Abstract
Background
High success rates are achievable for chronic total occlusion (CTO) percutaneous coronary intervention (PCI) using the hybrid approach, but periprocedural complications remain of concern. Although scores estimating success and efficiency in CTO PCI have been developed, there is currently no available score for estimation of the risk for periprocedural complications. We sought to develop a scoring tool for prediction of periprocedural complications during CTO PCI.
Methods and Results
We analyzed data from 1569 CTO PCIs in the Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS CTO) using a derivation and validation sampling ratio of 2:1. Variables independently associated with periprocedural complications in multivariable analysis in the derivation set were assigned points based on their respective odds ratios. Forty‐four (2.8%) patients experienced complications. Three factors were independent predictors of complications and were included in the score: patient age >65 years, +3 points (odds ratio, OR=4.85, CI 1.82‐16.77); lesion length ≥23 mm, +2 points (OR=3.22, CI 1.08‐13.89); and use of the retrograde approach +1 point (OR=2.41, CI 1.04‐6.05). The resulting score showed good calibration and discriminatory capacity in the derivation (Hosmer‐Lemeshow χ2 6.271, P=0.281, receiver‐operating characteristic [ROC] area=0.758) and validation (Hosmer‐Lemeshow χ2 4.551, P=0.473, ROC area=0.793) sets. Score values of 0 to 2, 3 to 4, and ≥5 were defined as low, intermediate, and high risk of complications (derivation cohort 0.4%, 1.8%, 6.5%, P<0.001; validation cohort 0.0%, 2.5%, 6.8%, P<0.001).
Conclusions
The PROGRESS CTO complication score is a useful tool for prediction of periprocedural complications in CTO PCI.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02061436.
Keywords: chronic total occlusion, complication, outcome, percutaneous coronary intervention, risk stratification
Subject Categories: Percutaneous Coronary Intervention, Revascularization, Chronic Ischemic Heart Disease, Complications
Introduction
Chronic total occlusion (CTO) percutaneous coronary intervention (PCI) success rates continue to improve as new techniques and tools develop to address the specific challenges in CTO PCI.1, 2, 3, 4 The occurrence of periprocedural complications, however, continues to impact risk‐benefit considerations, with a rate of 3.1% in a large contemporary meta‐analysis.1 Although scores predicting technical and procedural outcomes in CTO PCI have been developed (such as the Japanese Chronic Total Occlusion [J‐CTO] score,5 the Prospective Global Registry for the Study of Chronic Total Occlusion Intervention [PROGRESS CTO] score,6 and the Clinical and Lesion‐related [CL] score7), there is currently no specific tool to predict the risk of periprocedural complications in this setting. We sought to develop a scoring system to predict occurrence of periprocedural complications during CTO PCI.
Methods
Patient Population
We examined the clinical, angiographic, and procedural characteristics of 1569 consecutive CTO PCIs in 1569 patients who were included in the PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention, NCT02061436)2, 6, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 between January 2012 and March 2016 at 12 US centers. A list of the contributing centers can be found in Data S1. Procedures were entered retrospectively and prospectively into the database. Some centers only enrolled patients during part of the study period due to participation in other studies. Second CTO PCIs in a single patient were excluded from the analysis, as were procedures without data on technical success, procedural success, or periprocedural complications. The study was approved by the institutional review board of each center. A waiver of informed consent was obtained for this study.
Definitions
Coronary CTOs were defined as coronary lesions with thrombolysis in myocardial infarction (TIMI) grade 0 flow of at least 3 months’ duration. Estimation of the duration of occlusion was clinical, based on the first onset of angina, prior history of myocardial infarction in the target vessel territory, or comparison with a prior angiogram. Calcification was assessed by angiography as mild (spots), moderate (involving ≤50% of the reference lesion diameter), and severe (involving >50% of the reference lesion diameter). Moderate proximal vessel tortuosity was defined as the presence of at least 2 bends >70° or 1 bend >90°, and severe tortuosity as 2 bends >90° or 1 bend >120° in the CTO vessel. Blunt or no stump was defined as lack of tapering or lack of a funnel shape at the proximal cap. Interventional collaterals were defined as collaterals considered amenable to crossing by a guidewire and a microcatheter by the operator.
Technical success of CTO PCI was defined as successful CTO revascularization with achievement of <30% residual diameter stenosis within the treated segment and restoration of TIMI grade 3 antegrade flow. Procedural success was defined as the combination of technical success with no in‐hospital complications. In‐hospital complications included any of the following adverse events prior to hospital discharge: death, myocardial infarction, recurrent symptoms requiring urgent repeat target vessel revascularization with PCI or coronary artery bypass graft surgery (CABG), tamponade requiring either pericardiocentesis or surgery, and stroke. Myocardial infarction (MI) was defined using the Third Universal Definition of Myocardial Infarction (type 4 MI).19 Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease (MDRD) formula.
Score Development
The study population was divided with a ratio of 2:1 using random number generation, resulting in a derivation set of 1065 and a validation set of 504 CTO PCIs. Univariable analysis was performed on the derivation cohort to identify variables associated with the occurrence of in‐hospital complications. All variables available in the PROGRESS CTO registry were included in the univariable analysis. Variables associated with complications with P<0.10 were entered into a multivariable model in order to identify independent predictors of complications. Stepwise backward selection was performed until only variables with P<0.05 in the multivariable model remained. These variables were considered independent predictors of complications. Points were assigned to each independent predictor variable based on odds ratio to form a scoring system.
Statistical Analysis
Categorical variables are expressed as percentages and were compared using a Pearson chi‐squared test or Fisher exact test. Continuous variables are presented as mean±standard deviation or median (interquartile range, IQR) unless otherwise specified and were compared using the t test or Wilcoxon rank‐sum test, as appropriate. The calibration of the score was assessed using the Hosmer‐Lemeshow chi‐squared statistic. The discriminatory capacity was evaluated with receiver‐operating characteristic (ROC) curves and with the calculated area‐under‐the‐curve (AUC). Validation was performed by comparing the ROC curves in the derivation and validation cohorts. Differences in AUC between curves were tested using the method described by Hanley and McNeil.20, 21 The Cochran‐Armitage test was used to evaluate for trend. All statistical analyses were performed with JMP 12.0 (SAS Institute, Cary, NC), SPSS version 22.0 (IBM Corporation, Armonk, NY), and MedCalc version 16.2.1 (Ostend, Belgium). A 2‐sided P value of 0.05 was considered statistically significant.
Results
Patient Population and Procedural Outcomes
The study population consisted of 1569 CTO PCIs in 1569 patients. Mean age was 65±10 years; 84% were male; 36% had a history of CABG, and 66% had a prior PCI (Table 1). The right coronary artery was the most common target vessel (56%), followed by the left anterior descending coronary artery (23%) and the left circumflex coronary artery (21%). Retrograde techniques and collaterals used in the study population are summarized in Table 2. Overall technical success was 90%, and overall procedural success was 88%. Periprocedural complications occurred in 44 patients (2.8%). Sixteen patients experienced myocardial infarction; 15 patients developed tamponade requiring pericardiocentesis; 4 patients had a stroke; 4 patients required urgent repeat PCI; 1 patient required urgent CABG; 9 patients died before discharge from the hospital. Median procedure time was 129 minutes (IQR 88‐192), and median fluoroscopy time was 47 minutes (IQR 29‐77). Median patient air kinetic energy released per unit mass (kerma) dose was 3.2 Gray (IQR 2.0‐5.2), and median contrast volume was 270 mL (IQR 200‐370).
Table 1.
Clinical, Angiographic, Procedural Characteristics, and Outcomes in the Overall Study Population, Derivation Set, and Validation Set
| Variable | Overall | Derivation | Validation | P Value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, y | 65±10 | 66±10 | 65±10 | 0.35 |
| Age >65 y | 50 | 52 | 47 | 0.055 |
| Male | 84 | 84 | 85 | 0.57 |
| Body mass index, kg/m2 | 31±6 | 31±6 | 31±6 | 0.99 |
| Diabetes mellitus | 45 | 46 | 42 | 0.14 |
| Dyslipidemia | 95 | 95 | 94 | 0.42 |
| Hypertension | 90 | 90 | 89 | 0.57 |
| Prior myocardial infarction | 43 | 43 | 42 | 0.88 |
| Prior PCI | 66 | 64 | 68 | 0.13 |
| Prior CABG | 36 | 36 | 35 | 0.58 |
| Prior heart failure | 29 | 29 | 27 | 0.47 |
| Prior valve procedure | 3 | 4 | 2 | 0.11 |
| Cerebrovascular disease | 11 | 11 | 10 | 0.82 |
| Peripheral arterial disease | 17 | 15 | 19 | 0.061 |
| Chronic lung disease | 13 | 13 | 13 | 0.77 |
| Current tobacco use | 25 | 24 | 28 | 0.054 |
| eGFR, mL/min per 1.73 m2 | 72±26 | 72±25 | 71±27 | 0.67 |
| eGFR <60 mL/min per 1.73 m2 or currently on dialysis | 32 | 32 | 32 | 0.99 |
| Currently on dialysis | 3 | 3 | 4 | 0.31 |
| LV ejection fraction, % | 50±14 | 50±14 | 50±13 | 0.80 |
| LV ejection fraction <40% | 21 | 22 | 20 | 0.29 |
| Angiographic characteristics | ||||
| RCA target | 56 | 56 | 54 | 0.53 |
| LAD target | 23 | 23 | 24 | 0.72 |
| LCX target | 21 | 20 | 21 | 0.72 |
| Proximal segment target | 38 | 38 | 39 | 0.83 |
| Lesion length, mm | 30 (20‐45) | 30 (20‐40) | 30 (20‐50) | 0.63 |
| Length ≥20 mm | 77 | 77 | 76 | 0.92 |
| Length ≥23 mm | 66 | 66 | 65 | 0.92 |
| Proximal cap ambiguity | 32 | 31 | 33 | 0.62 |
| Side branch at proximal cap | 47 | 48 | 47 | 0.75 |
| Blunt/no stump | 53 | 54 | 52 | 0.51 |
| Distal cap at bifurcation | 32 | 31 | 33 | 0.47 |
| Good distal landing zone | 62 | 63 | 61 | 0.55 |
| Interventional collaterals | 59 | 60 | 57 | 0.41 |
| Moderate/severe calcification | 57 | 57 | 57 | 0.96 |
| Moderate/severe tortuosity | 36 | 36 | 38 | 0.45 |
| In‐stent restenosis | 15 | 14 | 17 | 0.16 |
| Prior CTO PCI attempt | 17 | 15 | 20 | 0.020 |
| J‐CTO score | 2.5±1.2 | 2.5±1.2 | 2.6±1.2 | 0.17 |
| PROGRESS CTO score | 1.3±1.0 | 1.3±1.0 | 1.4±1.0 | 0.13 |
| Procedural characteristics | ||||
| Radial access | 27 | 27 | 27 | 0.92 |
| Dual injection | 72 | 72 | 72 | 0.98 |
| Antegrade wire escalation used | 74 | 74 | 74 | 0.94 |
| ADR used | 35 | 35 | 34 | 0.65 |
| Retrograde approach used | 42 | 41 | 43 | 0.40 |
| IVUS used | 44 | 43 | 46 | 0.30 |
| Prophylactic LVAD | 2 | 2 | 3 | 0.45 |
| Procedural outcomes | ||||
| Technical success | 90 | 90 | 90 | 0.82 |
| Procedural success | 88 | 89 | 87 | 0.35 |
| Contrast volume, mL | 270 (200‐370) | 270 (200‐369) | 274 (200‐370) | 0.67 |
| Fluoroscopy time, minutes | 47 (29‐77) | 46 (28‐77) | 49 (30‐78) | 0.41 |
| Patient air kerma dose, Gy | 3.2 (2.0‐5.2) | 3.2 (2‐5.3) | 3.2 (1.9‐5.2) | 0.97 |
| Procedure time, minute | 129 (88‐192) | 126 (87‐192) | 139 (94‐199) | 0.052 |
| Periprocedural MACE | 2.8 | 2.6 | 3.2 | 0.54 |
| Death | 0.6 | 0.7 | 0.4 | 0.52 |
| Myocardial infarction | 1.0 | 0.8 | 1.6 | 0.12 |
| Re‐PCI | 0.3 | 0.2 | 0.4 | 0.44 |
| Emergency CABG | 0.1 | 0 | 0.2 | 0.15 |
| Stroke | 0.3 | 0.4 | 0 | 0.17 |
| Tamponade requiring pericardiocentesis | 1.0 | 0.9 | 1.0 | 0.92 |
Values are % or mean±standard deviation or median (interquartile range). ADR indicates antegrade dissection reentry; CABG, coronary artery bypass grafting; CTO, chronic total occlusion; eGFR, estimated glomerular filtration rate; IVUS, intravascular ultrasound; J‐CTO score, Multicenter CTO Registry of Japan score; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; LV, left ventricular; LVAD, left ventricular assist device; MACE, major adverse cardiovascular event; PCI, percutaneous coronary intervention; PROGRESS CTO, Prospective Global Registry for the Study of Chronic Total Occlusion Intervention; RCA, right coronary artery.
Table 2.
Retrograde Crossing Techniques and Collaterals Used in the Study Cohort
| Retrograde Technique Used | % |
|---|---|
| Retrograde true lumen puncture | 26 |
| Kissing wire | 1 |
| Just marker | 3 |
| Knuckle wire | 5 |
| CART | 4 |
| Reverse CART | 64 |
| Guideliner reverse CART | 2 |
| Collateral Channel Used | % |
|---|---|
| Septal | 62 |
| Epicardial | 35 |
| SVG | 16 |
| LIMA | 2 |
CART indicates controlled antegrade and retrograde subintimal tracking; LIMA, left internal mammary artery; SVG, saphenous vein graft.
Score Derivation
The derivation set included 1065 randomly assigned CTO PCIs, with technical success 90%, procedural success 89%, and periprocedural major adverse cardiovascular events (MACE) in 28 patients (2.6%) (Table 1). On univariable analysis in the derivation group, procedures that resulted in MACE were more likely to have been performed in patients over age 65 (85% vs 51%, P<0.001), with prior cardiac valve procedure or cardiac valve surgery (14% vs 4%, P=0.003), or in patients who required prophylactic use of a percutaneous left ventricular assist device (LVAD, 11% vs 2%, P=0.002). Periprocedural complications occurred more frequently in CTO PCIs that involved a CTO ≥23 mm in length (88% vs 65%, P=0.013), use of the retrograde approach (71% vs 40%, P=0.001), or in CTOs with a higher J‐CTO score (3.0±1.1 vs 2.5±1.2, P=0.012) (Table 3). Complications tended to occur in patients with prior heart failure (44% vs 29%, P=0.078), with a blunt or no stump at the proximal end of the CTO (72% vs 53%, P=0.066), and with the presence of interventional collaterals (76% vs 59%, P=0.089). The following binary variables that met the threshold of P<0.10 were entered into a multivariable model: patient age >65, prior heart failure, prior valve procedure or surgery, CTO length ≥23 mm, blunt or no stump, and use of the retrograde approach (Table 4). Three of these variables were independently associated with the occurrence of periprocedural complications; points were assigned to each variable based on the magnitude of the odds ratio (+3 points for age >65 [OR=4.85, CI 1.82‐16.77], +2 points for length ≥23 mm [OR=3.22, CI 1.08‐13.89], and +1 point for use of the retrograde approach [OR=2.41, CI 1.04‐6.05]). These points were summed together to form the PROGRESS CTO complications score (Figure 1). The PROGRESS CTO complications score performed well on receiver‐operating characteristics (ROC) curve analysis for prediction of complications (AUC 0.758, 95% CI 0.665‐0.850) (Figure 2). The score had good calibration (Hosmer‐Lemeshow χ2=6.271, P=0.281). The score was used to stratify the population into risk groups: low risk (0‐2 points), intermediate risk (3‐4 points), and high risk (≥5 points). The proportions of the study population in each stratum of the score were 34% low risk; 33% intermediate risk; and 34% high risk. In the derivation set, the probability of periprocedural complications in each of these groups was: 0.4%, 1.8%, and 6.5%, respectively (Cochran‐Armitage test for trend P<0.001).
Table 3.
Univariable Analysis of Clinical, Angiographic, and Procedural Characteristics in the Derivation Set
| Variable | Overall | Complications | No Complications | P Value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, y | 66±10 | 72±9 | 65±10 | <0.001 |
| Age >65 y | 52 | 85 | 51 | <0.001 |
| Male | 84 | 86 | 84 | 0.81 |
| Body mass index, kg/m2 | 31±6 | 30±5 | 31±6 | 0.64 |
| Diabetes mellitus | 46 | 39 | 46 | 0.45 |
| Dyslipidemia | 95 | 96 | 95 | 0.72 |
| Hypertension | 90 | 89 | 90 | 0.88 |
| Prior myocardial infarction | 43 | 56 | 42 | 0.17 |
| Prior PCI | 64 | 57 | 65 | 0.41 |
| Prior CABG | 36 | 36 | 36 | 0.94 |
| Prior heart failure | 29 | 44 | 29 | 0.078 |
| Prior valve procedure | 4 | 14 | 4 | 0.003 |
| Cerebrovascular disease | 11 | 14 | 11 | 0.55 |
| Peripheral arterial disease | 15 | 14 | 15 | 0.88 |
| Chronic lung disease | 13 | 22 | 12 | 0.13 |
| Current tobacco use | 24 | 14 | 24 | 0.23 |
| eGFR, mL/min per 1.73 m2 | 72±25 | 65±21 | 72±26 | 0.042 |
| eGFR <60 mL/min per 1.73 m2 or currently on dialysis | 32 | 42 | 32 | 0.31 |
| Currently on dialysis | 3 | 7 | 3 | 0.17 |
| LV ejection fraction, % | 50±14 | 46±15 | 50±14 | 0.27 |
| LV ejection fraction <40% | 22 | 41 | 22 | 0.033 |
| Angiographic characteristics | ||||
| RCA target | 56 | 63 | 56 | 0.49 |
| LAD target | 23 | 15 | 23 | 0.29 |
| LCX target | 20 | 22 | 20 | 0.82 |
| Proximal segment target | 38 | 43 | 38 | 0.60 |
| Lesion length, mm | 30 (20‐40) | 30 (27‐56) | 30 (20‐40) | 0.10 |
| Length ≥20 mm | 77 | 88 | 76 | 0.15 |
| Length ≥23 mm | 66 | 88 | 65 | 0.013 |
| Proximal cap ambiguity | 31 | 40 | 31 | 0.34 |
| Side branch at proximal cap | 48 | 56 | 47 | 0.40 |
| Blunt/no stump | 54 | 72 | 53 | 0.066 |
| Distal cap at bifurcation | 31 | 24 | 31 | 0.45 |
| Good distal landing zone | 63 | 52 | 63 | 0.25 |
| Interventional collaterals | 60 | 76 | 59 | 0.089 |
| Moderate/severe calcification | 57 | 67 | 57 | 0.32 |
| Moderate/severe tortuosity | 36 | 37 | 36 | 0.87 |
| In‐stent restenosis | 14 | 14 | 14 | 0.97 |
| Prior CTO PCI attempt | 15 | 21 | 15 | 0.38 |
| J‐CTO score | 2.5±1.2 | 3.0±1.1 | 2.5±1.2 | 0.012 |
| PROGRESS CTO score | 1.3±1.0 | 1.2±1.0 | 1.3±1.0 | 0.84 |
| Procedural characteristics | ||||
| Antegrade wire escalation used | 26 | 25 | 26 | 0.90 |
| ADR used | 35 | 43 | 35 | 0.41 |
| Retrograde approach used | 41 | 71 | 40 | 0.001 |
| IVUS used | 43 | 30 | 43 | 0.22 |
| Prophylactic LVAD | 2 | 11 | 2 | 0.002 |
Values are % or mean±standard deviation or median (interquartile range). ADR indicates antegrade dissection reentry; CABG, coronary artery bypass grafting; CTO, chronic total occlusion; eGFR, estimated glomerular filtration rate; IVUS, intravascular ultrasound; J‐CTO score, Multicenter CTO Registry of Japan score; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; LV, left ventricular; LVAD, left ventricular assist device; MACE, major adverse cardiovascular event; PCI, percutaneous coronary intervention; PROGRESS CTO, Prospective Global Registry for the Study of Chronic Total Occlusion Intervention; RCA, right coronary artery.
Table 4.
Multivariate Logistic Regression in the Derivation Set
| Variable | Odds Ratio | 95% CI | P Value | Points |
|---|---|---|---|---|
| Age >65 y | 4.85 | 1.82 to 16.77 | 0.001 | +3 |
| Prior heart failure | NS | |||
| Prior valve procedure | NS | |||
| Length ≥23 mm | 3.22 | 1.08 to 13.89 | 0.035 | +2 |
| Blunt/no stump | NS | |||
| Retrograde approach used | 2.41 | 1.04 to 6.05 | 0.041 | +1 |
CI indicates confidence interval; NS, statistically nonsignificant.
Figure 1.
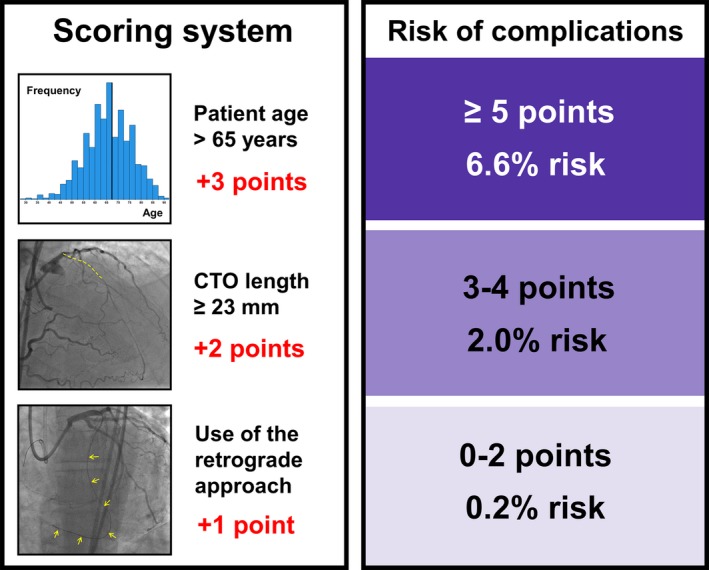
The PROGRESS CTO complications score. Summary of the PROGRESS CTO complications scoring system and risk groups for the overall cohort (validation cohort+derivation cohort). PROGRESS CTO indicates Prospective Global Registry for the Study of Chronic Total Occlusion Intervention.
Figure 2.
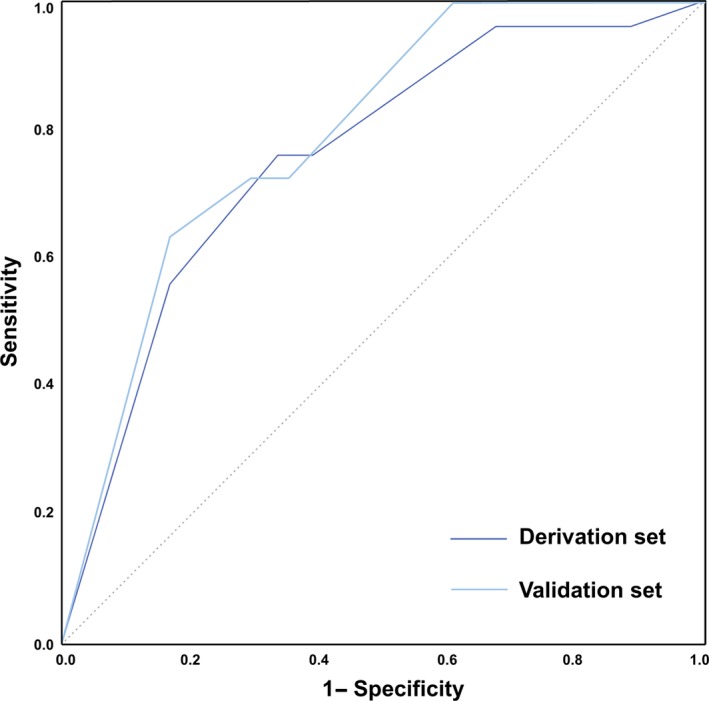
Comparison of the PROGRESS CTO complications score in the derivation and validation sets. The areas under the curves for the derivation and validation sets are 0.758 (95% CI 0.665‐0.850) and 0.793 (95% CI 0.682‐0.905), respectively. PROGRESS CTO indicates Prospective Global Registry for the Study of Chronic Total Occlusion Intervention.
Score Validation
The validation set included 504 randomly assigned CTO PCIs, in which 16 patients (3.2%) experienced periprocedural complications. There were no significant differences in clinical characteristics, angiographic characteristics, procedural characteristics, or outcomes between the derivation and validation groups, with the exception of prior failed CTO PCI, which occurred more frequently in the validation group than in the derivation group (20% vs 15%, P=0.020) (Table 1).
In the validation set and in the whole study cohort, stratification into risk groups using the PROGRESS CTO complications score was similar (test for trend P<0.001) (Figure 3). The AUC of the ROC for complications in the validation set was similar to that in the derivation set (0.793 [95% CI 0.682‐0.905]) (Figure 2). The score showed good calibration (Hosmer‐Lemeshow χ2=4.551, P=0.473). The difference between AUCs in the derivation and validation sets was Δ=0.035 (P=0.64).
Figure 3.
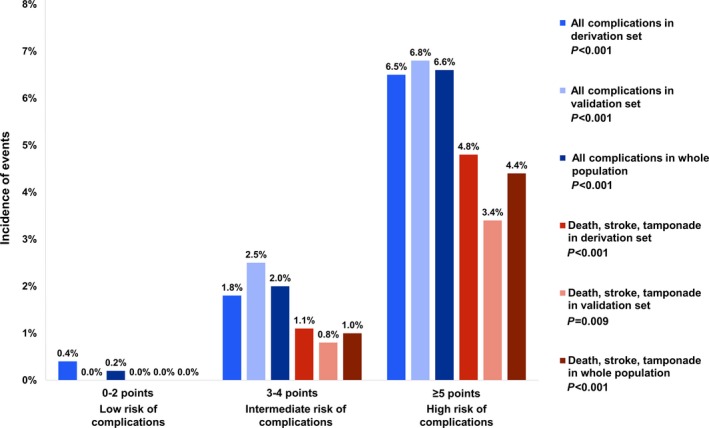
Incidence of periprocedural complications in strata of the PROGRESS CTO complications score. The incidence of all complications is represented by the blue bars; the incidence of the most serious complications (death, stroke, and tamponade requiring pericardiocentesis) is represented by the red bars. Differences in the incidence of events among strata were statistically significant in the derivation set, the validation set, and the whole study population. PROGRESS CTO indicates Prospective Global Registry for the Study of Chronic Total Occlusion Intervention.
In addition, the ability of the score to predict the most serious complications (death, stroke, and tamponade requiring pericardiocentesis) was assessed in the derivation and validation set using ROC analysis (AUC=0.833, 95% CI 0.681‐0.984); the score showed increasing incidence of these events at each stratum of the score (test for trend in derivation and validation sets P<0.001 and P=0.009, respectively) (Figure 3).
Sensitivity and specificity of the score analysis were calculated, showing stepwise alterations with change in PROGRESS CTO complications score (Figure 4).
Figure 4.
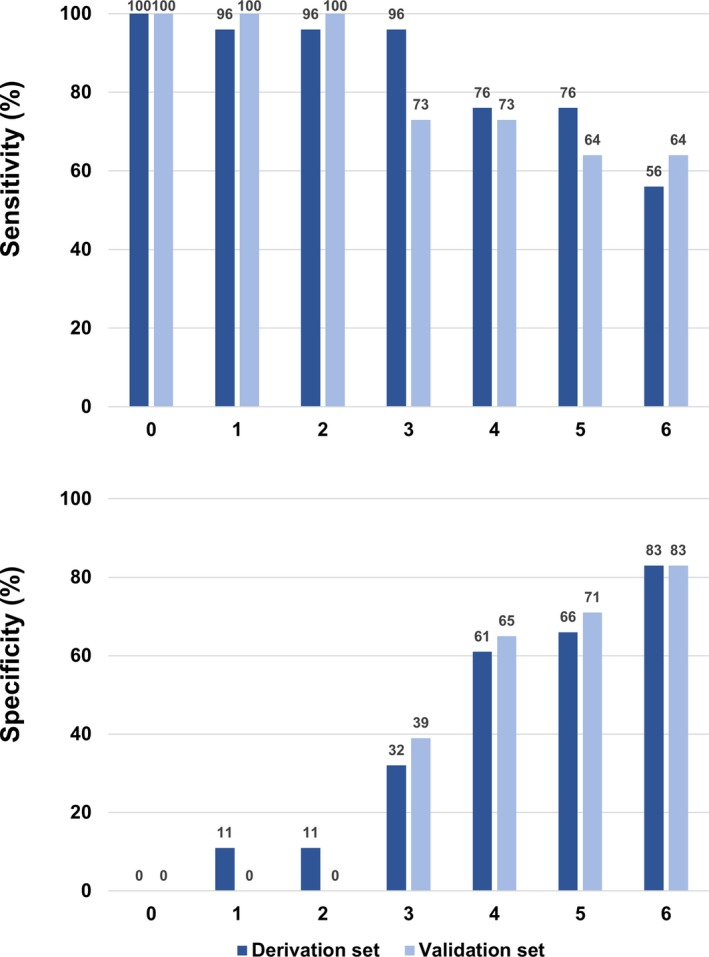
Sensitivity and specificity of the PROGRESS CTO complications score in the derivation and validation sets. PROGRESS CTO indicates Prospective Global Registry for the Study of Chronic Total Occlusion Intervention.
Comparison With Other CTO PCI Scores for Prediction of Complications
The performance of the PROGRESS CTO complications score for predicting occurrence of periprocedural MACE was compared with those of other CTO PCI scores. The J‐CTO score, the PROGRESS CTO score, and the CL score were compared with the PROGRESS CTO complications score for prediction of complications in the validation set (Figure 5). The AUCs were: PROGRESS CTO complications score 0.793 (95% CI 0.682‐0.905), J‐CTO score 0.676 (95% CI 0.560‐0.791), PROGRESS CTO score 0.501 (95% CI 0.379‐0.620), and CL score 0.776 (95% CI 0.669‐0.884), respectively. The differences in AUCs between the PROGRESS CTO complications score and other scores were J‐CTO score Δ=0.117 (P=0.15); PROGRESS CTO score Δ=0.292 (P<0.001); and CL score Δ=0.017 (P=0.83).
Figure 5.
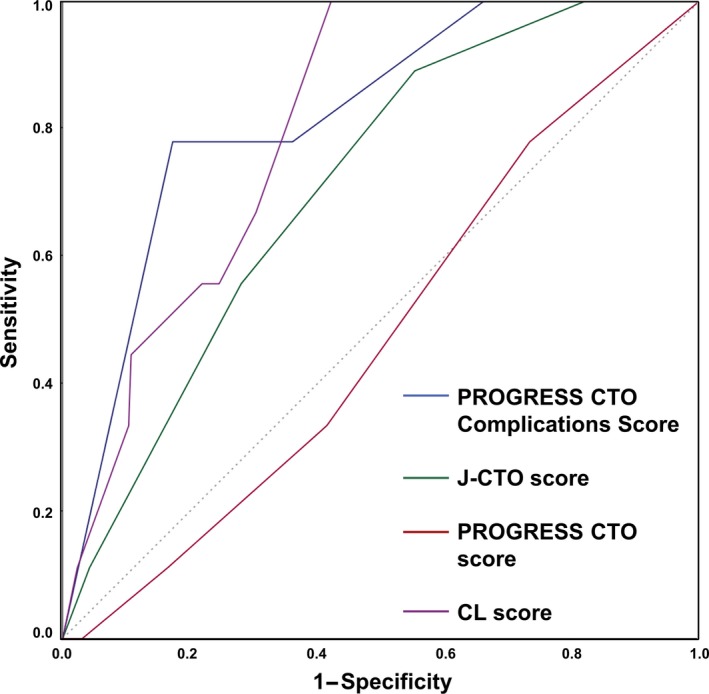
Comparison of the PROGRESS CTO complications score with other scoring systems. The PROGRESS CTO complications score is compared with the J‐CTO score, the PROGRESS CTO score, and the CL score in the validation set. The areas under the curves (AUCs) were PROGRESS CTO complications score 0.793 (95% CI 0.682‐0.905), J‐CTO score 0.676 (95% CI 0.560‐0.791), PROGRESS CTO score 0.501 (95% CI 0.379‐0.620), and CL score 0.776 (95% CI 0.669‐0.884), respectively. The differences in AUCs between the PROGRESS CTO complications score and other scores were as follows: J‐CTO score Δ=0.117, P=0.15; PROGRESS CTO score Δ=0.292, P<0.001; and CL score Δ=0.017, P=0.83. PROGRESS CTO indicates Prospective Global Registry for the Study of Chronic Total Occlusion Intervention.
Discussion
Our study demonstrates that a simple, 3‐component score can be used to determine the risk for periprocedural complications during CTO PCI. To the best of our knowledge, this is the first score specifically designed to predict complications during CTO PCI and may be of great value for procedural planning and discussion with the patient.
Several scores have been developed to predict the efficiency and success of CTO PCI,5, 6, 7 such as the CL score, which uses a combination of 6 clinical and angiographic characteristics to predict procedural failure.7 Although procedural failure is sometimes related to a complication,13 procedural outcomes may be related to distinct baseline characteristics. There is an association between technical outcome and complications (technical success among patients who experienced periprocedural complications was 64% vs 91% in those without complications), as some of the factors that may contribute to technical failure (angiographic factors such as calcification; clinical factors such as patient age) may also predispose to procedural complications. However, technical outcome is not known during planning for CTO PCI and thus was not included in the PROGRESS CTO complications score.
Although a failed attempt at CTO PCI is undesirable, some would consider a periprocedural complication potentially more undesirable. Hence, use of a simple, validated score specific for complications (in addition to scores predicting success and efficiency) can significantly aid physician and patient decision making by allowing accurate determination of the risk/benefit ratio for each procedure.22 In the context of other clinical factors, such a score could also help operators decide how aggressively to pursue angiographic success. Ultimately, an integrated approach that balances the desire for success with the risk for complications is critical for CTO PCI (or any PCI).
Older age was the most important predictor for complications in our study: the incidence of complications was 7% in patients aged >75 years versus 4% in patients aged 66 to 75 years versus 1% in patients aged ≤65 years (P<0.001). This finding is consistent with prior studies10, 23, 24 and is likely related to more complex coronary anatomy with increasing age, higher prevalence of tortuosity and calcification, higher prevalence of prior CABG, and possibly lower tolerance to inadvertent guidewire exits. Older patients are more likely to have diffuse aortic atheroma, predisposing them to strokes during coronary intervention. Moreover, older patients tend to have more comorbidities and likely have less reserve to tolerate a complication. Despite the association of age with the above comorbidities, age itself was a strong independent predictor of complications, indicating that these factors act synergistically to increase the risk of adverse outcomes.
CTO length was an independent predictor of complications, a finding that is in line with the CL score (≥20 mm length predictive of procedural failure)4 and other studies.13, 25 Longer lesion length may increase the complexity of the procedure and the need for advanced (and potentially more hazardous) crossing strategies, such as antegrade dissection/reentry and the retrograde approach.
Use of the retrograde approach was an independent predictor of complications in our cohort.26 Although judicious use of retrograde techniques is important for high technical success27, 28 and is integral to the hybrid algorithm,29 this specialized and potentially complex technique does carry increased risk for complications, such as donor vessel or collateral injury30 and donor vessel territory ischemia with increased risk for myocardial infarction.31, 32, 33 Device entrapment in collateral vessels may also occur.34 The retrograde approach also requires longer activated clotting time (ACT, >350 seconds) targets, potentially increasing the risk for bleeding.
The PROGRESS CTO complications score performed better than the J‐CTO and PROGRESS CTO score for predicting periprocedural MACE; however, the CL score (which was developed for predicting procedural success) performed comparably to the PROGRESS CTO complications score (difference in AUC 0.015), although it contains twice as many (6) input variables.
Limitations
Our study is limited by the observational design as well as by lack of independent angiographic and clinical event adjudication. Because quantitative coronary angiographic analysis was not performed, evaluation of angiographic characteristics may be subject to operator bias. Long‐term follow‐up data were not available for the entire study cohort; thus, no conclusions can be drawn about long‐term risk of major adverse cardiac events or the impact of periprocedural complications on longer‐term outcomes. The scoring model was developed using only cases with complete data, without imputation for missing values. The PROGRESS CTO registry contains data about procedures performed at high‐volume centers by highly experienced operators; as a result, conclusions drawn about this study cohort may not be broadly generalizable. Although only centers that contributed at least 40 cases are included in the analysis, some of these centers had more than 1 operator. Only variables collected as part of the registry were analyzed; some lesion and procedural characteristics that were not assessed could potentially be associated with the risk for complications. Additionally, data on contrast‐induced nephropathy were not collected. Because the incidence of complications was relatively low in our overall cohort (2.8%), our study may have limited power to identify predictors of complications. However, it is expected that in a larger cohort (or a cohort with higher incidence of complications), higher model diagnostic accuracy would result in increased statistical significance of the score components. External independent validation is needed to confirm these findings.
Conclusions
A simple score consisting of 1 clinical characteristic (age >65 years), 1 angiographic characteristic (CTO length ≥23 mm), and 1 procedural characteristic (use of the retrograde approach) may be useful to predict the occurrence of in‐hospital complications during CTO PCI. This tool can be used to assess patient risk and inform clinical decision‐making.
Sources of Funding
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1‐RR024982. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Disclosures
Dr Karmpaliotis reports speaker's fees from Abbott Vascular and MEDTRONIC; and consultant fees/honoraria from Asahi and Boston Scientific. Dr Alaswad reports consultant fees/honoraria from Asahi, Terumo, and Boston Scientific; and speaker's fees from Abbott Vascular. Dr Yeh reports a Career Development Award (1K23HL118138) from the National Heart, Lung, and Blood Institute; and consultant fees/honoraria from Boston Scientific and Gilead Sciences. Dr Jaffer reports consultant fees/honoraria from Abbott Vascular and Boston Scientific; and research grants from National Institutes of Health (HL‐R01‐108229), Kowa Ltd, Merck, and Siemens. Dr Mahmoud reports advisory board/consulting fees from Medtronic and Corindus; speaker's fees from Medtronic, Corindus, and Abbott Vascular; educational program fees from Abbott Vascular; and clinical events committee fees from St. Jude. Dr Wyman reports consultant fees/honoraria from Boston Scientific, Abbott Vascular, and Asahi. Dr Grantham reports consultant fees/honoraria from Abbott Vascular, Asahi, and Boston Scientific; and research grants from Boston Scientific, Asahi, Abbott Vascular, Medtronic, and Bridgepoint Medical. Dr Kandzari reports consultant fees/honoraria from Boston Scientific, Medicines Company, and Medtronic. Dr Lembo reports consultant fees/honoraria from Abbott Vascular, Boston Scientific, and Medtronic. Dr Garcia reports consulting fees from Medtronic and Surmodics. Dr Kirtane reports research grants from Boston Scientific, Medtronic, Abbott Vascular, Abiomed, St. Jude Medical, GlaxoSmithKline, and Eli Lilly. Dr Moses reports research grants from Abiomed. Dr Ali reports consultant fees/honoraria from St. Jude Medical, and AstraZeneca Pharmaceuticals; ownership interest/partnership/principal in Shockwave Medical and VitaBx Inc; and research grants from Medtronic and St. Jude Medical. Dr Rangan reports research grants from InfraRedX and Spectranetics. Dr Thompson reports salary from Boston Scientific. Dr Banerjee reports research grants from Gilead and the Medicines Company; consultant/speaker honoraria from Covidien and Medtronic; ownership in MDCARE Global (spouse); intellectual property in HygeiaTel. Dr Brilakis reports consultant fees/honoraria from Abbott Vascular, Asahi, Boston Scientific, Elsevier, Somahlution, St. Jude, and Terumo; research grants from Boston Scientific, and InfraRedX; and salary from Medtronic (spouse). The remaining authors have no disclosures to report.
Supporting information
Data S1. Contributing centers included in present analysis (>40 cases contributed each).
Acknowledgments
Study data were collected and managed using REDCap electronic data capture tools hosted at University of Texas Southwestern Medical Center. REDCap (Research Electronic Data Capture) is a secure, web‐based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
(J Am Heart Assoc. 2016;5:e004272 doi: 10.1161/JAHA.116.004272)
References
- 1. Patel VG, Brayton KM, Tamayo A, Mogabgab O, Michael TT, Lo N, Alomar M, Shorrock D, Cipher D, Abdullah S, Banerjee S, Brilakis ES. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta‐analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv. 2013;6:128–136. [DOI] [PubMed] [Google Scholar]
- 2. Christopoulos G, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Wyman RM, Lombardi WL, Menon RV, Grantham JA, Kandzari DE, Lembo N, Moses JW, Kirtane AJ, Parikh M, Green P, Finn M, Garcia S, Doing A, Patel M, Bahadorani J, Tarar MN, Christakopoulos GE, Thompson CA, Banerjee S, Brilakis ES. Application and outcomes of a hybrid approach to chronic total occlusion percutaneous coronary intervention in a contemporary multicenter US registry. Int J Cardiol. 2015;198:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galassi AR, Sianos G, Werner GS, Escaned J, Tomasello SD, Boukhris M, Castaing M, Buttner JH, Bufe A, Kalnins A, Spratt JC, Garbo R, Hildick‐Smith D, Elhadad S, Gagnor A, Lauer B, Bryniarski L, Christiansen EH, Thuesen L, Meyer‐Gessner M, Goktekin O, Carlino M, Louvard Y, Lefevre T, Lismanis A, Gelev VL, Serra A, Marza F, Di Mario C, Reifart N; Euro CTO Club . Retrograde recanalization of chronic total occlusions in Europe: procedural, in‐hospital, and long‐term outcomes from the multicenter ERCTO registry. J Am Coll Cardiol. 2015;65:2388–2400. [DOI] [PubMed] [Google Scholar]
- 4. Tanaka H, Morino Y, Abe M, Kimura T, Hayashi Y, Muramatsu T, Ochiai M, Noguchi Y, Kato K, Shibata Y, Hiasa Y, Doi O, Yamashita T, Morimoto T, Hinohara T, Fujii T, Mitsudo K. Impact of J‐CTO score on procedural outcome and target lesion revascularisation after percutaneous coronary intervention for chronic total occlusion: a substudy of the J‐CTO Registry (Multicentre CTO Registry in Japan). EuroIntervention. 2016;11:981–988. [DOI] [PubMed] [Google Scholar]
- 5. Morino Y, Abe M, Morimoto T, Kimura T, Hayashi Y, Muramatsu T, Ochiai M, Noguchi Y, Kato K, Shibata Y, Hiasa Y, Doi O, Yamashita T, Hinohara T, Tanaka H, Mitsudo K; J‐CTO Registry Investigators . Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J‐CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213–221. [DOI] [PubMed] [Google Scholar]
- 6. Christopoulos G, Kandzari DE, Yeh RW, Jaffer FA, Karmpaliotis D, Wyman MR, Alaswad K, Lombardi W, Grantham JA, Moses J, Christakopoulos G, Tarar MN, Rangan BV, Lembo N, Garcia S, Cipher D, Thompson CA, Banerjee S, Brilakis ES. Development and validation of a novel scoring system for predicting technical success of chronic total occlusion percutaneous coronary interventions: the PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) Score. JACC Cardiovasc Interv. 2016;9:1–9. [DOI] [PubMed] [Google Scholar]
- 7. Alessandrino G, Chevalier B, Lefevre T, Sanguineti F, Garot P, Unterseeh T, Hovasse T, Morice MC, Louvard Y. A clinical and angiographic scoring system to predict the probability of successful first‐attempt percutaneous coronary intervention in patients with total chronic coronary occlusion. JACC Cardiovasc Interv. 2015;8:1540–1548. [DOI] [PubMed] [Google Scholar]
- 8. Alaswad K, Menon RV, Christopoulos G, Lombardi WL, Karmpaliotis D, Grantham JA, Marso SP, Wyman MR, Pokala NR, Patel SM, Kotsia AP, Rangan BV, Lembo N, Kandzari D, Lee J, Kalynych A, Carlson H, Garcia SA, Thompson CA, Banerjee S, Brilakis ES. Transradial approach for coronary chronic total occlusion interventions: insights from a contemporary multicenter registry. Catheter Cardiovasc Interv. 2015;85:1123–1129. [DOI] [PubMed] [Google Scholar]
- 9. Christopoulos G, Karmpaliotis D, Alaswad K, Lombardi WL, Grantham JA, Rangan BV, Kotsia AP, Lembo N, Kandzari DE, Lee J, Kalynych A, Carlson H, Garcia S, Banerjee S, Thompson CA, Brilakis ES. The efficacy of “hybrid” percutaneous coronary intervention in chronic total occlusions caused by in‐stent restenosis: insights from a US multicenter registry. Catheter Cardiovasc Interv. 2014;84:646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christopoulos G, Karmpaliotis D, Wyman MR, Alaswad K, McCabe J, Lombardi WL, Grantham JA, Marso SP, Kotsia AP, Rangan BV, Garcia SA, Lembo N, Kandzari D, Lee J, Kalynych A, Carlson H, Thompson CA, Banerjee S, Brilakis ES. Percutaneous intervention of circumflex chronic total occlusions is associated with worse procedural outcomes: insights from a multicentre US registry. Can J Cardiol. 2014;30:1588–1594. [DOI] [PubMed] [Google Scholar]
- 11. Christopoulos G, Menon RV, Karmpaliotis D, Alaswad K, Lombardi W, Grantham A, Patel VG, Rangan BV, Kotsia AP, Lembo N, Kandzari D, Carlson H, Garcia S, Banerjee S, Thompson CA, Brilakis ES. The efficacy and safety of the “hybrid” approach to coronary chronic total occlusions: insights from a contemporary multicenter US registry and comparison with prior studies. J Invasive Cardiol. 2014;26:427–432. [PMC free article] [PubMed] [Google Scholar]
- 12. Christopoulos G, Menon RV, Karmpaliotis D, Alaswad K, Lombardi W, Grantham JA, Michael TT, Patel VG, Rangan BV, Kotsia AP, Lembo N, Kandzari DE, Lee J, Kalynych A, Carlson H, Garcia S, Banerjee S, Thompson CA, Brilakis ES. Application of the “hybrid approach” to chronic total occlusions in patients with previous coronary artery bypass graft surgery (from a contemporary multicenter US registry). Am J Cardiol. 2014;113:1990–1994. [DOI] [PubMed] [Google Scholar]
- 13. Sapontis J, Christopoulos G, Grantham JA, Wyman RM, Alaswad K, Karmpaliotis D, Lombardi WL, McCabe JM, Marso SP, Kotsia AP, Rangan BV, Christakopoulos GE, Garcia S, Thompson CA, Banerjee S, Brilakis ES. Procedural failure of chronic total occlusion percutaneous coronary intervention: insights from a multicenter US registry. Catheter Cardiovasc Interv. 2015;85:1115–1122. [DOI] [PubMed] [Google Scholar]
- 14. Christopoulos G, Wyman RM, Alaswad K, Karmpaliotis D, Lombardi W, Grantham JA, Yeh RW, Jaffer FA, Cipher DJ, Rangan BV, Christakopoulos GE, Kypreos MA, Lembo N, Kandzari D, Garcia S, Thompson CA, Banerjee S, Brilakis ES. Clinical utility of the Japan‐Chronic Total Occlusion score in coronary chronic total occlusion interventions: results from a multicenter registry. Circ Cardiovasc Interv. 2015;8:e002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karacsonyi J, Karatasakis A, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Wyman MR, Lombardi WL, Grantham JA, Kandzari DE, Lembo N, Moses JW, Kirtane AJ, Parikh MA, Green P, Finn M, Garcia S, Doing A, Patel M, Bahadorani J, Martinez Parachini JR, Resendes E, Rangan BV, Ungi I, Thompson CA, Banerjee S, Brilakis ES. Effect of previous failure on subsequent procedural outcomes of chronic total occlusion percutaneous coronary intervention (from a contemporary multicenter registry). Am J Cardiol. 2016;117:1267–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christakopoulos GE, Tarar MN, Brilakis ES. The impact of percutaneous coronary intervention of chronic total occlusions on left ventricular function and clinical outcomes. J Thorac Dis. 2015;7:1107–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nguyen‐Trong PK, Rangan BV, Karatasakis A, Danek BA, Christakopoulos GE, Martinez‐Parachini JR, Resendes E, Ayers CR, Luna M, Abdullah S, Kumbhani DJ, Addo T, Grodin J, Banerjee S, Brilakis ES. Predictors and outcomes of side‐branch occlusion in coronary chronic total occlusion interventions. J Invasive Cardiol. 2016;28:168–173. [PubMed] [Google Scholar]
- 18. Amsavelu S, Christakopoulos GE, Karatasakis A, Patel K, Rangan BV, Stetler J, Roesle M, Resendes E, Grodin J, Abdullah S, Banerjee S, Brilakis ES. Impact of crossing strategy on intermediate‐term outcomes after chronic total occlusion percutaneous coronary intervention. Can J Cardiol. 2016; pii: S0828‐282X(16)00060‐X. doi: 10.1016/j.cjca.2016.01.020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Joint ESC/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction , Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035.22923432 [Google Scholar]
- 20. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 21. McNeil BJ, Hanley JA. Statistical approaches to the analysis of receiver operating characteristic (ROC) curves. Med Decis Making. 1984;4:137–150. [DOI] [PubMed] [Google Scholar]
- 22. Brilakis E. Manual of Coronary Chronic Total Occlusion Interventions. Waltham, MA: Elsevier Inc; 2014. [Google Scholar]
- 23. Safley DM, House JA, Marso SP, Grantham JA, Rutherford BD. Improvement in survival following successful percutaneous coronary intervention of coronary chronic total occlusions: variability by target vessel. JACC Cardiovasc Interv. 2008;1:295–302. [DOI] [PubMed] [Google Scholar]
- 24. Andre R, Dumonteil N, Lhermusier T, Lairez O, Van Rothem J, Fournier P, Elbaz M, Carrie D, Boudou N. In‐hospital and long‐term outcomes after percutaneous coronary intervention for chronic total occlusion in elderly patients: a consecutive, prospective, single‐centre study. Arch Cardiovasc Dis. 2016;109:13–21. [DOI] [PubMed] [Google Scholar]
- 25. Noguchi T, Miyazaki MS, Morii I, Daikoku S, Goto Y, Nonogi H. Percutaneous transluminal coronary angioplasty of chronic total occlusions. Determinants of primary success and long‐term clinical outcome. Catheter Cardiovasc Interv. 2000;49:258–264. [DOI] [PubMed] [Google Scholar]
- 26. Karmpaliotis D, Karatasakis A, Alaswad K, Jaffer FA, Yeh RW, Wyman RM, Lombardi WL, Grantham JA, Kandzari DE, Lembo NJ, Doing A, Patel M, Bahadorani JN, Moses JW, Kirtane AJ, Parikh M, Ali ZA, Kalra S, Nguyen‐Trong PK, Danek BA, Karacsonyi J, Rangan BV, Roesle MK, Thompson CA, Banerjee S, Brilakis ES. Outcomes with the use of the retrograde approach for coronary chronic total occlusion interventions in a contemporary multicenter US registry. Circ Cardiovasc Interv. 2016;9:pii. e003434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karmpaliotis D, Michael TT, Brilakis ES, Papayannis AC, Tran DL, Kirkland BL, Lembo N, Kalynych A, Carlson H, Banerjee S, Lombardi W, Kandzari DE. Retrograde coronary chronic total occlusion revascularization: procedural and in‐hospital outcomes from a multicenter registry in the United States. JACC Cardiovasc Interv. 2012;5:1273–1279. [DOI] [PubMed] [Google Scholar]
- 28. Brilakis ES, Grantham JA, Thompson CA, DeMartini TJ, Prasad A, Sandhu GS, Banerjee S, Lombardi WL. The retrograde approach to coronary artery chronic total occlusions: a practical approach. Catheter Cardiovasc Interv. 2012;79:3–19. [DOI] [PubMed] [Google Scholar]
- 29. Brilakis ES, Grantham JA, Rinfret S, Wyman RM, Burke MN, Karmpaliotis D, Lembo N, Pershad A, Kandzari DE, Buller CE, DeMartini T, Lombardi WL, Thompson CA. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc Interv. 2012;5:367–379. [DOI] [PubMed] [Google Scholar]
- 30. Hashidomi H, Saito S. Dilation of the septal collateral artery and subsequent cardiac tamponade during retrograde percutaneous coronary intervention using a microcatheter for chronic total occlusion. J Interv Cardiol. 2011;24:73–76. [DOI] [PubMed] [Google Scholar]
- 31. Lo N, Michael TT, Moin D, Patel VG, Alomar M, Papayannis A, Cipher D, Abdullah SM, Banerjee S, Brilakis ES. Periprocedural myocardial injury in chronic total occlusion percutaneous interventions: a systematic cardiac biomarker evaluation study. JACC Cardiovasc Interv. 2014;7:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Werner GS, Coenen A, Tischer KH. Periprocedural ischaemia during recanalisation of chronic total coronary occlusions: the influence of the transcollateral retrograde approach. EuroIntervention. 2014;10:799–805. [DOI] [PubMed] [Google Scholar]
- 33. Stetler J, Karatasakis A, Christakopoulos GE, Tarar MN, Amsavelu S, Patel K, Rangan BV, Roesle M, Resendes E, Grodin J, Abdullah S, Banerjee S, Brilakis ES. Impact of crossing technique on the incidence of periprocedural myocardial infarction during chronic total occlusion percutaneous coronary intervention. Catheter Cardiovasc Interv. 2016;88:1–6. [DOI] [PubMed] [Google Scholar]
- 34. Utsunomiya M, Kobayashi T, Nakamura S. Case of dislodged stent lost in septal channel during stent delivery in complex chronic total occlusion of right coronary artery. J Invasive Cardiol. 2009;21:E229–E233. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Contributing centers included in present analysis (>40 cases contributed each).


