aortic valve surgery with proactive ascending aorta repair. J Thorac Cardiovasc Surg. 2011;142:622–629, 629.e1‐3. [DOI] [PubMed] [Google Scholar]
- 16. Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12:462–468. [DOI] [PubMed] [Google Scholar]
- 17. Coady MA, Rizzo JA, Hammond GL, Mandapati D, Darr U, Kopf GS, Elefteriades JA. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113:476–491; discussion 489‐491 [DOI] [PubMed] [Google Scholar]
- 18. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. [DOI] [PubMed] [Google Scholar]
- 19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 20. Klodas E, Enriquez‐Sarano M, Tajik AJ, Mullany CJ, Bailey KR, Seward JB. Optimizing timing of surgical correction in patients with severe aortic regurgitation: role of symptoms. J Am Coll Cardiol. 1997;30:746–752. [DOI] [PubMed] [Google Scholar]
- 21. Tribouilloy CM, Enriquez‐Sarano M, Schaff HV, Orszulak TA, Bailey KR, Tajik AJ, Frye RL. Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications. Circulation. 1999;99:400–405. [DOI] [PubMed] [Google Scholar]
- 22. Kaneko T, Vassileva CM, Englum B, Kim S, Yammine M, Brennan M, Suri RM, Thourani VH, Jacobs JP, Aranki S. Contemporary outcomes of repeat aortic valve replacement: a benchmark for transcatheter valve‐in‐valve procedures. Ann Thorac Surg. 2015;100:1298–1304; discussion 1304 [DOI] [PubMed] [Google Scholar]
- 23. Della Corte A, Bancone C, Buonocore M, Dialetto G, Covino FE, Manduca S, Scognamiglio G, D'Oria V, De Feo M. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovasc Imaging. 2013;6:1301–1310. [DOI] [PubMed] [Google Scholar]
- 24. Detaint D, Michelena HI, Nkomo VT, Vahanian A, Jondeau G, Sarano ME. Aortic dilatation patterns and rates in adults with bicuspid aortic valves: a comparative study with Marfan syndrome and degenerative aortopathy. Heart. 2014;100:126–134. [DOI] [PubMed] [Google Scholar]
- 25. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 26. Klodas E, Enriquez‐Sarano M, Tajik AJ, Mullany CJ, Bailey KR, Seward JB. Surgery for aortic regurgitation in women. Contrasting indications and outcomes compared with men. Circulation. 1996;94:2472–2478. [DOI] [PubMed] [Google Scholar]
- 27. Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol. 1999;34:618–620. [DOI] [PubMed] [Google Scholar]


