Abstract
Background
Lifestyle modifications are first‐line measures for cardiovascular disease prevention. Whether lifestyle intervention also preserves cardiovascular health is less clear. Our study examined the role of a Health Partner–administered lifestyle intervention on metrics of ideal cardiovascular health.
Methods and Results
A total of 711 university employees (48±11 years; 66% women, 72% Caucasian/22.5% African Americans) enrolled in a program that promoted healthier lifestyles at Emory University (Atlanta, GA). Anthropometric, laboratory, and physical activity measurements were performed at baseline and at 6 months, 1 year, and 2 years of follow‐up. Results were utilized by the Health Partner to generate a personalized plan aimed at meeting ideal health metrics. Compared to baseline, at each of the 6‐month, 1‐year, and 2‐year follow‐up visits, systolic blood pressure was lower by 3.6, 4.6, and 3.3 mm Hg (P<0.001), total cholesterol decreased by 5.3, 6.5, and 6.4 mg/dL (P<0.001), body mass index declined by 0.33, 0.45, and 0.38 kg/m2 (P<0.001), and the percentage of smokers decreased by 1.3%, 3.5%, and 3.5% (P<0.01), respectively. Changes were greater in those with greater abnormalities at baseline. Finally, the American Heart Association “Life's Simple 7” ideal cardiovascular health score increased by 0.28, 0.40, and 0.33 at 6 month, 1 year, and 2 years, respectively, compared to baseline visit.
Conclusions
A personalized, goal‐directed Health Partner intervention significantly improved the cardiometabolic risk profile and metrics of cardiovascular health. These effects were evident at 6 months following enrollment and were sustained for 2 years. Whether the Health Partner intervention improves long‐term morbidity and mortality and is cost‐effective needs further investigation.
Keywords: cardiovascular risk, health education, health partner, lifestyle, prevention
Subject Categories: Exercise, Cardiovascular Disease
Cardiovascular disease (CVD) afflicts nearly two thirds of US adults, and much of its premature morbidity and mortality is related to modifiable risk factors. Lifestyle modifications of dietary and exercise habits, weight management, and smoking cessation are primary goals for prevention of CVD, and even modest behavioral changes may significantly improve outcomes of established disease.1 However, because the effects of lifestyle changes accumulate over time, sustained adherence is key in accrual of CVD‐related benefits. Nevertheless, few subjects are able to achieve these goals. To this effect, various lifestyle and nonpharmacological interventions have been examined in cardiac and noncardiac patient populations and have used cognitive‐behavioral strategies, including motivational interviewing, goal setting, continued support, promotion of clinical self‐knowledge and efficacy, as well as self‐monitoring and management.2, 3, 4 These measures have been delivered by health care providers, computer programs, or online interfaces, predominantly in patients with established CVD or its risk factors.5, 6, 7, 8, 9, 10, 11
Effectiveness of these approaches with significant reductions in weight, blood pressure, and blood lipid concentrations, as well as improvements in physical activity and medication compliance have been reported.2 However, the relatively short duration of these studies precludes assessment of long‐term adherence to lifestyle changes, or whether these translate into gains in morbidity or mortality. Indeed, a study of over 5000 diabetics reported no benefits in CVD mortality with an intensive lifestyle intervention after a median follow‐up of nearly 10 years.12 In addition, the role of primordial and preventative lifestyle interventions aimed at preserving cardiovascular health are also understudied. For example, studies examining the effectiveness of lifestyle interventions directed at meeting the metrics of “ideal cardiovascular health” related to physical activity, body mass index (BMI), total cholesterol, fasting glucose, and smoking habits are lacking.2
To address these questions, the Emory University/Georgia Tech Predictive Health Institute (Atlanta, CA) established a novel program utilizing counseling delivered by a trained Health Partner (HP) as part of the Center for Health Discovery and Well Being (CHDWB). Here, we report on the effects of the HP‐administered lifestyle intervention on cardiovascular risk profile and metrics of ideal cardiovascular health in CHDWB participants over a 2‐year period. We hypothesized that the HP intervention will favorably affect the subjects’ risk profile and subclinical markers of CVD.
Methods
Subjects
Subjects were recruited into the CHDWB program in Atlanta, Georgia. Recruited subjects were selected from a random sample of employees of Emory University and Georgia Institute of Technology.13 The CHDWB utilized the human resources department to identify university employees who were employed for at least 2 years and covered by the university sponsored health insurance plans. A list of ≈10 000 employees was generated, and every 10th employee was sent an invitation e‐mail to participate. Around 30% of solicited employees agreed to be contacted for screening, and ≈10% were ultimately enrolled in the cohort.14 Subjects with an acute illness, recent hospitalization within the past year, pregnant women, and individuals with poorly controlled medical conditions were excluded, and subjects signed an informed consent that was approved by the Emory and Georgia Tech institutional review boards.15, 16
Subjects were followed with comprehensive evaluations at baseline, 6‐month, 1‐year, and 2‐year visits. Physical measurements included vital signs, height, weight, and waist‐hip ratio (WHR). Blood samples were collected for a complete serum metabolic panel and lipid profile. Participants completed questionnaires to obtain detailed information about dietary intake and physical activity.
Cardiovascular Risk Factors
Hypertension, hypercholesterolemia, and diabetes mellitus were defined according to the Joint National Committee, Adult Treatment Panel III, and American Diabetes Association criteria, respectively, and smoking habits were recorded.17, 18, 19 Ten‐year risks for coronary death or nonfatal myocardial infarction were estimated by the Framingham risk score (FRS), atherosclerotic cardiovascular disease (ASCVD) in adults.20, 21
Ideal Cardiovascular Health
The American Heart Association (AHA) “Life's Simple 7” (LS7) includes 7 modifiable behaviors and biological factors that represent the degree to which an individual's health behavior is in accord with ideal cardiovascular health.22 Specifically, an ideal cardiovascular health profile by AHA LS7 involves ideal physical activity (≥150 or 75 minutes/week of moderate‐ or vigorous‐intensity exercise, respectively), total cholesterol (<200 mg/dL), blood pressure (<120/80 mm Hg), fasting glucose (<100 mg/dL), BMI (<25 kg/m2), smoking (never smoker or quit >1 year ago), as well as diet score of 4 to 5.22 Each LS7 component was categorized as being poor, intermediate, or ideal by assigning 2, 1, and 0 points, respectively, and a composite LS7 score ranging between 0 and 14 was summed (Table 1).
Table 1.
Definitions of Subgroups for Each LS7 Metric Based on Participants’ Baseline Measurements
| Measure | Normal/Ideal | Borderline | Abnormal/Not Ideal |
|---|---|---|---|
| Systolic blood pressure, mm Hg | <120 | 120 to 140 | >140 |
| Diastolic blood pressure, mm Hg | <80 | 80 to 90 | >90 |
| Total cholesterol, mg/dL | <200 | 200 to 240 | >240 |
| High‐density lipoprotein, mg/dL | >60 |
40 to 60 (men) 50 to 60 (women) |
<40 (men) <50 (women) |
| Low‐density lipoprotein, mg/dL | <100 | 100 to 130 | >130 |
| Triglycerides, mg/dL | <150 | 150 to 200 | >200 |
| Fasting glucose, mg/dL | <100 | 100 to 126 | >126 |
| Insulin sensitivity index | ≥0.31 | NA | <0.31 |
| Body mass index, kg/m2 | 18 to 25 | 25 to 30 | >30 |
| Waist‐hip ratio |
≤0.9 (men) ≤0.85 (women) |
NA |
>0.9 (men) >0.85 (women) |
| Healthy diet score (0–5 components) | 4 to 5 | 2 to 3 | 0 to 1 |
| Moderate/vigorous physical activity, h/week | <9.5 | 9.5 to 31 | >31 |
LS7 indicates Life's Simple 7; NA indicates not applicable.
Insulin Sensitivity
Fasting insulin and glucose levels were used to calculate the quantitative insulin sensitivity check index (QUICKI). We assessed the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model.23
Physical Activity
Selected items from the Cross‐Cultural Activity Participation typical week physical activity survey were used to determine whether subjects met the 2008 Physical Activity Guideline for Americans, which is similar to AHA LS7 ideal level of physical exertion for achieving ideal cardiovascular health.22, 24
Dietary Intake
The 2005 Block Food Frequency Questionnaire (FFQ) was used to assess dietary intake during the past year.25 Using the FFQ summary data, we created a healthy diet score (HDS) for each participant. The HDS is the sum of 5 nutritional components according to AHA's diet recommendations.26 The HDS ranges from 0 to 5, with higher scores being healthier diet.
The HP Intervention
The Emory University/Georgia Tech Predictive Health Institute established a novel academic program in 2008 as the CHDWB at Emory University. CHDWB extensively phenotyped actively working “generally healthy” individuals and provided personalized, preventive care based on the serial comprehensive longitudinal data collected. Continued counseling was delivered by a trained HP focused on promoting clinical self‐knowledge and adoption of a healthier lifestyle. Major goals of this program included evaluating adherence to positive changes and whether this results in long‐term clinical and economic effectiveness, as well as the impact on cardiovascular health of the HP‐administered intervention.
HPs were required to have a minimum of a bachelor's degree in a health science or related field. A broad range of backgrounds and skills were also required to address the integrated complex issues of health definition and maintenance including a basic understanding of human biology. Each individual HP was trained by CHDWB to be the primary consultative and interpretive contact for each participant. Specifically, the HP was the primary contact for long‐term identification and maintenance of health issues. The comprehensive training of HP was performed at the CHDWB, which included several areas in predictive health history, goals, customer relations, basic physiology, test/survey results, lab safety, and institutional review board consent process. Involvement of the HP with the subjects in chronic disease management were driven by the constraints of medical care to educate, engage, or motivate the patients for optimal outcomes. The HP did not provide medical care, and participants needing medical care were referred to their primary physicians for further interventions. Further training on behavioral therapies were provided, including empathetic and active listening, motivational interviewing, collaborative goal setting, goal‐directed problem solving, and elements of coaching, mentoring, and supportive engagement. Further details on the role of the HP have been previously described.14
At the baseline visit, each subject was assigned 1 of 6 HPs, individuals who were specifically trained to utilize subjects’ data profiles and collaboratively generate a health goal and personalized action plan at each visit. The health action plans were self‐generated by subjects and included strategies aimed at improving metrics related to physical activity, body weight, cholesterol, fasting glucose, stress reduction, dietary patterns, and smoking habits. The HP advised subjects on specific tactical approaches for reaching their goals, and subjects were offered HP interim support in the form of weekly to monthly e‐mail or phone contact. Subjects met with their HP after each visit, and the action plan was recalibrated based on review of data and overall progress. The HP remained in contact with the participant by e‐mail or telephone at intervals according to the formulated action plan on the initial visit.
Clinical, psychological, anthropometric, dietary, laboratory, and vascular function profiles were generated for each participant at every visit and compared to those derived from a previously described “super healthy” cohort, selected for the absence of cardiovascular risk factors, diagnosed CVD, and absence of medications or supplements.27 Whenever a study measurement revealed an abnormality of clinical relevance (eg, elevated blood pressure, lipids, body weight, or BMI), the HP encouraged subjects to take appropriate action, including seeking medical attention.
Subjects were followed with comprehensive evaluations at baseline, 6‐month, 1‐year, and 2‐year visits. Physical measurements included vital signs, height, weight, and WHR. Blood samples were collected for a complete serum metabolic panel and lipid profile. Participants completed questionnaires to obtain detailed information about dietary intake and physical activity.
Statistical Analysis
Baseline characteristics for 711 participants were summarized as means±SD for continuous variables or as counts and proportions for categorical variables. Of 711 participants, 609 (86%) had at least 1 follow‐up visit. We compared characteristics (sex, race, hypertension, diabetes mellitus, and AHA LS7 measures) of participants with follow‐up data with those without using t tests for continuous variables and chi‐square tests for categorical variables. No significant differences were found. We assumed missing data were “missing at random.”
To investigate the effect of the HP intervention on LS7 measures, a linear mixed model was utilized for continuous variables, including systolic blood pressure (SBP), diastolic blood pressure (DBP), QUICKI, BMI, WHR, physical activity level (log transformed), HDS, fasting blood glucose concentration (log transformed), and total cholesterol, high‐density lipoprotein (HDL), low‐density lipoprotein (LDL), and triglycerides. A generalized linear mixed model was used to analyze binary data, including smoking (yes/no), and the improvement in HDS (yes/no). In all models, visit (baseline, 6 months, 1 year, and 2 years) was included as the independent variable. Subject‐specific random intercept was incorporated in the model to account for within‐subject correlation.
Given that the FRS and ASCVD risk scores increase with age, we compared the observed trajectory of the scores (intervention group) with a “theoretical” trajectory (control group) within subjects. The subject‐specific “theoretical” FRS/ASCVD at each follow‐up visit was computed by baseline risk factors and age at the particular time point. FRS/ASCVD was regressed on visit and visit‐by‐group interaction using linear mixed models with random intercepts.
To further examine whether changes depend on participants’ LS7 measures at baseline, participants were categorized into 2 to 3 groups based on clinical cutoffs or medians (for FRS, ASCVD, and HDS) at baseline. Group‐by‐visit interactions were considered. All analyses were performed using SAS software (version 9.3; SAS Institute Inc., Cary, NC). P≤0.05 was deemed statistical significance.
Results
Baseline Characteristics
A total of 711 employees were enrolled into the CHDWB with a mean age of 48±11 years, of which 66% were female, 72% were Caucasian, and 23% were African American. Prevalence of diabetes mellitus, hypertension, and smoking were 11%, 34%, and 5%, respectively. Median FRS and ASCVD risk scores were 5.5% and 2.6%, respectively (Table 2.
Table 2.
Demographic Characteristics
| Total (N=711) | |
|---|---|
| Age | 48.5 (11.1) |
| Male (%) | 245 (35) |
| Race (%) | |
| Caucasian | 512 (72) |
| African American | 160 (23) |
| Other | 39 (6) |
| College degree and above (%) | 581 (82) |
| Marital status (%) | |
| Single | 165 (23) |
| Married/partnered | 449 (63) |
| Divorced/widowed | 97 (14) |
| Body mass index (%) | |
| Normal (18.5–24.9) | 246 (35) |
| Overweight (25.0–29.9) | 259 (36) |
| Obese (30.0 and above) | 200 (28) |
| Smoking (%) | 39 (5) |
| Diabetes mellitus (%) | 77 (11) |
| Hypertension (%) | 239 (34) |
| Framingham risk score (%) | 5.5 (2.5, 9.8) |
| ASCVD risk score (%) | 2.6 (1.0, 5.8) |
| Life's Simple 7 score | 7.9 (1.9) |
ASCVD indicates atherosclerotic cardiovascular disease.
Mean (SD), or median (first quartile, third quartile), and counts (%) for continuous and categorical variables, respectively.
Changes in Cardiovascular Risk Factors
A total of 521, 498, and 426 participants returned for follow‐up visit at 6 months, 1 year, and 2 years. Table 3 shows the estimated mean changes in LS7 measures comparing each follow‐up visit to the baseline visit for each subgroup according to their baseline status (Tables 3 and 4).
Table 3.
Estimated Mean Differences in Study Variables Compared to Baseline
| Measure | Effect | Normal/Ideal | Borderline | Abnormal | All | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ∆ | SE | P Value | ∆ | SE | P Value | ∆ | SE | P Value | ∆ | SE | P Value | ||
| Systolic blood pressure, mm Hg | 6 months | 0.57 | 0.65 | 0.38 | −5.63 | 0.75 | <0.0001a | −13.37 | 1.37 | <0.0001a | −3.55 | 0.50 | <0.0001a |
| 1 year | −0.36 | 0.66 | 0.58 | −7.19 | 0.76 | <0.0001a | −13.43 | 1.33 | <0.0001a | −4.62 | 0.50 | <0.0001a | |
| 2 years | 1.25 | 0.69 | 0.07 | −4.81 | 0.79 | <0.0001a | −17.32 | 1.50 | <0.0001a | −3.33 | 0.53 | <0.0001a | |
| Diastolic blood pressure, mm Hg | 6 months | 0.33 | 0.42 | 0.42 | −5.24 | 0.67 | <0.0001a | −9.24 | 1.00 | <0.0001a | −2.15 | 0.36 | <0.0001a |
| 1 year | 0.04 | 0.42 | 0.93 | −5.38 | 0.69 | <0.0001a | −7.21 | 0.98 | <0.0001a | −2.18 | 0.36 | <0.0001a | |
| 2 years | 2.10 | 0.44 | <0.0001a | −4.28 | 0.73 | <0.0001a | −8.98 | 1.10 | <0.0001a | −0.79 | 0.38 | 0.04a | |
| Total cholesterol, mg/dL | 6 months | 0.99 | 1.63 | 0.54 | −10.52 | 2.04 | <0.0001a | −24.01 | 3.73 | <0.0001a | −5.27 | 1.27 | <0.0001a |
| 1 year | −0.21 | 1.41 | 0.88 | −11.16 | 1.82 | <0.0001a | −28.45 | 3.42 | <0.0001a | −6.54 | 1.12 | <0.0001a | |
| 2 years | −0.19 | 1.50 | 0.90 | −11.60 | 1.93 | <0.0001a | −22.95 | 3.59 | <0.0001a | −6.38 | 1.19 | <0.0001a | |
| High‐density lipoprotein, mg/dL | 6 months | −3.33 | 0.56 | <0.0001a | −0.25 | 0.76 | 0.75 | 2.41 | 1.18 | 0.04a | −1.52 | 0.43 | 0.001a |
| 1 year | −3.68 | 0.51 | <0.0001a | 0.60 | 0.63 | 0.34 | 2.71 | 1.03 | 0.01a | −1.41 | 0.38 | <0.001a | |
| 2 years | −4.32 | 0.53 | <0.0001a | −0.06 | 0.67 | 0.93 | 1.35 | 1.11 | 0.22 | −2.19 | 0.40 | <0.0001a | |
| Low‐density lipoprotein, mg/dL | 6 months | 3.44 | 1.64 | 0.04a | −2.33 | 1.85 | 0.21 | −17.16 | 1.87 | <0.0001a | −4.13 | 1.10 | <0.001 |
| 1 year | 1.74 | 1.45 | 0.23 | −3.54 | 1.56 | 0.02 | −17.12 | 1.71 | <0.0001a | −5.15 | 0.97 | <0.0001a | |
| 2 years | 5.02 | 1.54 | 0.001a | −3.43 | 1.65 | 0.04 | −16.44 | 1.80 | <0.0001a | −3.75 | 1.03 | <0.001 | |
| Triglycerides, mg/dL | 6 months | 3.37 | 2.27 | 0.14 | −1.11 | 6.59 | 0.87 | −49.47 | 8.77 | <0.0001a | −0.69 | 2.26 | 0.76 |
| 1 year | 6.38 | 2.01 | 0.002a | −15.76 | 5.90 | 0.01a | −75.01 | 7.02 | <0.0001a | −0.69 | 1.99 | 0.73 | |
| 2 years | 3.98 | 2.13 | 0.06 | −14.47 | 6.18 | 0.02a | −84.90 | 7.61 | <0.0001a | −3.18 | 2.11 | 0.13 | |
| Fasting glucose, % | 6 months | 1.94 | 0.67 | 0.003a | −7.63 | 1.79 | <0.0001a | −19.39 | 3.08 | <0.0001a | 0.40 | 0.65 | 0.54 |
| 1 year | 1.64 | 0.58 | 0.004a | −6.67 | 1.67 | <0.0001a | −16.99 | 3.18 | <0.0001a | 0.27 | 0.57 | 0.63 | |
| 2 years | 2.25 | 0.62 | <0.001a | −5.52 | 1.87 | 0.004a | −9.24 | 3.62 | 0.01a | 1.09 | 0.61 | 0.07 | |
| Quantitative insulin sensitivity check index (QUICKI) | 6 months | 0.02 | 0.02 | 0.15 | 0.00 | 0.00 | 0.52 | −0.001 | 0.003 | 0.79 | |||
| 1 year | 0.03 | 0.01 | 0.05a | 0.00 | 0.00 | 0.16 | 0.005 | 0.003 | 0.06 | ||||
| 2 years | 0.04 | 0.01 | 0.002a | 0.01 | 0.00 | 0.02a | 0.008 | 0.003 | 0.003a | ||||
| Body mass index, kg/m2 | 6 months | −0.08 | 0.11 | 0.47 | −0.32 | 0.11 | 0.003a | −0.66 | 0.13 | <0.0001a | −0.33 | 0.07 | <0.0001a |
| 1 year | −0.18 | 0.11 | 0.11 | −0.34 | 0.11 | 0.002a | −0.95 | 0.13 | <0.0001a | −0.45 | 0.07 | <0.0001a | |
| 2 years | −0.11 | 0.12 | 0.39 | −0.25 | 0.12 | 0.03a | −0.92 | 0.14 | <0.0001a | −0.38 | 0.07 | <0.0001a | |
| Waist‐hip ratio | 6 months | −0.004 | 0.002 | 0.05a | −0.03 | 0.00 | <0.0001a | −0.009 | 0.002 | <0.0001a | |||
| 1 year | −0.004 | 0.002 | 0.06 | −0.03 | 0.00 | <0.0001a | −0.010 | 0.002 | <0.0001a | ||||
| 2 years | 0.002 | 0.002 | 0.52 | −0.03 | 0.00 | <0.0001a | −0.006 | 0.002 | 0.004a | ||||
| Healthy diet score | 6 months | −0.87 | 0.07 | <0.0001a | 0.46 | 0.05 | <0.0001a | 0.06 | 0.04 | 0.15 | |||
| 1 year | −0.85 | 0.07 | <0.0001a | 0.44 | 0.05 | <0.0001a | 0.04 | 0.04 | 0.28 | ||||
| 2 years | −0.83 | 0.08 | <0.0001a | 0.48 | 0.05 | <0.0001a | 0.09 | 0.04 | 0.03a | ||||
| Moderate and vigorous activity, % | 6 months | −27.42 | 4.24 | <0.0001a | 4.02 | 4.04 | 0.29 | 59.00 | 9.17 | <0.0001a | 6.12 | 3.19 | 0.04 |
| 1 year | −22.93 | 4.57 | <0.0001a | 0.03 | 3.88 | 0.99 | 56.80 | 9.16 | <0.0001a | 4.42 | 3.16 | 0.14 | |
| 2 years | −29.64 | 4.58 | <0.0001a | −1.38 | 4.00 | 0.72 | 63.67 | 10.20 | <0.0001a | 2.81 | 3.31 | 0.37 | |
| AHA LS7 scoreb | 6 months | 0.13 | 0.06 | 0.02a | 0.83 | 0.12 | <0.0001a | 0.28 | 0.05 | <0.0001a | |||
| 1 year | 0.23 | 0.06 | <0.0001a | 1.04 | 0.12 | <0.0001a | 0.40 | 0.05 | <0.0001a | ||||
| 2 years | 0.15 | 0.06 | 0.01a | 0.99 | 0.13 | <0.0001a | 0.33 | 0.06 | <0.0001a | ||||
∆ indicates estimated change compared to baseline visit; AHA, American Heart Association; LS7, Life's Simple 7.
P values indicate significant changes after controlling false discovery rate at 0.05 level.
Borderline: AHA LS7 score between 7 and 12; abnormal: AHA LS7 score less than 7.
Table 4.
Changes in Descriptive Statistics of Study Variables Over Time Depending on Baseline Status
| Baseline | 6 Months | 1 Year | 2 Years | |
|---|---|---|---|---|
| Normal (or ideal) at baseline | ||||
| Systolic blood pressure, mm Hg | 108.10±7.70 | 108.83±10.47 | 107.96±10.17 | 109.86±10.58 |
| Diastolic blood pressure, mm Hg | 69.78±6.82 | 70.11±8.19 | 70.08±9.05 | 72.39±8.52 |
| Total cholesterol, mg/dL | 169.25±20.93 | 170.88±25.38 | 168.87±25.48 | 169.42±25.85 |
| High‐density lipoprotein, mg/dL | 76.88±13.30 | 73.29±15.06 | 73.20±15.34 | 72.92±14.89 |
| Low‐density lipoprotein, mg/dL | 79.75±14.55 | 83.07±19.65 | 81.37±19.40 | 84.72±20.28 |
| Triglycerides, mg/dL | 83.06±27.59 | 87.91±37.13 | 88.81±43.85 | 87.05±38.13 |
| Fasting glucose, mg/dL | 85.56±6.90 | 87.41±10.08 | 87.98±26.58 | 88.06±16.49 |
| Insulin sensitivity index (QUICKI) | 0.41 (0.36, 0.51) | 0.41 (0.36, 0.51) | 0.36 (0.41, 0.52) | 0.42 (0.36, 0.52) |
| Body mass index, kg/m² | 22.22±1.84 | 22.24±1.84 | 22.01±2.04 | 22.01±2.08 |
| Waist‐hip ratio | 0.79±0.06 | 0.78±0.07 | 0.78±0.07 | 0.79±0.07 |
| Moderate/vigorous physical activity, h/week | 45.38 (35.81, 61.94) | 37.25 (24.94, 50.63) | 37.63 (26.94, 55.31) | 34.75 (25.50, 53.00) |
| Borderline at baseline | ||||
| Systolic blood pressure, mm Hg | 128.04±5.68 | 122.50±11.76 | 120.83±11.86 | 122.89±12.82 |
| Diastolic blood pressure, mm Hg | 84.26±2.98 | 78.98±8.52 | 78.75±8.49 | 79.98±7.73 |
| Total cholesterol, mg/dL | 216.93±11.06 | 205.44±33.96 | 205.42±26.06 | 206.14±24.84 |
| High‐density lipoprotein, mg/dL | 50.93±5.30 | 50.31±7.52 | 51.51±9.29 | 50.93±8.58 |
| Low‐density lipoprotein, mg/dL | 113.78±8.47 | 111.66±21.73 | 110.22±21.77 | 109.88±22.28 |
| Triglycerides, mg/dL | 170.53±14.08 | 168.39±104.07 | 152.71±49.80 | 152.65±56.49 |
| Fasting glucose, mg/dL | 106.06±6.57 | 98.54±10.84 | 100.59±21.25 | 101.63±13.10 |
| Body mass index, kg/m² | 27.04±1.35 | 26.68±1.70 | 26.73±1.86 | 26.83±1.99 |
| Healthy diet score | 2.20±0.40 | 1.79±0.76 | 1.79±0.77 | 1.85±0.69 |
| Moderate/vigorous physical activity, h/week | 16.50 (9.50, 31.00) | 17.50 (10.50, 30.75) | 17.50 (9.75, 30.00) | 16.50 (10.00, 27.88) |
| Abnormal (or not ideal) at baseline | ||||
| Systolic blood pressure, mm Hg | 150.34±9.21 | 136.89±15.47 | 137.03±13.36 | 133.73±11.67 |
| Diastolic blood pressure, mm Hg | 95.29±5.07 | 85.80±8.58 | 88.10±9.36 | 85.70±7.96 |
| Total cholesterol, mg/dL | 261.29±17.55 | 237.65±35.21 | 233.30±31.61 | 239.61±33.55 |
| High‐density lipoprotein, mg/dL | 40.63±5.85 | 42.89±9.03 | 43.89±11.37 | 43.04±12.02 |
| Low‐density lipoprotein, mg/dL | 149.63±17.82 | 131.51±29.36 | 132.02±25.99 | 133.86±28.09 |
| Triglycerides, mg/dL | 265.52±86.12 | 239.32±85.97 | 191.77±83.09 | 178.25±76.30 |
| Fasting glucose, mg/dL | 173.65±50.34 | 144.30±52.16 | 157.20±66.69 | 165.00±55.96 |
| Insulin sensitivity index (QUICKI) | 0.29 (0.28, 0.30) | 0.31 (0.29, 0.32) | 0.31 (0.29, 0.32) | 0.32 (0.29, 0.35) |
| Body mass index, kg/m² | 35.76±5.86 | 34.50±5.40 | 34.75±5.51 | 34.50±5.54 |
| Waist‐hip ratio | 0.94±0.07 | 0.91±0.07 | 0.91±0.07 | 0.90±0.07 |
| Healthy diet score | 0.85±0.36 | 1.32±0.74 | 1.28±0.81 | 1.33±0.75 |
| Moderate/vigorous physical activity, h/week | 6.25 (4.19, 8.00) | 9.63 (5.44, 15) | 9.00 (5.13, 15.13) | 9.38 (6.31, 14.88) |
| All participants | ||||
| Systolic blood pressure, mm Hg | 120.87±16.02 | 117.12±14.95 | 116.32±14.89 | 117.42±14.23 |
| Diastolic blood pressure, mm Hg | 76.37±10.92 | 74.02±10.01 | 74.30±10.90 | 75.56±9.49 |
| Total cholesterol, mg/dL | 194.11±36.04 | 190.24±37.46 | 187.51±34.58 | 188.77±35.45 |
| High‐density lipoprotein, mg/dL | 63.27±18.06 | 62.67±17.83 | 61.87±17.76 | 61.78±17.65 |
| Low‐density lipoprotein, mg/dL | 110.42±31.65 | 106.66±31.17 | 105.20±30.32 | 107.04±30.63 |
| Triglycerides, mg/dL | 102.69±60.03 | 104.46±65.42 | 102.50±57.36 | 99.79±52.25 |
| Fasting glucose, mg/dL | 89.60±17.72 | 90.22±16.39 | 90.57±29.23 | 90.82±21.23 |
| Insulin sensitivity index | 0.40 (0.36, 0.51) | 0.40 (0.35, 0.51) | 0.41 (0.35, 0.52) | 0.36 (0.42, 0.52) |
| Body mass index, kg/m² | 27.83±6.38 | 27.28±5.78 | 27.13±5.93 | 27.17±5.89 |
| Waist‐hip ratio | 0.94±0.07 | 0.91±0.07 | 0.91±0.7 | 0.90±0.07 |
| Healthy diet score | 1.48±0.77 | 1.54±0.79 | 1.51±0.83 | 1.57±0.77 |
| Moderate/vigorous physical activity, h/week | 16.50 (9.50, 31.00) | 17.50 (10.50, 30.75) | 17.50 (9.75, 30.00) | 16.50 (10.00, 27.88) |
Mean±SD or median (first quartile, third quartile) are reported. Note that none of the participants scored more than 3 in the healthy diet score at baseline.
Effects on systemic arterial pressure
Compared to baseline, mean systolic BP decreased by 3.6, 4.6, and 3.3 mm Hg at the 6‐month, 1‐year, and 2‐year visits, respectively (all P<0.001). Similarly, DBP decreased by 2.2, 2.2, and 0.8 mm Hg at 6 months (P<0.0001), 1 year (P<0.0001), and 2 years (P=0.04), respectively, compared to baseline measurements. Changes in BP were significantly different among the 3 subgroups (P<0.0001). Specifically, participants with abnormal SBP and/or DBP at baseline exhibited the greatest improvement at follow‐up visits (17.3 and 9.0 mm Hg reduction in mean systolic and diastolic BP by 2 years, respectively), participants with borderline BP demonstrated moderate improvement, whereas those with normal BP remained normal at follow‐up visits (Figure 1A and 1B). Even though DBP increased marginally in those with normal values at baseline, the level remained normal (mean=72.4 mm Hg) in this group (Table 4).
Figure 1.
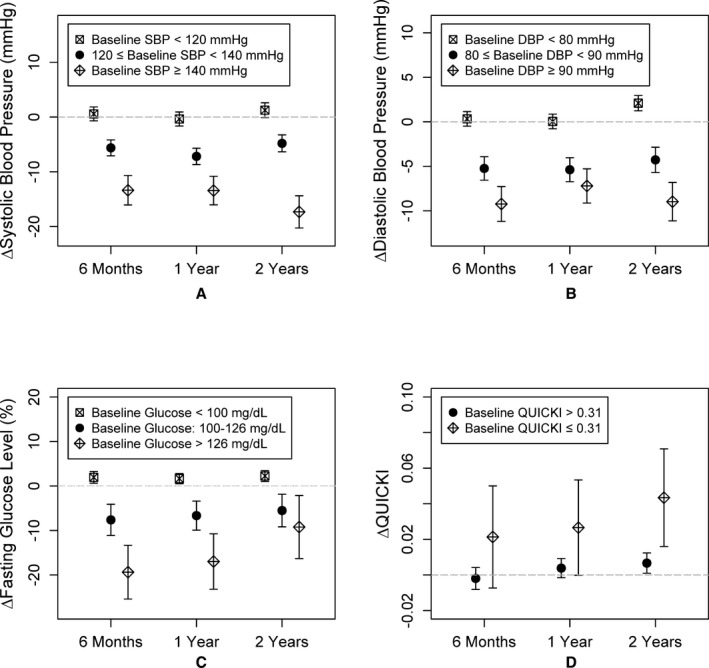
Change in (A) systolic blood pressure (mm Hg), (B) diastolic blood pressure (mm Hg), (C) fasting glucose (%), and (D) insulin sensitivity index (QUICKI) at 6 months, 1 year, and 2 years compared to the baseline visit. Mean changes and the corresponding 95% CIs at each visit for each subgroup are shown. DBP indicates diastolic blood pressure; SBP, systolic blood pressure.
Effects on glucose and insulin sensitivity
Although the overall fasting blood glucose level did not change significantly during follow‐up, participants with borderline and abnormal fasting glucose levels showed significant reductions at all follow‐up visits (all P≤0.01; Figure 1C). Overall level of QUICKI improved at 2 years (P=0.003) compared to baseline values (Figure 1D). Importantly, fasting glucose level improved in those with both borderline and high levels. There was a small, but statistically significant, increase in those with normal fasting glucose levels (Table 3).
Effects on blood lipids
The average total cholesterol level decreased by 5.3, 6.5, and 6.4 mg/dL from baseline at the 6‐month, 1‐year, and 2‐year of follow‐up, respectively (all P<0.001). Similar reductions were observed in the entire cohort for LDL and HDL levels (all P<0.001), but no significant changes in triglyceride levels were observed (Table 3). However, in subgroup analyses, participants with abnormal lipid levels at baseline exhibited substantial improvement at follow‐up visits (23‐, 16‐, and 85‐mg/dL reductions in mean total cholesterol, LDL, and triglyceride levels, respectively, after 2 years (Table 3; Figure 2). Interestingly, in the normal/ideal group, plasma LDL cholesterol levels increased and HDL concentrations decreased after 2 years. However, both LDL and HDL levels remained well within the normal range (Table 4).
Figure 2.
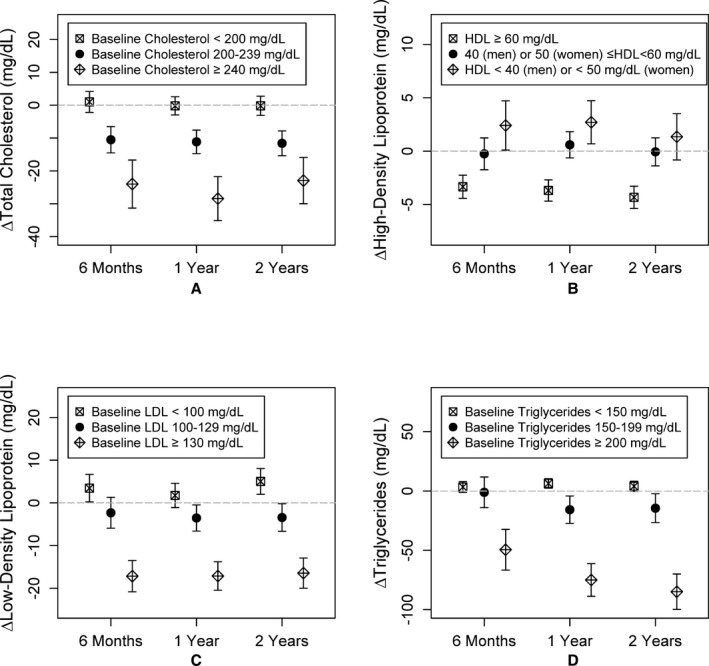
Change in (A) total cholesterol, (B) high‐density lipoprotein (HDL), (C) low‐density lipoprotein (LDL), and (D) triglycerides at 6 months, 1 year, and 2 years compared to baseline visit. Mean changes and the corresponding 95% CIs at each visit for each subgroup are shown.
Anthropometric changes
An average and sustained BMI reduction of 0.33, 0.45, and 0.38 kg/m2 (all P<0.001) was achieved at the 6‐months, 1‐year and 2‐year visits, respectively. WHR similarly improved modestly, reaching a reduction of 0.006 (P=0.004) at 2‐year follow‐up. Importantly, significant improvements were found only in the subgroups with baseline abnormal or borderline BMI and WHRs (Figure 3A and 3B).
Figure 3.
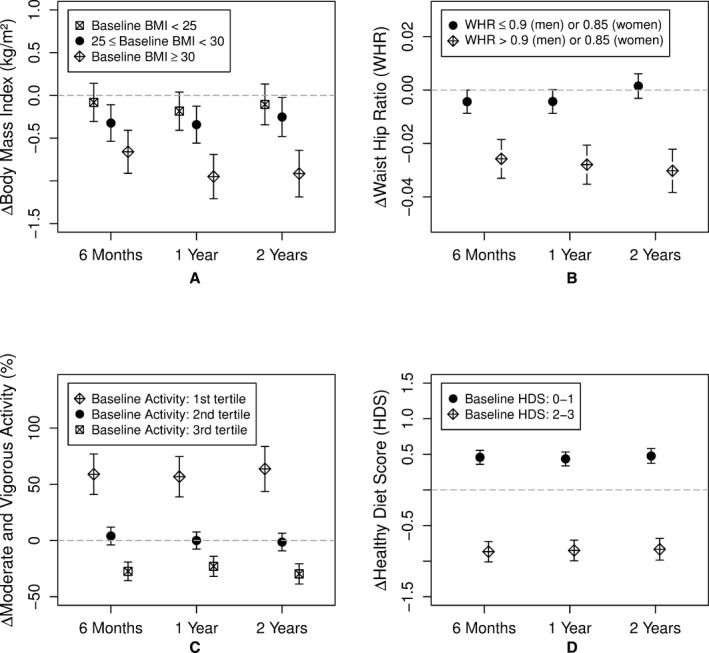
Change in (A) body mass index (BMI), (B) waist‐hip ratio (WHR), (C) moderate and vigorous activity level, and (D) healthy diet score at 6 months, 1 year, and 2 years compared to baseline visit. Mean changes and the corresponding 95% CIs at each visit for each subgroup are shown. Responses to the food frequency questionnaire were used to calculate the healthy diet score (HDS)—number of components of the 5 diet goals met; fruits and vegetables 8 to 10 servings/day; grains 6 to 8 servings/day; fish ≥2 servings/week; sodium ≤2400 mg/day; and added sugar ≤6 teaspoons or 100 calories/day for women and ≤9 teaspoons or 150 calories a day for men.
Smoking cessation
Of the 39 participants who reported active smoking at baseline, 9 subjects (23%) quit smoking by the 2‐year follow‐up visit, whereas 3 nonsmoking (at baseline) participants reported smoking at the follow‐up visits (P=0.15).
Physical activity
A total of 536 (75%) participants did not meet the suggested 150 minutes a week of moderate‐intensity or 75 minutes a week of vigorous‐intensity aerobic physical activity at the baseline visit.28, 29 Moderate and vigorous exercise increased by 6% at the 6‐month visit (P=0.04), but was not sustained during future follow‐up. In subgroup analyses, sedentary individuals increased time spent undertaking moderate/vigorous exercise by 59%, 57%, and 64% at the 6‐month, 1‐year, and 2‐year visits, respectively (all P<0.001; Table 3; Figure 3C. In contrast, the most physically active individuals exhibited a 20% to 30% decrease in moderate/vigorous physical activity at follow‐up visits (all P<0.001), but remained above the 2008 Physical Activity Guideline for Americans (Tables 3 and 4).
Dietary changes
None of the participants met all the AHA‐recommended requirements for a healthy diet (HDS of 4–5). Nonetheless, the average HDS increased by 0.46, 0.44, and 0.48 for the subgroup with baseline poor diet score (0–1) at the 6‐month, 1‐year, and 2‐year visits, respectively (all P<0.001). In contrast, the group with borderline HDS (2–3) showed a significant decrease in the HDS by 0.87, 0.85, and 0.83 at the 6‐month, 1‐year and 2‐year visits, respectively (all P<0.001; Table 3; Figure 3D).
Ten‐year CVD risk scores
In the entire cohort, there was a 12% reduction in FRS and ACVD risk score over 2 years (P<0.0001), after accounting for aging effect on risk scores. The cohort was divided into those with low (<median) and high risk scores (≥median; Figure 4. Both risk scores improved significantly in those with high, but not in those with low, risk scores at baseline (P<0.0001 between groups).
Figure 4.
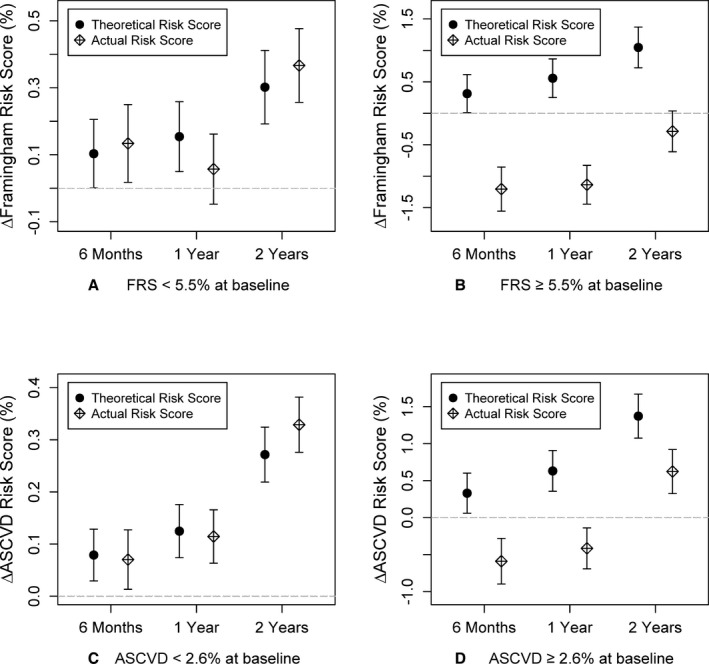
Change in Framingham risk score (FRS) and atherosclerotic cardiovascular disease (ASCVD) risk scores for participants with (A) baseline FRS <5.2% (2.6±1.3%), (B) baseline FRS ≥5.2% (11.4±7.1%), (C) baseline ASCVD <2.4% (1.0±0.6%), and (D) baseline ASCVD ≥2.4% (3.1±1.1%) at 6 months, 1 year, and 2 years compared to baseline visit. Mean changes and the corresponding 95% CIs at each visit are shown.
AHA LS7 score
The mean LS7 score was 7.93 (SD=1.98), with 70% of the participants having a LS7 score between 7 and 10 at baseline. Overall, the mean AHA LS7 score increased by 0.28, 0.40, and 0.33 at 6 months, 1 year, and 2 years, compared to baseline. The change in LS7 score in those with baseline LS7 <7 (N=156) at baseline was greater than in those with baseline LS7 ≥7 (N=555; P<0.0001). Although the LS7 score increased by 0.83, 1.04, and 0.99 at 6 months, 1 year, and 2 years, respectively, compared to baseline in those with lower scores, there was no consistent change in LS7 scores among those with higher scores at baseline.
Effect of Therapy
Eighteen subjects started antihypertensive and/or lipid‐lowering therapy, and 4 subjects started on diabetes mellitus treatment during the observation period. When these subjects were removed from the analysis, there were slight (<10%) decreases in the estimated changes in blood pressure and cholesterol levels in the group with abnormal values at baseline, and the significant findings remained unchanged.
Discussion
The Emory‐Georgia Tech CHDWB implemented an intervention with the goal of engaging participants in a process of personal health discovery with a larger goal of understanding and better defining human health. Herein, we demonstrate that individuals’ awareness of their biological health profile, together with a goal‐oriented HP intervention, is associated with improved cardiovascular risk factors and cardiovascular risk profile in otherwise healthy employed subjects without known CVD. The data‐driven HP intervention was effective in improving the majority of cardiovascular risk factors and cardiovascular risk in those with abnormal values at baseline and in achieving the AHA LS7 goals for ideal cardiovascular health. These beneficial effects were observed within 6 months of initiation and were sustained during the 2‐year follow‐up period and included broad improvements in blood pressure, lipid profile, insulin resistance, weight, dietary habits, and physical activity. Among subjects with ideal health characteristics at baseline, there was continued maintenance of health.
Our findings are consistent with previous trials utilizing lifestyle interventions in subjects with specific risk factors or with known CVD.30, 31, 32, 33, 34, 35 However, these studies have not been performed in an otherwise relatively healthy population where the goal was not prevention of disease, but to maintain ideal health. The extent to which the risk factors, when present, were modified in our cohort was comparable to the intervention groups in previously published studies. Thus, there was sustainable weight loss in those who were overweight,36, 37 lowering of blood pressure in those with high levels,38 improvement of the lipid profile and insulin resistance in those with baseline abnormalities,33, 39 and promotion of a healthy diet and exercise habits,33, 40, 41 findings similar to observations in high‐risk populations.42, 43 Importantly, no perceptible decline among those with relatively normal values was observed, indicating that health was maintained in this cohort.
It is known that aggressive and focused intervention on diet and exercise can reduce risk of diabetes mellitus.30, 32, 44 Often, these interventions require intense dietary and lifestyle modifications that are time‐consuming and expensive to implement because they require trained staff and their long‐term cost‐effectiveness remains debatable.45, 46, 47 Previous trials have utilized a variety of lifestyle interventions that included self‐help programs with counseling and resources, commercial weight loss programs, office‐based counseling, mailed health education materials, online lessons, and computer‐automated feedback.2 In contrast, we provided subjects data on their risk profile integrated with an individually tailored lifestyle program using the HP intervention. The term “HP” is used to emphasize engagement of participants in the program as partners in their own health care. Our program created an individually focused intervention utilizing multiple cognitive behavioral strategies, including goal setting, tailored contact, feedback, reinforcement, and self‐efficacy enhancement.48 The relationship encouraged a trusting joint effort to alter behaviors through frequent contacts between the HP and participant to monitor individual progress and perceptions regarding the participant's ability to carry out the proposed health plan.49, 50 The frequency of contact was based on the participant's needs, and it had no significant impact on the improvement of health outcomes. The communication between the HP and subject allowed implementation of problem‐solving strategies, including motivational interviewing to navigate challenges in behavior change.51
Potential mechanisms underlying the observed improvements include increased use of pharmacotherapy for hypertension, hypercholesterolemia, and hyperglycemia. Although a minority of subjects were started on these medications, our findings remained unchanged after removing these subjects from analysis. Whether providing detailed information about health status alone, or in combination with the HP, motivated the observed lifestyle changes remains to be determined.
We studied a population of employees recruited from large metropolitan academic centers and thus included a higher proportion of educated, insured, and prosperous individuals. Whether a HP intervention will produce similar benefits in the general population remains to be studied. In order to address this, we measured changes in those with lower socioeconomic status (SES). Participants in the lowest tertile of both education and income (N=132), on average, had higher BMI and lower LS7 scores at baseline compared to the remaining sample. There was no significant difference in improvements in cardiovascular risk factors between those with lower SES and those with higher SES. Thus, the HP intervention appeared to be helpful in reducing CVD risk factors over a wide range of SES strata in our cohort.
There are several limitations of our study. The study was not randomized, and there was no control group for comparison to the HP intervention cohort. Thus, it is possible that these beneficial changes may have occurred by merely being enrolled in a study and obtaining health status information. However, our findings are comparable to those of other lifestyle intervention trials where control groups were included and often deteriorated over time.12, 32, 35 Moreover, we found that improvements were observed in those with abnormalities relevant to CVD risk at baseline, whereas there was maintenance of healthy parameters in those with normal parameters at enrollment. There was 14% loss to follow‐up for a variety of reasons, including relocation. However, there were no demographic differences between those who completed follow‐up and those who were lost‐to follow‐up. We were unable to examine influence of individual HPs on participants’ outcome because participants may have switched HPs during the study period. However, the frequency of contact between HPs and participants did not have a significant impact on health metrics.
Conclusion
A personalized, goal‐directed HP intervention improved the cardiovascular risk factor profile, including blood pressure, weight, dyslipidemia, insulin resistance, and cardiovascular risk scores. These effects were greater in subjects with a higher risk burden and were sustained after 2 years. Moreover, subjects without risk factors maintained their health. Whether a HP intervention improves long‐term morbidity and mortality, and whether it is cost‐effective, needs further investigation.
Sources of Funding
This work was supported by the Marcus and Woodruff Foundations (Atlanta, GA); the Emory University/Georgia Tech Predictive Health Institute; and awards UL1 RR025008 and UL1 TR000454 from the Clinical and Translational Science Award Program, National Institutes of Health, National Center for Research Resources and National Center for Advancing Translational Sciences. Dr Quyyumi is supported by NIH grants 5P01HL101398‐02, 1P20HL113451‐01, 1R56HL126558‐01, 1RF1AG051633‐01, R01 NS064162‐01, R01 HL89650‐01, HL095479‐01, 1U10HL110302‐01, 1DP3DK094346‐01, and 2P01HL086773 and American Heart Association Grant No. 0000031288. Dr Mohamed Kelli is supported by the Abraham J. and Phyllis Katz Foundation and METRIC T32 training grant.
Disclosures
None.
(J Am Heart Assoc. 2016;5: e004217 doi: 10.1161/JAHA.116.004217)
References
- 1. Stuart‐Shor EM, Berra KA, Kamau MW, Kumanyika SK. Behavioral strategies for cardiovascular risk reduction in diverse and underserved racial/ethnic groups. Circulation. 2012;125:171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Artinian NT, Fletcher GF, Mozaffarian D, Kris‐Etherton P, Van Horn L, Lichtenstein AH, Kumanyika S, Kraus WE, Fleg JL, Redeker NS, Meininger JC, Banks J, Stuart‐Shor EM, Fletcher BJ, Miller TD, Hughes S, Braun LT, Kopin LA, Berra K, Hayman LL, Ewing LJ, Ades PA, Durstine JL, Houston‐Miller N, Burke LE; American Heart Association Prevention Committee of the Council on Cardiovascular N . Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:406–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Waure C, Lauret GJ, Ricciardi W, Ferket B, Teijink J, Spronk S, Myriam Hunink MG. Lifestyle interventions in patients with coronary heart disease: a systematic review. Am J Prev Med. 2013;45:207–216. [DOI] [PubMed] [Google Scholar]
- 4. Orozco LJ, Buchleitner AM, Gimenez‐Perez G, Roque IFM, Richter B, Mauricio D. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;3:CD003054. [DOI] [PubMed] [Google Scholar]
- 5. Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e‐mail counseling, computer‐automated tailored counseling, and no counseling in an Internet weight loss program. Arch Intern Med. 2006;166:1620–1625. [DOI] [PubMed] [Google Scholar]
- 6. Saffi MA, Polanczyk CA, Rabelo‐Silva ER. Lifestyle interventions reduce cardiovascular risk in patients with coronary artery disease: a randomized clinical trial. Eur J Cardiovasc Nurs. 2014;13:436–443. [DOI] [PubMed] [Google Scholar]
- 7. Glozier N, Christensen H, Naismith S, Cockayne N, Donkin L, Neal B, Mackinnon A, Hickie I. Internet‐delivered cognitive behavioural therapy for adults with mild to moderate depression and high cardiovascular disease risks: a randomised attention‐controlled trial. PLoS One. 2013;8:e59139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pengpid S, Peltzer K, Skaal L. Efficacy of a church‐based lifestyle intervention programme to control high normal blood pressure and/or high normal blood glucose in church members: a randomized controlled trial in Pretoria, South Africa. BMC Public Health. 2014;14:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maruthur NM, Wang NY, Appel LJ. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER Trial. Circulation. 2009;119:2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maron DJ, Boden WE, O'Rourke RA, Hartigan PM, Calfas KJ, Mancini GB, Spertus JA, Dada M, Kostuk WJ, Knudtson M, Harris CL, Sedlis SP, Zoble RG, Title LM, Gosselin G, Nawaz S, Gau GT, Blaustein AS, Bates ER, Shaw LJ, Berman DS, Chaitman BR, Weintraub WS, Teo KK; Group CTR . Intensive multifactorial intervention for stable coronary artery disease: optimal medical therapy in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial. J Am Coll Cardiol. 2010;55:1348–1358. [DOI] [PubMed] [Google Scholar]
- 11. Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, Kumanyika S, Schmitz KH, Diewald LK, Barg R, Chittams J, Moore RH. A two‐year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365:1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak‐Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi‐Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jospe MR, Fairbairn KA, Green P, Perry TL. Diet app use by sports dietitians: a survey in five countries. JMIR Mhealth Uhealth. 2015;3:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rask KJ, Brigham KL, Johns MM. Integrating comparative effectiveness research programs into predictive health: a unique role for academic health centers. Acad Med. 2011;86:718–723. [DOI] [PubMed] [Google Scholar]
- 15. Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, Kavtaradze N, Uphoff I, Hooper C, Tangpricha V, Alexander RW, Brigham K, Quyyumi AA. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol. 2011;58:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al Mheid I, Veledar E, Martin GS, Vaccarino V, Quyyumi AA. Functional health and well‐being, arterial stiffness and vascular dysfunction in healthy adults. Int J Cardiol. 2014;174:729–730. [DOI] [PubMed] [Google Scholar]
- 17. Lenfant C, Chobanian AV, Jones DW, Roccella EJ; Joint National Committee on the Prevention DE and Treatment of High Blood P . Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41:1178–1179. [DOI] [PubMed] [Google Scholar]
- 18. Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. [DOI] [PubMed] [Google Scholar]
- 19. Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P; Expert Committee on the D and Classification of Diabetes M . Follow‐up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. [DOI] [PubMed] [Google Scholar]
- 20. Albert MA, Glynn RJ, Ridker PM. Plasma concentration of C‐reactive protein and the calculated Framingham Coronary Heart Disease Risk Score. Circulation. 2003;108:161–165. [DOI] [PubMed] [Google Scholar]
- 21. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 22. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; American Heart Association Strategic Planning Task F and Statistics C . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 23. Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes. 2005;54:1914–1925. [DOI] [PubMed] [Google Scholar]
- 24. Williams PT. Reductions in incident coronary heart disease risk above guideline physical activity levels in men. Atherosclerosis. 2010;209:524–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data‐based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. [DOI] [PubMed] [Google Scholar]
- 26. Eckel RH, Jakicic JM, Ard JD, De Jesus JM, Miller NH, Hubbard VS, Lee I‐M, Lichtenstein AH, Loria CM, Millen BE. AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;2014:63. [DOI] [PubMed] [Google Scholar]
- 27. Brigham KL. Predictive health: the imminent revolution in health care. J Am Geriatr Soc. 2010;58:S298–S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. US Department of Health and Human Services . 2008 physical activity guidelines for Americans. 2015.
- 29. Kay MC, Carroll DD, Carlson SA, Fulton JE. Awareness and knowledge of the 2008 Physical Activity Guidelines for Americans. J Phys Act Health. 2014;11:693–698. [DOI] [PubMed] [Google Scholar]
- 30. Look ARG, Wing RR. Long‐term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four‐year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH Jr, Kostis JB, Kumanyika S, Lacy CR, Johnson KC, Folmar S, Cutler JA. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279:839–846. [DOI] [PubMed] [Google Scholar]
- 32. Lindstrom J, Peltonen M, Eriksson JG, Ilanne‐Parikka P, Aunola S, Keinanen‐Kiukaanniemi S, Uusitupa M, Tuomilehto J; Finnish Diabetes Prevention S . Improved lifestyle and decreased diabetes risk over 13 years: long‐term follow‐up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia. 2013;56:284–293. [DOI] [PubMed] [Google Scholar]
- 33. Giannuzzi P, Temporelli PL, Marchioli R, Maggioni AP, Balestroni G, Ceci V, Chieffo C, Gattone M, Griffo R, Schweiger C, Tavazzi L, Urbinati S, Valagussa F, Vanuzzo D; Investigators G . Global secondary prevention strategies to limit event recurrence after myocardial infarction: results of the GOSPEL study, a multicenter, randomized controlled trial from the Italian Cardiac Rehabilitation Network. Arch Intern Med. 2008;168:2194–2204. [DOI] [PubMed] [Google Scholar]
- 34. Castaldo JE, Reed JF III. The Lowering of Vascular Atherosclerotic Risk (LOVAR) program: an approach to modifying cerebral, cardiac, and peripheral vascular disease. J Stroke Cerebrovasc Dis. 2008;17:9–15. [DOI] [PubMed] [Google Scholar]
- 35. Jennings C, Kotseva K, De Bacquer D, Hoes A, de Velasco J, Brusaferro S, Mead A, Jones J, Tonstad S, Wood D; Group EPS . Effectiveness of a preventive cardiology programme for high CVD risk persistent smokers: the EUROACTION PLUS varenicline trial. Eur Heart J. 2014;35:1411–1420. [DOI] [PubMed] [Google Scholar]
- 36. Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer‐Davis EJ, Pi‐Sunyer X, Regensteiner J, Venditti B, Wylie‐Rosett J. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Look ARG. Eight‐year weight losses with an intensive lifestyle intervention: the Look AHEAD study. Obesity (Silver Spring). 2014;22:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller ER III, Erlinger TP, Young DR, Jehn M, Charleston J, Rhodes D, Wasan SK, Appel LJ. Results of the diet, exercise, and weight loss intervention trial (DEW‐IT). Hypertension. 2002;40:612–618. [DOI] [PubMed] [Google Scholar]
- 39. Ilanne‐Parikka P, Eriksson JG, Lindstrom J, Peltonen M, Aunola S, Hamalainen H, Keinanen‐Kiukaanniemi S, Laakso M, Valle TT, Lahtela J, Uusitupa M, Tuomilehto J; Finnish Diabetes Prevention Study G . Effect of lifestyle intervention on the occurrence of metabolic syndrome and its components in the Finnish Diabetes Prevention Study. Diabetes Care. 2008;31:805–807. [DOI] [PubMed] [Google Scholar]
- 40. Wood DA, Kotseva K, Connolly S, Jennings C, Mead A, Jones J, Holden A, De Bacquer D, Collier T, De Backer G, Faergeman O; Group ES . Nurse‐coordinated multidisciplinary, family‐based cardiovascular disease prevention programme (EUROACTION) for patients with coronary heart disease and asymptomatic individuals at high risk of cardiovascular disease: a paired, cluster‐randomised controlled trial. Lancet. 2008;371:1999–2012. [DOI] [PubMed] [Google Scholar]
- 41. Lindstrom J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, Uusitupa M, Tuomilehto J; Finnish Diabetes Prevention Study G . The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3‐year results on diet and physical activity. Diabetes Care. 2003;26:3230–3236. [DOI] [PubMed] [Google Scholar]
- 42. Unick JL, Beavers D, Bond DS, Clark JM, Jakicic JM, Kitabchi AE, Knowler WC, Wadden TA, Wagenknecht LE, Wing RR; Look ARG . The long‐term effectiveness of a lifestyle intervention in severely obese individuals. Am J Med. 2013;126:236–242, 242.e1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knowler WC, Barrett‐Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research G . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Unick JL, Beavers D, Jakicic JM, Kitabchi AE, Knowler WC, Wadden TA, Wing RR; Look ARG . Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care. 2011;34:2152–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wylie‐Rosett J, Herman WH, Goldberg RB. Lifestyle intervention to prevent diabetes: intensive and cost effective. Curr Opin Lipidol. 2006;17:37–44. [DOI] [PubMed] [Google Scholar]
- 46. Group DPPR . The 10‐year cost‐effectiveness of lifestyle intervention or metformin for diabetes prevention an intent‐to‐treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35:723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bertram M, Lim S, Barendregt J, Vos T. Assessing the cost‐effectiveness of drug and lifestyle intervention following opportunistic screening for pre‐diabetes in primary care. Diabetologia. 2010;53:875–881. [DOI] [PubMed] [Google Scholar]
- 48. Strecher VJ, Seijts GH, Kok GJ, Latham GP, Glasgow R, DeVellis B, Meertens RM, Bulger DW. Goal setting as a strategy for health behavior change. Health Educ Behav. 1995;22:190–200. [DOI] [PubMed] [Google Scholar]
- 49. Jeffery RW, Wing RR, Thorson C, Burton LR. Use of personal trainers and financial incentives to increase exercise in a behavioral weight‐loss program. J Consult Clin Psychol. 1998;66:777. [DOI] [PubMed] [Google Scholar]
- 50. Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, New Jersey: Prentice‐Hall, Inc; 1986. [Google Scholar]
- 51. Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta‐analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71:843. [DOI] [PubMed] [Google Scholar]


